Abstract
Nuclear hormone receptors are hormone-regulated transcription factors that play critical roles in chordate development and homeostasis. Aberrant nuclear hormone receptors have been implicated as causal agents in a number of endocrine and neoplastic diseases. The syndrome of Resistance to Thyroid Hormone (RTH) is a human genetic disease characterized by an impaired physiological response to thyroid hormone. RTH is associated with diverse mutations in the thyroid hormone receptor β-gene. The resulting mutant receptors function as dominant negatives, interfering with the actions of normal thyroid hormone receptors coexpressed in the same cells. We report here that RTH receptors interact aberrantly with a newly recognized family of transcriptional corepressors variously denoted as nuclear receptor corepressor (N-CoR), retinoid X receptor interacting protein-13 (RIP-13), silencing mediator for retinoid and thyroid hormone receptors (SMRT), and thyroid hormone receptor-associating cofactor (TRAC). All RTH receptors tested exhibit an impaired ability to dissociate from corepressors in the presence of thyroid hormone. Two of the RTH mutations uncouple corepressor dissociation from hormone binding; two additional RTH mutants exhibit an unusually strong interaction with corepressor under all hormone conditions tested. Finally, artificial mutants that abolish corepressor binding abrogate the dominant negative activity of RTH mutants. We suggest that an altered corepressor interaction is likely to play a critical role in the dominant negative potency of RTH mutants and may contribute to the variable phenotype in this disorder.
INTRODUCTION
Nuclear hormone receptors are hormone-regulated transcription factors and include the thyroid hormone receptors (encoded by two different loci, T3Rα and T3Rβ), steroid receptors, and retinoid receptors (reviewed in Refs. 1–6). All nuclear hormone receptors contain a variety of motifs involved in DNA binding, hormone binding, receptor dimerization, and interactions with the transcriptional machinery (Fig. 1). On binding to specific target DNA sequences, nuclear hormone receptors modulate the expression of adjacent target genes (1–6). The nature of the transcriptional response (activation or repression) is dictated by cell type, promoter context, and hormone status (1–10). In most contexts T3Rs are transcriptional repressors in the absence of cognate hormone (T3) and activators in its presence (2, 7, 8, 11–15).
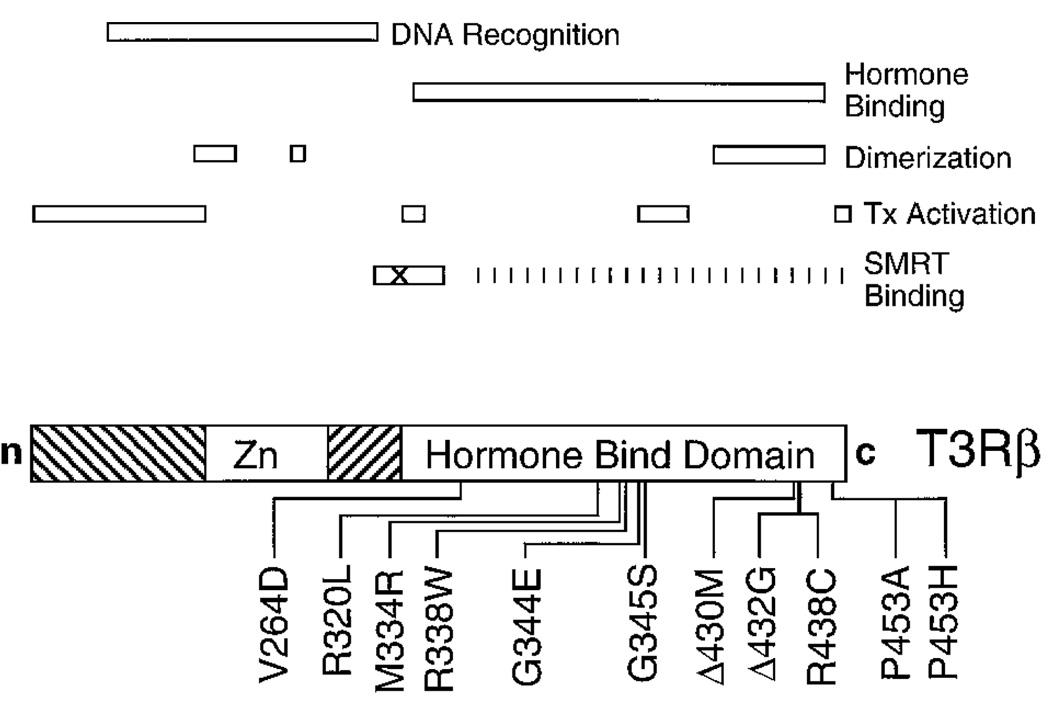
Fig. 1. Schematic Representation of the Human T3Rβ Protein and the Location of the RTH Mutations.
The T3Rβ is presented schematically from the N (n) terminus to C (c) terminus, with a central zinc-finger domain (Zn) indicated. The locations of the RTH mutations analyzed in this manuscript are indicated below. The regions of the wt receptor implicated in DNA recognition, hormone binding, receptor dimerizaton, transcriptional (Tx) activation, and SMRT corepressor binding are depicted above. X indicates the location of the site-directed mutations introduced to disrupt SMRT association (T3Rsad mutations; see text).
Some aspects of this transcriptional regulation are probably mediated by direct interactions between nuclear hormone receptors and the general transcriptional machinery (5). However, a series of specific ancillary proteins (denoted coactivators and corepressors) also appear to play critical roles in transcriptional control. For example, the transcriptional repression exhibited by T3Rs in the absence of hormone appears to require an association of the receptor with a family of corepressor proteins (variously denoted TRACs, SMRT, RIP13, or N-CoR) (11–18). T3R mutants unable to associate with these corepressors are unable to repress (11–15). Addition of T3 hormone converts T3Rs into transcriptional activators, a process that correlates with dissociation of the corepressors from the receptor and a corresponding recruitment of a novel set of proteins believed to function as coactivators (11–15, 19–25).
A variety of neoplastic and endocrine disorders are the consequence of aberrations in nuclear hormone receptor function (26–31). The syndrome of Resistance to Thyroid Hormone (RTH) is an autosomal dominant human endocrine disease (reviewed in Refs. 32–34). Individuals with RTH exhibit a failure to respond to elevated circulating thyroid hormone. This disorder is associated with diverse mutations at the T3Rβ locus, resulting in the synthesis of abnormal receptors that are impaired in hormone binding and in transcriptional activation (35–39). As a result, the RTH-T3Rs appear to function as dominant negatives, and interfere with the actions of the normal T3Rs synthesized from the unaffected T3Rα- and β-alleles (40–50). More than 30 different T3Rβ mutations have been associated with the RTH syndrome.
Nonetheless, the precise mechanism(s) by which RTH-T3Rs act as dominant negatives remains uncertain. In addition, a given RTH-T3R mutation can have very different phenotypic consequences in different individuals or in different tissues in the same individual, suggesting a potential involvement of other factors modulating dominant negative function and the degree of resistance (32, 48, 51–54). We report here evidence that the RTH syndrome is associated with an aberrant interaction between the RTH-T3Rs and the SMRT/TRAC corepressors and that this corepressor interaction is important in the ability of RTH-T3Rs to act as dominant negative inhibitors. Furthermore, different RTH-T3Rs exhibit distinct corepressor interactions. Conceivably, differential interactions with, expression expression of, or genetic polymorphisms within the corepressors may contribute to the highly variable presentation observed for RTH disease.
RESULTS
RTH-T3Rβ Mutants Exhibit Aberrant Interactions with SMRT Corepressor
We wished to investigate whether corepressors play a role in the dominant negative effect that is postulated to be the molecular basis of RTH. We first tested the ability of wild type (wt) T3Rβ and a panel of RTH-T3Rβ mutants to associate with corepressor in vitro. We employed a glutathione-S-transferase (GST) fusion of SMRT, and 35S-radiolabeled T3Rs obtained by in vitro transcription and translation. All of our T3Rβ alleles (wt or mutant) produced three main forms of translation product in vitro: a full-length molecule at approximately 55 kDa (p55) and two truncated forms at approximately 48 and 33 kDa (p48, p33) (Fig. 2). The truncated T3R species are likely to the products of translational initiation on internal ATG codons and have been reported previously for both in vitro and in vivo expression systems (26, 27, 55).
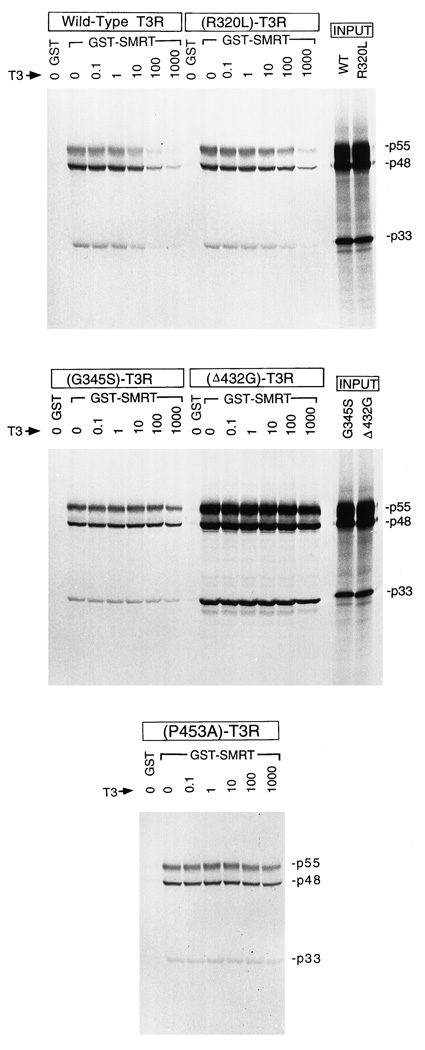
Fig. 2. Binding of wt and RTH Mutant Receptors to SMRT Corepressor.
Wild type and four different RTH mutant T3Rβs were tested for the ability to bind to an immobilized GST-SMRT fusion protein or to a nonrecombinant GST construct employed as a negative control, as indicated above each figure. Radiolabeled T3Rs was obtained by in vitro translation and were incubated with the immobilized GST or GST-SMRT under the hormone conditions indicated (expressed as nm T3 in the incubation buffer). The GST or GST-TRAC agarose was then extensively washed, and the radiolabeled receptor remaining bound to the matrix was eluted and was analyzed by SDS-PAGE and autoradiography. Input lanes display the in vitro translation products used for the binding experiments; all receptors were expressed at virtually identical levels and represent equivalent specific activities.
Wild-type T3Rβ bound to the GST-SMRT construct in the absence of hormone, but not to nonrecombinant GST employed as a negative control (Fig. 2). Addition of increasing amounts of T3 hormone strongly inhibited binding of the wtT3Rβ to GST-SMRT, with a 50% inhibition observed at 56 nm hormone (Fig. 2, and quantified in Fig. 3 and Table 1). This interaction of wtT3Rβ with SMRT closely resembles that observed previously for wtT3Rα (11, 14, 15). Note that all three receptor translation products (p55, p48, and p33) were bound by the GST-SMRT construct and were released congruently by hormone, indicating that the use of alternative initiation sites did not alter the interaction of T3Rβ with corepressor (Fig. 2).
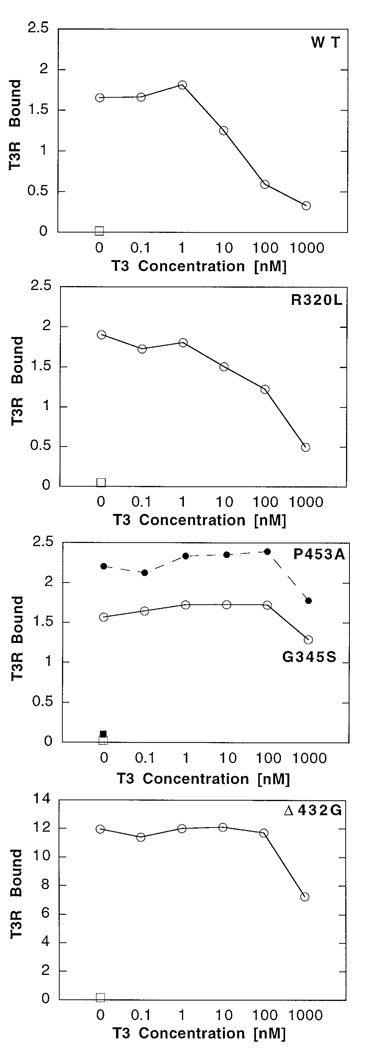
Fig. 3. Quantification of wt and RTH-T3Rβ Binding to SMRT.
The electrophoretograms shown in Fig. 3 were quantified by PhosphoImager (Molecular Dynamics, Sunnyvale, CA) analysis. The amount of T3R bound to GST (squares) or to GST-SMRT (circles) at a given hormone concentration is expressed as a percentage of the total radiolabeled T3R used in the binding reaction. Data for two different RTH-mutants, G345S (open symbols) and P453A (closed symbols), are shown in the third panel. Note the change in scale of the ordinate for the Δ432G mutant.
Table 1.
Clinical Phenotype, T3 Binding Affinity, and SMRT Association Properties of wt and Mutant T3Rβs
| T3Rβ Allele | Index Clinical Phenotypea | Ka for T3b | [T3] for 50% Inhibition of SMRT Associationc |
|---|---|---|---|
| Wild type | Normal | 2.2 | 56 ± 14 |
| V264D | GRTH | <0.02 | >1000 |
| R320L | GRTH | 0.21 | 245 ± 13 |
| M334R | PRTH | 0.11 | 690 ± 22 |
| R338W | PRTH | 0.21 | 290 ± 144 |
| G344E | GRTH | N.D. | >1000 |
| G345S | GRTH | <0.02 | >1000 |
| Δ430M | PRTH | N.D. | >1000d |
| Δ432G | PRTH | N.D. | >1000d |
| R438C | GRTH | 0.67 | 103 ± 17 |
| P453A | PRTH | 0.38 | >1000 |
| P453H | GRTH | 0.51 | >1000 |
Clinical phenotype refers to the index case from each kindred, with PRTH representing a diagnosis of pituitary resistance to thyroid hormone, and GRTH representing a diagnosis of generalized resistance to thyroid hormone (48). Note, however, that certain mutants (e.g. R320L and R338W) have been independently isolated from both PRTH and GRTH patients.
Apparent association constants for T3 (expressed as × 1010 m−1) were previously summarized in Ref. 48 The G345S mutant bound T3 but at levels too low to quantify, whereas no binding was detected (N.D.) for the G344E, Δ430M, and Δ432G mutants.
The amount of hormone (nm) required to inhibit SMRT association by 50% was determined for each mutant using binding assays as in Fig. 3. Each value represents the average and range of at least two different determinations.
Mutants that exhibited highly elevated levels of SMRT association relative to wt at all hormone concentrations tested.
The RTH-T3Rβ mutants also associated with GST-SMRT in the absence of hormone, but in contrast to wtT3Rβ, all the RTH-T3Rβs tested exhibited aberrations in their ability to dissociate on T3 treatment. The results for representative mutants are presented in detail in Fig. 2 and Fig. 3 and are summarized, along with data for additional mutants, in Table 1. Nine of the 11 RTH mutants analyzed here associated normally with SMRT in the absence of hormone (i.e. at levels comparable to wtT3R). Four RTH mutants in this group of nine (R320L, M334R, R338W, and R438C) were eventually displaced from SMRT by T3, but only at hormone levels significantly greater than those required for the wt receptor/SMRT dissociation. For the remaining five of these nine mutants (V264D, G344E, G345S, P453A, and P453H), little or no receptor/SMRT dissociation (less than 50%) was observed at up to 1 µM T3; notably the P453A and p453H mutants fail to dissociate from SMRT even though these mutant receptors are able to bind thyroid hormone (see Discussion).
The last two of the 11 RTH mutants analyzed (Δ430 M and Δ432G) were not only impaired in their dissociation from SMRT in the presence of T3, but also exhibited a dramatically enhanced SMRT association at all hormone concentrations (Fig. 2 and Fig. 3 and Table 1). These mutants bound to GST-SMRT in the absence or presence of T3 at levels some 8- to 10-fold greater than that seen with wtT3Rβ or the other RTH mutants (note the change in ordinate in Fig. 3); equal inputs of receptor were employed in all experiments. All mutant and wt receptors were transcribed and translated at equal efficiencies, utilizing identical conditions, and therefore represent equal specific activities. Additionally, the enhanced binding observed for mutants Δ430 M and Δ432G was consistently seen in multiple experiments utilizing different preparations of receptor and of GST-SMRT. Intriguingly, both of these mutants represent single amino acid deletions mapping to the same α-helix of the receptor and present clinically in a similar fashion (see Discussion).
We conclude that a common hallmark of the RTH receptors analyzed here is an aberrant interaction with corepressor. The phenotypes of these RTH mutants could be divided into three general categories: 1) normal levels of SMRT association, but requiring higher than normal levels of T3 for dissociation, 2) normal levels of SMRT association, but with little or no T3- mediated dissociation observed under the conditions employed, and 3) a dramatically elevated level of SMRT association at all hormone concentrations tested.
Altered Corepressor Association Correlates with the Dominant Negative Properties of the RTH Mutants
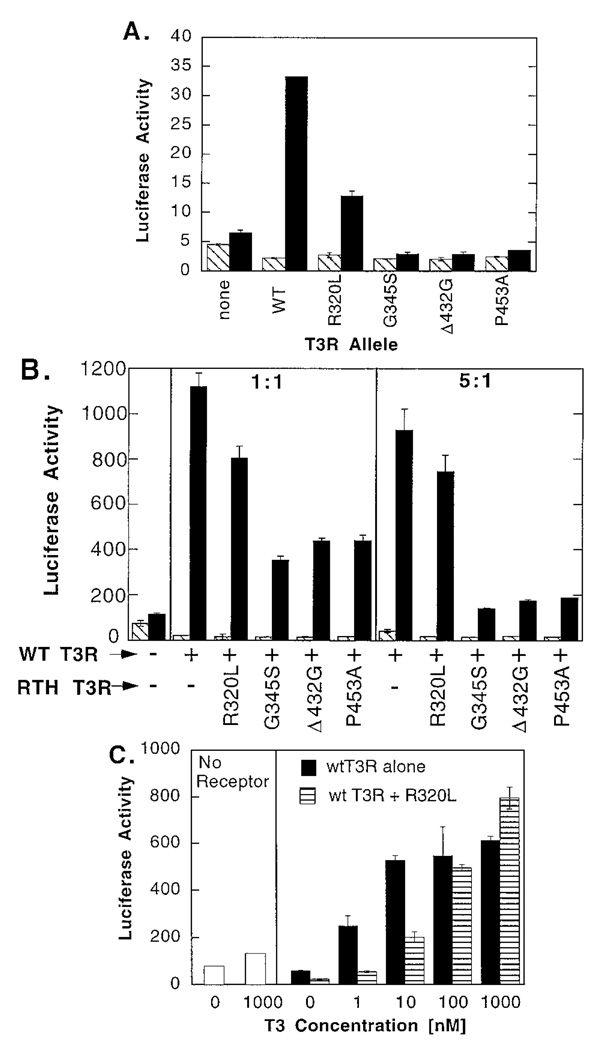
We next tested the transcriptional properties of representative RTH mutants from the categories defined above, using transient transfections of CV-1 cells. We initially introduced the various receptor mutants individually (Fig. 4A). In the absence of hormone, all five receptors tested functioned as repressors, inhibiting reporter gene expression some 50% relative to that seen in the absence of exogenous T3R. As expected, addition of 100 nm T3 converted the wtT3Rβ from a repressor into a strong transcriptional activator (Fig. 4A). In contrast, the R320L mutant exhibited an impaired ability to activate reporter gene expression in the presence of 100 nm T3, and the G345S, Δ432G, and P453A mutants functioned as constitutive repressors unable to induce significant activation of the reporter in response to T3 (Fig. 4A).
Fig. 4. Transcriptional Properties of wt and RTH-T3Rβs.
A, Transcriptional properties of the receptors when analyzed individually. CV-1 cells were transfected by lipofection with a DR4 luciferase reporter together with a pSG5 plasmid expressing either wt or mutant T3Rβ, as indicated (none indicates an empty pSG5 vector). The cells were incubated in the absence (hatched bars) or presence (filled bars) of 100 nm T3, and the cells were harvested. The resulting luciferase activity was determined and normalized relative to the activity of a pCH110-lac Z plasmid employed as an internal control. Data represent the average and range of duplicate experiments. B, Dominant negative activities of the different RTH-T3Rβ mutants when cointroduced with wt receptor. CV-1 cells were transfected by lipofection with the DR4 luciferase reporter, together with different combinations of wt and RTH mutant receptors, as indicated below the panel. Either a 1:1 or 5:1 ratio of mutant to wt receptor were used, with the wt receptor at 100 ng per plate in all cases. Empty pSG5 was employed to keep the total amount of expression vector identical in all samples. The cells were incubated in the absence (hatched bars) or presence (filled bars) of 100 nm T3, and the relative luciferase activity was determined as for panel A. Data represents the average and range of duplicate experiments. C, Effect of hormone on dominant negative properties of the R320L mutant. The same general experimental design as in panel B was used, but employing no exogenous receptor (open bars), wtT3Rβ alone (filled bars) or a 5:1 ratio of R320L to wtT3Rβ (horizontal striped bars) under a range of T3 concentrations (indicated below the figure). A calcium phosphate transfection method was used. Data represent the average and range of duplicate experiments.
We next examined the abilities of the RTH mutants to act as dominant negatives when cointroduced together with the wt receptor (Fig. 4B). The G345S and Δ432G mutants were strong dominant negatives in this cotransfection assay and significantly inhibited the functions of the wt receptor in a dose-dependent manner. In contrast, the R320L mutant acted only as a weak inhibitor of wtT3Rβ at 100 nm T3. We retested the dominant negative properties of R320L at a range of hormone concentrations (Fig. 4C). Consistent with the effects of hormone on the SMRT/R320L-T3R association in vitro, the R320L mutant functioned in cells as a strong dominant negative at low T3 concentrations (i.e. at hormone levels that fail to displace SMRT) but lost dominant negative activity at higher T3 concentrations (presumably reflecting the release of SMRT). We conclude that the RTH-T3Rs that exhibited a strong constitutive association with SMRT in vitro manifested the strongest dominant negative phenotypes, whereas the mutant receptor that could be partially dissociated from SMRT by T3 exhibited weaker, T3-sensitive dominant negative properties.
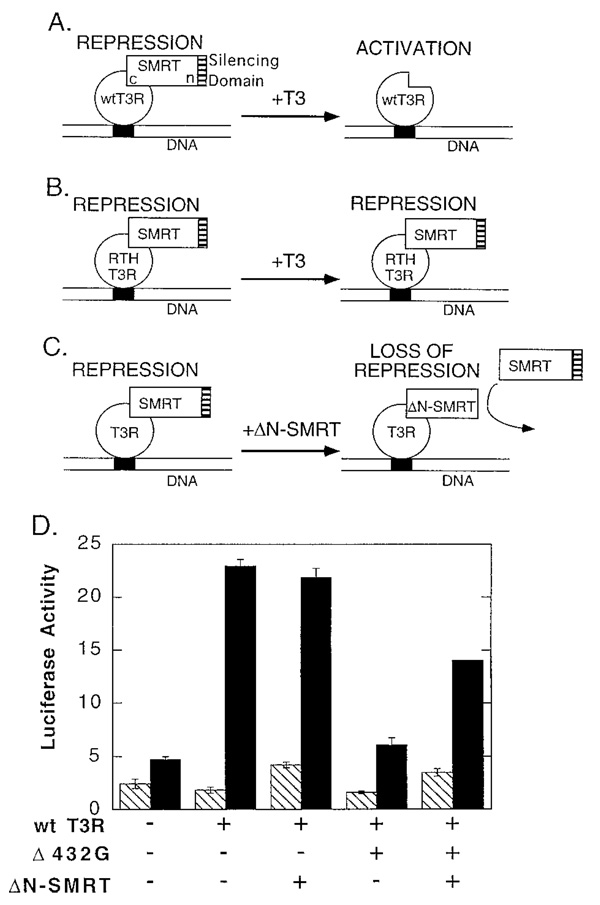
Exogenously Introduced SMRT Derivatives Can Interfere with the Dominant Negative Phenotype of the RTH-T3Rs
The N terminus of SMRT is required for transcriptional repression, whereas the C terminus contains the domains necessary for T3R association (Fig. 5A and Refs. 11–15). As a consequence, ectopic expression of an N-terminally truncated SMRT (ΔN-SMRT) interferes with the wtT3R-mediated repression observed in the absence of hormone (Refs. 11 and 14 and Fig. 5D), presumably by displacing endogenous full-length SMRT from the receptor (Fig. 5C). In contrast, expression of ΔN-SMRT has little or no effect on the transcriptional activation observed for wtT3Rβ in the presence of T3 (Fig. 5D and Ref. 14), conditions in which endogenous SMRT is not bound to the wt receptor (Fig. 5A). If the dominant negative actions of the RTH-T3Rs reflect a hormone-resistant association with SMRT (Fig. 5B), cointroduction of a ΔN-SMRT deletion should counteract the dominant negative RTH phenotype and partially restore the thyroid hormone response (Fig. 5C).
Fig. 5. ΔN-SMRT Expression Reverses the Dominant Negative Actions of the RTH-T3Rβs.
A, Model of the actions of SMRT corepressor on wtT3R. SMRT is bound to receptor in the absence of T3 and mediates transcriptional repression, but dissociates under physiological T3 levels to permit transcriptional activation. The N-terminal (n) SMRT domain required for transcriptional repression (hatched) and the C-terminal (c) domain required for receptor association are depicted. B, Model of the actions of SMRT on RTH-T3Rs. SMRT is bound to receptor in the absence of T3, but remains bound under physiological T3 conditions. As a result, the RTH-T3R behaves as a constitutive repressor and interferes in a dominant fashion with the actions of the wtT3R. C, Model of the actions of ΔN-SMRT derivatives on receptor-mediated repression. ΔN-SMRT retains the ability to bind receptor, but lacks the N-terminal domain required to silence transcription. As a result, the ectopic expression of ΔN-SMRT displaces endogenous full-length SMRT from T3R and interferes with receptor-mediated repression. D, ΔN-SMRT interference with the dominant negative phenotype of the Δ432G T3 mutant. CV-1 cells were transfected with the DR-4 luciferase reporter and with various combinations of pSG5 vector expressing wtT3Rβ (0.5 µg per plate), the Δ432G mutant (2.5 µg per plate), or ΔN-SMRT (2.5 µg per plate), as indicated below the panel. A calcium phosphate precipitation procedure was employed. Empty pSG5 was introduced where necessary to keep the total amount of expression vector the same for all samples. The cells were incubated in the absence (hatched bars) or presence (filled bars) of 100 nm T3, and the relative luciferase activity was determined as for Fig. 4. Data represent the average and range of duplicate experiments.
We tested this hypothesis using the Δ432G mutant (which exhibited both a very strong dominant negative phenotype in vivo and an enhanced association with SMRT in vitro). As before, introduction of the Δ432G mutant severely inhibited T3-mediated gene activation by wtT3Rβ (Fig. 5D). However, these dominant negative properties of the Δ432G mutant were strongly counteracted by the cointroduction of the ΔN-SMRT derivative, resulting in significant restoration of reporter gene activation by the wtTR3 (Fig. 5D). These effects were proportional to the amount of ΔN-SMRT expression vector introduced and were not observed for an empty pSG5 vector (Fig. 5D and data not shown).
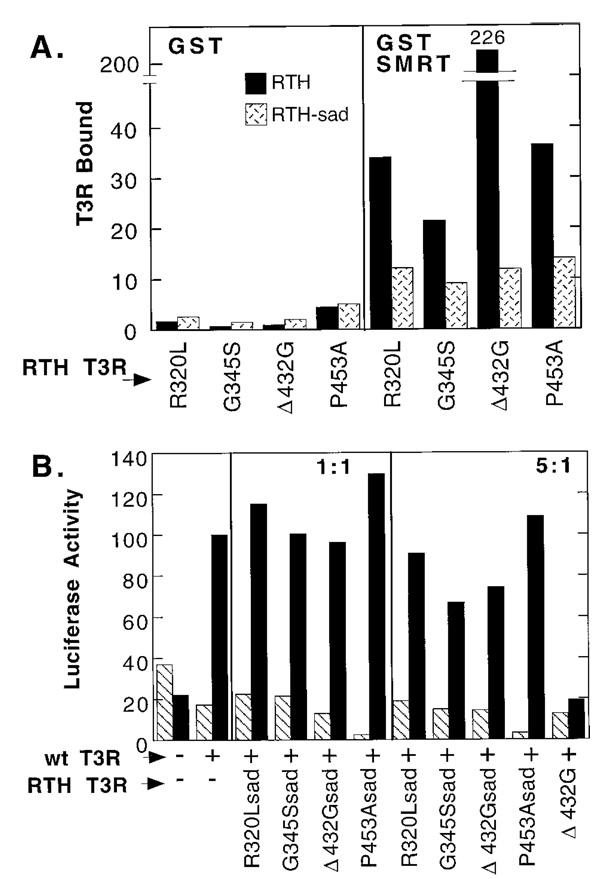
Artificial Mutants of RTH-T3Rs That Are Impaired in SMRT Association Are Also Impaired in Dominant-Negative Function
Although several distinct receptor domains interact with corepressor, a conserved amino acid sequence in the receptor ligand-binding domain is particularly important for corepressor association (Fig. 1 and Refs. 11–15). Receptors bearing mutations in this conserved sequence are impaired for corepressor association in vitro and for transcriptional repression in vivo, but retain the ability to activate transcription (Refs. 11 and 14 and data not shown). To ask whether impairment of SMRT association results in impairment of the RTH dominant negative phenotype, we engineered analogous SMRT-association disruptive (sad) mutations into four of our representative RTH mutant clones (R320L, G345S, Δ432G, and P453A). When tested in vitro, these RTH-T3Rsad mutants were all significantly reduced in their ability to bind to GST-SMRT relative to the parental receptors (Fig. 6A). The residual SMRT binding displayed by the T3Rsad proteins (Fig. 6A) has been noted before and is likely mediated by additional points of contact between SMRT and receptor that are not altered in this mutagenesis scheme (14).
Fig. 6. Effects of Mutations That Inhibit SMRT Association on the Properties of RTH-T3Rβs.
A, Inhibition of T3Rβ binding to SMRT in vitro by SMRT association-disruptive (sad) mutations. The RTH (solid bars) or RTHsad (speckled bars) T3R derivatives were tested for the ability to bind in vitro to nonrecombinant GST (left panel) or GST-SMRT (right panel). The amount of radiolabeled T3R bound was determined as in Fig. 2, quantified by Phospho-Imager analysis, and is presented in arbitrary units. B, Impairment of dominant negative activities of RTH-T3Rβs by sad mutations. The dominant negative assay described in Fig. 4 was repeated, but employing the RTH-T3Rsad mutants, as indicated below the panel. Unmodified Δ432G RTH-T3Rβ (last lane) was also included as a control. Wild type T3Rβ activity was defined as 100
In keeping with our hypothesis of an obligatory role for corepressor in RTH dominant negative function, the RTH-T3Rsad mutants were all severely compromised in their ability to interfere with wtT3R function (compare Fig. 6B to Fig. 4B). Little or no inhibition of T3-mediated reporter gene activation was detected when T3Rsad versions of R320L, G345S, Δ432G, or p453A were introduced at 1:1 or 5:1 ratios relative to wtT3R (Fig. 6B). This contrasts with the strong inhibition of wtT3Rβ activation observed for the cointroduction of the unmodified G345S, Δ432G, or P453A mutants (Fig. 6B, last lane, and Fig. 4B). Note that repression of the reporter gene in the absence of hormone, mediated by the unmodified wtT3Rβ, is still observed in Fig. 6B, confirming that the defects in transcriptional repression are specific for the receptors bearing the sad mutations. The analogous sad mutant form of the wtT3Rβ fails to repress, but retains the ability to activate, transcription when introduced individually into CV-1 cells (data not shown), indicating that the sad lesion does not abolish receptor expression, nuclear localization, or DNA binding.
DISCUSSION
The RTH Syndrome Is a Complex Genetic Disorder Characterized by a Diminished Physiological Response to Thyroid Hormone
More than 300 RTH kindreds have been identified, with most of the known genetic lesions mapping to two domains (codons 310 to 349 and 429 to 461) within the T3Rβ locus (32–34, 38). These domains of the receptor play multiple roles in wtT3Rβ function, including hormone binding, receptor dimerization, and interactions with the transcriptional machinery (1–6); as a result an elucidation of the precise molecular basis of the RTH phenotype has been complex. Significantly, the vast majority of RTH mutants display a diminished or complete loss of affinity for T3 hormone. Apparently as a consequence, many RTH-T3Rs can repress gene transcription, but are defective in gene activation in response to hormone (40–50). Thus, it has been proposed that RTH-T3Rs function as dominant negative inhibitors, interfering in trans with the actions of the normal T3Rα and -β (40–50).
However, many features of RTH remain poorly understood, and its precise molecular mechanism remains elusive. Various models by which RTH-receptors could function as dominant negatives have been proposed, including 1) the formation of inactive dimmers between RTH-T3Rs and wtT3Rs, 2) a competition between RTH and wt receptors for essential cofactors, or 3) a competition between RTH and wt receptors for DNA-binding sites. This complexity is paralleled by the clinical phenotype of RTH syndrome, which varies in its severity and characteristics from kindred to kindred. Thus, the known biochemical properties of the RTH receptors do not invariably predict the severity of the disease state, and individuals with the same genetic lesion can display different symptoms (32–34, 48, 49, 51, 52). It has been suggested that additional, independently inherited factors may be involved in RTH (32, 49, 53, 54).
We therefore asked whether the recently elucidated SMRT/N-CoR family of corepressors might be involved in the pathogenesis of RTH. In this manuscript, we present evidence that RTH-T3Rβ mutants are indeed altered in their interactions with SMRT and that these alterations in corepressor association appear to play a role in the dominant negative properties of the RTH receptors and may therefore influence the clinical manifestations of the disease.
RTH Mutant Receptors Exhibit Defects in T3-Mediated Dissociation from Corepressor
Wild type T3Rβ binds to SMRT in the absence of T3 but dissociates on binding hormone, a process paralleled by a conversion of the receptor from a transcriptional repressor to an activator (11–15). The RTH-T3Rs analyzed here share this ability to associate with SMRT in the absence of T3 but are notably impaired in the ability to dissociate from corepressor on addition of hormone. Four of the RTH mutants tested required higher than normal levels of hormone to dissociate from SMRT, whereas seven others failed to dissociate significantly from SMRT at even 1 µM hormone. Given the strong transcriptional silencing properties of SMRT (11, 14), the RTH-T3R/SMRT complexes would be predicted to repress transcription under hormone conditions in which wtT3Rβ activates, a prediction consistent with the dominant negative properties of the RTH mutants. Indeed, the RTH receptors that were constitutively bound to SMRT exhibited strong, hormone-refractory dominant negative properties in our transfection studies. In contrast, an RTH-receptor (R320L) that exhibited impaired, but detectable, release of corepressor at high hormone concentrations displayed a similarly hormone-labile dominant negative phenotype.
Two RTH Mutations Appear to Uncouple SMRT Dissociation from Hormone Binding
The failure of most of our RTH mutants to release from SMRT in the presence of T3 could be attributed to the impaired affinities of the mutant receptors for this hormone. For example, the R338W mutant exhibits a 10-fold reduction in affinity for T3 relative to wtT3Rβ and requires some 6-fold more hormone for SMRT dissociation; similarly the G345S mutant is virtually unable to bind hormone in vitro and fails to significantly dissociate from SMRT at even 1 µM T3. However, two striking exceptions were noted to this general correlation between affinity for T3 and SMRT release. The P453A and P453H mutants are only mildly impaired in hormone binding in vitro (possessing even higher affinities for T3 than the R338W mutant), yet fail to dissociate from SMRT at any hormone concentration tested. Protease sensitivity assays (56) confirm that the P453A and P453H mutant receptors are indeed occupied by hormone at these T3 concentrations that fail to displace SMRT (data not shown). Notably, the P453A and P453H mutants represent different amino acid substitutions at the same codon, in a region of the receptor that is proposed to undergo a conformational change on binding hormone (57, 58). Codon 453 may therefore define a receptor domain critical for corepressor release after hormone binding, and lesions at this site may confer RTH by preventing SMRT dissociation in a manner independent of hormone occupancy.
Consistent with these concepts, mutants at codon 453 possess dominant negative properties that are relatively refractory to T3 (48). This region of the receptor has been suggested to operate as a hinge, permitting a C-terminal tail of the receptor to rotate from an exposed position in the absence of hormone to a new position nested against the body of the receptor in the presence of hormone (57, 58). Our preliminary evidence, employing protease sensitivity as a probe of structure, supports the concept that this hinge function may be impaired in the P453A and P453H mutants (B. Lin, S. Yoh, and M. L. Privalsky, manuscript in preparation).
It should be noted that the hormone concentrations required for inhibition of T3R/SMRT binding in our GST assay were higher than the association constants (Ka) for T3 reported for the same receptors (Table 1). This phenomenon was consistently observed in multiple assays, and was also seen for wtT3Rα,-β, and for retinoic acid receptors. Intriguingly, analogous discrepancies exist between the Ka values of these receptors and the significantly higher hormone concentrations required for transcriptional activation in transient transfection assays (e.g. Refs. 47 and 48). These differences may simply be technical in origin, i.e. due to sequestration of hormone by components of the transfection/binding assays (47). On the other hand, this phenomenon may reflect a physiologically relevant process. For example, SMRT association may alter the affinity of T3Rs for hormone, analogous to the effects reported for heterodimer formation with retinoid X receptor (59, 60).
Two RTH Mutants Also Exhibit Dramatically Enhanced Levels of SMRT Association
Two of the nine RTH mutants analyzed (Δ430 M and Δ432G) were not only impaired in T3-mediated dissociation from SMRT, but also exhibited a strongly elevated association with SMRT even in the absence of hormone. Consistent with this enhanced SMRT binding, the Δ430 M and Δ432G mutants exert dominant negative phenotypes that are among the strongest observed for any of RTH mutants tested; this is particularly evident with certain promoters and under high hormone concentrations (48). Notably, these two, independently derived mutations represent in-frame, single-codon deletions that map to an α-helical domain in the T3Rβ C terminus (based on the crystallographic model of T3Rα; Ref. 57). This helix appears to play important roles in the conformational changes associated with hormone binding, in transcriptional activation, and in dimer formation with other receptors, but has not been previously identified as a direct contact site for corepressor. It appears that by shortening or rotating this region by one amino acid residue, the binding of corepressor can be greatly enhanced, either by directly affecting an interaction surface or by a more indirect effect on the global conformation of the receptor C terminus.
Corepressor Association Appears to be Required for the Dominant Negative Actions of the RTH-T3Rs
Correlating with their dominant negative properties, all 11 RTH mutants interacted with corepressor under hormone conditions in which the wtT3R does not. Furthermore, mutations introduced into RTH-T3Rs that disrupt SMRT association, or transfection of ΔN-SMRT derivatives that interfere with SMRT function, disrupted the dominant negative phenotype. These results strongly implicate corepressors in the ability of RTH-T3Rs to act as dominant negative inhibitors of the T3 response. In these properties, the RTH-T3Rβ mutants closely parallel v-Erb A, an oncogenic allele of T3Rα that both acts as a dominant negative allele and exhibits hormone-independent corepressor association (11, 14). The ability of v-Erb A to repress gene transcription and to function in oncogenesis closely correlates with SMRT association (11, 14). However, it should be noted that corepressors are a diverse family of protein factors, and the dominant negative properties observed for v-Erb A and for RTH-T3Rs may be mediated by SMRT itself, N-CoR, or some, as yet unidentified, corepressor-like entity.
A Correlation May Exist between the SMRT Association Properties of RTH-T3Rs in Vitro and the Clinical Manifestations of RTH
Clinically, RTH has been broadly divided into a pituitary form (PRTH) characterized by a predominant pituitary resistance but preserved peripheral response to T3, vs. a generalized form (GRTH) characterized by T3 resistance at the level of both pituitary and peripheral tissues (reviewed in Refs. 32–34). Despite these different clinical manifestations, receptors isolated from PRTH and GRTH are often similar or identical to one another in their biochemical properties. It is therefore interesting that the RTH-T3Rs that display a highly elevated SMRT association (Δ430M and Δ432G) in the current study were both isolated from patients presenting with PRTH. It is possible that this constitutively elevated SMRT association may play a role peculiar to the PRTH phenotype. However, the converse did not appear to be true: not all PRTH-T3R mutants also displayed elevated SMRT association in vitro (e.g. R338W).
Notably, none of the experiments described here were performed in different tissues or in the genetic background of the original patients. Therefore, our results do not address whether alterations in corepressor, rather than in receptor, may also contribute to the RTH syndrome. It is tempting to speculate that differences in corepressor expression, perhaps coupled to genetic polymorphisms at the corepressor loci, might account for the variable resistance of the RTH syndrome in different individuals of the same kindred. Similarly, differences in corepressor expression in different tissues could contribute to the varying organ-specific effects of RTH. Clearly, however, RTH is a complex clinical disease and is associated not only with a failure to activate expression of certain genes in response to hormone, as tested here, but also as a failure to suppress expression of others, most notably that of pituitary TSH (32–34). Further work will be necessary to determine the contributions of corepressors or related factors to these different manifestations of this endocrine disorder.
MATERIALS AND METHODS
Molecular Clones
Wild type T3Rβ was the generous gift of R. M. Evans; the origins of the RTH mutant clones have been described previously (7, 48). All T3Rβ alleles were introduced as BamHI to BamHI fragments into a pSG5 vector for expression in vitro and for transient transfections. The T3Rsad mutants (equivalent to an A223G, H224G, T227A triple mutant, using the numbering system of Ref. 12) were created by a PCR protocol (61). Appropriate restriction fragments of the resulting PCR product was used to replace the corresponding sequences within the pSG5 clones of the RTH-T3Rβ alleles. Mutations were confirmed by restriction digestion/DNA sequence analysis.
Assay of the SMRT/T3Rs Interaction in Vitro
GST-SMRT (identical to the GST-TRAC-1 construct described previously) was isolated from transformed Escherichia coli and was immobilized by binding to glutathione agarose, as previously described (14). 35S-Labeled receptors were synthesized by in vitro transcription from pSG5 templates using T7 RNA polymerase, coupled to in vitro translation using rabbit reticulocyte lysates (TNT kit, Promega, Madison, WI). Each receptor was subsequently mixed with approximately 2 µg immobilized GST-SMRT (bound to 20 µl glutathione-agarose) in 200–400 ml HEMG buffer (14) containing 10 mg/ml BSA and a protease inhibitor cocktail (Complete, Boehringer-Mannheim, Indianapolis, IN). T3 hormone (or an equivalent volume of ethanol carrier) was included in the binding reactions where indicated. The binding reactions were incubated for 60–90 min at 4 C with gentle rocking, and the agarose matrix was subsequently washed with 4 × 1 ml changes of HEMG buffer. Bound proteins were eluted in 35 µl of 50 mm Tris-Cl (pH 7.6) containing 10 mm glutathione, resolved by SDS-PAGE, and visualized by autoradiography (14). Quantification of the binding experiments were performed with a Molecular Biosystems Storm phosphoimaging system (Sunnyvale, CA).
Transient Transfections
For calcium phosphate transfections, CV-1b cells (2 × 105 per 60-mm plate) were propagated overnight at 37 C in a 5% CO2 atmosphere in DMEM containing 10% heat-inactivated FBS and penicillin-streptomycin (GIBCO/BRL, Gaithersburg, MD). The cells were subsequently transferred to hormone-depleted medium and incubated an additional 6 h. Calcium phosphate/DNA precipitates, prepared by standard protocol (62), were then added, and the cells were incubated for an additional 16–18 h. Typically, each plate received 1 µg pCH110-lacZ (employed as an internal standard), 1 µg of a DR4-luciferase reporter (63), various combinations of empty pSG5, pSG5-T3Rβ, or pSG5-ΔN-SMRT (as indicated), and sufficient pUC18 or pUC19 to bring the total DNA to 10 µg. After an overnight incubation with precipitate, the cells were washed and transferred to fresh medium lacking or containing T3. The cells were harvested 30 h later and lysed in 100 µl Reporter Lysis Buffer (Promega) per plate. Luciferase activity was determined by mixing 30 µl of extract with 100 µl Promega luciferase reagent in an MGM luminometer (MGM Instruments, Cambridge, MA); β-galactosidase activity was determined by colorimetric assay (14, 64).
Lipofections were performed using 3 × 104 to 5 × 104 cells seeded per 1 cm-well in 24-well microtiter plates. Lipofectin reagent (GIBCO/BRL) was diluted 10-fold into serum-free medium, incubated 30–45 min at room temperature, then mixed with the DNA and incubated an additional 10 min. Typically, 300 ng pCH110, 100 ng DR4-luciferase reporter, and 100 ng pSG5-T3R construct were employed per well, with sufficient pUC19 DNA to bring the total DNA concentration to 1 µg. The cells were overlayed with the DNA and Lipofectin mixture, incubated 6 h at 37 C, subsequently incubated 18 h with medium containing 20% FBS, and then incubated 24–48 h in medium containing 10% FBS with or without hormone. Cells were lysed and assayed for luciferase and β-galactosidase activity in a similar manner as that described for the calcium phosphate method.
Acknowledgments
We thank M. d. M. Vivanco Ruiz and R. M. Evans for generously providing the luciferase reporter and wtT3Rβ molecular clones.
This work was supported by Public Health Services/NIH Grant R37 CA-53394.
Contributor Information
Sunnie M. Yoh, Section of Microbiology (S.M.Y., M.L.P.), Division of Biological Sciences, University of California at Davis, Davis, California 95616
V. K. K. Chatterjee, Department of Medicine (V.K.K.C.), University of Cambridge, Level 5, Addenbrooke’s Hospital, Cambridge, CB2 2QQ, United Kingdom
Martin L. Privalsky, Section of Microbiology (S.M.Y., M.L.P.), Division of Biological Sciences, University of California at Davis, Davis, California 95616
REFERENCES
- 1.Carlson-Jurica MA, Schrader WT, O’Malley BW. Steroid receptor family: structure and function. Endocr Rev. 1990;11:201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- 2.Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. Overview: the nuclear receptor superfamily: the second decade. Cell. 1995;83:835–840. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 5.Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–858. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 6.Kastner P, Mark M, Chambon P. Nonsteroidal nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–870. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 7.Damm K, Thompson CC, Evans RM. Protein encoded by v-Erb A functions as a thyroid hormone receptor antagonist. Nature. 1989;339:593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- 8.Sap J, Munoz A, Schmitt H, Stunnenberg H, Vennstrom B. Repression of transcription mediated by a thyroid hormone response element by the v-Erb A oncogene product. Nature. 1989;340:242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- 9.Privalsky ML, Sharif M, Yamamoto KR. The viral Erb A oncogene protein, a constitutive repressor in animal cells, is a hormone-regulated activator in yeast. Cell. 1990;63:1277–1286. doi: 10.1016/0092-8674(90)90423-c. [DOI] [PubMed] [Google Scholar]
- 10.Miner JN, Diamond MI, Yamamoto KR. Transcriptional factor interactions: selectors of positive or negative regulation from a single DNA element. Cell Growth Differ. 1991;2:525–530. [Google Scholar]
- 11.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 12.Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamel Y, Soderstrom M, Glass CK, Rosenfeld MG. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 13.Kurokawa R, Soderstrom M, Horlein A, Halachmi S, Brown M, Rosenfeld MG, Glass CK. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 14.Sande S, Privalsky ML. Identification of TRACs (T3 Receptor-associating cofactors) a family of cofactors that associate with, and modulate the activity of, nuclear hormone receptors. Mol Endocrinol. 1996;10:813–825. doi: 10.1210/mend.10.7.8813722. [DOI] [PubMed] [Google Scholar]
- 15.Chen JD, Umesono K, Evans RM. SMRT isoforms mediate repression and anti-repression of nuclear receptor heterodimers. Proc Natl Acad Sci USA. 1996;93:7567–7571. doi: 10.1073/pnas.93.15.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baniahmad A, Kohne AC, Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-Erb A oncogene product, and in the retinoic acid receptor. EMBO J. 1992;11:1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casanova J, Helmer E, Selmi-Ruby S, Qi JS, Au-Flieger M, Desai-Yajnik V, Koudinova N, Yarm F, Raaka BM, Samuels HW. Functional evidence for ligand-dependent dissociation of thyroid hormone and retinoid acid receptors from an inhibitory cellular factor. Mol Cell Biol. 1994;14:5756–5765. doi: 10.1128/mcb.14.9.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baniahmad A, Leng X, Burris TP, Tsai SY, Tsai MJ, O’Malley BW. The t4 activation domain of the thyroid hormone receptor is required for release of a putative corepressor(s) necessary for transcriptional silencing. Mol Cell Biol. 1995;15:76–86. doi: 10.1128/mcb.15.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavailles V, Dauvois S, L’Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptors. EMBO J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Estrogen receptor-associated proteins- possible mediators of hormone-induced transcription. Science. 1994;264:1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- 21.Le Douari B, Zechel C, Garnier JM, Lutz Y, Tora L, Pierrat B, Heery D, Gronemeyer H, Chambon P, Losson R. The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function (AF2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO J. 1995;9:2020–2033. doi: 10.1002/j.1460-2075.1995.tb07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JW, Ryan F, Swaffield JC, Johnston SA, Moore DD. Interaction of thyroid hormone receptor with a conserved transcriptional mediator. Nature. 1995;374:91–94. doi: 10.1038/374091a0. [DOI] [PubMed] [Google Scholar]
- 23.Soel W, Choi WS, Moore DD. Isolation of proteins that interact specificially with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol. 1995;9:72–85. doi: 10.1210/mend.9.1.7760852. [DOI] [PubMed] [Google Scholar]
- 24.Onate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 25.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 26.Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennstrom B. The c-erbA protein is a high affinity receptor for thyroid hormone. Nature. 1986;324:635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. The c-erbA gene encodes a thyroid hormone receptor. Nature. 1986;234:641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- 28.Kakizuka A, Miller WH, Umesono K, Warrell RP, Frankel SR, Murty VVS, Dmitrovsky E, Evans R. Chromosomal translocation t(15:17) in human acute promyelocytic leukemia fuses RARα with a novel putative transcription factor. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- 29.de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RARα fusion mRNA encodes a funcionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 30.McPhaul MJ, Marcelli M, Zoppi S, Griffin JE, Wilson JD. Genetic basis of endocrine disease 4: the spectrum of mutations in the androgen receptor gene that cause androgen resistance. J Clin Endocrinol Metab. 1993;76:17–23. doi: 10.1210/jcem.76.1.8421085. [DOI] [PubMed] [Google Scholar]
- 31.Hughes MR, Malloy PJ, Kieback DG, Kesterson RA, Pike JW, Feldman D, O’Malley BW. Point mutations in the vitamin D receptor associated with hypocalcemic rickets. Science. 1988;242:1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- 32.Beck-Peccoz P, Chatterjee VKK. The variable clinical phenotype in thyroid hormone resistance syndrome. Thyroid. 1994;4:225–232. doi: 10.1089/thy.1994.4.225. [DOI] [PubMed] [Google Scholar]
- 33.Refetoff S, Weiss RE, Usala S. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- 34.Kopp P, Kitajima K, Jameson JL. Syndrome of resistance to thyroid hormone: insights into thyroid hormone action. Proc Soc Exp Biol Med. 1996;211:49–61. doi: 10.3181/00379727-211-43951. [DOI] [PubMed] [Google Scholar]
- 35.Usala SJ, Bale AE, Gesundheit N, Weinberger C, Lash RW, Wondisford FE, Accili D, McBride OW, Weintraub BD. Tight linkage between the syndrome of generalized thyroid hormone resistance and the human c-erb Aβ gene. Mol Endocrinol. 1988;2:1217–1220. doi: 10.1210/mend-2-12-1217. [DOI] [PubMed] [Google Scholar]
- 36.Sakurai AK, Takeda K, Ain K, Ceccarelli P, Nakai A, Seino S, Bell GI, Refetoff S, DeGroot LJ. Generalized resistance to thyroid hormone associated with a mutation in the ligand-binding domain of the human thyroid hormone receptor β. Proc Natl Acad Sci USA. 1989;86:8977–8991. doi: 10.1073/pnas.86.22.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Usala SJ, Tennyson GE, Bale AE, Lash RW, Gesundheit N, Wondisford FE, Accili D, Hauser P, Weintraub BD. A base mutation of the c-erb Aβ thyroid hormone receptor in a kindred with generalized thyroid hormone resistance Molecular heterogeneity in two other kindreds. J Clin Invest. 1990;85:93–100. doi: 10.1172/JCI114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parrilla R, Mixson AJ, McPherson JA, McClaskey JH, Weintraub BD. Characterization of seven novel mutations of the c-erb Aβ gene in unrelated kindreds with generalized thyroid hormone resistance: evidence for two “hot spot” regions of the ligand binding domain. J Clin Invest. 1991;88:2123–2130. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda K, Weiss RE, Refetoff S. Rapid localization of mutations in the thyroid hormone receptor β gene by denaturing gel electrophoresis in 18 families with thyroid hormone resistance. J Clin Endocrinol Metab. 1992;74:712–719. doi: 10.1210/jcem.74.4.1548332. [DOI] [PubMed] [Google Scholar]
- 40.Chatterjee VKK, Nagaya T, Madison LD, Datta S, Rentoumis A, Jameson JL. Thyroid hormone resistance syndrome: inhibition of normal receptor function by mutant thyroid hormone receptors. J Clin Invest. 1991;87:1977–1984. doi: 10.1172/JCI115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier CA, Dickstein BM, Ashizawa K, McClaskey JH, Muchmore P, Ransom SC, Menke JB, Hao E-H, Usala SJ, Bercu BB, Cheng S-Y, Weintraub BD. Variable transcriptional activity and ligand binding of mutant β1 3,5,3′ triiodothyronine receptors from four families with generalized resistance to thyroid hormone. Mol Endocrinol. 1992;6:248–258. doi: 10.1210/mend.6.2.1569968. [DOI] [PubMed] [Google Scholar]
- 42.Sakurai A, Miyamoto T, Refetoff S, DeGroot LJ. Dominant negative transcriptional regulation by a mutant thyroid hormone receptor-β in a family with generalized resistance to thyroid hormone. Mol Endocrinol. 1990;4:1988–1994. doi: 10.1210/mend-4-12-1988. [DOI] [PubMed] [Google Scholar]
- 43.Baniahmad A, Tsai SY, O’Malley BW, Tsai M-J. Kindred S thyroid hormone receptor is an active and constitutive silencer and a repressor for thyroid hormone and retinoic acid responses. Proc Natl Acad Sci USA. 1992;89:10633–10637. doi: 10.1073/pnas.89.22.10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagaya TN, Madison LD, Jameson JL. Thyroid hormone receptor mutations that cause resistance to thyroid hormone: evidence for receptor competition for DNA sequences in target genes. J Biol Chem. 1992;267:13014–13019. [PubMed] [Google Scholar]
- 45.Yen PM, Sugawa A, Refetoff S, Chin WW. New insights on the mechanism(s) of the dominant negative effect of mutant thyroid hormone receptors in generalized resistance to thyroid hormone. J Clin Invest. 1992;90:1825–1831. doi: 10.1172/JCI116058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagaya T, Eberhardt NL, Jameson JL. Thyroid hormone resistance syndrome: correlation of dominant negative activity and location of mutations. J Clin Endocrinol Metab. 1993;77:982–990. doi: 10.1210/jcem.77.4.8408475. [DOI] [PubMed] [Google Scholar]
- 47.Zavacki AM, Harney JW, Brent GA, Larsen PR. Dominant negative inhibition by mutant thyroid hormone receptors is thyroid hormone response element and receptor isoform specific. Mol Endocrinol. 1993;7:1319–1330. doi: 10.1210/mend.7.10.8264663. [DOI] [PubMed] [Google Scholar]
- 48.Collingwood TN, Adams M, Tone Y, Chatterjee VKK. Spectrum of transcriptional, dimerization, and dominant negative properties of twenty different mutant thyroid hormone β receptors in thyroid hormone resistance syndrome. Mol Endocrinol. 1994;8:1262–1277. doi: 10.1210/mend.8.9.7838159. [DOI] [PubMed] [Google Scholar]
- 49.Adams M, Matthews C, Collingwood TN, Tone Y, Beck-Peccoz P, Chatterjee KK. Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone. J Clin Invest. 1994;94:506–515. doi: 10.1172/JCI117362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yen PM, Chin WW. Molecular mechanisms of dominant negative activity by nuclear hormone receptors. Mol Endocrinol. 1994;8:1450–1454. doi: 10.1210/mend.8.11.7877614. [DOI] [PubMed] [Google Scholar]
- 51.Pohlenz J, Wirth S, Winterpacht A, Wemme H, Zabel B, Schonberger W. Phenotypic variability in patients with generalized resistance to thyroid hormone. J Med Genet. 1995;32:393–395. doi: 10.1136/jmg.32.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayashi Y, Weiss RE, Sarne DH, Yen PM, Sunthornthepvarakul T, Marcocci C, Chin WW, Refetoff S. Do clinical manifestations of resistance to thyroid hormone correlate with the functional alteration of the corresponding mutant thyroid hormone-beta receptors? J Clin Endocrinol Metab. 1995;80:3246–3256. doi: 10.1210/jcem.80.11.7593433. [DOI] [PubMed] [Google Scholar]
- 53.Weiss RE, Marcocci C, Bruno-Bossio G, Refetoff S. Multiple genetic factors in the heterogeneity of thyroid hormone resistance. J Clin Endocrinol Metab. 1993;76:257–259. doi: 10.1210/jcem.76.1.8421095. [DOI] [PubMed] [Google Scholar]
- 54.Wong R, Zhu XG, Pineda MA, Cheng SY, Weintraub BD. Cell type-dependent modulation of the dominant negative action of human mutant thyroid hormone beta 1 receptors. Mol Med. 1995;1:306–319. [PMC free article] [PubMed] [Google Scholar]
- 55.Bigler J, Eisenman RN. c-erbA encodes multiple proteins in chicken erythroid cells. Mol Cell Biol. 1988;8:4155–4161. doi: 10.1128/mcb.8.10.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keidel S, LeMotte P, Apfel C. Different agonist- and antagonist-induced conformational changes in retinoic acid receptors analyzed by protease mapping. Mol Cell Biol. 1994;14:287–298. doi: 10.1128/mcb.14.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner RL, Apriletti JW, McGrath ME, West BL, Baxter JD, Fletterick RJ. A structural role for hormone in the thyroid hormone receptor. Nature. 1995;378:690–697. doi: 10.1038/378690a0. [DOI] [PubMed] [Google Scholar]
- 58.Renaud J-P, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D. Crystal structure of the RAR-γ ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378:681–689. doi: 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- 59.Forman BM, Umesono K, Chen J, Evans RM. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 1995;81:541–550. doi: 10.1016/0092-8674(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 60.Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld MG, Heyman RA, Glass CK. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994;371:528–531. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- 61.Ausubel FM, Brent R, Kinston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley & Sons Press; pp. 8.5.7–8.5.9. [Google Scholar]
- 62.Ausubel FM, Brent R, Kinston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: Wiley & Sons Press; pp. 9.1.1–9.1.3. [Google Scholar]
- 63.Vivanco Ruiz MdM, Bugge TH, Hirschmann P, Stunnenberg HG. Functional characterization of a natural retinoic acid responsive element. EMBO J. 1991;10:3829–3838. doi: 10.1002/j.1460-2075.1991.tb04952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vivanco Ruiz MdM, Johnson R, Galante PE, Hanahan D, Yamamoto KR. A transistion in transcriptional activation by the glucocorticoid and retinoic acid receptors at the tumor stage of dermal fibrosarcoma development. EMBO J. 1995;14:2217–2228. doi: 10.1002/j.1460-2075.1995.tb07216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]