Abstract
This report describes a detailed analysis how donor-specific HLA class II epitope mismatching affects antibody reactivity patterns in 75 solid organ transplant recipients with an in situ allograft and who were considered for retransplantation. Sera were tested for antibodies in a sensitive antigen-binding assay (Luminex) with single class II alleles. Their reactivity was analyzed with HLAMatchmaker, a structural matching algorithm that considers so-called eplets to define epitopes recognized by antibodies. Only 24% of the patients showed donor-specific anti-DRB1 antibodies and there was a significant correlation with a low number of mismatched DRB1 eplets. This low detection rate of anti-DRB1 antibodies may also be due to allograft absorption. In contrast, antibodies to DRB3/4/5 mismatches were more common. Especially, 83% of the DRB4 (DR53) mismatches resulted in detectable antibodies against an eplet uniquely found on DR53 antigens.
Donor-specific DQB mismatches led to detectable anti-DQB antibodies with a frequency of 87%. Their specificity correlated with eplets uniquely found on DQ1-4. The incidence of antibodies induced by 2-digit DQA mismatches was 64% and several eplets appeared to play a dominant role. These findings suggest that both α and β chains of HLA-DQ heterodimers have immunogenic epitopes that can elicit specific antibodies. About one-third of the sera had anti-DP antibodies; they reacted primarily with two DPB eplets and an allelic pair of DPA eplets.
These data demonstrate that HLA class II reactive sera display distinct specificity patterns associated with structurally defined epitopes on different HLA-D alleles.
Introduction
Humoral immune responses to class II HLA antigens affect the outcome of various types of organ transplants. Preformed anti-donor class II antibodies increase the risk of transplant failure [1–9] and the post-transplant development of anti-class II antibodies is associated with a higher incidence of acute and chronic rejection [10–19]
Current class II matching strategies for kidney transplantation consider only the HLA-DR antigens controlled by the DRB1 locus but mismatching for HLA-DQ and HLA-DP may also lead to lower graft survival rates [20–25]. Newer serum screening methods such as ELISA, Flow Cytometry and Luminex have greatly enhanced the detection of anti-HLA-DQ and HLA-DP antibodies and their association with transplant rejection [2, 7, 26–29]. Nevertheless, the clinical relevance of these anti-class II antibodies has remained a controversial issue.
Antibodies react with epitopes on antigenic molecules and a characterization of the antibody response to class II epitopes rather than antigens seems important for the management of sensitized patients considered for retransplantation. In this report we address the question whether in the presence of the allograft, circulating antibodies can be detected that are specific for epitopes on donor HLA-DR, HLA-DQ and HLA-DP mismatches. Class II antigens have generally lower levels of tissue expression than class I antigens and this may affect the ability of the allograft to absorb donor-specific anti-class II antibodies. Serum testing for antibodies was done with a highly sensitive antibody-binding assay with single allele panels using the Luminex platform [30]. Antibody reactivity patterns were analyzed with HLAMatchmaker, a structural matching algorithm that considers amino acid residue polymorphisms to define epitopes recognized by antibodies. We have applied a recent version that uses so-called eplets defined by molecular surface-exposed polymorphic residues surrounded by residues within a three-Angstrom radius as previously described [31, 32]. The data demonstrate distinct antibody specificity patterns associated with eplets on donor class II antigens encoded by the different HLA-D loci.
Patients and Methods
Patients
This analysis was done for 75 class II sensitized patients with different types of failed allografts including sixty kidney, four liver, four heart, two lung, two pancreas and three small bowel transplants. All patients had become candidates for retransplantation and their transplants were still present. A second group consisted of 38 class II sensitized patients who did not have a transplant, including 9 patients from whom the allograft had been removed. This study was approved by the Institutional Review Board of the University of Pittsburgh Medical Center.
Determination of HLA-DR, -DQ and -DP types
HLA typings of patients and donors were done by standard DNA-based methods and considered only alleles reported as most common in the US population [33]. Since the HLAMatchmaker analysis requires high-resolution (4-digit) types, we have typed as many possible subjects at this level for DRB1, 3, 4, 5 and DQB1. In other cases, the HLAMatchmaker program can assign 4-digit types on the basis of most frequent DRB1-DRB3/4/5-DQB1 combinations according to recently published data about HLA class II haplotype frequencies in different populations [34–36]. The same linkage disequilibrium-based approach was used for assigning 4-digit DQA1 types. An analysis of 59 class II typings has shown that at the 2-digit level, 98% of the predicted DQA1 alleles agreed with the actual typing results and there was a 91% concordance at the 4-digit level (data not shown). We conclude that the prediction model to assign DQA1 alleles is highly reliable. A small group of patients (N=34) and donors (N=9) were DNA-typed for HLA-DPB1 because these patients had shown anti-DP antibodies. No typing was done for DPA.
Serum Reactivity Assays
All sera showed anti-class II antibody activity determined by screening with HLA antigen mixtures in Elisa and/or Luminex assays by standard methods. Antibody specificity was determined with Luminex assays using single allele kits supplied by two commercial vendors (One Lambda, Inc., Canoga Park, CA; Tepnel Life Codes Corporation, Stamford, CT). This combination offers two advantages. First is the opportunity to compare the reactivity pattern for each allele shared by each kit. This antibody detection technology is rather new and it is possible that certain allele preparations give aberrant results. Indeed, our experience has shown major discrepancies for one DRB3*0101 preparation which had a contaminating DRB3*04 allele and one DQB1*0301 preparation had weak reactivity; they were excluded from our analysis. Other preparations showed minor discrepancies such as comparatively low or high reactivity but this did not interfere with our antibody specificity analysis. The second advantage was that one kit had allelic combinations that were not present in the other kit; this applied especially to the DQ and DP preparations. As shown in Table 1, the combined sets had 26 distinct DRB alleles, 33 unique DQA-DQB heterodimers and 27 unique DPA-DPB heterodimers. For many sera, this combination allowed a more precise analysis of antibody specificity than one kit alone.
Table 1.
Class II allele distribution in two commercial Luminex kits
| Class II Gene Product | Both Kits | Tepnel a | One Lambda b | Total |
|---|---|---|---|---|
| Unique DRB allele | 20 | 0 | 6 | 26 |
| Unique DQA-DQB heterodimer | 2 | 15 | 16 | 33 |
| Unique DPA-DPB heterodimer | 9 | 14 | 4 | 27 |
Tepnel LifeCodes LSATM Class II Lot 01207
OneLambda LABScreenTM Lot #004
HLAMatchmaker Analysis of Serum Reactivity with Class II Panels
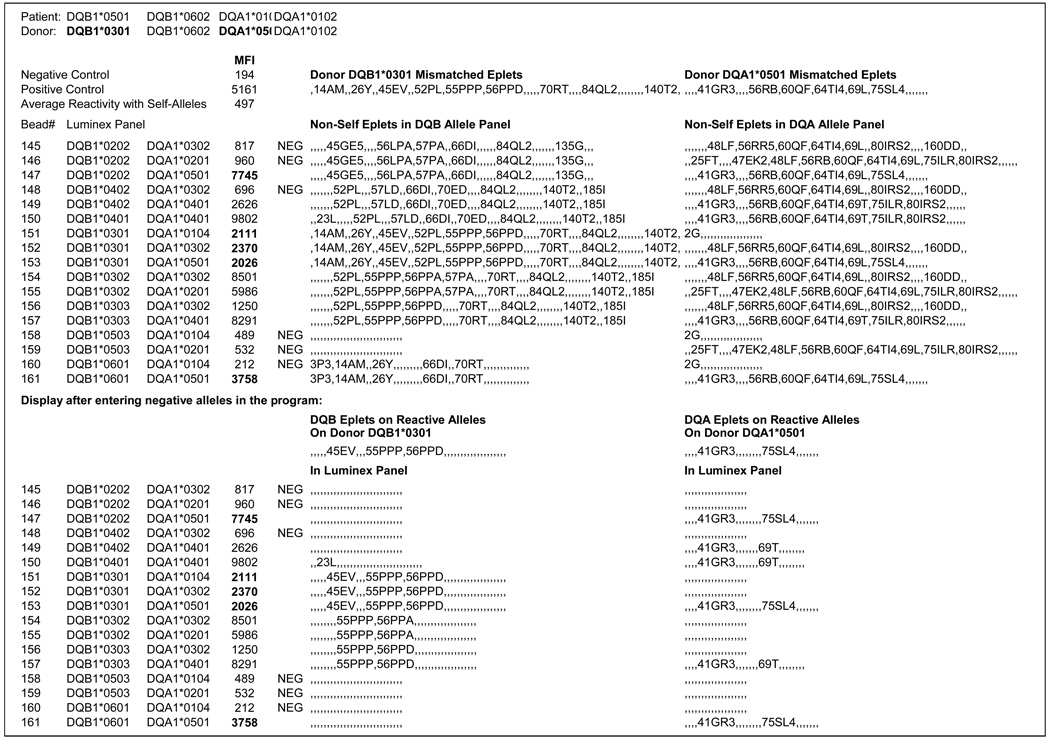
Different HLAMatchmaker programs can be downloaded from the www.tpis.edu website. We have used a program to analyze serum reactivity patterns with Luminex single class II alleles. Figure 1 is an example of a reactivity pattern with DQ heterodimers in the Tepnel panel. The patient who typed as DQB1*0501, 0602; DQA1*0101, 0102 had received a kidney transplant from a one-haplotype matched related donor with a mismatched DQB1*301, DQA1*0501 combination. The mismatched DQB eplets are 14AM, 26Y, 45EV, 52PL, 55PPP, 56PPD, 70RT, 84QL2 and 140T2 and DQA eplets are 41GR3, 56RB, 60QF, 64TI4, 69L and 75SL4. (See Footnote1). Any of these eplets may have the potential of inducing specific antibodies. This was determined by analyzing the antibody reactivity with the panel. Serum reactions are shown as MFI values and those above two times the average reactivity with self-alleles (in this case 2 ×497) were considered positive. The panel had 17 DQ heterodimers and Figure 1 shows for each one which eplets are non-self for this patient. Six heterodimers gave negative reactions; their non-self eplets were considered non-reactive. The negative DQB and DQA alleles were recorded and the computer program then deleted the non-reactive eplets from the donor and panel alleles. The bottom half of Figure 1 shows the remaining alleles on the reactive alleles. It can be readily seen that DQB1*0301 (DQ7), *0302 (DQ8) and *0303 (DQ9) share 55PPP, an eplet uniquely found on all DQ3 molecules. DQB1*0302 was especially informative because it shared only 55PPP with the immunizing DQB1*0301. Two eplets 45EV (unique for DQ7) and 56PPD (shared between DQ7 and DQ9) are also on reactive alleles but no informative DQB alleles were in the overall panel to rule out antibodies against these eplets. We conclude that DQ7, 8 and 9 are unacceptable mismatches because of anti-55PPP reactive antibodies. No antibody reactivity was seen with other eplets on the immunizing DQB1*0301 namely, 14AM, 26Y, 70RT, 84QL2 and 140T2.. These eplets are acceptable mismatches.
Figure 1.
Example of an HLAMatchmaker analysis of serum reactivity with a Luminex panel of HLA-DQ heterodimers. This patient had a DQB1*0301, DQA1*0501 mismatched kidney allograft. The upper half of this figure shows eplets on the donor and panel HLA-DQ antigens that are non-self for the patient. Spaces between two commas (, ,) indicate locations of self-eplets. Serum reactivity is shown as MFI (Mean Fluorescence Intensity) values. Reactivity of Luminex preparations that share donor DQ alleles are depicted in bold font. After the negatively reacting alleles have been recorded, the program removes all their presumably no-reactive eplets from the donor and panel alleles. The remaining eplets on reactive alleles are shown in the bottom half of this figure.
This serum had also donor-specific anti-DQA1 reactivity and there were two eplets on reactive alleles, namely 41GR3 (shared by DQA1*04, *05 and *06) and 75SL4 (on DQA1*05). This suggests that DQA1*04, *05 and *06 are unacceptable mismatches for this patient. The remaining DQA eplets 56RB, 60QF, 64TI4 and 69L appear to be acceptable mismatches.
These findings illustrate that the antibody response generally involve a limited repertoire of eplets on the immunizing allele. The characterization of epitope specificity provides a more affirmative and comprehensive assessment of mismatch acceptability.
Statistical methods
Differences in serum reactivity patterns and eplet numbers were compared using two-tailed Student t-test and Fischer's exact test.
Results
Incidence of HLA-DR, -DQ and –DP antibodies in HLA-class II sensitized patients
The initial analysis was done on two groups of HLA class II sensitized patients. The first had antibodies induced during pregnancy, after blood transfusion and/or a previous transplant that had been removed. There were 38 cases and the frequencies of antibodies to HLA-DR, HLA-DQ and HLA-DP were 92%, 84% and 39%, respectively (Table 2). The second group consisted of 75 patients in whom the transplanted organ was still present, 32 of them had a primary allograft and no detectable pre-transplant antibodies.
Table 2.
Incidence of Anti-HLA-DR, -DQ and -DP Antibodies in HLA Class II Sensitized Patients with or without a Transplant Present
| Number of Cases | Anti-HLA-DR antibodies |
Anti-HLA-DQ antibodies |
Anti-HLA-DP antibodies |
|
|---|---|---|---|---|
| Transplant absent | 38 | 92%* | 84% | 39% |
| Transplant present | 75 | 63%* | 92% | 32% |
| Donor -Specific Responses | 29/75 (39%)** | 58/74 (78%)** |
Chi Square=10.99, p= 0.001
Chi Square= 24.2, p<0.0001
The incidence of anti-HLA-DR antibodies was lower in the group of patients with a transplant (63% vs 92%, p=0.001). On the other hand, antibody reactivity with HLA-DQ and HLA-DP had similar incidences in both groups.
In patients with an allograft, donor-specific anti-HLA-DR antibodies are much less readily detected than donor-specific anti-HLA-DQ antibodies (39%.vs 78%, p<0.0001). This lower incidence was seen for all types of transplanted organs and appeared unrelated to the time post-transplantation.
The next step of this analysis was the determination of how structurally defined epitope differences may affect the formation of donor-specific HLA class II antibodies.
Eplet differences and antibodies to DRB1, DRB3, DRB4 and DRB5 mismatches
This HLAMatchmaker analysis has shown that in transplant recipients, the detection of donor-specific anti-DRB antibodies correlates with the number of mismatched donor DRB1/3/4/5 eplets (Table 3). Patients with DRB antibodies were exposed to twice as many mismatched eplets than those who did not show antibodies (21.4 ± 8.0 vs10.6 ± 8.0, p<0.0001). An analysis of the individual DRB loci yielded data that indicated different contributions to the reactivity patterns of class II antibodies. There were 96 mismatched DRB1 antigens (as defined by UNOS criteria) but only 23 (24%) reacted with patient antibodies and they had significantly higher numbers of mismatched eplets than the DRB1 mismatches without detectable antibodies (8.5 ± 2.7 vs 6.2 ± 3.7, p=0.003).
Table 3.
Effect of eplet mismatching and the Incidence of antibodies to donor DRB1, DRB3,DRB4 and DRB5 mismatches
| Mismatch | Donor-Specific Antibodies |
No Donor-Specific Antibodies |
Significance* |
|---|---|---|---|
| DRB1/3/4/5 | 29 (39%) | 46 (61%) | |
| Nr of Mismatched Eplets | 21.4 ± 8.0 | 10.6 ± 7.8 | p<0.0001 |
| DRB1 | 23/96 (24%) | 73/96 (76%) | |
| Nr of Mismatched Eplets | 8.5 ± 2.7 | 6.2 ± 3.7 | p=0.003 |
| DRB5 (DR51) | 4/8 (50%) | 4/8 (50%) | |
| Nr of Mismatched Eplets | 9.5 ± 1.3 | 10.0 ± 3.6 | p=0.80 (NS) |
| DRB3 (DR52) | 6/13 (46%) | 7/13(54%) | |
| Nr of Mismatched Eplets | 11.5 ± 1.2 | 11.0 ± 0.8 | p=0.42 (NS) |
| Within DRB3 | 1/17 (6%) | 16/17 (94%) | |
| Nr of Mismatched Eplets | 3 | 5 .0 ± 1.2 | NS |
| DRB4 (DR53) | 15/18 (83%) | 3/18 (17%) | |
| Nr of Mismatched Eplets | 12.6 ± 2.1 | 13.4 ± 2.3 | p=0.64 (NS) |
Student's t-test assuming unequal variances, NS: not significant
About one-half of the DR51 (DRB5) and DR52 (DRB3) mismatches showed detectable antibodies. Their numbers of mismatched eplets were similar tot those with DR51 and DR52 mismatches showing undetectable antibodies. Table 3 shows also that DR51 and DR52 antigen mismatches have higher numbers of mismatched eplets than DRB1 antigen mismatches (p<0.0001). DRB3 has three 2-digit alleles DRB3*01, *02 and *03 and mouse monoclonal antibodies have been produced to two of them, DRB3*01 (DR52a) and DRB3*02 (DR52b) [37, 38]. These antibodies react with epitopes associated with unique eplets, namely 183A2 and 51R2, respectively [32]. An analysis of 17 cases whereby the donor was mismatched within DRB3, mostly DRB3*01 into DRB3*02 or vice versa, but only one patient had detectable antibodies. A possible explanation for this low antibody incidence might be that intra-DRB3 mismatches involve only about five eplets.
Most striking was the 83% incidence of antibodies against donor DR53 (DRB4) mismatches (Table 3). The numbers of mismatched eplets was significantly higher than for the DRB1 mismatches (p<0.0001), or the DR51/52mismatches (p=0.0006). DR53 has seven unique eplets, 25HWN, 44NL, 48YQ, 98QM, 180MM and 187Q, [32]; they are collectively referred to as 48YQ7. Five cases showed antibody reactivity with only 48YQ7, but no informative alleles were available to determine which of the seven DR53 eplets were recognized. There were 10 cases with antibody reactivity with other DR53 eplets besides 48QY7. For instance, the eplet 4Q is shared between DR53, DR7 and DR9; this means that a 4Q-specific antibody reacts with all three antigens. There were 7 such cases with antibodies to 4Q and for 6 of them, the donor typed also for DR7 or DR9. It is possible that this DR7/9 reactivity is due to antibodies to the 4Q epitope shared with and perhaps induced by DR53. This could mean that the incidence of detectable antibodies induced by donor DRB1 antigens might be even lower that the 24% shown in Table 3.
Altogether, the donor-specific DRB antibody reactivity patterns suggest a predominant recognition of DR53 followed by DR51 and DR52, whereas anti-DRB1 antibodies are less readily detectable. These data indicate also an association between the number of mismatched eplets and the detection of antibodies reacting with donor antigens. They are similar to the reported correlations between HLA antibody responses and the number of structurally defined mismatched epitopes (triplets or amino acid residues) on transplant donor antigens [39–41]. However, antibody absorption by the allograft may also explain the low detection rate of donor-specific anti-DR antibodies. Removal of the allograft is associated with an increase of circulating donor-specific anti-class I antibodies [42] and our preliminary studies still in progress, have shown that this is also the case for donor-specific anti-DR antibodies (data not shown). Such studies may reveal information which DRB epitopes are likely to induce specific antibody responses.
Eplet mismatching and anti-HLA-DQ antibodies
This analysis showed an overall 78% incidence of donor-specific anti-HLA-DQ antibodies in transplant recipients (Table 2). We addressed the question how often such antibodies reacted with donor DQB and DQA alleles and what eplets might be dominantly involved. Table 4 shows that at the 2-digit level, DQB mismatches induced more antibody responses than DQA mismatches (87% vs 64%; chi-square= 9.82, p= 0.002). The incidence of antibodies to DQ1, DQ2, DQ3 and DQ4 was comparable as were the numbers of mismatched eplets in each group. The absence of anti-DQ antibodies did not correlate with lower numbers of mismatched eplets. There were 18 cases with intra-DQ1 mismatches, i.e. within DQ5 and/or DQ6, and they had fewer mismatched eplets than the overall 2-digit DQB mismatch group (5.7 ± 3.6 vs 10.2 ± 3.3, p<0.0001). Not surprisingly, donor-specific antibodies were detected in only 4 cases (22%) This analysis has also revealed a rather high frequency (64%) of antibodies against donor DQA alleles (Table 4). It seemed higher for DQA1*04 and *05 than for DQA1*01, *02 and *03 mismatches, but there were no significant differences between the numbers of mismatched eplets in these groups. There were 16 cases with intralocus DQA1*01 mismatches; they involved low numbers of eplets and showed a very low incidence (13%) of antibodies.
Table 4.
Eplet mismatching and the incidence of donor-specific anti-HLA-DQ antibodies
| Donor DQ mismatch | Nr of cases | Antibody Incidence | Number of mismatched Eplets |
|---|---|---|---|
| All 2 digit DQB alleles | 62 | 87% | 10.2 ± 3.3 |
| DQ1 (DQB1*05/06) | 18 | 89% | 10.0 ± 4.3 |
| Within DQB1*05/*06 | 18 | 25% | 5.7 ± 3.6 * |
| DQ2 (DQB1*02) | 24 | 88% | 9.9 ± 2.3 |
| DQ3 (DQB1*03) | 14 | 79% | 11.8 ± 2.4 |
| DQ4 (DQB1*04) | 6 | 100% | 8.7 ± 3.7 |
| All 2 digit DQA alleles | 74 | 64% | 11.4 ± 4.9 |
| DQA1*01 | 22 | 59% | 13.6 ± 5.5 |
| within DQA1*01 | 16 | 13% | 2.9 ± 1.0 ** |
| DQA1*02 | 16 | 56% | 9.8 ± 4.4 |
| DQA1*03 | 10 | 50% | 11.6 ± 4.9 |
| DQA1*04 | 6 | 83% | 9.0 ± 5.6 |
| DQA1*05 | 19 | 74% | 10.9 ± 3.9 |
p<0.0001
p<0.0001
These findings demonstrate that donor-specific anti-HLA-DQ antibodies are readily detectable in sera from patients with an allograft in situ. To address the structural basis of HLA-DQ immunogenicity we have also determined which mismatched DQ eplets were most frequently associated with donor-specific antibody reactivity. Table 5 shows the number of cases when a donor eplet was mismatched and how often this eplet correlated with antibody reactivity. Four DQB eplets 52PQ3, 45GE5, 55PPP and 79ED2 correspond to the originally defined serologically defined specificities DQ1-DQ4 and they were most frequently found on antibody reactive alleles, about 80% antibody incidence or higher. They appear to represent the most immunodominant DQB epitopes. Four eplets seemed to have an intermediate level of immunogenicity. Two of them, 14GL5 and 70GT, are on DQ1 subtypes DQ5 and DQ6 and their frequency on antibody reactive alleles was 50% and 68%, respectively. The 45EV eplet uniquely found on DQ7 had a 62% frequency of antibody reactivity. DQ2 and DQ8 share 57PA which had a 50% antibody incidence. Six eplets displayed low immunogenicity as indicated by the 20–30% frequencies of reactive antibodies. The remaining DQB eplets had antibody reactivity frequencies of less than 15%, and they were considered relatively non-immunogenic.
Table 5.
Frequencies of antibodies to donor DQ eplet mismatches
| DQB Eplet | Eplet on | Nr of cases | Antibody Frequency | DQA Eplet | Eplet on | Nr of cases | Antibody Frequency |
|---|---|---|---|---|---|---|---|
| 79ED2 | DQ4 | 6 | 100% | 160AE | DQA1*0501/5 | 15 | 80% |
| 52PQ3 | DQ1 | 18 | 89% | 41GR3 | DQA1*04 *05 *06 | 23 | 74% |
| 45GE5 | DQ2 | 24 | 88% | 75SL4 | DQA1*05 | 21 | 67% |
| 55PPP | DQ3 | 14 | 79% | 47EK2 | DQA1*02 | 16 | 56% |
| 70GT | DQB1*0602/3 | 19 | 68% | 50EF11 | DQA1*01 | 21 | 45% |
| 45EV | DQ7 | 16 | 63% | 48LF | DQA1*02 *03 | 14 | 43% |
| 57PA | DQ2,8 | 24 | 50% | 56RR5 | DQA1*03 | 10 | 40% |
| 14GL5 | DQ5 | 12 | 50% | 69L | DQA1*02 *03 *05 | 17 | 35% |
| 77DR | DQ2,5 | 27 | 30% | 60QF5 | DQA1*02 *03 *04 *05 *06 | 22 | 32% |
| 45GV | DQ1,4,8,9 | 14 | 29% | 80IRS2 | DQA1*02 *03 *04 *06 | 20 | 30% |
| 74SV2 | DQ4,5 | 11 | 29% | 75ILR | DQA1*02 *04 *06 | 17 | 29% |
| 140A2 | DQ2,6 | 15 | 27% | 56RB | DQA1*02 *04 *05 *06 | 31 | 23% |
| 52PL3 | DQ3,4 | 15 | 27% | 47ERW | DQA1*0101/4/5 | 10 | 10% |
| 84QL2 | DQ2,3,4 | 13 | 23% | 34HE | DQA1*0101/4/5 *02 *03 | 15 | 7% |
| 74EL2 | DQ3,6 | 14 | 14% | 25YT | DQA1*01/2/4 *04 *05 | 24 | 4% |
| 14GM | DQ2,4,6,8 | 15 | 13% | 41ER | DQA1*0101/2/4/5 *02 *03 | 12 | 0% |
| 26L | DQ2,8,9 DQB1*0602/3/4/9 | 18 | 11% | 160AD | DQA1*01 *02 *0301 *04 *06 | 16 | 0% |
| 66DI | DQ2,4,6s | 25 | 8% | ||||
| 70RT | DQB1*0602 *0603 | 15 | 0% | ||||
| 26YL3 | DQB1*03 *0601/4/9 | 12 | 0% |
DQA eplets displayed somewhat lower levels of immunogenicity. Mismatches for 180AE, 41GR3, 75SL4 and 47EK2 resulted in the highest incidence of specific antibodies, about 65–80% (Table 5). They appear to be the most immunogenic among DQA eplets. Interestingly, three of them are on DQA1*05. Eight eplets showed an incidence of antibody reactivity ranging from 23–45%. For five eplets, the antibody incidence was 10% or less. They were considered largely non immunogenic. Interestingly, two allelic eplets displayed opposite immunogenicity: a 160AE mismatch led to antibodies with an 80% frequency but a 160AD mismatch was not immunogenic.
In conclusion, these data demonstrate that HLA-DQ mismatching involves a repertoire of about 8–10 eplets that represent epitopes with considerable ability of inducing specific antibodies. Most immunodominant eplets appear to be equivalent to the serologically defined DQ specificities or the 2-digit DQA alleles. With notable exception for 41GR3 present on DQA1*04, *05 and *06, most eplets shared between multiple DQB or DQA antigens seem less immunodominant role in terms of antibody formation. This suggests that serological cross-reactivity might be less prevalent for DQ antigens.
Eplet specificity analysis of anti-DP antibodies
About 35% of the overall set of sera with class II antibodies reacted with DP alleles in the Luminex assay (Table 2). Epitope specificity analysis was done for 34 DPB-typed patients including 9 cases with DPB-mismatched donors. Their DPB antibody specificity patterns were almost always associated with the presence of the 84DEAV eplet and/or one or both 56DE/56EE eplets. Antibodies against 84DEAV reacted with 11 /15 (73%) different DPB alleles represented by the combined Luminex kits. The DPB types of 22 patients had DPB alleles that lack 84DEAV, namely DPB1*0201, *0401 and *0402 that carry 84GGPM and DPB1*1501 that has 84VGPM. Anti-84DEAV reactivity was detected in 17 (or 77%) of these patients including 5 of 6 cases whereby the donor was mismatched for a 84DEAV-carrying allele.(Table 6).
Table 6.
Predominant Eplets Reacting with anti-HLA-DP Antibodies
| Locus | Mismatched Eplet |
Eplet Carrying Alleles | Antibody Frequency |
Donor- Specific Antibody Frequency |
|---|---|---|---|---|
| DPB | 84DEAV | DPB1 *01 *03 *05 *06 *09 *10 *11 *13 *14 *16 *17 *19 *20 *21 *30 |
18/23 (78%) | 5/6 (83%) |
| DPB | 56ED/56EE | DPB1 *03 *06 *09 *14 *17 *20/ / *0201 *0402 *10 *16 *18 |
15/19 (79%) | 5/6 (83%) |
| DPB | 84DEAV and/or 56ED/56EE |
31/34 (91%) | 11/11 (100%) | |
| DPA | 51RA,83A | DPA1 *02 *04 | 18/56 (32%) | nd |
| DPA | 51QA,83T | DPA1 *01 *03 | 5/56/ (9%) | nd |
| DPA | Other Eplets | 0/33 (0%) | nd |
Position 56 has three eplets 56AE (on DPB1*01, *0202, *0401, *05, *11, *13, *15, *19, *21, *23, *30 and *40), 56DE (on DPB1*03, *06, *09, *14, *17 and *20) and 56EE (on DPB1*0201, *0402, *10, *16 and *18). There were 19 patients whose DPB alleles carried only 56AE, 15 of them (79%) had antibodies that reacted with 56DE and/or 56EE. They included 8 cases reacting with 84DEAV-positive alleles but the combined DPB panel could not distinguish between 84DEAV and 56DE specific antibodies. All of them reacted also with DRB1*1101 which shares 56DE with DPB alleles [43]. It seems that 56DE and 56EE represent cross-reacting epitopes: exposure to one of them may result in antibodies that react with both of them. In several cases however, the antibodies react with only 56DE or 55EE.
Altogether, specificity for 84DEAV and or 56DE/56EE accounted for the anti-DPB reactivity of in 31/34 (91%) cases. These findings suggest an immunodominance of these two DPB epitopes. The remaining cases showed antibody reactivity with three or four additional eplets (data not shown).
There are four two-digit DPA alleles, DPA1*01-*04 and they have considerably less amino acid sequence polymorphism than DPB and DQA. Patients with anti-DP antibodies show reactivity with DPA alleles that share one of two sets of DPA alleles (Table 6). The 51RA, 81A eplet combination is carried by DQA1*02 and DQA1*04 alleles and reacts with 12/52 (23%) of DP-reactive sera. The 51QA, 83T eplet combination present on DPA1*01 and DPA1*03 alleles reacts with 5/52 (10%) anti-DP sera. Since none of the remaining anti-DP sera react with other DPA1 eplets, it seems that anti-DPA antibody recognition involves a bi-allelic epitope.
Discussion
This is the first detailed analysis of how donor-specific HLA class II epitope mismatching affects antibody response patterns in transplant recipients considered for retransplantation. The application of a sensitive antibody test with a comprehensive panel of single alleles has yielded informative, donor-specific antibody reactivity patterns that were analyzed with a computer algorithm. HLAMatchmaker uses structurally defined eplets to describe epitopes that can react with specific antibodies [31, 32]. This analysis focused on the class II- specific antibody response although most patients also showed anti-class I antibodies (data not shown). The data have several features that provide a better understanding of the complexity of class II antibody reactivity patterns induced by a transplant. Our findings for the different class II loci can be summarized as follows:
DRB
Donor-specific, anti-DRB1 epitope antibodies were much less frequently detected than antibodies against other class II epitopes. Terasaki’s group has recently reported a similar observation [44]. The correlation between antibody absence and a low number of mismatched DRB1 eplets (Table 3) suggests many DR antigen mismatches may have a limited potential of inducing a humoral immune response. Another explanation for the low detection rate of circulating anti-HLA-DR antibodies is that they have been absorbed by the allograft which has been shown to express HLA-DR antigens on its endothelium and parenchymal cells [45–48] and that DRB antibodies can be eluted from rejected transplants [49]. Moreover, anti-DRB antibodies are more readily detected in class II sensitized patients in the absence of a transplanted organ (Table 2), and their frequency of antibody detection is similar to that of HLA-DQ.
Antibodies against donor-specific DRB3, 4 and 5 mismatches were more often detected. Especially striking is the high frequency of antibodies against DRB4 (DR53) eplets. Other studies have also shown the prevalence of anti-DR53 antibodies in transplant patients [28]. The frequent antibody response to DR53 is not really surprising because DR53 mismatches involves a large array of eplets including seven (represented by 48YQ7) that are all unique [32]. These findings suggest that DR53 is very immunogenic
Thus, the donor-specific, anti-DRB response in transplant recipients appears to have a certain hierarchy: DRB4 > DRB3 and DRB5 >DRB1. The current study cannot determine what eplets play a primary role in antibody responses to DRB mismatches because of the likelihood that many donor-specific anti-DRB antibodies might be undetectable because of absorbance by the allograft. Informative data would become available after graft removal. Under auspices of the 15th International Histocompatibility Workshop, a multi-laboratory collaborative study is underway to analyze donor-specific antibody reactivity patterns in patients who have undergone allograft nephrectomy. These studies are expected to provide a better understanding of DRB eplet immunogenicity in relation to the profound effect of HLA-DR compatibility on kidney retransplant outcome [50, 51] and the increased risk of graft failure due to anti-HLA-DR antibodies [44, 52].
DQB
Antibodies against HLA-DQ were much more common and this finding is consistent with data reported by other investigators [2, 7, 28, 53]. Our data indicate that 87% of class II antibody responses comprise antibodies to donor-specific, 2-digit DQB mismatches corresponding to DQ1-DQ4 but only 24% for the 2-digit DRB1 mismatches. This high anti-DQB reactivity correlates with more mismatched eplets for DQ1-DQ4 than for DRB1 (p<0.0001). An analysis of anti-DQB antibody reactivity patterns revealed the immunodominance of eplets uniquely present on these serologically defined DQ antigens. We identified four eplets, 52PQ3 (on DQ1), 45GE5 (on DQ2), 55PPP (on DQ3) and, 79ED2 (on DQ4) that are highly immunogenic. Four additional eplets 14GL5 (on DQ5), 47EV (on DQ7), 57PA (on DQ2) and DQ8) and 70GT (on DQB1*0602/3) are less immunogenic. On the other hand, many eplets shared between groups of DQB antigens appear not very immunogenic. This finding may suggest a lack of serological cross-reactivity between DQ antigens
DQA
Specific antibody detection was less common for DQA than DQB (64% vs 87%, p=0.002). Unpublished data from other investigators have also reported anti-DQA antibodies in transplant patients. Although there are no serological equivalents for DQA, we could readily identify at least seven eplets that are often enough associated with anti-DQA antibody reactivity. Most common eplets included 41GR3 (shared by DQA1*04, *05 and *06), 75SL4 (on DQA1*05), 47EK2 (on DQA1*02), 50EF11 (on DQA1*01) and.55RR5 (on DQA1*03). These findings suggest that both α and β chains of HLA-DQ heterodimers have immunogenic epitopes that can elicit specific antibodies.
DPB
Although complete HLA-DPB typing information was available for only 34 patients, this analysis has revealed distinct antibody reactivity patterns against structurally defined DPB epitopes. Two eplets dominated donor-specific anti-DPB antibody detection; 84DEAV and or 56DE/56EE reacted in more than 90% of the cases. These eplets correspond to well-defined serological epitopes recognized by monoclonal antibodies [43, 54–56]. Youngs has also reported a high frequency of anti-84DEAV antibodies in transplant patients [27]. Other DPB eplets seem less prevalent but more informative cases are needed to define their immunogenicity.
The immunodominant role of 84DEAV and 56DE/56EE seems relevant to DPB antigen mismatching and antibody formation. There are almost one-hundred 4-digit DPB1 alleles and about 25 are common in the United States [33]. One might expect that many donor-recipient combinations will be DPB mismatched at the allele level. However, many DPB allele mismatches will be compatible for these immunodominant epitopes and therefore, may not elicit anti-DPB antibody responses. This may explain why anti-DP antibodies have a lower detection rate than anti-DQ antibodies (see Table 2).
DPA
The HLA-DPA1locus has much less amino acid sequence polymorphism; there are only thirteen alleles in four groups: DPA1*01-04. Although no DPA typing was done for this analysis, the antibody reactivity with the combined Luminex panel showed two distinct specificity patterns against allelic eplet pairs 51RA/83A (on DPA1*02 and *04) and 51QA/83T (on DPA1*01 and *03). This suggests that DPA antigens have a simple bi-allelic epitope configuration similar to the Bw4/6 system of HLA-B. Accordingly, one might expect that patients with DPA types indicating homozygosity for these epitopes can, and those who are heterozygous cannot make anti-DPA antibodies.
Sufficient numbers of cases were available to determine antibody formation after exposure to mismatches within DRB3, DQ1 or DQA1*01. All three groups showed a low incidence of specific antibodies and they had relatively low numbers of mismatched eplets. These findings suggest intra-mismatching may result in fewer antibody responses.
Altogether, these data demonstrate that HLA class II reactive sera display distinct specificity patterns associated with structurally defined epitopes on different HLA-D alleles. Clinical studies are needed to see whether the determination of acceptable mismatches from epitope specificity patterns is a clinically useful approach to identify suitable donors for patients in need of a retransplant.
The epitope mismatch approach may also be useful in lowering the incidence of transplant failure due to humoral rejection. At present, class II compatibility considers only DRB1 antigens but each DRB mismatch has additional mismatches for DRB3/4/5, DQB/DQA and DPB/DPA Since these mismatches can now be assessed at the epitope level, it has now become possible to identify class II mismatches with low numbers of mismatched eplets. Such mismatches may reduce the class II specific antibody responses and perhaps improve transplant survival.
Acknowledgments
Funding Source: R01 grant AI-55933 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Certain eplets show a number at the end of their notation; it indicates that such eplet represents two or more eplets shared by the same antigen or group of antigens. For instance, 84QL2 represents two eplets 84QL and 90ETT; both are on DQ2, DQ3 and DQ4. For a patient with anti-82QL2 antibodies it is unknown whether they react with 84QL and/or 90ETT. We can conclude however that such antibodies react with the 84QL2 eplet shared by DQ2, DQ3 and DQ4 and these antigens should be considered unacceptable mismatches.
References
- 1.Takemoto S, Zeevi A, Feng S, Colvin R, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery R, Nickerson P, Platt J, Rabb H, Thistlethwaite R, Tyan D, Delmonico F. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004 Jul;4(7):1033–1041. doi: 10.1111/j.1600-6143.2004.00500.x. 2004. 4: p. 1033-1041. [DOI] [PubMed] [Google Scholar]
- 2.Schoenemann C, Groth J, Leverenz S, May G. HLA class I and class II antibodies:monitoring before and after kidney transplantation. Transplantation. 1998;65:1519–1524. doi: 10.1097/00007890-199806150-00024. [DOI] [PubMed] [Google Scholar]
- 3.Scornik JC, Zander DS, Baz MA, Donnelly WH, Staples ED. Susceptibility of lung transplants to preformed donor-specific HLA antibodies as detected by flow cytometry. Transplantation. 1999;68(10):1542–1546. doi: 10.1097/00007890-199911270-00018. [DOI] [PubMed] [Google Scholar]
- 4.Mahoney RJ, Taranto S, Edwards E. B-Cell Crossmatching and Kidney Allograft Outcome in 9031 United States Transplant Recipients. Hum Immunol. 2002;63:324–335. doi: 10.1016/s0198-8859(02)00363-4. [DOI] [PubMed] [Google Scholar]
- 5.Gebel H, Bray R, Nickerson P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: contraindication vs. risk. Amer. J. Transplant. 2003;3:1488–1500. doi: 10.1046/j.1600-6135.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 6.Susal C, Opelz G. Kidney graft failure and presensitization against HLA class I and class II antigens. Transplantation. 2002;73(8):1269–1273. doi: 10.1097/00007890-200204270-00014. [DOI] [PubMed] [Google Scholar]
- 7.Iniotaki-Theodoraki AG, Boletis JN, Trigas G, Kalogeropoulou HG, Kostakis AG, Stavropoulos-Giokas CG. Humoral immune reactivity against human leukocyte antigen (HLA)-DQ graft molecules in the early posttransplantation period. Transplantation. 2003;75(9):1601–1603. doi: 10.1097/01.TP.0000061611.51612.09. [DOI] [PubMed] [Google Scholar]
- 8.Itescu S, Tung TC, Burke EM, Weinberg A, Moazami N, Artrip JH, Suciu-Foca N, Rose EA, Oz MC, Michler RE. Preformed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of hear transplantation. Circulation. 1998;98(8):786–793. doi: 10.1161/01.cir.98.8.786. [DOI] [PubMed] [Google Scholar]
- 9.Pollinger HS, Stegall MD, Gloor JM, Moore SB, Degoey SR, Ploeger NA, Park WD. Kidney Transplantation in Patients with Antibodies against Donor HLA Class II. American Journal of Transplantation. 2007;7:857–863. doi: 10.1111/j.1600-6143.2006.01699.x. [DOI] [PubMed] [Google Scholar]
- 10.Terasaki PI. Humoral Theory of Transplantation. Amer. J. Transplant. 2003;3:665–673. doi: 10.1034/j.1600-6143.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee PC, Terasaki PI, Takemoto SK, Lee PH, Hung CJ, Chen YL, Tsai A, Lei HY. All chronic rejection failures of kidney transplants were preceded by the development of HLA antibodies. Transplantation. 2002;74(8):1192–1194. doi: 10.1097/00007890-200210270-00025. [DOI] [PubMed] [Google Scholar]
- 12.Vasilescu ER, Ho EK, de la Torre L, Itescu S, Marboe C, Cortesini R, Suciu-Foca N, Mancini D. HLA antibodies in heart transplantation. Transplant Immunol. 2004;12:177–183. doi: 10.1016/j.trim.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Muller-Steinhardt M, Fricke L, Kirchner H, Hoyer J, Kluter H. Monitoring of anti-HLA class I and II antibodies by flow cytometry in patients after first cadaveric kidney transplantation. Clinical Transplantation. 2000;14(1):85–89. doi: 10.1034/j.1399-0012.2000.140116.x. [DOI] [PubMed] [Google Scholar]
- 14.Zachary A, Montgomery R, Leffell M. Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum. Immunol. 2005;66:364–370. doi: 10.1016/j.humimm.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 15.Harmer AW, Heads AJ, Vaughn RW. Detection of HLA class I- and class II-specific antibodies by flow cytometry and PRA-STAT screening in renal transplant recipients. Transplantation. 1997;63(12):1828–1832. doi: 10.1097/00007890-199706270-00021. [DOI] [PubMed] [Google Scholar]
- 16.Reinsmoen N, Nelson K, Zeevi A. Anti-HLA antibody analysis and crossmatching in heart and lung transplantation. Transplant Immunology. 2004 doi: 10.1016/j.trim.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Tambur A, Pamboukian S, Costanzo M, Herrera N, Dunlap S, Montpetit M, Heroux A. The presence of HLA-directed antibodies after heart transplantation is associated with poor allograft outcome. Transplantation. 2005;80:1019–1025. doi: 10.1097/01.tp.0000180564.14050.49. [DOI] [PubMed] [Google Scholar]
- 18.Langan LL, Park LP, Hughes TL, Irish A, Luxton G, Witt CS, Christiansen FT. Post-Transplant HLA class II Antibodies and High Soluble CD30 Levels are Independently Associated with Poor Kidney Graft Survival. American Journal of Transplantation. 2007;7:847–856. doi: 10.1111/j.1600-6143.2006.01691.x. [DOI] [PubMed] [Google Scholar]
- 19.Campos E, Tedesco-Silva H, Machado P, Franco M, Medina-Pestana J, Gerbase-Lima M. Post-Transplant Anti0HLA Class II Antibodies as Risk Factor for Late Kideny Allograft Failure. Amer J Transplant. 2007;6:2316–2320. doi: 10.1111/j.1600-6143.2006.01503.x. [DOI] [PubMed] [Google Scholar]
- 20.Duquesnoy RJ, Annen K, Marrari M, Kauffman H.M. J. Association of MB compatibility with successful intrafamilial kidney transplantation. New England Journal of Medicine. 1980;302:821–825. doi: 10.1056/NEJM198004103021501. [DOI] [PubMed] [Google Scholar]
- 21.Matsuno N, Hidetoshi I, Ando A, T N, Sato T, Ichikawa S, Sonoda T, Tsuji K. Importance of DQB as indicator in living related kidney transplant. Transplantation. 1990;49:208–213. doi: 10.1097/00007890-199001000-00046. [DOI] [PubMed] [Google Scholar]
- 22.Tong JY, Hsia S, Parris GL, Nghiem DD, Cottington EM, Rudert WA, Trucco M. Molecular compatibility and renal graft survival--the HLA DQB1 genotyping. Transplantation. 1993;55(2):390–395. doi: 10.1097/00007890-199302000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Middleton D, Mytilineos D, Savage D, Ferrara GB, Angelini G, Ameroso A, Trainor F, Gaweco A, Mazzola G, Delfino L, Berrino M, Opelz G. Matching for HLA-DPB1 alleles in zero mismatched HLA-A, -B and -DR Renal Transplants. Transpl. Proc. 1992;24:2439–2440. [PubMed] [Google Scholar]
- 24.Mytilineos J, Deufel A, Opelz G. Clinical relevance of HLA-DPB locus matching for cadaver kidney retransplants: a report of the Collaborative Transplant Study. Transplantation. 1997;63(9):1351–1354. doi: 10.1097/00007890-199705150-00025. [DOI] [PubMed] [Google Scholar]
- 25.Laux G, Mansmann U, Deufel A, et al. A new epitope-based HLA-DP matching approach for cadaveric kidney transplantation. Transplantation. 2003;75:1527–1532. doi: 10.1097/01.TP.0000061759.57702.8A. [DOI] [PubMed] [Google Scholar]
- 26.Arnold M-L, Pei R, Spriewald B, Wassmuth R. Anti-HLA class II antibodies in kidney retransplant patients. Tissue Antigens. 2005;65:370–379. doi: 10.1111/j.1399-0039.2005.00382.x. [DOI] [PubMed] [Google Scholar]
- 27.Youngs D. DP alloantibodies. ASHI Quarterly. 2004(2):60–62. [Google Scholar]
- 28.Worthington JE, Martin S, Al-Husseini DM, Dyer PA, Johnson RW. Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation. 2003;75(7):1034–1040. doi: 10.1097/01.TP.0000055833.65192.3B. [DOI] [PubMed] [Google Scholar]
- 29.Leech SH, Mather PJ, Eisen HJ, Pina IL, Margulies KB, Bove AA, Jeevanandam V. Donor-Specific HLA Antibodies After Transplantation are Associated with Deterioration in Cardiac Function. Clinical Transplantation. 1996;10(6 Part 2):639–645. [PubMed] [Google Scholar]
- 30.Pei R, Lee JH, Shih NJ, Chen M, Terasaki PI. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation. 2003;75(1):43–49. doi: 10.1097/00007890-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 31.Duquesnoy R. A Structurally Based Approach to Determine HLA Compatibility at the Humoral Immune Level. Human Immunol. 2006;67:847–862. doi: 10.1016/j.humimm.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duquesnoy RJ, Askar M. HLAMatchmaker: A Molecularly Based Algorithm for Histocompatibility Determination V. Eplet Matching for HLA-DR, HLA-DQ and HLA-DP. Human Immunol. 2007;68:12–25. doi: 10.1016/j.humimm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cano P, Klitz W, Mack S, Maiers M, Marsh S, Noreen H, Reed E, Senitzer D, Setterholm M, Smith A, Fernandez-Vina M. Common and Weill-Documented HLA Alleles. Report of the Ad-Hoc Committee of the American Society fro Histocompatibility and Immunogenetics. Human Immunol. 2007;68:392–417. doi: 10.1016/j.humimm.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Vina M, Moraes JR, Moraes ME, Miller S, Stastny P. HLA class II haplotypes in Amerindians and in black North and South Americans. Tissue Antigens. 1991;38(5):235–237. doi: 10.1111/j.1399-0039.1991.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 35.Clayton J, Lonjou C, Whittle D. DRB1-DQA1-DQB1 haplotype frequencies. In: Charron D, editor. HLA Genetic diversity of HLA. Functional and Medical Implication. Paris, France: EDK; 1997. pp. 769–772. [Google Scholar]
- 36.Klitz W, Maiers M, Spellman S, Baxter-Lowe LA, Schmeckpeper B, Williams TM, Fernandez-Vina M. New HLA haplotype frequency reference standards: high-resolution and large sample typing of HLA DR-DQ haplotypes in a sample of European Americans. Tissue Antigens. 2003;62(4):296–307. doi: 10.1034/j.1399-0039.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 37.Fuggle SV, Carter C, Watts F, Kirkley J, Morris PJ. Monoclonal antibody definition of multiple polymorphic epitopes on HLA-DRw52. Human Immunol. 1987;19:255–263. doi: 10.1016/0198-8859(87)90107-8. [DOI] [PubMed] [Google Scholar]
- 38.Taylor CJ, Ugozolli L, Tanigaki N, Tosi G, Bunce M, Ting A, Ferrara GB. Antigen Sociey #29 Report (DRw52) In: Dupont B, editor. Immunobiology of HLA. Volume I: Histocompatibility Testing 1987. 1987. pp. 273–275. [Google Scholar]
- 39.Dankers MKA, Witvliet MD, Roelen DL, De Lange P, Korfage N, Persijn GG, Duquesnoy RJ, Doxiadis IIN, Claas FHJ. The Number of Amino Acid Triplet Differences between Patient aand Donor is Predictive for the Antibody Reactivity Against Mismatched HLA Antigens. Transplantation. 2004;I28:1236–1239. doi: 10.1097/01.tp.0000120385.03278.28. [DOI] [PubMed] [Google Scholar]
- 40.Mihaylova A, Baltadjieva D, Boneva P, Ivanova M, Penkova K, Marinova D, Mihailova S, Paskalev E, Simeonov P, Naumova E. Clinical Relevance of Anti-HLA Antibodies Detected by Flow-Cytometry Bead-Based Assays—Single-Center Experience. Human Immunology. 2006;67:787–794. doi: 10.1016/j.humimm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Adorno D, Canossi A, Piazza A, Poggi E, Papola F, Di Rocco M, Liberatore G, Del Beato T, Ozzella G, Anaclerio M, Casciani CU. The Role of beta-Pleated Sheet DRB1 Differences in Acute Rejection After Cadaveric Renal Transplant. Transplantation Proceedings. 1999;31:730–733. doi: 10.1016/s0041-1345(98)01745-x. [DOI] [PubMed] [Google Scholar]
- 42.Adeyi OE, Girnita A, Awadalla Y, Askar M, Shapiro R, Howe J, Martell J, Zeevi A, Nalesnik M, Rhandawa P, Demetris AJ, Duquesnoy RJ. Serum Analysis After Kidney Transplant Nephrectomy Reveals Restricted Antibody Specificity Patterns Against Donor HLA Class I Antigens. Transpl. Immunol. 2005;14:53–62. doi: 10.1016/j.trim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Drover S, Codner D, Gamburg J, Hutchings L, Marshall W. A Site-specific Anti-HLA-DP Monoclonal Antibody Recognizes Molecules Bearing 'DE' at Positions 55 and 56 on the Beta Chain. Tissue Antigens. 1991;38:37–40. doi: 10.1111/j.1399-0039.1991.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 44.Cai J, Terasaki P, Mao Q, Pham T, El-Awar N, Lee J-H, Rebellato L. Development of Nondonor-Specific HLA-DR Antibodies in allograft Recipients is Associated with Shared Epitopes with Mismatched Donor DR Antigens. Amer J Transplant. 2006;6:2947–2954. doi: 10.1111/j.1600-6143.2006.01560.x. [DOI] [PubMed] [Google Scholar]
- 45.Häyry P, Von Willebrand E. The Influence of the Pattern of Inflammation and Administration of Steroids on Class II MHC Antigen Expression in Renal Transplants. Transplantation. 1986;42:358–364. doi: 10.1097/00007890-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Manfro RC, Gonçalves LFS, Rauber M, Moura LAR. Analysis of ICAM-1 and HLA-DR expression on renal allograft aspirates. Clinical Transplantation. 1996;10:379–383. [PubMed] [Google Scholar]
- 47.Muczynski K, Ekle D, Coder D, Anderson S. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells. characterization, isolation, and regulation of MHC class II expression. J Am Soc Nephrol. 2003;14:1336–1348. doi: 10.1097/01.asn.0000061778.08085.9f. [DOI] [PubMed] [Google Scholar]
- 48.Michaels P, Espejo M, Kobashigawa J, Alejos J, Burch C, Takemoto S, Reed E, Fishbein MC. Humoral rejection incardiac transplantation: risk factors, hemodynamic consequences and relationship to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:58–69. doi: 10.1016/s1053-2498(02)00472-2. [DOI] [PubMed] [Google Scholar]
- 49.Scornik J, Lefor W, Cicciarelli J, et al. Hyperacute and acute kidney graft rejection associated with antibodies to B cells. Transplantation. 1992;54:61–64. doi: 10.1097/00007890-199207000-00010. [DOI] [PubMed] [Google Scholar]
- 50.Cecka JM, Terasaki PI. Repeating HLA antigen mismatches in renal retransplants--a second class mistake? Transplantation. 1994;57(4):515–519. [PubMed] [Google Scholar]
- 51.Thompson J, Thacker L, Krishnan G. Human leukocyte antigens DR and AB and kidney retransplantation. Transplantation. 2003;75:718–723. doi: 10.1097/01.TP.0000048376.79803.C1. [DOI] [PubMed] [Google Scholar]
- 52.Fuller A, Profaizer T, Roberts L, Fuller TC. Repeat donor HLA-DR mismatches in renal transplantation: is the increased failure rate caused by noncytotoxic HLA-DR alloantibodies? Transplantation. 1999;68(4):589–591. doi: 10.1097/00007890-199908270-00027. [DOI] [PubMed] [Google Scholar]
- 53.Taylor C, Chapman J, Fuggle S, Ting A, Morris P. A positive B cell crossmatch due to IgG anti-HLA-DQ antibody present at the time of transplantation in a successful renal allograft. Tissue Antigens. 1987;30:104–112. doi: 10.1111/j.1399-0039.1987.tb01605.x. [DOI] [PubMed] [Google Scholar]
- 54.Bodmer JG, Bodmer W, Heyes Jea. Identification of HLA-DP Polymorphism with DPβ and DPα Probes and Monoclonal Antibodies: Correlation with Primed Lymphocyte Typing. Proc. Nat.Acad. Sci. USA. 1987;84:4596–4600. doi: 10.1073/pnas.84.13.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu WY, Watts R, Karr RW. Identification of amino acids in HLA-DPw4β and -DR5β1 chains that are involved in antibody binding epitopes using site-directed mutagenesis and DNA-mediated gene transfer. Human Immunology. 1990;27(2):122–135. doi: 10.1016/0198-8859(90)90109-3. [DOI] [PubMed] [Google Scholar]
- 56.Marshall WH, Drover S, Codner D, Gamberg J, Copp MD, Liu HW, Deng LT, Younghusband HB. HLA-DP epitope typing using monoclonal antibodies. Human Immunology. 1998;59(3):189–197. doi: 10.1016/s0198-8859(98)00003-2. [DOI] [PubMed] [Google Scholar]