Abstract
Macrophages are cells that function as a first line of defence against invading microorganisms. One of the hallmarks of macrophages is their ability to become activated in response to exogenous ‘danger signals’. Most microbes have molecular patterns (PAMPS) that are recognized by macrophages and trigger this activation response. There are many aspects of the activation response to PAMPS that are recapitulated when macrophages encounter endogenous danger signals. In response to damaged or stressed self, macrophages undergo physiological changes that include the initiation of signal transduction cascades from germline-encoded receptors, resulting in the elaboration of chemokines, cytokines and toxic mediators. This response to endogenous mediators can enhance inflammation, and thereby contribute to autoimmune pathologies. Often the overall inflammatory response is the result of cooperative activation signals from both exogenous and endogenous signals. Macrophage activation plays a critical role, not only in the initiation of the inflammatory response but also in the resolution of this response. The clearance of granulocytes and the elaboration of anti-inflammatory mediators by macrophages contribute to the dissolution of the inflammatory response. Thus, macrophages are a key player in the initiation, propagation and resolution of inflammation. This review summarizes our understanding of the role of macrophages in inflammation. We pay particular attention to the endogenous danger signals that macrophages may encounter and the responses that these signals induce. The molecular mechanisms responsible for these responses and the diseases that result from inappropriately controlled macrophage activation are also examined.
Keywords: cancer, inflammation, inflammatory bowel disease, Macrophages, NF-κB, toll-like receptors
Introduction
Cornelius Celsus is generally regarded as the first person to describe the heat, redness, swelling and pain that accompany inflammation. This description was made around the time of the birth of Christ. We now know that inflammation is a complex biological response that involves many different cell types and mediators, which exists to protect the host by eliminating or sequestering the injurious stimuli. In most tissue, granulocytes are among the first cells to migrate to the sites of inflammation. These cells are endowed with a potent antimicrobial armamentarium that makes them particularly adept at dispensing with the inciting stimuli. They are also quite active in recruiting other immune cells to the lesion, such as monocyte-derived macrophages. These cells contribute to the sustained localized elaboration of toxic mediators that comprises the inflammatory focus. These metabolites can include lipid mediators, oxygen and nitrogen radicals, chemokines and cytokines. It can generally be surmised that the stronger the stimuli, the greater the host response will be. Eventually the insult will be eliminated and the process of resolution will ensue. Monocyte recruitment into the lesion continues long after the resolution phase has started, and this continued recruitment is a critical component of the resolution phase.
The resolution of acute inflammation is a highly coordinated process controlled by many distinct mechanisms. Despite the fact that the synthesis of many inflammatory cytokines is transient and the half-lives of mRNAs encoding these inflammatory cytokines are generally very short [1], the simple depletion of pro-inflammatory mediators is not sufficient to curtail inflammation. The active removal of granulocytes and their secreted products must also occur. The most important mechanism to locally eliminate inflammatory cells is via phagocytosis by recruited monocyte-derived macrophages. Monocytes recognize cells undergoing apoptosis and efficiently and rapidly remove them. This process not only eliminates inflammatory cells, but the removal process itself can result in the production of TGFβ by macrophages [2]. This cytokine contributes to the resolution of inflammation and to the initiation of wound healing, by inducing the production of extracellular matrix components. The production of other anti-inflammatory mediators in the lesion by tissue macrophages can also contribute to the resolution of inflammation. IL-10 is an important anti-inflammatory cytokine that inhibits the synthesis and action of many inflammatory cytokines [3,4]. Paradoxically, TNF, a major macrophage-derived inflammatory cytokine, can also contribute to the resolution of inflammation by virtue of its ability to bind to the death-domain-containing TNF-R1, thereby inducing cellular apoptosis [5].
When these immunomodulatory processes are intact, the inflammatory process is self-restricting and will successfully resolve without causing excessive tissue damage. When failures in these processes occur, chronic inflammation with consequent tissue-damage can ensue. The list of inflammatory diseases in which inflammatory macrophages are key mediators is long and diverse, and includes inflammatory bowel disease, rheumatoid arthritis, asthma and even atherosclerosis. Understanding the roles of macrophages in the induction of the inflammatory response and in the resolution of this response has the potential to lead to new approaches to the design of therapeutics that can be added to our arsenal of weapons to combat inflammatory diseases.
Monocyte-derived macrophages
Monocytes differentiate from progenitor cells in the bone marrow prior to their release into the blood stream. Monocytes are relatively long-lived cells that can differentiate further into macrophages or dentritic cells (DCs) as they migrate into tissue. In simplified terms, monocyte migration into tissue can occur in response to an inflammatory stimulus, or it can be a constitutive process that occurs at a much lower level in the absence of any apparent cues. There is now ample evidence that there exist functionally distinct subpopulations of monocytes that can give rise to tissue macrophages [6].
In the absence of inflammation, tissue-resident macrophages are generally differentiated from a Gr-1lowCX3CR1high CCR2−CD62L− subpopulation of murine monocytes [6,7]. These cells correspond functionally to the CD14+CD16+CX3CR1high CCR2− CD62L− subpopulation of human monocytes. The constitutive migration of these ‘resident’ monocytes into tissue under steady state can not only function as sensors to monitor inflammatory or other danger signals as they patrol the blood vessels [8], but also contribute to the maintenance of macrophages in tissue. However, a substantial contribution to the maintenance of the local tissue macrophage populations is also made by localized tissue-resident colony-forming cells. Together, local progenitor cells and newly infiltrating ‘resident’ monocytes maintain a relatively constant number of cells in tissue during steady state.
During inflammation, the dynamics of macrophage accumulation is dramatically changed and a substantial contribution to the expansion of tissue macrophages is made from newly migrating immature blood monocytes, which rapidly differentiate into macrophages. These newly migrating ‘inflammatory’ monocytes are typically a Gr-1highCX3CR1lowCCR2+ CD62L+ subpopulation in mice or CD14highCD16− CX3CR1lowCCR2+CD62L+ monocytes in humans [6,7]. In the murine system, they appear to be able to differentiate not only into macrophages but also into inflammatory DCs, including those which can produce TNFα and inducible nitric oxide [9]. Based on CD14, CD16 and CD64 expression, an additional subset of monocyte populations has been described in humans [10]. These CD14+CD16+CD64+ cells may represent an intermediate phenotype between monocytes and DCs with specific immunoregulatory functions [10–12].
In tissue, macrophages can undergo profound physiological changes in response to the combination of cytokines and inflammatory stimuli. These physiological changes allow these cells to much more efficiently kill and degrade microbes. These macrophages also up-regulate MHC Class II and the co-stimulatory molecules CD80 and CD86 [13–15]. Therefore, in lesions both macrophages and DC can function as antigen-presenting cells to propagate local adaptive (antigen-specific) immune responses. For many years it was assumed that all activated macrophages shared similar capabilities, pertaining to their enhanced ability to elaborate toxic mediators and kill microbes. These cells are now called ‘classically activated’ macrophages [12]. We now appreciate that macrophages are remarkably plastic cells that can respond to a variety of different stimuli and undergo distinct physiological changes in response to different environmental cues. As the environment in which a macrophage resides changes, so too will the functional and physiological properties of these cells change. This plasticity is particularly relevant to the resolution of inflammation, where the macrophages that had previously been the major contributor to the inflammatory process can later participate in its resolution.
Macrophage activation — the basics
It is generally accepted that classically activated macrophages develop in response to two signals. The first signal is IFNγ and the second signal is provided by what has been termed ‘pathogen-associated molecular patterns’ (PAMPS) [16]. Early in the immune response, IFNγ can be produced by innate immune cells, such as NK or NKT cells, but as the immune response develops the most important source of IFNγ becomes the antigen-specific Th1 T cells [17]. In most descriptions of macrophage activation, the second signal is provided by a microbe that expresses one or more PAMPs, which stimulate macrophage activation through toll-like receptors (TLRs). The term PAMP is actually a misnomer, since many of these molecules can be expressed on host cells as well as on microbes. The prototypical PAMPs, which stimulate macrophage activation responses, are LPS or LTA from the surface of Gram-negative or -positive bacteria, respectively. These stimuli generally induce the production of TNF by macrophages. The combination of TNF and IFNγ results in optimal macrophage activation. These classically activated macrophages become strongly microbicidal and they are important immune effector cells [18].
There have been myriad reviews on the toll-like receptors (TLRs) that recognize the various PAMPS and the signalling pathways that emanate from these receptors. The genes that are activated subsequent to receptor ligation and the transcription factors that mediate this induction have also been reviewed [19]. The present review focuses on endogenous danger signals that can replace the PAMPS and signal a similar macrophage activation response. In order to understand how these endogenous stimuli work, it is necessary to provide some brief background about the pathways that allow macrophages to respond to exogenous stimuli.
‘Sensors’ to detect exogenous danger signals: pattern-recognition receptors (PRRs)
PAMPs are detected by the immune cells via a set of limited number of germ line-encoded pattern recognition receptors (PRRs) [20–22] (Table 1). These receptors are essential for the host to initiate an immune response. PPRs are not limited to the plasma membrane of immune cells. They can also be found in the extracellular space (secreted), on intracellular vesicles or in the cytoplasm (Table 2). The secreted PRRs can mediate opsonization, activation of complement pathways and the transfer of PAMPs to other PRRs. The intracellular PRRs can induce cellular apoptosis and activate cytokine secretion. PRRs on the cell membrane may contribute to pathogen uptake by phagocytosis and they may even promote phagolysosomal maturation. They may facilitate the presentation of antigen to immune cells and, importantly, they can initiate signalling events that lead to cellular activation.
Table 1.
Membrane-bound innate receptors and their ligands
| Receptors and their ligands | Locations | Adaptors | |
|---|---|---|---|
| TLRs and ligands | |||
| TLR1 | Tri-acyl lipoprotein | Cell surface | MyD88/Mal |
| TLR2 | Gram-positive peptidoglycan and lipoteichoic acid; fungi zymosan; mycobacteria lipoarabinomannan; Trypanosoma cruzi glycoinositolphospholipids; Treponema maltophilum glycolipids; Neisseria porins; viral glycoproteins |
Cell surface | MyD88/Mal |
| TLR3 | Virus double-stranded RNA; poly I : poly C; poly I [150] | Endosomal | TRIF |
| TLR4 | Gram-negative LPS; MMTV envelope proteins; RSV fusion proteins; toxol | Cell surface | MyD88/Mal/Trif/Tram |
| TLR5 | Bacteria flagellin | Cell surface | MyD88 |
| TLR6 | Mycoplasma di-acyl lipopeptides | Cell surface | MyD88/Mal |
| TLR7 | Virus single-stranded RNA; small synthetic compounds—bropirimine, imidazoquinoline; loxoribine |
Endosomal | MyD88 |
| TLR8 | Virus single-stranded RNA; imidazoquinoline | Endosomal | MyD88 |
| TLR9 | Bacterial and viral unmethylated CpG DNA; malaria pigment haemozoin | Endosomal | MyD88 |
| TLR10 | Unknown (human only) | Cell surface | Unknown |
| TLR11 | Toxoplasma gondi profilin-like protein [151] | Cell surface | MyD88 |
| TLR12 | Unknown (mice only) | Unknown | Unknown |
| TLR13 | Unknown (mice only) | Unknown | Unknown |
| C-type lectin receptors | |||
| MR* | Ligands bearing mannose, fucose, or N-acetyl glucosamine | Cell surface | Cdc42, Rho [152] |
| DC-SIGN | ICAM2/3; HIVgp120; M. tuberculosis ManLAM | Cell surface | Unknown |
| Dectin-1 | Fungus β-glucans; zymosan | Cell surface | Src; Syk; Tec [153] |
| Scavenger receptors | |||
| CD36 | Apoptotic cells; collagen types I and IV; erythrocyte membrame protein 1; oxidized LDL; Plasmodium falciparum GPI [154]; platelet-agglutinating protein p37; thrombospondin |
Cell surface | Lyn; MEKK2 [155] |
| MARCO | LPS, LTA (lipoteichoic acid); acetylated LDL; uteroglobin-related protein 1 | Cell surface | Unknown |
| SRA* | Apoptotic cells; LPS; LTA; oxidized LDL; polyribonucleic acids | Cell surface | Unknown |
| Complement receptors | |||
| CR3, CR4 | iC3b; β-glucan; fibrinogen | Cell surface | Cytohesin-1 [156] |
| C5aR | C5a | Cell surface | Unknown |
| gC1qR | C1q | Cell surface | CD59 [157] |
Table 2.
Cytoplasmic pattern recognition receptors
| Receptors and major ligands | Live infectious bacteria and virus | Leukocytes |
|---|---|---|
| NLR family | ||
| CARD subfamily | ||
| NOD1 GM-tripeptide; meso-lanthionine; meso-DAP; |
Chlamydia spp.; enteroinvasive E. coli; |
Monocytes; macrophages; |
| γ –D-Glu-DAP; | Helicobacter pylori; Listeria monocytogenes; | DCs* |
| FK156 (d-lactyl-l-ala-γ-Glu-meso-DAP-Gly); |
Pseudomonas spp.; and Shigella flexneri |
|
| FK565 (Heptanoly-γ-Glu-meso-DAP-d-ala) | ||
| NOD2 M-TRILys (MurNAc-L-Ala-γ-d-Glu-l-Lys); MDP | L. monocytogenes; Salmonella flexneri; | Monocytes; mast cells; DCs; |
|
S. typhimurium; and Streptococcus pneumoniae |
granulocytes | |
| IPAF Bacterial flagellin from Coxiella, Legionella and Salmonella | S. typhimurium | Monocytes; macrophages; DCs; B cells |
| Pyrin subfamily (14 NALPs) | ||
| NALP1 LeTx (anthrax lethal toxin); MDP | Staphylococcus aureus and L. monocytogenes | Monocytes; DCs; B and T cells |
| NALP3 ATP; bacterial mRNA; maitotoxin; nigericin; uric acid crystal; R848 | Monocytes; DCs; T cells | |
| BIR subfamily | ||
| NAIP Flagellin from Bacillus, Legionella and Salmonella | Legionella pneumophila | |
| Macrophages | ||
| RNA helicases | ||
| RIG-1 Uncapped 5′-triphosphate-RNA | Newcastle disease virus, Sendai virus, influenza virus, vesicular stomatitis virus, Japanese encephalitis virus |
Conventional DCs |
| MDA5 Encephalomyocarditis virus dsRNA; poly (I :C) | Encephalomyocarditis virus; picornaviruses; Thyler’s virus and Mengo virus |
Conventional DCs |
Non-leukocytes expressing NODs include epithelioid and fibroblastoid cells. dsRNA-dependent protein kinase (PKR) and R proteins are not listed [158].
Toll-like receptors (TLRs)
The TLRs are the most well-characterized PRRs and, because these receptors play a major role in the recognition of both endogenous as well as exogenous stimuli [23], their biology will be briefly reviewed. There are some 13 TLRs found in mice and 11 in humans (Table 1). TLRs are expressed predominantly, but not exclusively, by professional antigen-presenting cells [24]. A portion of the cytoplasmic portion of TLRs was shown to have homology with the interleukin (IL)-1 receptor family [25], and this domain was named the Toll/IL-1 receptor (TIR) domain. TIR domains are conserved among all TLRs except for TLR3, and this domain associates with other TIR domain-containing molecules, including the adaptors that mediate TLR signalling. The four best-characterized TIR domain-containing activating adaptors include myeloid differentiation primary response gene 88 (MyD88), TIR domain-containing adaptor protein (TIRAP)/MyD88-adaptor-like (Mal), TIR domain-containing adaptor inducing IFNβ (TRIF)/TIR domain-containing adaptor molecule-1 (TICAM-1) and TRIF-related adaptor molecule (TRAM) [24]. All of these proteins are expressed in myeloid cells and all play important roles in activating innate signalling events. MyD88 is an essential adaptor that is used by all TLRs except TLR3. The early production of inflammatory cytokines is largely dependent on the presence of MyD88. MyD88-knockout mice exhibit defective production of IL-12 and TNFα in response to TLR activation. TIRAP/Mal is required for TLR4-and MyD88-dependent responses and signals primarily via TLR2. TRIF/TICAM-1 mediates responses to ligation of TLR3 and it can also mediate MyD88-independent TLR4 responses. TRAM is also required for TLR4-mediated responses independent of MyD88. The fifth TIR domain-containing protein, sterile and HEAT/Armadillo motifs-containing protein (SARM), has been shown to negatively regulate TRIF-dependent TLR signalling [26]. Recently, it has been reported that SARM is primarily expressed in neurons and appears to play a regulatory role in neuronal survival via interaction with JNK3 in mitochondria [27].
Following ligand binding to TLRs, the engagement of the IRAKs by MyD88 causes the activation of a complex containing TNF receptor-associated factor (TRAF)-6 and TAB2. This complex activates TGFβ-activated kinase (TAK)-1. TAK-1 then serves as a branch point, leading to the activation of both NF-κB and mitogen-activated protein kinases (MAPK) signalling pathways. The recruitment of specific adaptor proteins to various TLRs may determine the character and magnitude of cellular signalling in response to TLR activation.
Lipopolysaccharide (LPS), which binds to TLR4, is one of the most well-studied PAMPs. TLR4 requires MD-2 to recognize the active moiety of LPS. MD-2 is expressed as a dimer with TLR4 on the surface of immune cells. Lipid A and its two derivatives, monophosphoryl lipid A and lipid IVa, bind directly to MD-2. Each lipid molecule binds MD-2 and induces a unique conformational change in TLR4, leading to the formation of specific docking sites at cytoplasmic regions of TLR4 for adaptors and subsequent activation of specific signalling pathways. Lipid A engagement results in activation of both MyD88-dependent and TRIF-dependent (MyD88-independent) signalling events [28], whereas monophosphoryl lipid A causes primarily activation of TRIF-mediated signalling pathways [29]. Binding of Lipid IVa to MD-2–TLR4 complex inhibits signalling events mediated by MyD88 and TRIF [30].
Preferential activation of selective signalling pathways can also be influenced by receptors that associate with TLRs. Recently, Coyle and colleagues showed that class A CpG-containing oligodeoxynucleotides can form a complex with HMGB1 (see below) and signal through both TLR9 and receptor for advanced glycation end-products (RAGE), a receptor for HMGB1 [31]. This signalling is distinct from that induced by TLR9 alone. Thus, TLR signalling can variously depend on the association of receptors with cytosolic adaptors, on the oligomerization of TLRs with themselves, or the co-ligation of TLRs with other receptors to influence the quality and quantity of TLR signalling.
The NOD-like receptor (NLR) family of receptors
In addition to TLRs, other PRRs can play similar roles to detect the danger signals in the cytoplasm. The NLR family is the largest identified family of intracellular PRRs, which include both nucleotide-binding oligomerization domains (NODs) and neuronal apoptosis inhibitory protein/MHC class II transcription activator/incompatibility locus protein form Podospora anserina/telemerase-associated protein (NALPs/NACHT). Three structural domains are characteristics of these intracellular PRRs. (a) a LRR domain that is homologous to those found in TLRs can be found at the C-terminus. Similar to TLRs, this domain is essential for ligand recognition. (b) The central localized NACHT domain contains ATPase activity; its ologmerization is necessary and essential for the N-terminal effector domain to transduct signals. (c) The N-terminal effector domain can be a PYRIN domain, a caspase recruitment domain (CARD), or a baculovirus inhibitor of apoptosis protein repeat (BIR) domain. Based on the N-terminal effector domains, the NLR family of receptors can be classified into three major subfamilies: the CARD subfamily (LRR, NACHT and CARD domain-containing proteins), the pyrin subfamily (LRR, NACHT and PYD-containing proteins) and the BIR subfamily (LRR, NACHT and BIR domain-containing protein) (Table 2).
The molecular mechanisms for NLR activation are incompletely understood. It has been suggested that they may be similar to what has been described for the activation of Apaf-1 of apoptosome [32]. Normally, the NLR proteins are present in the cytoplasm in an inactive and auto-repressed conformation. Upon binding to a PAMP, the inactive NLR protein changes conformation to expose the NACHT oligomerization domain. Through oligomerization, NLR proteins form hexamers or heptamers, while the N-terminal effector domains, such as PYDs or CARDs, become accessible for homophilic interaction with downstream PYD-or CARD-containing effector molecules, including adaptor proteins, caspases or kinases.
In the past few years, much attention has centred on deciphering the signalling pathways initiated by NOD1 and NOD2 [33]. NOD1 is encoded by the caspase-recruitment domain 4 (CARD4) gene, whereas NOD2 is encoded by the caspase-recruitment domain 15 (CARD15) gene. NOD1 and NOD2 are expressed in APCs, and also on epithelial cells, where they may play an important role in the pathogenesis of gastrointestinal diseases. The NOD1 and NOD2 ligands are the PGN (peptidoglycan)-derived peptides d-glutamyl-meso-diaminopimelic acid (iE-DAP) and muramyl dipeptide (MDP), respectively. MDP is present in the PGN derived from both Gram-positive and Gram-negative bacteria. Thus, NOD2 functions as a general sensor of most, if not all, bacteria, whereas NOD1 senses primarily products yielded from Gram-positive bacteria. In Drosophila, PGN-recognition proteins (PGRPs) are required to recognize bacterial PGN. PGRP-SA and PGRP-SD identify lysine-containing PGN that is produced by Gram-positive bacteria, whereas PGRP-LC and PGRP-LE recognize meso-diaminopimelic acid-containing PGN produced by Gram-negative bacteria [34]. PGRP-LE has no transmembrane domain and can be found in the cytoplasm, but it can function as an extracellular PGN receptor with function analogous to CD14 to present PGN to PGRP-LC. As an intracellular receptor, PGRP-LE may directly recognize intracellular PGN and activate Imd pathway independently of PGRP-LC [35].
Upon oligomerization of NOD1 or NOD2, the recruitment of the serine/threonine kinase receptor-interacting serine/threonine kinase (RICK; also known as RIP2 or CARDIAK) occurs through a homophilic CARD–CARD interaction. RICK, in turn, is cross-activated to transduce downstream signals leading to NF-κB activation. This NF-κB activation appears to be exclusively mediated by RICK, since transfection of RICK-deficient fibroblasts with constructs encoding NOD1 or NOD2 results in severely defective NF-κB activation [36]. It is noteworthy that RICK may also be a downstream molecule for TLR2 and TLR4, since RICK-deficient macrophages have reduced production of cytokines following TLR2 or TLR4 activation by extracellular agonists [36]. The kinase activity of RICK is not directly essential for NF-κB activation. Instead, upon activation by NOD2, RICK induces K63-linked polyubiquitylation of IKKγ /NEMO at a unique ubiquitylation site (Lysine-285) [37]. It has been known that K63-linked polyubiquitylation is associated with activation of the NF-κB pathway [38]. The interaction of RICK and IKKγ /NEMO leads to translocation of NF-κB transcription factors to the nucleus. The effect of RICK on K63-linked polyubiquitylation may be due to activation of an E3 ubiquitin ligase or inhibition of an enzyme that can deubiquitinate K63-linked polyubiquitinated proteins. In addition to RICK, gene associated with retinoid-IFN-induced mortality 19 (GRIM19) is another intracellular molecule that has been associated with optimal NF-κB activation induced by NOD2 but not NOD1 [39].
Similar to TLR-mediated signalling cascades, NOD1 and NOD2 activation can also lead to activation of MAPK pathways. Activation of ERK and p38 MAPK has been shown to occure in wild-type macrophages, but not in NOD2-deficient macrophages stimulated by MDP, a NOD2 ligand [39,40]. JNK (JUN N-terminal kinase) activation has been reported following NOD1 activation by invasive Shigella flexneri [41]. The detailed molecular mechanism of NOD-mediated activation of the MAPK pathway remains unclear.
Similar to TLR receptor cooperativity described above, NLRs can exhibit cross-talk with TLRs. For example, PGN is a ligand for cell surface TLR2, but upon uptake by macrophages, PGN can be processed to yield MDP, a ligand for cytosolic NOD2. Interestingly PGN induces more IL-12 in NOD2-deficient macrophages than wild-type macrophages [42]. This inhibitory effect of NOD2 on IL-12p40 and p35 may be linked to its effect on nuclear translocation of NF-κB. In macrophages deficient in NOD2, activation-induced translocation of NF-κB is more pronounced relative to wild-type cells [42]. Therefore, the responses to PGN by macrophages can be a combined effect of activation (and in some cases inhibition) via both TLR2 and NOD2.
RIG-I-MDA5 family of CARD helicases
During viral infections, cellular cytoplasmic sensors will signal the presence of viral RNA in the cell cytoplasm. Retinoid acid-inducible protein I (RIG-1) and melanoma differentiation associated gene-5 (MDA5) are two cytoplasmic CARD helicases that have this capability. Both proteins have similar structural features, with a DExD/H box RNA helicase domain that is responsible for ligand recognition and two CARDs that are essential for downstream signalling. RIG-1, but not MDA5, can recognize uncapped 5′-triphosphate RNA (termed 3pRNA) present in viruses that can only be generated by viral polymerases, while MDA5 is the principle cytoplasmic receptor for synthetic poly(I : C) [43,44]. RIG-I is critical in response to RNA viruses, including paramyxoviruses, influenza virus and Japanese encephalitis virus, whereas MDA5 is essential for picornavirus detection [45]. In addition to the direct effect by viral RNA, both RIG-I and MDA5 can recognize small self-RNA molecules that are generated by anti-viral endoribonuclease, RNase L [46]. Briefly, viral RNAs can activate OSA (2′-5′-oligoadenylate synthestase) yields 2–5A (2′,5′-linked oligoadenylate) from ATP. 2–5A activates RNase L to produce small RNA cleavage products from cellular self-RNA and some viral RNA. Clearly, this unique process will be beneficial for the host to initiate and amplify antiviral response and also make RNase L as a possible drug target for antiviral therapy. RIG-I may have a role in controlling inflammatory bowel disease (IBD). RIG-I-null mice develop a colitis-like phenotype with increasing susceptibility to dextran sulphate sodium-induced colitis, possibly through downregulation of Gαi2 and disrupted T cell homeostasis [47].
The adaptor protein for RIG-1 and MDA5 is named MAVS (VISA, CARDIF or IPS-1) [48]. MAVS contains a CARD domain at the N-terminus and a mitochondiral localization domain at the C-terminus. MAVS is recruited and bound to RIG-1 or MDA5 via CARD–CARD interactions. RIG-1-or MDA5-mediated signalling cascade and anti-viral responses are compromised in MAVS-deficient mice, confirming the essential adaptor role of MAVS for RIG-I and MDA5 activation. MAVS knockout mice are highly susceptible to systemic EMCV infection (MDA5-mediated) or intranasal VSV infection (RIG-I-mediated). MAVS-deficient cDCs fail to produce type I IFNs or proinflammatory cytokines in response to infections with RNA viruses. TLR-mediated type I IFN production, however, is normal in MAVS-deficient cells, indicating that there are two independent pathways for type I IFN production.
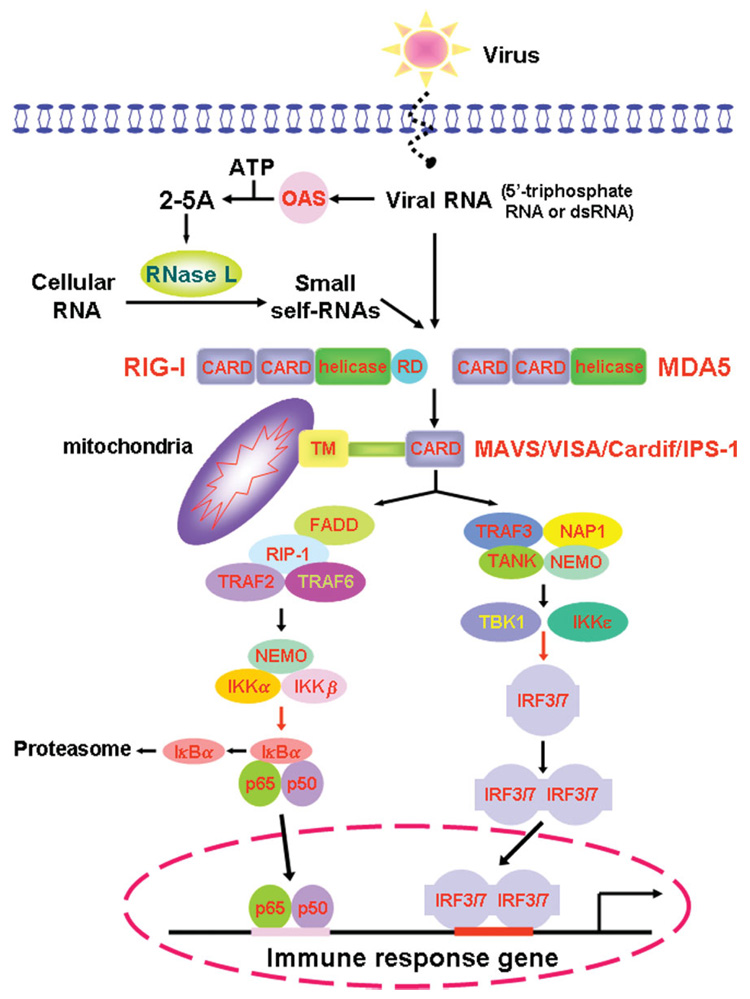
Activation of IRF3 or IRF7 and the subsequent production of type I IFNs, and activation of NF-κB and the production of inflammatory cytokines have been observed upon activation of RIG-I and MDA5. TRAF3 has been shown to interact with MAVS, and be essential for MAVS-mediated IFNα production and anti-viral responses. On the other hand, MAVS-mediated activation of IKK complex can lead to NF-κB activation. TBK1/IKKi appear to be the convergence point for TLRs via the TLR–TRIF pathway and CARD helicases via MAVS–TRIF interaction. This provides another example of the cross-talk between the TLR and CARD signalling pathways. A brief schematic presentation of RIG-1- and MDA5-mediated signalling pathway is shown in Figure 1.
Figure 1. Overview of RIG-I- and MDA5-mediated signalling pathway.
Viral ssRNA with 5′-triphosphate group and dsRNA are detected by the cytoplasmic pathogen recognition receptors, RIG-I and MDA5, respectively. Viral RNAs also activate 2′,5′-oligoadenylate synthetase (OAS) to synthesize 2′,5′-linked oligoadenylate (2–5A) from ATP. 2–5A activates RNase L to cleavage cellular RNAs to yield small self-RNA molecules, which are also recognized by RIG-I and/or MDA5. RIG-I and MDA5 contain N-terminal CARD and C-terminal RNA helicase motifs. In addition, RIG-I contains a repressor domain (RD). The RNA-mediated interaction induces conformational changes of RIG-1 and MDA5, which leads to the CARD–CARD interaction of RIG-I and MDA5 with their adaptor, CARD-containing MAVS (also known as VISA, Cardif and IPS-1) in the mitochondrial membrane. These events relay the signal through activation and translocation of NF-κB p65–p50 heterodimer and IRF3 as well as IRF7 into the nucleus to activate downstream genes that encode α- and β-interferon. The exact roles of TRAF2, TRAF6, FADD and RIP1 in this pathway are yet to be defined. Activation of RIG-I by the ubiquitin ligase TRIM25-mediated lysine-63-linked ubiquitination is not shown, neither is a MAVS-mediated MAP kinase cascade. Red arrows indicate phosphorylation
Endogenous danger signals
In addition to the exogenous danger signals that can be introduced into the body, the immune system can also sense distress cues released from damaged or stressed tissue [49]. Thus, the immune system can not only discriminate ‘self from non-self’ but also ‘healthy from damaged self’. The host has evolved a system of surveillance and sensing that is coupled to inflammation and tissue repair. The injurious stimuli can be quite diverse, and the host response to these ‘domestic’ danger signals can share many of the features of the response to PAMPs. In fact, many of the endogenous signals related to tissue stressors bind to the same PPRs that PAMPs signal through (Table 3).
Table 3.
Endogenous danger signals and their PPRs
| Ligands | PPRs |
|---|---|
| Biglycan; EDN; gp60; Hsp60; Hsp70; HMGB1; hyaluronan fragments |
TLR2 |
| Endogenous mRNA [159] | TLR3 |
| αA crystalline; biglycan; fibrinogen; Fn-EDA; gp60; HMGB1; Hsp60; Hsp70; HSPB8; hyaluronan fragments; mmLDL; Mrp8; Mrp8–Mrp14 complex; murine β-defensin 2; polysaccharide fragments of heparan sulphate; SP-A |
TLR4* |
| HMGB1 | TLR9 |
| Apoptotic cells; oxidized LDL | CD36 |
| Modified LDL | MARCO |
| Apoptotic cells; oxidized LDL | Scavenger receptor A |
| C1q | gC1qR |
| Fibrinogen | CR3, CR4 |
| C5a | C5aR |
| Extracellular ATP | NALP3 |
Concerns of possible LPS contamination remain for some of these ligands [160].
The endogenous stress signals can be released in response to a variety of tissue trauma resulting from burns, cold, chemical insults, radiation, oxygen deprivation, auto-immune tissue destruction and nutrient depletion. Tumours and xenobiotics can also signal cells to release endogenous mediators. A convenient and instructive term, ‘alarmins’, has been assigned to some of these endogenous stress signals [50]. Several characteristics of alarmins have been summarized by Bianchi [51]: (a) alarmins are molecules that should be released immediately after non-programmed cell death; (b) alarmins can also be released from immune cells without dying, via specialized secretion systems; (c) alarmins can recruit and activate innate immune cells, such as macrophages and dendritic cells, to promote adaptive immune responses; and (d) alarmins should also restore homeostasis by promoting the reconstruction of the tissue that was destroyed, because of either the direct insult or the secondary effects of inflammation. Thus, alarmins and other endogenous danger molecules have been proposed to constitute a family of so-called ‘damage-associated molecular patterns’ (DAMPs) [51].
In response to these endogenous danger signals, macrophages can undergo profound physiological changes. Some of the endogenous danger signals to which macrophages can respond are listed in Table 3 and described below.
Heat-shock proteins (HSPs)
HSPs are perhaps the most diverse of the endogenous danger signals [52]. There are many HSPs, which reside in several cellular compartments and which can perform myriad functions with regard to immunity and inflammation. HSP22 and HSP70 can be found in the cytosol and the nucleus. In the mitochondria, HSPs consist mainly of HSP60 and HSP70, while gp96 is localized in the endoplasmic reticulum. In addition to their obvious roles in promoting correct protein folding, HSPs represent an important initiator of innate immune responses to cellular stress. These proteins are released from damaged cells and they can activate innate immune cells, probably by binding to one or more of the Toll-like receptors (TLRs). In macrophages, HSP60 induces the production of NO and TNFα via TLR4 [53]. TLR4 is also critical for HSP70 induction of IL-12 production [54]. Gp96-induced production of pro-inflammatory cytokines and other co-stimulation factors from bone marrow-derived DCs is also dependent on TLR4 but it may also involve TLR2 [55]. Interestingly, the endocytosis of gp60 is mediated by the surface receptor, CD91, which is required for gp96-mediated DC activation [56]. αA crystalline and HSPB8 belong to the small HSP family, both of which can activate DC in a TLR4-dependent fashion [57].
One potential complication regarding the activation of immune cells by HSPs is their frequent contamination with bacterial products, such as LPS or flagellin [58]. Furthermore, like many of the endogenous factors, HSPs are not exclusively inflammatory in character. They have been reported to induce the production of regulatory T cells and even protect cells from oxygen radical damage, thereby inhibiting inflammation [59].
In addition to their role in activating innate immune cells, HSPs can also facilitate an adaptive immune response. The association of antigens with some HSPs can result in improved antigen presentation and enhanced immunogenicity. Thus, HSPs have been considered as experimental adjuvants that may be particularly useful for poorly immunogenic substrates [60]. Unfortunately, HSPs may play prominent roles in the propagation and exacerbation of autoimmune disease. The abundant expression of HSPB8 in synovial tissue of rheumatoid arthritis is consistent with its role in promoting the inflammatory process and contributing to autoimmune diseases.
Hyaluronan
Hyaluronan is a major component of the extra-cellular matrix (ECM), with a structure of non-sulphated glycosaminoglycan that consists of repeating polymetric disaccharides d-glucuronic acid and N-acetyl glucosamine [61]. Normally, hyaluronan exists as a higher molecular polymer that can reach 2 × 104 kDa in size. This large polymer has actually been shown to have anti-inflammatory activity in some settings [62], possibly due to its association with other glycosaminoglycan-binding anti-inflammatory proteins [63]. Hyaluronan has also been shown to have cytoprotective activity, preserving epithelial cell integrity during inflammation, possibly by activating NF-κB. During tissue injury and/or inflammation, however, hyaluronan is progressively cleaved by a series of enzymatic reactions that yield hyaluronic acids of smaller sizes. CD44 is the major cell surface receptor for hyaluronan, but recent evidence indicates that hyaluronan can also bind to and signal through TLR2 and TLR4. This later signalling response is thought to mediate the inflammatory response of innate immune cells to hyaluronan fragments [64]. Hyaluronan fragments isolated from individuals with acute lung injuries can activate macrophages to produce chemokines in a TLR2- and TLR4-dependent fashion [65]. Impaired transepithelial migration of immune cells has been observed in both MyD88−/− and TLR4−/−TLR2−/− mice. Survival of these mice after acute lung injury is decreased, possibly due to enhanced apoptosis of epithelial cells, which suggests a possible protective role of HA during lung injury.
In addition to activating TLRs, or perhaps because of it, hyaluronan release has also been tied to DC maturation and even DC migration [66]. Thus, these fragments of hyaluronic acid can contribute to the initiation of an adaptive immune response by a variety of different mechanisms.
Biglycan is another small proteoglycan that is rich in leucine and can be released from the extracellular matrix (ECM). Biglycan acts on macrophages to produce TNFα and MIP-2 in a TLR2- and TLR4-dependent manner [67]. The biglycan-dependent production of cytokines by macrophages is significantly reduced in either TLR2−/− or TLR4−/− mice, but completely abolished in double knockout (TLR2−/−TLR4−/−) mice. Biglycan-null mice are resistant to LPS- or zymosan-induced sepsis, due to a reduced level of TNFα. Thus, this ECM component can make a positive contribution to inflammation by binding to and activating macrophage TLRs.
HMGB1(high-mobility group box 1)
HMGB1 may be one of the best examples of an endogenous mediator that can amplify inflammatory responses to exogenous PAMPs [68]. HMGBs are a highly conserved family of proteins that consists of an A-box and B-box protein with a carboxy-terminal acidic tail. HMGB proteins are typically located in the nucleus. These proteins bind to the minor groove of DNA with little sequence specificity and regulate many transcriptional events, primarily by increasing the binding affinity of transcription factors to their corresponding DNA binding sites via bending or distortion of the double helix.
HMGB1 can be released from cells dying by necrosis but not apoptosis, and its acetylated form can be actively secreted from appropriately stimulated innate immune cells, such as monocytes/macrophages and DCs [63]. In a model of hepatic ischaemia reperfusion injury, HMGB1 served as an early mediator of inflammation and organ damage [69]. Ischaemia reperfusion-induced hepatic injury was less severe in TLR4-defective C3H/Hej mice than in control C3H/HeOuj mice, suggesting that HMBG1 was an agonist for TRL4. A neutralizing antibody against HMGB1 had no effect on hepatic ischaemia reperfusion injury in C3H/Hej mice, but it significantly reduced hepatic damage in C3H/HeOuj mice.
Perhaps the most striking example of synergy between endogenously produced HMGB1 and an exogenous PAMP has been observed during sepsis [70]. HMGB1 is synthesized relatively late in sepsis by LPS-activated macrophages. This molecule contributes to the late lethality seen in sepsis. HMGB1 levels rise prior to lethal sepsis, and blocking HMGB1 with either antibody or inhibitors can prevent lethality. HMGB1 may either signal through multiple receptors or associate with multiple ligands to signal through different receptors. TLR4, TLR2 and TLR9 have all been reported to respond to HMGB1 [71]. The immunoglobulin ‘superfamily’ member RAGE has also been reported to be a receptor for HMGB1 [72]. Recently, Coyle and colleagues showed that class A CpG-containing oligodeoxynucleotides (ODNs) and HMGB1 can form a complex. The resultant complex induced association of TLR9 and RAGE to activate plasmacytoid DCs to produce IFNα. These types of interactions may have relevance to autoimmunity, where there is data showing that immune complexes containing HMGB1 and cellular DNA are found in SLE patients, and that these complexes can also bind to TLR9 via RAGE [31]. Thus, HMGB1 may amplify and sustain inflammatory or autoimmune pathologies by chaperoning endogenous stimuli to innate immune cells.
Fibronectin fragments
Exposure of monocytes to inflammatory stimuli can induce the expression of several matrix metalloproteases (MMPs). These proteases can cleave components of the extracellular matrix, and some of these cleaved components can activate macrophages. Fragments of fibronectin are generated in this fashion, and some of these fragments are potent stimuli for macrophages [73]. Fibronectin degradation can occur in response to tissue injury in pathologies such as rheumatoid arthritis, wound healing, epithelial fibrosis, vascular intimal proliferation or inflammation. An exhaustive characterization of the various Fn fragments is beyond the scope of this review, but the most well-characterized major 110–120 kDa fragment of fibronectin has been shown to bind to macrophages via the α5β1 integrin receptor and transduce signals that either activate cells directly or prime them for activation by other ligands [74]. An enhancement of macrophage phagocytosis, especially via complement receptors, results from the interaction of macrophages with fibronectin fragments [75].
There is now ample evidence that integrin receptors can associate with other receptors on macrophages and cooperate with them. This appears to be the case with the extradomain A fragment of fibronectin (Fn-EDA) [76], which is a potent macrophage activator. An alternative splicing event in the fibronectin exon encoding the type III repeat yields Fn-EDA. Recombinant Fn-EDA, but not other domains of fibronectin, was demonstrated to induce MMP-9 expression from human macrophages via TLR4. This TLR4-dependent activation, like LPS, depends on MD-2 expression. Fn-EDA also activates NF-κB in macrophages. FN-EDA is synthesized by the synovial lining in the activated rheumatoid synovium [77,78]. The role of FN-EDA-mediated TLR4 activation has been implicated in the pathologies of rheumatoid arthritis [79]. In fact FN-EDA, as an endogenous ligand for TLR4, has been investigated as a potential adjuvant [80].
β-Defensins
Mammalian defensins are cysteine-rich endogenous antibiotic peptides of the innate immune system. In addition to their antimicrobial and immunomodulatory effects, defensins also possess antiviral and toxin-neutralizing properties [81]. They belong to the family of eukaryotic anitmicrobial peptides with cationic and amphiphilic sequences of 12–50 amino acids. Mammalian defensins have a β-sheet structure that is stabilized by two or three intramolecular disulphide bonds. They can be divided into three classes on the basis of their structural features: α-defensins, β-defensins and θ-defensins. In response to microbial infection, the α-defensins are mainly produced from neutrophils and Paneth cells of small intestine, whereas β-defensins are produced from both leukocytes and epithelial cells in mucosal tissue and skin. α-Defensins can also be produced from human NK cells [82]. Generally, monocytes and macrophages do not produce defensins, despite earlier studies in which rabbit lung macrophages were the sources for the isolation of defensins [83]. However, they can signal epithelial cells to induce the synthesis of β-defensins, and this signalling is mediated by TLRs or cytoplasmic NODs [84,85]. Divergent signalling events are involved in governing gene expression of different forms of defensins. For example, human β-defensin 2 expression is dependent on NOD1, whereas β-defensin 3 expression is NOD1-independent but dependent on EGFR and the ERK pathway [86]. IL-17R and PAR-2 have also been implicated in the control of defensin gene expression [87]. On the other hand, it has been reported that human β-defensin 2 modulates TLR7 expression in colon and breast epithelial cells [88]. Mammalian defensins exert their effects via multiple receptors in immune cells. β-Defensins interact with CCR6 on the cell surface of both dendritic and T cells to bridge innate and adaptive immunity [83,89]. In a TLR4-dependent manner, murine β-defensin 2 acts directly on immature DCs to induce expression of co-stimulatory molecules and DC maturation [90]. It is noteworthy that human neutrophil-derived defensins were reported to induce production of TNFα and IL-1 by monocytes/macrophages activated with Staphylococcus aureus or PMA [91]. β-Defensins can also interfere with the effects of LPS on macrophages [92].
Modified low-density lipoproteins
There is now consensus that atherosclerosis is an inflammatory disease. T cells and macrophages accumulate in plaques and contribute to the growth of the plaque and even to plaque dissolution, leading to thrombo-embolic disorders. The generation of oxidized low-density lipoproteins has been directly associated with the pathogenesis of atherosclerosis. Macrophages take up oxidized LDL and become foam cells. These cells assume an inflammatory phenotype and attract other immune cells, including monocytes, to the lesion. OxLDL induces the production of TNF and IL-8 from monocytes, and primes macrophages to produce more cytokines in response to conventional stimuli. Furthermore, immune complexes consisting of Ox-LDL activate complement and contribute to inflammation. Ox-LDL itself, along with a variety of lipid-associated mediators, appears to be responsible for the activated phenotype that promotes these lipid-laden cells to secrete MMPs, growth factors and cytokines. The receptors for Ox-LDL are in the scavenger receptor family, and include CD36 and a newly described family member termed lectin-like oxidized lipoprotein scavenger receptor (LOX-1) [93]. A similar accumulation of oxidized lipoproteins may also occur in age-related macular degeneration (AMD), where macrophages expressing high levels of scavenger receptors accumulate and secrete immunoreactive mediators [94]. Minimally modified LDL (mmLDL) has also been shown to accumulate in macrophages during atherosclerosis. Unlike OxLDL, mmLDL is not recognized by scavenger receptors. However, like OxLDL, mmLDL can be a potent pro-atherogenic and pro-inflammatory lipoprotein [95]. mmLDL induces actin polymerization and spreading of macrophages, which leads to inhibition of phagocytosis of apoptotic cells but an enhancement of OxLDL uptake. The CD14–TLR4–MD-2 complex is critical for the stimulatory effects of mmLDL. Macrophages from C3H/HeJ mice, which have a defect in TLR4-mediated responses, respond poorly to mmLDL and fail to spread on substrates. Macrophages with CD14 deficiency exhibited lower mmLDL binding and a reduced F-actin response. Chinese hamster ovary cells transfected with human TLR4/MD-2 have a significantly higher F-actin response. Thus, mmLDL could influence macrophage phagocytosis and physiology, and it may play a role in atherogenesis via its interaction with TLR4.
Extracellular ATP
High amounts of ATP can be released from activated platelets at the site of inflammation and injury, where dying cells release ATP into the extracellular space. Extracellular ATP induces a variety of physiological responses in various cell types via G protein-coupled P2Y receptors, P2X7 receptor, and a pattern recognition receptor, NALP3 [96]. Normally, biological responses triggered by extracellular ATP are modulated or terminated by ectoenzymes that have ATPase activity.
It has been shown that LPS stimulation of macrophages and other cells is accompanied by the release of ATP [97]. Extracellular ATP appears to be required for LPS-induced production of IL-1β and IL-18 from macrophages. In the presence of apyrase, the release of IL-1 from LPS-treated cells was effectively blocked. LPS-induced IL-1α release was significantly attenuated by over-expression of ectonucleotidase. NALP3 (also known as cryopyrin, PYPAF1 and CIAS1) is composed of an N-terminal LRR receptor domain, a central NACHT domain and a C-terminal PYD domain. It has been reported that NALP3 gene expression in primary human monocytes is induced by TNFα and TLR ligands. In addition to ATP, other molecules have the potential to interact with NALP3, including nigericin, maitotoxin, Staphylococcus aureus and Listeria monocytogenes, and RNA released from dying cells [96]. Upon interaction with its ligands, NALP3 recruits ASC via PYD–PYD interactions, and CARDINAL via NACHT–FIIND interactions, respectively. By interaction with ASC, NALP3 triggers the activation of caspase-1 and subsequent release of the proinflammatory cytokines IL-1β and IL-18. Thus, NALP3, with the adaptor protein ASC, functions as a critical component of the inflammasone, a cytosolic complex of proteins that activates caspase-1 to process IL-1β and IL-18. Purified NALP3 contains ATPase activity that is specific to ATP, dATP and ATP–agarose, but not CTP, GTP or UTP [98].
It has been shown that mutations in NALP3 are linked to diseases with excessive production of IL-1β, including familial cold autoinflammatory syndrome, Muckle–Wells syndrome and neonatal onset multisystem inflammatory disease. Macrophages of NALP3-deficient mice cannot activate caspase-1 in response to TLR agonists plus ATP, whereas ATP-mediated P2X7 receptor activation, as shown by reduction in intracellular K+ levels, is unaffected [96]. ASC- and NALP3-deficient mice have an impaired IL-1β-dependent contact hypersensitivity response to the hapten trini-trophenylchloride, because macrophages from these mice cannot efficiently secrete caspase-1-dependent cytokines IL-1α, IL-1β and IL-18 [99].
Mrp8 (myeloid-related protein-8) and Mrp14 (myeloid-related protein-14)
Mrp8 and Mrp14 are the members of the calgranulin family of S100 proteins. They are the most abundant cytoplasmic proteins of neutrophils and are specifically released upon activation or phagocytosis. The formation of Mrp8 and Mrp14 complexes are correlated with the pathogenesis of numerous inflammatory diseases, such as sepsis, rheumatoid arthritis, inflammatory bowel disease and cancer [100]. Recently, Vogl et al demonstrated a pivotal role of these proteins in enhancing LPS-induced phagocyte activation via their interaction with TLR4 in models of LPS-induced shock and Escherichia coli-induced abdominal sepsis [101]. Mrp8 specifically interacts with the TLR4–MD2 complex to induce intracellular translocation of MyD88 and activation of IRAK-1 and NF-κB, leading to transcriptional up-regulation of TNF gene expression. Mice without Mrp8 and Mrp14 are relatively resistant to the challenge with bacterial LPS and galactosamine, with less TNF production.
Macrophages and the resolution of inflammation
The well-orchestrated resolution of an inflammatory response is necessary to maintain homeostasis. Neutrophils are typically the first cells to respond to pathogenic stimuli. These cells rapidly migrate to and accumulate at inflammatory sites. Neutrophils are highly phagocytic and represent the first line of defence against invading microbes. Neutrophil phagocytosis is associated with the release of oxygen radicals, antimicrobial peptides and lysosomal granule contents, all of which can be toxic, not only to microbes but also to the otherwise healthy host cells in this environment. This results in the death of neutrophils and the local release of heat shock proteins and alarmins from PMNs, as well as from the surrounding tissue. An important function of inflammatory macrophages is to clean the inflammatory site of cell debris, including the removal of necrotic neutrophils and their secreted products. This interaction with endogenous danger signals released from cells within the inflammatory site results in a population of locally activated macrophages. As inflammation progresses, these cells are replaced by a population of cells that can participate in the resolution phase of inflammation.
It has been appreciated for many years that macrophages play a key role in the resolution of inflammation and in wound healing. In a guinea-pig wound model in the 1970s, the depletion of neutrophils, under ‘sterile conditions’, had only a minor effect on tissue repair. However, macrophage depletion by antisera and steroids led to a failure to clear dead and damaged cells and other debris at the wound site. This resulted in a marked delay in the healing process. This role of macrophages was confirmed in PU.1-null mice [102]. Due to the lack of both neutrophils and macrophages in these mice, the task of ‘janitor’ was passed to fibroblastic phagocytes, which were much slower and less efficient at cleaning the inflammatory site of debris and spent cells. Other knockout mouse models have shown that a deficiency in the molecules essential for macrophage infiltration, such as P- and E-selectin and ICAM-1, have all resulted in a delay in inflammation resolution and wound closure [103,104]. Thus, monocytes continue to migrate into resolving inflammatory sites, where they transform into macrophages with a physiology that is distinct from the classically activated macrophages that had originally populated the inflammation site.
Components present in the local microenvironment can prime macrophages to adopt unique activation phenotype to promote the resolution of inflammation. It is clear that the resolving phase of inflammation is not a passive process in which toxic mediators simply dissolve, but rather an active process that involves the release of specific mediators that prevent ongoing inflammation and contribute to its resolution. Some of the secreted mediators can include glucocorticoids, prostaglandin derivatives, adenosine, anti-inflammatory cytokines and growth factors. In response to these signals, macrophages begin to produce cytokines, growth factors and angiogenic factors that play important roles in the resolution of inflammation resolution and subsequent tissue repair.
Glucocorticoids (GC)
GCs have been used to treat inflammatory diseases for almost 50 years. They exert a profound inhibitory effect on immune cells, by inhibiting inflammatory cytokine production from antigen-presenting cells and by inhibiting the proliferation of stimulated T and B cells. Recently, Ehrchen et al demonstrated that GC treatment leads to the differentiation of a specific subset of monocytes with anti-inflammatory phenotype, but without a global suppression of monocyte effector functions [105]. These monocytes express high levels of formyl-peptide receptor 1 (FPR1), which, when ligated, suppresses the production of pro-inflammatory cytokines and removes damaged cells [106]. GCs have been known to promote macrophage phagocytosis of apoptotic neutrophils [107], thereby inhibiting the release of pro-inflammatory cytokines [108]. The expression of IL-10 and other anti-inflammatory molecules was increased in monocytes exposed to GC. Thus, the release of endogenous glucocorticoids may mimic what is observed when exogenous high-dose GCs are administered to patients, and induce the production of monocytes that may play an important role in the resolution of inflammation.
Adenosine
Adenosine is released from platelets and accumulates in inflammatory sites. It can also be generated locally from extracellular ATP by the ectoenzymes ATPase–ADPase/5′ –nucleotidase system expressed on leukocytes and endothelial cells. It has been demonstrated that adenosine receptors, particularly the A2 type, are expressed during differentiation of monocytes to macrophages [109]. Adenosine may play a critical role in the resolution of inflammation. Treatment of macrophages with an agonist of the adenosine A2A receptor (A2AR) inhibits TNF production [110], whereas agonists of A2BR increase IL-10 production by a post-translational mechanism [111]. Furthermore, the co-stimulation of macrophages with agonists of TLRs and A2AR results in an increase in VEGF production by macrophages [112]. Thus, adenosine inhibits the production of inflammatory cytokines and increases the production of IL-10, while inducing the production of growth factors necessary for angiogenesis and wound healing.
Resolvins
The metabolism of the C20 fatty acid eicosapentaenoic acid by COX2 can result in the formation of so-called resolvins, which were named because of their ability to contribute to the resolution of inflammation. Resolvins, along with lipoxins derived from arachidonic acid, can contribute to resolution phase of inflammation by inhibiting PMN chemotaxis and by promoting the uptake of apoptotic cells by macrophages [113,114]. These lipid derivatives inhibit leukocyte migration and may also inhibit inflammatory cytokine production.
Cytokines
T cell-derived cytokines in the lesion can exert a dramatic affect on macrophage physiology. So-called ‘alternatively-activated’ macrophages are formed in the presence of IL-4 and/or IL-13. These cytokines are produced primarily by Th2 cells. They induce macrophages to express high levels of the mannose receptor with diminished levels of inflammatory cytokines [115]. One distinct feature of these macrophages is the expression of high levels of arginase I, which can shift arginine utilization from the production of nitric oxide by iNOS in the classically activated macrophages to the production of polyamines and proline. This shift in arginine metabolism has the potential to contribute to wound healing by alternatively activated macrophages. Thus, these macrophages produce lower amounts of proinflammatory cytokines but they can contribute to the synthesis of matrix components which may lead to the repair of damaged tissue.
Immune complexes
One important feature that frequently appears in the later stages of inflammation is the presence of high levels of antigen-specific antibodies. These antibodies neutralize pathogens by opsonization and initiate ADCC and CDCC to eliminate the pathogens. Immune complex formed by opsonization with antibody can also alter the activation status of macrophages [116]. In concert with microbial stimuli such as LPS or any of the various endogenous danger signals described above, immune complexes can exert profound effects on cytokine production [116–118]. In the presence of immune complexes, macrophages produce high amounts of IL-10 and little to no detectable IL-12. These cells can skew T helper cell responses by inducing T cells to produce IL-4 [119]. Thus, these cells can contribute to the resolution of inflammation because they produce a high amount of IL-10, they are highly phagocytic and so can remove cellular debris, and they can present antigen to T cells and induce them to produce IL-4.
Macrophages in inflammation-related pathogenesis
Many diseases have been linked to uncontrolled inflammatory cytokine production by macrophages. In most of these cases, the diseases are caused by an inability to terminate an appropriate cellular response. The failure to resolve the inflammatory process or the inability of macrophages to respond to resolving stimuli can result in a variety of autoimmune diseases. In this section we have selected two different diseases to illustrate the divergent roles that macrophage activation and deactivation can play. In one of the diseases, inflammatory bowel disease, healthy gut macrophages producing anti-inflammatory cytokines are replaced by activated macrophages producing inflammatory cytokines. These inflammatory cytokines produced by activated macrophages can contribute to pathology and disease progression. In the other disease, cancer, the opposite type of macrophage reprogramming occurs. Initially activated macrophages produce inflammatory cytokines and toxic radicals that can cause DNA breaks, leading to the onset of cancer. As tumours progress, however, macrophages receive further differentiative cues, probably from the tumour itself, and begin to produce anti-inflammatory cytokines. These tumour-associated macrophages and the anti-inflammatory cytokines they produce can induce a state of immunosuppression that prevents tumour rejection. Thus, in both of these diseases and in many others, the responses of macrophages to their microenvironment can determine disease outcome. Manipulating these responses may represent a therapeutic target to reverse diseases or prevent pathology.
Inflammatory bowel disease (IBD)
IBD comprises the two chronic disorders that are characterized by inflammation of the bowel: ulcerative colitis and Crohn’s disease [120]. The exact causes of IBD are not known, but genetic factors are probably involved, since from 15–30% of people with IBD have a relative with the disease. Despite the fact that our intestinal mucosa is in constant contact with the resident microflora, we typically fail to mount an inflammatory response to this flora. The lack of response appears to be due to an active suppression of innate immunity, but the mechanism(s) behind this suppression remain largely conjectural.
The key trigger to inflammation in the gut appears to be the exposure of the resident flora to the submucosa and the immune cells in this region. During IBD there is an increased number of activated macrophages that express up-regulated co-stimulatory molecules (eg CD80/CD86), with a cytokine profile favouring a type I pro-inflammatory response [121]. Depletion of intestinal macrophages by poly-d,l-lactic acid microspheres containing dichloromethylene diphosphonate significantly suppresses the development of chronic colitis in an animal model of human IBD. IL-23, which is a heterodimer of p40 and p19, is a pro-inflammatory cytokine that is over-expressed in inflamed tissues from IBD mice [122,123]. Deletion of IL-23 significantly reduced inflammation of the bowel, with little impact on systemic T cell immunity. In mice lacking B and T lymphocytes, IL-23 can still induce intestinal inflammation, confirming the important role of the innate immune system, including macrophages, in IBD.
One of the bacterial components in the microflora is PGN, a proteoglycan found on most bacteria, particularly Gram-positive bacteria, and a ligand for TLR2 on the surface of macrophages. There have been reports that TLR2 engagement in the gut actively suppresses immunity in the bowel [124]. PGN contains MDP, a ligand for NOD2, and NOD2 activation may limit robust TLR2 responses. There have also been reports that TLR2 ligation of DCs preferentially leads to increased IL-10 production [125]. Card15 is the gene that encodes NOD2. Card15 mutations have been implicated in IBD pathogenesis [126]. Thus, the NOD2-mediated signalling pathway appears to be a critical element in the control of IBD.
The cells of our gastrointestinal system also produce a number of antimicrobial substances which appear to counter IBD [127]. RegIIIγ and its human homologue, HIP/PAP, is one such substance produced from Paneth cells. RegIIIγ can bind to a carbohydrate component of PGN to kill the bacteria. Other antibacterial substances include lysozyme, secretory phospholipase A2, angiogenin 4 and α-defensins. Decreased production of α-defensins has been found in IBD patients who carry mutations in the Card15 gene [128].
It has been known that mice deficient in IL-10 can develop pathological changes and symptoms that are very similar to those of human IBD [129]. Administration of IL-10 has provided therapeutic benefit, not only to IL-10 deficient mice but also in other murine models of human IBD [130,131]. IL-10 exerts its compensatory activity either through the down-regulation of type-I pro-inflammatory cytokines and/or possible modulation of Th17 cells [123]. Th17 T cells belong to a novel group of CD4+ T helper cells that are found highly up-regulated in the gut of IBD mice and which have been implicated in the pathology of IBD. The IL-6 that is secreted in the gut promotes T cell accumulation by preventing T cell apoptosis. These persistent inflammatory T cells produce TNF, IL-12, IL-23 and IL-27 in addition to IL-17. Thus, disease progression appears to be the result of a positive feedback loop in which inflammatory macrophages give rise to activated T cells, which in turn propagate macrophage activation.
Two clinical trials with recombinant human IL-10 in the treatment of patients with Crohn’s disease were reported by the IL-10 Inflammatory Bowel Disease Cooperative Study Group [132,133]. A group of 95 patients with mild to moderately active Crohn’s disease treated with 5 µg/kg subcutaneous IL-10 showed some clinical and endoscopic improvement [132]. However, subcutaneous treatment of a group of therapy-refractory patients failed to mediate remission. A tendency toward clinical improvement was observed in the high-dose group [133]. The systemic route of subcutaneous administration of recombinant IL-10 could be one of many concerns regarding to this failure [134]. A clinical Phase I trial with transgenic Lactococcus lactis to produce human IL-10 has shown efficacy in 10 patients with Crohn’s disease [135], suggesting that induction of IL-10 in the local intestinal mucosa may be a better therapeutic option. Thus, we propose that the therapeutic modulation of local intestinal macrophages, to induce them to produce IL-10, would be a logical approach to treat or even prevent these diseases.
Cancer-associated macrophages
The connection between inflammation and the induction of cancer has been well established, and is based not only on repeated anecdotal observations but also on many subsequent clinical studies. It has long been thought that chronic and non-healing wounds due to unresolved inflammation present a risk for cancer [136]. Activated macrophages and the toxic metabolites they produce have been implicated in this process. The molecules and the mechanisms for tumour induction in chronic wounds remain elusive, but oxygen and nitrogen radicals have been implicated. The presence of human papillomavirus infection is often found in squamous cell carcinoma that is highly correlated with chronic wounds [137]. Approximately 15% of tumour incidents are associated with infectious agents [138]. Inflammatory bowel disease is a high-risk factor for the development of colorectal cancer. An inflammatory microenvironment is often found at the tumour site of breast cancer [139]. Non-steroidal anti-inflammatory drugs are under investigation for their use as possible anti-cancer compounds, and these drugs have already been prescribed for protection against several forms of cancers. Thus, classically activated macrophages and the inflammatory products they produce have been associated with the onset of tumours.
Tumour progression, however, has been associated with a macrophage phenotype that is very differenmt from the classically activated macrophage. In both primary and secondary tumours, macrophages represent a major component of the inflammatory infiltrate. These cells, which have been termed tumour-associated macrophages (TAMs), exhibit clear functional distinctions from classically activated macrophages. Although there is likely a variety of different macrophage phenotypes associated with different stages of tumour growth, some generalization about TAMs appears valid. These cells often have a higher expression of IL-10 and lower levels of pro-inflammatory cytokines [140–142]. TAMs are generally better at scavenging debris from apoptotic cells, promoting angiogenesis, and repairing and remodelling wounded or damaged tissues. TAMs are usually poor at presenting antigen to T cells, and they can sometimes produce factors that suppress T cell functions. An increase in the accumulation of TAMs in some tumours is often considered an indicator of a poor prognosis [143]. The signals that induce an immunosuppressive population of macrophages in tumours are not known. Certainly tumour-derived factors have been considered [143]. Antibodies may actually be another of these anti-inflammatory factors. While it is clear that in some experimental systems antibodies to tumour-associated antigens can be protective, there are also a number of instances in which antibody depletion actually leads to reduced tumour growth [144,145].
IL-10 production by tumour-associated macrophages appears to be one of the most important factors to subvert tumour-specific immunity [142]. IL-10 not only subverts the development of classical macrophage activation responses but also stimulates macrophages to express B7-H4, a novel member of the B7 family of T cell co-stimulator molecules [146]. B7-H4 inhibits T cell proliferation, cell cycle progression, and cytokine production. B7-H4+ macrophages induce immuno-suppression independent of B7-H1, arginase and iNOS. Gene expression profiling of tumour-associated macrophages isolated from a murine fibrosarcoma reveals characteristic alterations in gene expression, which are consistent with some alternative type of macrophage activation. A higher expression of IL-10 and a lower expression of IL-12 and the IFNγ -inducible chemokines, CXCL9, CXCL10 and CXCL16, suggest that tumour-associated macrophages exhibit a transcriptional reprograming [147,148].
Angiogenesis is one important feature of cancer and a requirement for tumour progression [149]. The tumour itself can stimulate new blood vessels, but TAMs can also make major contributions to tumour angiogenesis by stimulating the production of angiogenic factors such as VEGF. Chemokines produced either from TAMs or local tumour sites can also play a role to modulate angiogenesis. Interleukin-8 is a well-characterized pro-angiogenic factor, whereas IP-10 possesses anti-angiogenic activity. The balance between pro-angiogenic and antiangiogenic factors, rather than their absolute amounts, may be the critical element to determining tumour angiogenesis. There is little doubt that angiogenic factors produced by macrophages associated with tumours can contribute to tumour progression.
Conclusions
Activated macrophages have long been considered amongst the most potent inflammatory cells in the body. However, we now know that macrophages can also be activated by different stimuli to give rise to cells that have potent anti-inflammatory activity. These anti-inflammatory macrophages are important contributors to the resolution of inflammation and the initiation of wound healing. Understanding how these cells vacillate between inflammatory and antiinflammatory mediators represents a key to being able to manipulate immune/inflammatory responses. The macrophage receptors that respond to environmental cues and initiate these divergent responses, and the signalling pathways that are activated by each receptor, all represent targets for manipulating macrophage activation states. Preventing unwanted macrophage activation or inducing a population of anti-inflammatory macrophages may be a way to minimize autoimmune pathologise. Conversely, preventing the induction of immunosuppressive ‘wound healing’- or ‘resolving’-type macrophages may lead to new and better vaccines and prevent the immunosuppression associated with cancer and other chronic diseases.
Footnotes
No conflicts of interest were declared.
Teaching materials
Power Point slides of the figures from this Review may be found at the web address http://www.interscience.wiley.com/jpages/0022-3417/suppmat/path.2284.html
References
- 1.Adams DO, Hamilton TA. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 2.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect Dis. 2006;6:557–569. doi: 10.1016/S1473-3099(06)70577-1. [DOI] [PubMed] [Google Scholar]
- 4.O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 5.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 6.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 7.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 9.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defence against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 10.Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64+/CD16+ blood monocytes. Eur J Immunol. 2001;31:48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69:11–20. [PubMed] [Google Scholar]
- 12.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 13.Menendez-Benito V, Neefjes J. Autophagy in MHC class II presentation: sampling from within. Immunity. 2007;26:1–3. doi: 10.1016/j.immuni.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 15.Harding CV, Ramachandra L, Wick MJ. Interaction of bacteria with antigen presenting cells: influences on antigen presentation and antibacterial immunity. Curr Opin Immunol. 2003;15:112–119. doi: 10.1016/s0952-7915(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 16.Schnare M, Holt AC, Takeda K, Akira S, Medzhitov R. Recognition of CpG DNA is mediated by signalling pathways dependent on the adaptor protein MyD88. Curr Biol. 2000;10:1139–1142. doi: 10.1016/s0960-9822(00)00700-4. [DOI] [PubMed] [Google Scholar]
- 17.Young HA. Unraveling the pros and cons of interferon-gamma gene regulation. Immunity. 2006;24:506–507. doi: 10.1016/j.immuni.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, et al. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. TLR signalling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Delbridge LM, O’Riordan MX. Innate recognition of intracellular bacteria. Curr Opin Immunol. 2007;19:10–16. doi: 10.1016/j.coi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 22.Franchi L, McDonald C, Kanneganti TD, Amer A, Nunez G. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defence. J Immunol. 2006;177:3507–3513. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 24.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 25.Gay NJ, Gangloff M. Structure and function of toll receptors and their ligands. Annu Rev Biochem. 2007;76:141–165. doi: 10.1146/annurev.biochem.76.060305.151318. [DOI] [PubMed] [Google Scholar]
- 26.Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signalling. Nat Immunol. 2006;7:1074–1081. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Zhou P, Qian L, Chuang JZ, Lee J, Li C, et al. MyD88-5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J Exp Med. 2007;204:2063–2074. doi: 10.1084/jem.20070868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishitani C, Mitsuzawa H, Sano H, Shimizu T, Matsushima N, Kuroki Y. Toll-like receptor 4 region Glu24-Lys47 is a site for MD-2 binding: importance of CYS29 and CYS40. J Biol Chem. 2006;281:38322–38329. doi: 10.1074/jbc.M606904200. [DOI] [PubMed] [Google Scholar]
- 29.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 30.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 31.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 32.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol. 2008 doi: 10.1189/jlb.0607402. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 34.Kaneko T, Silverman N. Bacterial recognition and signalling by the Drosophila IMD pathway. Cell Microbiol. 2005;7:461–469. doi: 10.1111/j.1462-5822.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- 35.Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi K, Inohara N, Hernandez LD, Galán JE, Núñez G, Janeway CA, et al. RICK/Rip2/CARDIAK mediates signaling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 37.Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Wertz I, O’Rourke K, Ultsch M, Seshagiri S, Eby M, et al. Bcl10 activates the NF-κB pathway through ubiquitination of NEMO. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 40.Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23:7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, et al. CARD4/Nod1 mediates NF-κB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2001;2:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 43.Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 44.Pichlmair A, Schulz O, Tan CP, Näslund TI, Liljeström P, Weber F, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 45.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 46.Malathi K, Dong B, Gale M, Jr, Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448:816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Zhang HX, Sun YP, Liu ZX, Liu XS, Wang L, et al. Rig-I−/− mice develop colitis associated with downregulation of Gαi2. Cell Res. 2007;17:858–868. doi: 10.1038/cr.2007.81. [DOI] [PubMed] [Google Scholar]
- 48.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 50.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 52.Tsan MF, Gao B. Heat shock protein and innate immunity. Cell Mol Immunol. 2004;1:274–279. [PubMed] [Google Scholar]
- 53.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 54.Vabulas RM, Hmad-Nejad P, Ghose S, Kirschning CJ, Issels RD. Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 55.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 56.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 57.Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, et al. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol. 2006;176:7021–7027. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- 58.Ye Z, Gan YH. Flagellin contamination of recombinant heat shock protein 70 is responsible for its activity on T cells. J Biol Chem. 2007;282:4479–4484. doi: 10.1074/jbc.M606802200. [DOI] [PubMed] [Google Scholar]
- 59.Wieten L, Broere F, van der ZR, Koerkamp EK, Wagenaar J, van EW. Cell stress induced HSP are targets of regulatory T cells: a role for HSP inducing compounds as anti-inflammatory immuno-modulators? FEBS Lett. 2007;581:3716–3722. doi: 10.1016/j.febslet.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 60.Reimann J, Schirmbeck R. DNA vaccines expressing antigens with a stress protein-capturing domain display enhanced immunogenicity. Immunol Rev. 2004;199:54–67. doi: 10.1111/j.0105-2896.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 61.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Cantor JO, Nadkarni PP. Hyaluronan: the Jekyll and Hyde molecule. Inflamm Allergy Drug Targets. 2006;5:257–260. doi: 10.2174/187152806779010936. [DOI] [PubMed] [Google Scholar]
- 63.Osterloh A, Kalinke U, Weiss S, Fleischer B, Breloer M. Synergistic and differential modulation of immune responses by Hsp60 and lipopolysaccharide. J Biol Chem. 2007;282:4669–4680. doi: 10.1074/jbc.M608666200. [DOI] [PubMed] [Google Scholar]
- 64.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 65.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 66.Mummert ME. Immunologic roles of hyaluronan. Immunol Res. 2005;31:189–206. doi: 10.1385/IR:31:3:189. [DOI] [PubMed] [Google Scholar]
- 67.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris HE, Raucci A. Alarmin(g) news about danger: work-shop on innate danger signals and HMGB1. EMBO Rep. 2006;7:774–778. doi: 10.1038/sj.embor.7400759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischaemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 71.Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917–C924. doi: 10.1152/ajpcell.00401.2005. [DOI] [PubMed] [Google Scholar]
- 72.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol. 2005;26:381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Barilla ML, Carsons SE. Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum. 2000;29:252–265. doi: 10.1016/s0049-0172(00)80012-8. [DOI] [PubMed] [Google Scholar]
- 74.Trial J, Rubio JA, Birdsall HH, Rodriguez-Barradas M, Rossen RD. Monocyte activation by circulating fibronectin fragments in HIV-1-infected patients. J Immunol. 2004;173:2190–2198. doi: 10.4049/jimmunol.173.3.2190. [DOI] [PubMed] [Google Scholar]
- 75.Wright SD, Meyer BC. Fibronectin receptor of human macrophages recognizes the sequence Arg–Gly–Asp–Ser. J Exp Med. 1985;162:762–767. doi: 10.1084/jem.162.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwarzbauer JE, Patel RS, Fonda D, Hynes RO. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987;6:2573–2580. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hino K, Shiozawa S, Kuroki Y, Ishikawa H, Shiozawa K, Sekiguchi K, et al. EDA-containing fibronectin is synthesized from rheumatoid synovial fibroblast-like cells. Arthritis Rheum. 1995;38:678–683. doi: 10.1002/art.1780380516. [DOI] [PubMed] [Google Scholar]
- 78.Chevalier X, Claudepierre P, Groult N, Zardi L, Hornebeck W. Presence of ED-A containing fibronectin in human articular cartilage from patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 1996;23:1022–1030. [PubMed] [Google Scholar]
- 79.Gondokaryono SP, Ushio H, Niyonsaba F, Hara M, Takenaka H, Jayawardana ST, et al. The extra domain A of fibronectin stimulates murine mast cells via Toll-like receptor 4. J Leukoc Biol. 2007;82:657–665. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 80.Lasarte JJ, Casares N, Gorraiz M, Hervás-Stubbs S, Arribillaga L, Mansilla C, et al. The extra domain A from fibronectin targets antigens to TLR4-expressing cells and induces cytotoxic T cell responses in vivo. J Immunol. 2007;178:748–756. doi: 10.4049/jimmunol.178.2.748. [DOI] [PubMed] [Google Scholar]
- 81.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 82.Chalifour A, Jeannin P, Gauchat JF, Blaecke A, Malissard M, N’Guyen T, et al. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood. 2004;104:1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 83.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defence. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 84.Vora P, Youdim A, Thomas LS, Fukata M, Tesfay SY, Lukasek K, et al. β-Defensin-2 expression is regulated by TLR signalling in intestinal epithelial cells. J Immunol. 2004;173:5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 85.Voss E, Wehkamp J, Wehkamp K, Stange EF, Schroder JM, Harder J. NOD2/CARD15 mediates induction of the antimicrobial peptide human β-defensin-2. J Biol Chem. 2006;281:2005–2011. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 86.Boughan PK, Argent RH, Body-Malapel M, Park JH, Ewings KE, Bowie AG, et al. Nucleotide-binding oligomerization domain-1 and epidermal growth factor receptor: critical regulators of β-defensins during Helicobacter pylori infection. J Biol Chem. 2006;281:11637–11648. doi: 10.1074/jbc.M510275200. [DOI] [PubMed] [Google Scholar]
- 87.Froy O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol. 2005;7:1387–1397. doi: 10.1111/j.1462-5822.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 88.Stroinigg N, Srivastava MD. Modulation of toll-like receptor 7 and LL-37 expression in colon and breast epithelial cells by human β-defensin-2. Allergy Asthma Proc. 2005;26:299–309. [PubMed] [Google Scholar]
- 89.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. β-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 90.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by β-defensin 2. Science. 2002;298:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 91.Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV, Voitenok NN. Neutrophil α-defensin human neutrophil peptide modulates cytokine production in human monocytes and adhesion molecule expression in endothelial cells. Eur Cytokine Netw. 2000;11:257–266. [PubMed] [Google Scholar]
- 92.Motzkus D, Schulz-Maronde S, Heitland A, Schulz A, Forss-mann WG, Jübner M, et al. The novel β-defensin DEFB123 prevents lipopolysaccharide-mediated effects in vitro and in vivo. FASEB J. 2006;20:1701–1702. doi: 10.1096/fj.05-4970fje. [DOI] [PubMed] [Google Scholar]
- 93.Vohra RS, Murphy JE, Walker JH, Ponnambalam S, Homer-Vanniasinkam S. Atherosclerosis and the Lectin-like Oxidized low-density lipoprotein scavenger receptor. Trends Cardiovasc Med. 2006;16:60–64. doi: 10.1016/j.tcm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 94.Kamei M, Yoneda K, Kume N, Suzuki M, Itabe H, Matsuda K, et al. Scavenger receptors for oxidized lipoprotein in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:1801–1807. doi: 10.1167/iovs.06-0699. [DOI] [PubMed] [Google Scholar]
- 95.Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 96.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 97.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-κB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 98.Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signalling. Proc Natl Acad Sci USA. 2007;104:8041–8046. doi: 10.1073/pnas.0611496104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al. Critical role for NALP3/CIAS1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 100.Roth J, Vogl T, Sorg C, Sunderkotter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003;24:155–158. doi: 10.1016/s1471-4906(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 101.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 102.Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, et al. Wound healing in the PU.1 null mouse — tissue repair is not dependent on inflammatory cells. Curr Biol. 2003;13:1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- 103.Subramaniam M, Saffaripour S, Van De Water L, Frenette PS, Mayadas TN, Hynes RO, et al. Role of endothelial selectins in wound repair. Am J Pathol. 1997;150:1701–1709. [PMC free article] [PubMed] [Google Scholar]
- 104.Nagaoka T, Kaburagi Y, Hamaguchi Y, Hasegawa M, Takehara K, Steeber DA, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or l-selectin expression. Am J Pathol. 2000;157:237–247. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ehrchen J, Steinmüller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, et al. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109:1265–1274. doi: 10.1182/blood-2006-02-001115. [DOI] [PubMed] [Google Scholar]
- 106.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Cousin JM, Hughes J, Van Damme J, Seckl JR, Haslett C, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J Immunol. 1999;162:3639–3646. [PubMed] [Google Scholar]
- 108.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eppell BA, Newell AM, Brown EJ. Adenosine receptors are expressed during differentiation of monocytes to macrophages in vitro. Implications for regulation of phagocytosis. J Immunol. 1989;143:4141–4145. [PubMed] [Google Scholar]
- 110.Ezeamuzie CI, Khan I. The role of adenosine A(2) receptors in the regulation of TNF-α production and PGE(2) release in mouse peritoneal macrophages. Int Immunopharmacol. 2007;7:483–490. doi: 10.1016/j.intimp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 111.Németh ZH, Lutz CS, Csóka B, Deitch EA, Leibovich SJ, Gause WC, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ramanathan M, Pinhal-Enfield G, Hao I, Leibovich SJ. Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2A receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the VEGF promoter. Mol Biol Cell. 2007;18:14–23. doi: 10.1091/mbc.E06-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 115.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 116.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 117.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intra-cellular pathogen Leishmania. J Exp Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang Z, Mosser DM, Zhang X. Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J Immunol. 2007;178:1077–1085. doi: 10.4049/jimmunol.178.2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Anderson CF, Mosser DM. Cutting edge: biasing immune responses by directing antigen to macrophage Fcγ receptors. J Immunol. 2002;168:3697–3701. doi: 10.4049/jimmunol.168.8.3697. [DOI] [PubMed] [Google Scholar]
- 120.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 121.Rugtveit J, Bakka A, Brandtzaeg P. Differential distribution of B7.1 (CD80) and B7.2 (CD86) costimulatory molecules on mucosal macrophage subsets in human inflammatory bowel disease (IBD) Clin Exp Immunol. 1997;110:104–113. doi: 10.1046/j.1365-2249.1997.5071404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 125.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–928. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, et al. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet. 2001;357:1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- 127.Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol. 2007;19:70–83. doi: 10.1016/j.smim.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 128.Ferguson LR, Shelling AN, Browning BL, Huebner C, Petermann I. Genes, diet and inflammatory bowel disease. Mutat Res. 2007;622:70–83. doi: 10.1016/j.mrfmmm.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 129.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 131.Rogy MA, Beinhauer BG, Reinisch W, Huang L, Pokieser P. Transfer of interleukin-4 and interleukin-10 in patients with severe inflammatory bowel disease of the rectum. Hum Gene Ther. 2000;11:1731–1741. doi: 10.1089/10430340050111386. [DOI] [PubMed] [Google Scholar]
- 132.Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473–1482. doi: 10.1053/gast.2000.20229. [DOI] [PubMed] [Google Scholar]
- 133.Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119:1461–1472. doi: 10.1053/gast.2000.20196. [DOI] [PubMed] [Google Scholar]
- 134.Bickston SJ, Cominelli F. Recombinant interleukin 10 for the treatment of active Crohn’s disease: lessons in biologic therapy. Gastroenterology. 2000;119:1781–1783. doi: 10.1053/gast.2000.20822. [DOI] [PubMed] [Google Scholar]
- 135.Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:754–759. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 136.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 137.Garven TC, Thelmo WL, Victor J, Pertschuk L. Verrucous carcinoma of the leg positive for human papillomavirus DNA 11 and 18: a case report. Hum Pathol. 1991;22:1170–1173. doi: 10.1016/0046-8177(91)90272-q. [DOI] [PubMed] [Google Scholar]
- 138.Parkin DM, Pisani P, Muñoz N, Ferlay J. The global health burden of infection associated cancers. In: Newton R, Bersal V, Weiss R, editors. Cancer Surveys, vol 33, Infections and Human Cancer. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 139.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 140.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 141.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-κB activation in tumor-associated macrophages. J Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 143.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 144.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 145.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 146.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 148.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, et al. p50 nuclear factor-κB over-expression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- 149.Bian XW, Chen JH, Jiang XF, Bai JS, Wang QL, Zhang X. Angiogenesis as an immunopharmacologic target in inflammation and cancer. Int Immunopharmacol. 2004;4:1537–1547. doi: 10.1016/j.intimp.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 150.Marshall-Clarke S, Downes JE, Haga IR, Bowie AG, Borrow P, Pennock JL, et al. Polyinosinic acid is a ligand for toll-like receptor 3. J Biol Chem. 2007;282:24759–24766. doi: 10.1074/jbc.M700188200. [DOI] [PubMed] [Google Scholar]
- 151.Yarovinsky F, Zhang D, Andersen JF, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 152.Zhang J, Zhu J, Bu X, Cushion M, Kinane TB, Avraham H, et al. Cdc42 and RhoB activation are required for mannose receptor-mediated phagocytosis by human alveolar macrophages. Mol Biol Cell. 2005;16:824–834. doi: 10.1091/mbc.E04-06-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fuller GL, Williams JA, Tomlinson MG, Eble JA, Hanna SL, Pöhlmann S, et al. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signalling cascade. J Biol Chem. 2007;282:12397–12409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Patel SN, Lu Z, Ayi K, Serghides L, Gowda DC, Kain KC. Disruption of CD36 impairs cytokine response to Plasmodium falciparum glycosylphosphatidylinositol and confers susceptibility to severe and fatal malaria in vivo. J Immunol. 2007;178:3954–3961. doi: 10.4049/jimmunol.178.6.3954. [DOI] [PubMed] [Google Scholar]
- 155.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signalling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sendide K, Reiner NE, Lee JS, Bourgoin S, Talal A, Hmama Z. Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria: regulation by phosphatidylinositol 3-kinase and cytohesin-1. J Immunol. 2005;174:4210–4219. doi: 10.4049/jimmunol.174.7.4210. [DOI] [PubMed] [Google Scholar]
- 157.Ghiran I, Klickstein LB, Nicholson-Weller A. Calreticulin is at the surface of circulating neutrophils and uses CD59 as an adaptor molecule. J Biol Chem. 2003;278:21024–21031. doi: 10.1074/jbc.M302306200. [DOI] [PubMed] [Google Scholar]
- 158.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [DOI] [PubMed] [Google Scholar]
- 159.Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 160.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]