Abstract
Introduction
There are 1.1 billion smokers worldwide. Traditional smoking cessation methods, such as nicotine replacement therapy and smoking cessation groups, yield between 14% and 27% abstinence rates at 6 months. Evidence-based Internet interventions with comparable abstinence rates could be a powerful global tool to reduce tobacco-related morbidity and mortality.
Methods
We report a randomized control trial in which 500 Spanish-speaking and 500 English-speaking adult Internet users, smoking at least 5 cigarettes/day and intending to quit in the next month, were recruited online from 68 countries. Consenting participants who completed baseline measures, logged cigarettes smoked on 3 days within a week, and set a quit date were randomized to four conditions. Each condition added new elements: Condition 1 was the “Guía Para Dejar de Fumar,” a static National Cancer Institute evidence-based stop smoking guide; Condition 2 consisted of Condition 1 plus E-mail reminders to return to the site; Condition 3 consisted of Condition 2 plus mood management lessons; and Condition 4 consisted of Condition 3 plus a “virtual group” (an asynchronous bulletin board). Main outcome measures were 7-day point prevalence abstinence at 1, 3, 6, and 12 months after initial quit date.
Results
There were no significant differences among the four conditions. The overall 12-month 7-day abstinence rates were 20.2% for Spanish speakers and 21.0% for English speakers when those with missing data were assumed to be smoking.
Discussion
Internet smoking cessation interventions with such abstinence rates provided globally in additional languages could contribute substantially to tobacco control efforts.
Introduction
There are 1.1 billion smokers worldwide (World Bank, 1999), and there were 5.4 million tobacco attributable deaths in 2005 (Mathers & Loncar, 2006). International approaches to smoking cessation are needed to help those with less access to traditional smoking cessation methods. The Internet provides one means of providing such interventions. Traditional smoking cessation methods, such as nicotine replacement therapy or smoking cessation groups, yield abstinence rates in the range of 14%–27% at 6 months (Fiore, Smith, Jorenby, & Baker, 1994; Lando, McGovern, Barrios, & Etringer, 1990; Schroeder, 2005). Newer medications, such as varenicline (Gonzales et al., 2006) and long-term combination approaches (Hall, Humfleet, Reus, Muñoz, & Cullen, 2004), can increase and maintain abstinence rates to 30% or higher levels. However, these approaches consist of consumable methods, which can only be used once. In contrast, Internet interventions can be used repeatedly by thousands of users. It is important, therefore, to determine their effectiveness.
Automated, self-help Internet smoking cessation interventions tested in randomized control trials (RCTs) have yielded abstinence rates ranging from 8% to 20% using the “missing = smoking” convention, in which participants with missing data at follow-ups are considered to be smoking (An et al., 2008; Brendryen & Kraft, 2008; Clark et al., 2004; Etter, 2005; Feil, Noell, Lichtenstein, Boles, & McKay, 2003; Japuntich et al., 2006; Lenert, Muñoz, Perez, & Banson, 2004; Lenert et al., 2003; McKay, Danaher, Seeley, Lichtenstein, & Gau, 2008; Mermelstein & Turner, 2006; Muñoz et al., 2006; Patten et al., 2006; Pike, Rabius, McAlister, & Geiger, 2007; Rabius, Pike, Wiatrek, & McAlister, 2008; Stoddard et al., 2005; Strecher, Shiffman, & West, 2005; Strecher et al., 2008; Swartz, Noell, Schroeder, & Ary, 2006; Wilborg, Hanewinkel, Isensee, & Horn, 2004; Woodruff, Conway, Edwards, Elliott, & Crittenden, 2007). With few exceptions (Brendryen & Kraft, 2008; Clark et al., 2004; Patten, et al., 2006; Pike et al., 2007; Rabius et al., 2008; Strecher et al., 2008), the duration of follow-up has been limited to 90 days. Attrition has been a problem in Internet studies (Eysenbach, 2005).
The current study examines abstinence rates of an Internet smoking cessation intervention and whether providing additional elements to a static Internet stop-smoking guide increases them. The study examined quit rates for four conditions, including two previously reaching 20% and 26% abstinence rates at 6 months (Muñoz et al., 2006). Innovative characteristics of this study include international online recruitment of Spanish- and English-speaking participants, stratification by depression status, a requirement to return to the site three times to log daily cigarettes prior to randomization, follow-up assessments up to 12 months, and methods to reduce follow-up attrition.
Methods
Participants
Spanish- and English-speaking participants were recruited using Google AdWords campaigns targeted at users worldwide. Smokers came to our site (http://www.stopsmoking.ucsf.edu or http://www.dejardefumar.ucsf.edu) via search engines (after entering relevant key words), links from other Web sites, media stories, or word of mouth. The Web site was described as a “Free online University of California Stop Smoking Study.” Visitors were asked brief demographic questions. A description of the study was presented: “This program is for smokers who are ready to quit. The course will take approximately 8 weeks to complete. You will also be contacted at 1, 3, 6, and 12 months after study entry to answer a brief questionnaire about your progress with quitting and your use of the site.” Those interested clicked on an enroll button and were asked eligibility questions. To be eligible, participants had to report being ≥18 years old, smoking ≥5 cigarettes/day, using E-mail at least once weekly, and intending to quit in the next month. Those meeting criteria provided an E-mail address to obtain a password. They used the password to “sign” the online informed consent form approved by our local Institutional Review Board. Consenting participants continued to the online baseline questionnaire. Those not eligible or not consenting could access the smoking cessation guide online.
Study procedures
The baseline questionnaire provided feedback on nicotine dependence and depression status, including suggestions to use nicotine replacement therapy and/or consult a physician regarding treatment for depression. To screen out those merely browsing and unlikely to return, potential participants who completed the baseline questionnaire were asked to log daily cigarette use on an online cigarette counter on three separate days within the following week. E-mail reminders were sent daily until the third entry or the seventh day, and, after the third entry, participants were asked to set their initial quit date within the next 30 days. Follow-ups were keyed to the initial quit date, although users could change it later. Participants were then randomized and taken to their individualized home page. They could access their designated interventions throughout the 12-month study. Participants returning after the 7-day period were not randomized into the study but were provided access to Condition 3.
Cohort maintenance procedures
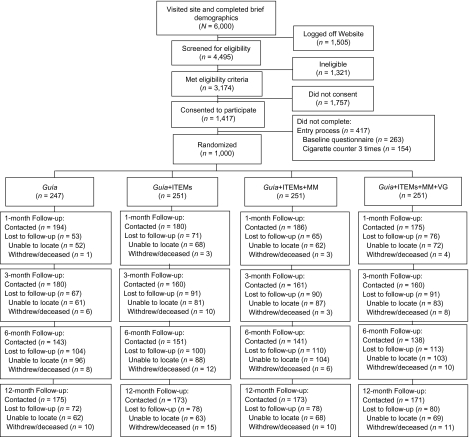
A welcome letter on university letterhead was sent via surface mail after randomization. A similar letter was sent before each follow-up, asking participants to look for an E-mail with a link to the follow-up surveys. These E-mails were sent every 2 days for 1 week to participants 1, 3, 6, and 12 months after initial quit date. If they did not respond, a research assistant sent individualized E-mails requesting completion of the online survey and made up to 10 attempts to obtain follow-up data by phone. Research assistants were blind to assigned condition, requested participants not to disclose their condition assignment, and limited their interaction to obtaining data. Figure 1 shows each step in the recruitment process and the progression of participants across follow-up assessments.
Figure 1.
CONSORT diagram for progression of participants through UCSF/SFGH Internet World Health Research Center Internet Stop Smoking Trial (2005–2007). UCSF = University of California, San Francisco; SFGH = San Francisco General Hospital; Guía = smoking cessation guide; ITEMs = Individually Timed Educational Messages (sent via E-mail with link to site); MM = mood management intervention (eight-lesson cognitive-behavioral course); and VG = virtual group (an asynchronous bulletin board that allowed participants to provide each other mutual support and information).
Interventions
Four Web-based intervention conditions were compared using a parallel-groups randomized trial design. Condition 1 was the Guía para Dejar de Fumar (Guide to Stop Smoking, referred to as “Guía” below), a National Cancer Institute evidence-based intervention initially developed for Spanish-speaking smokers (Muñoz, Marín, Posner, & Pérez-Stable, 1997; National Cancer Institute, 2002; Pérez-Stable, Sabogal, Marín, Marín, & Otero-Sabogal, 1991). The three other conditions added elements incrementally. Labels used for each condition in the Muñoz et al. (2006) study are in parentheses.
Condition 1 (“Guía alone”): the online static “Guía,” a cigarette counter, and an online journal to record experiences while quitting. The Guía covered reasons to quit, cessation strategies, relapse prevention and management, pharmacological aids, and how to help a smoker quit.
Condition 2 (“Guía + ITEMs”): the “Guía” plus “Individually Timed Educational Messages (ITEMs),” that is, automated E-mails with links to sections of the Guía keyed to quit date (Lenert et al., 2004).
Condition 3 (“Guía + ITEMs + MM”): the Guía, ITEMs, plus an eight-lesson cognitive-behavioral mood management course (an extended version of an intervention tested previously; Muñoz et al., 1997).
Condition 4 (“Guía + ITEMs + MM + VG”): the Guía, ITEMS, mood intervention, and a “virtual group” (an asynchronous bulletin board for mutual support and suggestions).
Hypotheses
The randomized trial compared cessation rates for the four conditions. We tested three hypotheses using planned comparisons: (a) Conditions 2, 3, and 4 versus the static Condition 1; (b) incremental quit rates per condition (4 > 3 > 2 > 1); and (c) mood management Conditions 3 and 4 versus Conditions 1 and 2. We also tested whether Conditions 3 and 4 yielded higher quit rates than Conditions 1 and 2 for smokers with a history of major depressive episodes (MDEs).
Measures
Baseline questionnaires included (a) demographics (gender, race/ethnicity, education, employment, income, and marital status); (b) smoking patterns (age first smoked, age regular smoker, cigarettes smoked per day, confidence in quitting on 10-point scale, and methods used to quit in the last 6 months); and (c) the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
The MDE Screener (“Mood Screener,” Muñoz, 1998) inquired about the nine MDE symptoms in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association, 1994). Participants screened positive for an MDE if they experienced five or more of the nine symptoms (including Symptom 1 or 2) for 2 weeks or more and the symptoms interfered with their life or activities a lot at any point during their lifetime (Past MDE) or in the last 2 weeks (Current MDE). The MDE Screener is validated and reliable in English and Spanish speakers (Muñoz, McQuaid, González, Dimas, & Rosales, 1999; Vázquez, Muñoz, Blanco, & López, 2008) and showed good predictive validity for quit rates in participants with past or current major depression (Muñoz et al., 1997, 2006). Three diagnostic groups were defined: (a) No MDE history, never having an MDE; (b) Past MDE, history of MDEs but no Current MDE; and (c) Current MDE, meeting MDE criteria in last 2 weeks.
The Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977) is a 20-item scale yielding a continuous score (0–60), with higher scores signifying greater depression. CES-D scores are related to smoking status in Latinos (Pérez-Stable, Marín, Marín, & Katz, 1990) and predict cessation (Anda et al., 1990).
Web site utilization measures.
The cigarette counter and the journal were available to all participants and served as a proxy for site utilization. For the mood management conditions (3 and 4), we examined tool use (logging of mood, activities, thoughts, and people contacts), as well as number of lessons completed.
Main outcome measures.
Self-reported 7-day point prevalence abstinence was defined as a “no” response to “Have you smoked 1 or more cigarettes in the last 7 days?” Prolonged abstinence was defined as a “no” response to the following questions: “During the past [period since last assessment], have you smoked at least part of a cigarette every day for 7 consecutive days?” and “During [period since last assessment], have you smoked part of a cigarette at least once a week for 2 consecutive weeks?” (Hughes et al., 2003). “Observed” rates are based only on participants who completed follow-ups. Missing = smoking rates impute missing follow-up data as smoking.
Randomization
Stratified randomization using gender and history of MDEs was implemented using an automated algorithm programmed into the Web site.
Data analyses
Sample size estimates were based on statistical power analysis using procedures as described by Rochon (1998) and O’Brien and Muller (1998) under a Type I error rate of .05 (two-tailed) and a minimal power level of .80. Estimates of the effect sizes were based on results from our prior research (Muñoz et al., 2006) and extant literature in this field (Feil et al., 2003). The primary outcome was the proportion who quit smoking, and estimates were computed for each hypothesis that ranged from 18% to 56% in our observed data. The methodology used allowed for anticipated participant retention, which was also based on our most recent data and rates found in the literature (Feil et al., 2003). With 250 per group and a 10% abstinence base rate, the study was able to detect a difference of 9 percentage points (i.e., between 10% and 19%), corresponding to an odds ratio of 2.11. Final sample sizes required ranged from a total sample of 300 up to 480 per language group.
Statistical methods
Descriptive statistics for all measures were computed and tabled separately by language. Data were examined for potential errors, outliers, and missing data. Analyses were conducted twice, once using data as observed and once using the missing = smoking convention. The three hypotheses were tested by comparing abstinence rates for the conditions, individually or combined, as specified in each hypothesis, using Pearson’s chi-square test for proportions. This analysis was extended to test for changes in time by using a linear regression model estimated via the generalized estimating equation (GEE) approach to account for the repeated measurement implemented in the SAS GENMOD Proc. Tests for Hypothesis 2 were conducted by specifying contrasts among the treatment conditions. We examined the secondary hypothesis using the same methods.
Results
Sample
Participants represented 68 countries. English speakers were predominantly from the United States (n = 220), India (n = 57), South Africa (n = 30), the United Kingdom (n = 25), Mexico (n = 19), Australia and Ireland (n = 18, each), Canada (n = 10), and Spain and United Arab Emirates (n = 8, each); Spanish speakers were from Spain (n = 123), Argentina (n = 91), Chile (n = 80), Mexico (n = 59), Venezuela (n = 35), Colombia (n = 30), Uruguay (n = 17), Peru (n = 11), Guatemala (n = 7), and Brazil (n = 6).
Demographic descriptors are displayed in Table 1. More than half of participants were men, 70% were White, two thirds were employed full time, and just more than 40% were married. Smoking and depression history appear in Table 2. Spanish and English speakers are similar in terms of cigarettes smoked and FTND scores. Spanish speakers are less likely to have used other cessation methods. Thirty percent reported enough symptoms for either Current or Past MDEs. English speakers reported more past episodes and Spanish speakers more current episodes. Spanish speakers had significantly higher CES-D scores, although both language groups scored about 1 SD (8.4) above the M (8.7) in the general adult U.S. population (Sayetta & Johnson, 1980).
Table 1.
Demographic characteristics for English- and Spanish-speaking participants of the UCSF/SFGH Internet stop smoking trial (2005–2007)
| All participants (N = 1,000) |
English (n = 500) |
Spanish (n = 500) |
||
| Variable | % |
Group test p | ||
| Sex (N = 1,000)a | .374 | |||
| Men | 55.0 | 56.4 | 53.6 | |
| Women | 45.0 | 43.6 | 46.4 | |
| Ethnicity (n = 975) | <.0001 | |||
| Hispanic/Latino | 52.9 | 10.6 | 94.3 | |
| Not Hispanic/Latino | 47.1 | 89.4 | 5.7 | |
| Race (n = 992) | <.0001 | |||
| European descent | 70.0 | 70.9 | 69.0 | |
| Asian descent | 8.3 | 16.2 | 0.4 | |
| African descent | 1.4 | 2.6 | 0.2 | |
| Indigenous descent | 0.3 | 0.4 | 0.2 | |
| Multiethnic | 3.0 | 2.6 | 3.4 | |
| Other | 6.2 | 4.8 | 7.6 | |
| Mestizob | 10.8 | 2.4 | 19.1 | |
| Education (n = 992) | .079 | |||
| High school or less | 17.1 | 15.9 | 18.3 | |
| Some college | 39.5 | 36.6 | 42.3 | |
| College grad | 28.7 | 31.6 | 25.8 | |
| Graduate degree | 14.7 | 15.9 | 13.5 | |
| Employed (n = 997) | .011 | |||
| Full time | 64.0 | 62.6 | 65.4 | |
| Part time | 11.5 | 9.7 | 13.4 | |
| Not currently | 19.2 | 20.5 | 17.8 | |
| Not ever | 5.3 | 7.2 | 3.4 | |
| Income, US $ (n = 958) | <.0001 | |||
| <10,000 | 31.8 | 21.9 | 41.9 | |
| 10,000–15,000 | 10.0 | 8.1 | 12.0 | |
| 15,000–20,000 | 9.8 | 6.6 | 13.1 | |
| 20,000–35,000 | 14.8 | 14.7 | 14.9 | |
| 35,000–50,000 | 10.1 | 12.6 | 7.6 | |
| 50,000–75,000 | 9.1 | 14.5 | 3.6 | |
| 75,000–100,000 | 7.2 | 11.6 | 2.7 | |
| >100,000 | 7.1 | 9.9 | 4.2 | |
| Marital status (n = 999) | .232 | |||
| Single | 33.1 | 32.3 | 34.0 | |
| Living with | 11.4 | 11.8 | 11.0 | |
| Married | 42.7 | 42.5 | 43.0 | |
| Separated | 4.2 | 3.2 | 5.2 | |
| Divorced | 7.1 | 8.8 | 5.4 | |
| Widowed | 1.4 | 1.4 | 1.4 | |
Note. UCSF = University of California, San Francisco; SFGH = San Francisco General Hospital.
Denotes total N for each variable. Some figures are less than 1,000 because of missing values. Valid percents reported only.
Mestizo, person of mixed Spanish and Indigenous ancestry.
Table 2.
Smoking history, history of major depression, and depression symptoms for English- and Spanish-speaking participants of the UCSF/SFGH Internet stop smoking trial (2005–2007)
| All participants (N = 1,000) | English (n = 500) | Spanish (n = 500) | Group test p | |
| Smoking history | M (SD) | |||
| Age (years) | 37.9 (11.3) | 38.0 (11.9) | 36.1 (10.8) | .010 |
| Age, first cigarette | 15.6 (3.3) | 15.7 (3.3) | 15.6 (3.4) | .675 |
| Age, regular smoker | 18.3 (4.0) | 17.8 (3.5) | 18.7 (4.5) | .001 |
| Cigarettes per day | 19.8 (10.1) | 19.6 (10.1) | 20.0 (10.2) | .525 |
| FTND | 5.2 (2.5) | 5.3 (2.4) | 5.2 (2.5) | .465 |
| Quit confidence | 6.8 (2.0) | 7.0 (2.0) | 6.7(2.0) | .039 |
| Methods used to quit in last 6 months | % Using | |||
| Nicotine gum | 14.6 | 19.2 | 10.0 | <.001 |
| Nicotine patch | 16.3 | 24.2 | 8.4 | <.001 |
| Nicotine inhaler | 2.6 | 3.8 | 1.4 | .017 |
| Nicotine spray | 0.5 | 0.6 | 0.4 | .654 |
| Buproprion (Zyban) | 8.3 | 11.0 | 5.6 | .002 |
| Other antidepressant | 2.2 | 2.8 | 1.6 | .196 |
| Stop smoking group | 2.0 | 1.4 | 2.6 | .175 |
| Hypnosis | 3.1 | 4.6 | 1.6 | .006 |
| Acupuncture | 2.9 | 2.8 | 3.0 | .851 |
| Motivational tapes | 3.2 | 4.2 | 2.2 | .072 |
| Other self-help | 5.6 | 5.2 | 5.8 | .783 |
| Other Web sites | 6.2 | 5.6 | 6.8 | .431 |
| Other | 5.0 | 5.2 | 4.8 | .772 |
| None | 58.6 | 52.5 | 64.8 | <.001 |
| Depression variable | % | |||
| Major depressive episodes | .001 | |||
| No MDE history | 69.7 | 70.7 | 68.7 | |
| Past MDE | 17.3 | 20.1 | 14.6 | |
| Current MDE | 12.9 | 9.2 | 16.6 | |
| CES-D score M (SD) | 16.0 (11.6) | 15.0 (11.0) | 17.0 (12.0) | .006 |
Note. Table based on those with no missing data in the relevant variables. CES-D = Center for Epidemiological Studies Depression Scale; FTND = Fagerström Test for Nicotine Dependence; UCSF = University of California, San Francisco; SFGH = San Francisco General Hospital; and MDE = major depressive episode.
Attrition
Ten percent provided no follow-up data at any of the four follow-ups, leaving 90% responding to at least one follow-up (14% to one, 18% to two, 20% to three, and 38% to all four). No differences in number of assessments were found between language groups, Pearson’s χ2 (1, N = 1,000) = 5.41, p = .25. The largest difference was in those not providing follow-ups, with fewer Spanish speakers (8.2%) doing so than English speakers (12.4%). No significant differences in number of completed assessments were found based on treatment condition, sex, or MDE history status.
Satisfaction with Web site
Ratings of the site at 1 month were: “not helpful,” 7.2%; “somewhat helpful,” 40.0%; “quite helpful,” 35.7%, and “extremely helpful,” 17.0%. Similar ratings at 3 months were: 8.2%, 43.8%, 34.7%, and 13.3%; at 6 months: 11.9%, 40.7%, 34.2%, and 13.2%; and at 12 months: 10.2%, 47.9%, 29.4%, and 12.5%. There were significant differences in satisfaction across Conditions at 1, 3, 6, and 12 months, with Conditions 3 and 4 generally reporting greater satisfaction at each timepoint.
Smoking abstinence
Table 3 displays self-reported 7-day rates for participants by condition and follow-up time. The observed rates varied from 21.7% to 34.3%. The missing = smoking rates varied from 12.7% to 22.7%. Post-hoc comparisons of 7-day quit rates across languages showed that the Spanish language sample rates were significantly lower than the English language sample at 1-, 3-, and 6-month follow-ups but not at the 12-month follow-up. MDE status at baseline was not significantly related to abstinence.
Table 3.
Self-reported 7-day smoking abstinence per intervention by follow-up method in percentages for the UCSF/SFGH Internet stop smoking trial (2005–2007)
| UCSF/SFGH IWHRC Internet smoking cessation trial |
||||
| Condition |
Guía |
Guía + ITEMs |
Guía + ITEMs + MM |
Guía + ITEMs + MM + VG |
| Follow-up point | Observed |
|||
| 1-Month total | 22.5 | 27.0 | 21.7 | 22.2 |
| Automated | 14.7 | 17.4 | 14.1 | 12.3 |
| Live | 7.9 | 9.6 | 7.6 | 9.9 |
| 3-Month total | 24.1 | 29.0 | 23.0 | 26.1 |
| Automated | 17.1 | 17.4 | 14.5 | 17.0 |
| Live | 7.1 | 11.6 | 8.6 | 9.2 |
| 6-Month total | 25.7 | 29.0 | 26.1 | 23.7 |
| Automated | 22.1 | 21.4 | 18.8 | 15.6 |
| Live | 3.6 | 7.6 | 7.2 | 8.1 |
| 12-Month total | 28.3 | 28.2 | 30.1 | 34.3 |
| Automated | 18.5 | 12.9 | 14.5 | 16.3 |
| Live | 9.8 | 15.3 | 15.6 | 18.1 |
| Missing = smoking | ||||
| 1-Month total | 17.4 | 19.1 | 15.9 | 15.1 |
| Automated | 11.3 | 12.4 | 10.4 | 8.3 |
| Live | 6.0 | 6.8 | 5.6 | 6.7 |
| 3-Month total | 16.6 | 17.9 | 13.9 | 15.9 |
| Automated | 11.7 | 10.8 | 8.7 | 10.3 |
| Live | 4.8 | 7.1 | 5.2 | 5.6 |
| 6-Month total | 14.5 | 16.7 | 14.3 | 12.7 |
| Automated | 12.5 | 12.3 | 10.3 | 8.3 |
| Live | 2.0 | 4.3 | 3.9 | 4.4 |
| 12-Month total | 19.8 | 19.1 | 20.7 | 22.7 |
| Automated | 12.9 | 8.7 | 9.9 | 10.7 |
| Live | 6.9 | 10.3 | 10.7 | 11.9 |
Note. Seven day abstinence was defined as a “no” answer to: “Have you smoked 1 or more cigarettes in the past 7 days?” Automated and live follow-up figures do not always add up to the total because of rounding. UCSF = University of California, San Francisco; SFGH = San Francisco General Hospital; IWHRC = Internet World Health Research Center. Automated follow-ups were done via programmed E-mail reminders (up to three) to return to the site at 1, 3, 6, and 12 months after initial quit date. Live follow-ups consisted of phone calls by blinded interviewers if participants did not respond to the automated E-mail after 7 days. Observed rates were calculated as the number reporting 7-day abstinence divided by the number of participants who responded at each follow-up point. Missing = smoking rates were calculated as the number reporting 7-day abstinence divided by the total number of participants assigned to the condition. Guía refers to the smoking cessation guide. ITEMs refer to Individually Timed Education Messages, that is, E-mails reminding participants to return to the site and read specific sections of the materials selected according to their quit date. MM (mood management) refers to an eight-session MM intervention designed to help participants regulate their mood to increase the likelihood of quitting. VG (virtual group) refers to an asynchronous bulletin board that allowed participants to interact and provide each other with information and encouragement.
Based on tests of the estimates of the GEE-based statistical models, none of the three primary hypotheses were supported (all p values > .05). The proportion of participants abstinent at each timepoint and across time did not differ statistically among groups using either observed data or missing = smoking data and using either 7-day or prolonged abstinence. Finally, the two conditions with mood management methods did not yield higher abstinence rates in those with past or current MDE.
Site utilization and abstinence rates
We examined the relationship between abstinence rates and three measures of site utilization within 30 days postrandomization: 26.9% of participants across all conditions used neither the cigarette counter nor the journal, 25% used either, and 48.1% used both. The mood management tools were used by 66.7% of the participants in Conditions 3 and 4. The ITEMs in Conditions 2, 3, and 4 increased utilization of the cigarette counter, χ2 (2, N = 1,000) = 78.01, p < .001, and the online journal, χ2 (1, N = 1,000) = 6.51, p = .011, above static Condition 1. The mood management Conditions (3 and 4) increased cigarette counter use over Condition 2, χ2 (2, n = 753) = 25.64, p < .001. Greater abstinence at 1-month assessment was significantly related with using the cigarette counter two or more times, χ2 (1, n = 722) = 6.99, p = .0082, and using the journal (vs. not), χ2 (1, n = 722) = 4.00, p = .0456. While the use of the cigarette counter was not related to abstinence at later assessments, journal use was related to greater abstinence rates at both the 3-month, χ2 (1, n = 649) = 3.99, p = .0485, and 6-month assessments, χ2 (1, n = 558) = 10.49, p = .0012. In the mood management conditions, tool use was not related to abstinence. Abstinence was not significantly related to completion of more mood management lessons. No significant differences were seen among treatment conditions when controlling for site use, and none of the interaction terms were significant.
Impact of method of obtaining follow-up data
We examined whether the phone follow-up procedure might have yielded higher quit rates because of social desirability effects. At 1-, 3-, 6-, and 12-month follow-ups, observed online 7-day quit rates were 14.7%, 16.0 %, 19.5 %, and 15.5%, and phone 7-day quit rates were 8.7%, 8.8%, 6.6%, and 14.7%. Thus, online self-reported quit rates are actually higher than phone self-reported quit rates.
Discussion
This study shows that it is possible to conduct a smoking cessation RCT via the Internet with an international sample of English- and Spanish-speaking smokers. The overall 12-month 7-day abstinence rates were 30.2% based on participants with follow-up data and 20.6% when those with missing data were assumed to be smoking. Because such public health smoking cessation interventions can reach a geographically wider audience of smokers than traditional face-to-face or pharmacological interventions, they should be developed and tested in additional languages.
Our results show that an empirically supported print intervention adapted to the Web and provided to Spanish- and English-speaking smokers throughout the world with or without additional elements yielded good results. English speakers quit at significantly higher rates at 1-, 3-, and 6-month follow-ups, but Spanish speakers caught up by the 12-month follow-up. We are intrigued by this increase in abstinence rates at 1 year for Spanish speakers (which underscores the advisability of follow-up assessments beyond 6 months). We speculate that local norms for smoking may have helped English speakers quit sooner but that the follow-up E-mails throughout the year may have reminded participants of their initial intention to quit and may have helped them recommit to quitting.
On hindsight, a major limitation of this study is the lack of an adequate control condition. We conceptualized the Guía alone as a control condition because earlier studies (e.g., Muñoz et al., 2006) had yielded lower rates for the Guía in one-condition studies. However, we had not compared the Guía alone with the current study’s Conditions 2 and 3. Our current results show that this static condition can be as efficacious as more interactive conditions. There is no evidence of increased abstinence rates with additional elements in either language, even though utilization of the interactive aspects of the site was significantly higher for the three conditions that added elements to the static condition, and utilization of the tools available to all participants was significantly related to abstinence.
An alternative interpretation is that the elements added to the static Guía were not effective. Cochrane reviews of self-help smoking cessation interventions (Lancaster & Stead, 2005; Lancaster, Stead, Silagy, & Sowden, 2000) have reported that self-help materials have not been found to be very helpful, “although there is likely to be a small benefit for people given no other support” (Lancaster & Stead, 2005, p. 2).
We have considered the possibility that the lack of differences among conditions is due to the requirement to log daily cigarettes three times before randomization. This may have limited participants to those motivated enough to quit at about the same rate (20%), no matter what the intervention received. We examined whether this requirement might have resulted in a large proportion of participants becoming abstinent before randomization. We found that 6.8% indicated smoking no cigarettes the day they were randomized (i.e., the third day they logged cigarettes). This rate is similar to the quit rates of smokers motivated enough to join face-to-face smoking cessation trials using the patch, who report 6-month placebo patch rates of only 5%–9% (Fiore et al., 1994; Schroeder, 2005). Thus, high motivation does not yield 20% quit rates even in smokers motivated enough to physically travel to clinical research sites and receive brief counseling interventions. In terms of Internet studies, a wait-list control condition (Swartz et al., 2006) yielded a 5% quit rate at 90 days. This is comparable to the nicotine patch placebo control rates above and does not approximate the 20% abstinence rate found in the present study. Finally, the missing = smoking cessation rates reported by Spanish-speaking smokers in this study at 1, 3, 6, and 12 months were 13%, 14.2%, 12.4%, and 20.2%, compared with 20.8%, 18.0%, 16.8%, and 21.0% for the English speakers. Thus, smoking cessation rates provided by our participants were not uniform across the board. As in our earlier article (Muñoz et al., 2006), we are left with the possibility that adding more interactive content is not necessarily better than an effective, though static, smoking cessation guide.
Abstinence rates observed were very different from those used in our power calculations and may have underpowered the trial, thus constraining our ability to find differences among the conditions. Our results are similar to those of the American Cancer Society’s QuitLink study (Pike et al., 2007). This study compared six interactive sites with a static site that provided a brochure online. The study sample was composed of 6,451 English-speaking adult U.S. smokers. Follow-up rates were 54% at 4 months and 44% at 7 months. Prolonged intent-to-treat abstinence rates were not significantly different and, on average, 11.0% and 9.9% for the interactive sites at 4 and 7 months, compared with 10.9% and 9.5% for the static site. Thirteen-month rates were, again, not significant (Rabius et al., 2008). The QuitLink study suggests that both tailored Web interventions and an online brochure are moderately efficacious. A recent study by McKay et al. (2008) also showed no significant differences between an active intervention and an attention placebo. The field needs to determine whether a Web site (static or interactive) yields higher abstinence rates than not having access to any site or whether participants who join online smoking cessation trials are so motivated that they all quit at about the same rates as long as the Internet interventions contain quality, empirically based information. Until this issue has been adequately examined, Web-assisted tobacco intervention trials should include no-intervention delayed conditions. Follow-up surveys should inquire whether control participants used other smoking cessation methods, including other Web sites, of course.
Attrition at follow-ups was reduced by (a) requiring that participants return to the site three times within a week prior to randomization, (b) sending surface mail letters at randomization and prior to each follow-up period, (c) sending E-mails with links to the follow-up surveys, and (d) calling participants on the phone up to 10 times if they did not respond to the E-mails. With this approach, we were able to contact 692 of the 1,000 participants in the trial (69.2%) at the 12-month follow-up, of whom 206 out of 682 who answered the 7-day abstinence question reported having quit.
The overall findings for each condition from this randomized trial are similar to our earlier findings (Muñoz et al., 2006). For example, for Study 4 in the 2006 report, which was done in Spanish, the Guía + ITEMs condition 12-month missing = smoking rate was 22.6%, compared with 17.3% for Spanish speakers in the current study, and the Guía + ITEMs + MM condition was 20.4%, compared with 19.8% for Spanish speakers in the current study. The new Guía + ITEMs + MM + VG condition (Condition 4 in the current study) yielded 12-month missing = smoking abstinence rates of 21.3% in English and 24.2% in Spanish. These rates reinforce the potential benefit of making these sites (http://www.stopsmoking.ucsf.edu and http://www.dejardefumar.ucsf.edu) available to smokers worldwide at no charge to users, to create similar sites in other languages, and to invite national and international health organizations to publicize these sites and make them accessible to smokers interested in quitting. If we can help 20% of those who come to such Internet sites to stop smoking at 1 year, these sites could have substantial impact on reducing the suffering and death caused by this major source of morbidity and mortality worldwide.
Funding
This research was supported by grant 13RT-0050 from the Tobacco-Related Disease Research Program (Muñoz PI), which helped establish the University of California, San Francisco/San Francisco General Hospital Internet World Health Research Center (www.health.ucsf.edu), and by an infrastructure grant from the University of California Committee on Latino Research to the UCSF/SFGH Latino Mental Health Research Program, directed by Muñoz. Dr. Pérez-Stable's time was funded by grant no. TW05935 from the Tobacco Research Network Program, Fogarty International Center, National Cancer Institute, National Institute on Drug Abuse, National Institutes of Health, and by a grant from the National Cancer Institute, Redes en Acción, U01-CA86117.
Declaration of Interests
None declared.
Supplementary Material
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- An LC, Klatt C, Perry CL, Lein EB, Hennrikus DJ, Pallonen UE, et al. The RealU online cessation intervention for college smokers: A randomized controlled trial. Preventive Medicine. 2008;47:194–199. doi: 10.1016/j.ypmed.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Anda RF, Williamson DF, Escobedo LG, Mast EE, Giovino GA, Remington PL. Depression and the dynamics of smoking: A national perspective. Journal of the American Medical Association. 1990;12:1541–1545. [PubMed] [Google Scholar]
- Brendryen H, Kraft P. Happy ending: A randomized controlled trial of a digital multi-media smoking cessation intervention. Addiction. 2008;103:478–484. doi: 10.1111/j.1360-0443.2007.02119.x. [DOI] [PubMed] [Google Scholar]
- Clark MM, Sanderson Cox L, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Effectiveness of smoking cessation self-help materials in a lung cancer screening population. Lung Cancer. 2004;44:13–21. doi: 10.1016/j.lungcan.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Etter J. Comparing the efficacy of two Internet-based computer-tailored smoking cessation programs: A randomized trial. Journal of Medical Internet Research. 2005;7:e2. doi: 10.2196/jmir.7.1.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenbach G. The law of attrition. Journal of Medical Internet Research. 2005;7:e11. doi: 10.2196/jmir.7.1.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil EG, Noell J, Lichtenstein E, Boles SM, McKay HG. Evaluation of an Internet-based smoking cessation program: Lessons learned from a pilot study. Nicotine & Tobacco Research. 2003;5:189–194. doi: 10.1080/1462220031000073694. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation: A meta-analysis. Journal of the American Medical Association. 1994;271:1940–1947. [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. Journal of the American Medical Association. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Reus VI, Muñoz RF, Cullen J. Extended nortriptylene and psychological treatment for cigarette smoking. American Journal of Psychiatry. 2004;161:2100–2107. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Japuntich SJ, Zehner ME, Smith SS, Jorenby DE, Valdez JA, Fiore MC, et al. Smoking cessation via the Internet: A randomized clinical trial of an Internet intervention as adjuvant treatment in a smoking cessation intervention. Nicotine & Tobacco Research. 2006;8(Suppl. 1):S59–S67. doi: 10.1080/14622200601047900. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2005;3 doi: 10.1002/14651858.CD001118.pub2. CD001118. doi: 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Stead L, Silagy C, Sowden A. Effectiveness of interventions to help people stop smoking: Findings from the Cochrane Library. British Medical Journal. 2000;321:355–358. doi: 10.1136/bmj.321.7257.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando HA, McGovern PG, Barrios FX, Etringer BD. Comparative evaluation of American Cancer Society and American Lung Association smoking cessation clinics. American Journal of Public Health. 1990;80:554–559. doi: 10.2105/ajph.80.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenert L, Muñoz RF, Perez J, Banson A. Automated E-mail messaging as a tool for improving quit rates in an Internet smoking cessation intervention. Journal of the American Medical Informatics Association. 2004;11:235–240. doi: 10.1197/jamia.M1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenert L, Muñoz RF, Stoddard J, Delucchi K, Bansod A, Skoczen S, et al. Design and pilot evaluation of an Internet smoking cessation program. Journal of the American Medical Informatics Association. 2003;10:16–20. doi: 10.1197/jamia.M1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PloS Medicine. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay HG, Danaher BG, Seeley JR, Lichtenstein E, Gau JM. Comparing two Web-based smoking cessation programs: Randomized controlled trial. Journal of Medical Internet Research. 2008;10:e40. doi: 10.2196/jmir.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein R, Turner L. Web-based support as an adjunct to group-based smoking cessation for adolescents. Nicotine & Tobacco Research. 2006;8(Suppl. 1):S69–S76. doi: 10.1080/14622200601039949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz RF. 1998. The Mood Screener. Retrieved 28 July 2008, from http://www.medschool.ucsf.edu/latino/manuals.aspx. [Google Scholar]
- Muñoz RF, Lenert LL, Delucchi K, Stoddard J, Pérez JE, Penilla C, et al. Toward evidence-based Internet interventions: A Spanish/English Website for international smoking cessation trials. Nicotine & Tobacco Research. 2006;8:77–87. doi: 10.1080/14622200500431940. [DOI] [PubMed] [Google Scholar]
- Muñoz RF, Marín B, Posner SF, Pérez-Stable EJ. Mood management mail intervention increases abstinence rates for Spanish-speaking Latino smokers. American Journal of Community Psychology. 1997;25:325–343. doi: 10.1023/a:1024676626955. [DOI] [PubMed] [Google Scholar]
- Muñoz RF, McQuaid JR, González GM, Dimas J, Rosales VA. Depression screening in a women's clinic: Using automated Spanish- and English-language voice recognition. Journal of Consulting and Clinical Psychology. 1999;67:502–510. doi: 10.1037//0022-006x.67.4.502. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Guia para dejar de fumar. (NIH Publication No. 02-3001) Washington, DC: U.S. Government Printing Office; 2002. Retrieved 2 August 2008, from http://dccps.cancer.gov/tcrb/No_FumarC.pdf. [Google Scholar]
- O’Brien RG, Muller KE. Proceedings of the 23rd SAS Users Group International Conference. Cary, NC: SAS Institute; 1998. A tour of UnifyPow: A SAS module/macro for sample-size analysis; pp. 1346–1355. [Google Scholar]
- Patten CA, Croghan IT, Meis TM, Decker PA, Pingree S, Colligan RC, et al. Randomized clinical trial of an Internet-based versus brief office intervention for adolescent smoking cessation. Patient Education and Counseling. 2006;64:249–258. doi: 10.1016/j.pec.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Pérez-Stable EJ, Marín G, Marín B, Katz MH. Depressive symptoms and cigarette smoking among Latinos in San Francisco. American Journal of Public Health. 1990;80:1500–1502. doi: 10.2105/ajph.80.12.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Stable EJ, Sabogal F, Marín G, Marín B, Otero-Sabogal R. Evaluation of “Guía para dejar de fumar,” a self-help guide in Spanish to quit smoking. Public Health Reports. 1991;106:564–570. [PMC free article] [PubMed] [Google Scholar]
- Pike KJ, Rabius V, McAlister A, Geiger A. American Cancer Society's QuitLink: Randomized trial of Internet assistance. Nicotine & Tobacco Research. 2007;9:415–420. doi: 10.1080/14622200701188877. [DOI] [PubMed] [Google Scholar]
- Rabius V, Pike KJ, Wiatrek D, McAlister AL. Comparing Internet assistance for smoking cessation: 13-month follow-up of a six-arm randomized controlled trial. Journal of Medical Internet Research. 2008;10:e45. doi: 10.2196/jmir.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Statistics in Medicine. 1998;17:1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Sayetta RB, Johnson DP. Basic data on depressive symptomatology: United States, 1974–1975. Vital and Health Statistics. Series 11, Number 216 (DHEW Publication No. PHS 80-1666) Hyattsville, MD: National Center for Health Statistics; 1980. [DOI] [PubMed] [Google Scholar]
- Schroeder SA. What to do with a patient who smokes. Journal of the American Medical Association. 2005;294:482–487. doi: 10.1001/jama.294.4.482. [DOI] [PubMed] [Google Scholar]
- Stoddard J, Delucchi KL, Muñoz RF, Collins NM, Pérez-Stable EJ, Augustson E, et al. Smoking cessation research via the Internet: A feasibility study. Journal of Health Communication. 2005;10:27–41. doi: 10.1080/10810730590904562. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, McClure J, Alexander G, Chakraborty B, Nair V, Konkel J, et al. The role of engagement in a tailored Web-based smoking cessation program: Randomized controlled trial. Journal of Medical Internet Research. 2008;10:e36. doi: 10.2196/jmir.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web-based computer-tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction. 2005;100:682–688. doi: 10.1111/j.1360-0443.2005.01093.x. [DOI] [PubMed] [Google Scholar]
- Swartz LH, Noell JW, Schroeder SW, Ary DV. A randomised control study of a fully automated Internet based smoking cessation programme. Tobacco Control. 2006;15:7–12. doi: 10.1136/tc.2003.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez FL, Muñoz RF, Blanco V, López M. Validation of Muñoz's Mood Screener in a nonclinical Spanish population. European Journal of Psychological Assessment. 2008;24:57–64. [Google Scholar]
- Wilborg G, Hanewinkel R, Isensee B, Horn WR. Development, implementation, and evaluation of a program for the cessation of smoking for adolescents and young adult smokers [Abstract] Gesundheitswesen. 2004;66:433–438. doi: 10.1055/s-2004-813108. [DOI] [PubMed] [Google Scholar]
- Woodruff SI, Conway TL, Edwards CC, Elliott SP, Crittenden J. Evaluation of an Internet virtual world chat room for adolescent smoking cessation. Addictive Behavior. 2007;32:1769–1786. doi: 10.1016/j.addbeh.2006.12.008. [DOI] [PubMed] [Google Scholar]
- World Bank. Global trends in tobacco use. 1999. Retrieved 2 August 2008, from http://www1.worldbank.org/tobacco/book/html/chapter1.htm. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.