Abstract
Introduction
Recent genetic evidence has implicated nicotine as a possible cause of cancer, suggesting the need to examine the potential contributions of nicotine itself to cancer versus the confounding effects of addiction and thus exposures to known carcinogens. The objective of this study was to examine the relationship between nicotine replacement therapy, smoking, and cancer outcomes.
Methods
The Lung Health Study enrolled 5,887 participants in a randomized trial to prevent chronic obstructive pulmonary disease. The present study used surveillance data on 3,320 intervention participants who enrolled in the Lung Health Study for 5 years and who were then followed by the Lung Cancer Substudy for 7.5 years. Nicotine replacement therapy use and smoking exposure were recorded during the 5-year Lung Health Study trial. Surveillance for lung cancer, gastrointestinal cancer (including oral cancers), and all cancers began following the Lung Health Study.
Results
Adjusted Cox proportional hazards regressions assessed the hazards of nicotine replacement therapy and smoking for each diagnosis group. In the adjusted models for lung cancer, nicotine replacement therapy alone was not a significant predictor (p = .57), while smoking during the Lung Health Study was a significant predictor (p = .03). When nicotine replacement therapy and smoking were entered in the same model, nicotine replacement therapy remained not significant (p = .25) and smoking was clearly significant (p = .02). Nicotine replacement therapy and smoking were not significant predictors of cancer in the models for gastrointestinal cancer or all cancers.
Discussion
Although the surveillance time was short, smoking predicted cancer in this analysis and nicotine replacement therapy did not.
Introduction
The key rationale for the current research is based on the findings of genome-wide studies of single nucleotide polymorphisms in lung cancer cases and controls in Europeans and others of European ancestry (Amos et al., 2008; Hung et al., 2008; Thorgeirsson et al., 2008). Three recent studies found a locus in chromosome region 15q25 that was strongly associated with lung cancer. The association region includes genes that encode nicotinic acetylcholine receptor subunits (CHRNA5 and CHRNA3). This evidence suggests the need to examine the potential contributions of nicotine itself to cancer versus the confounding effects of addiction and thus exposures to known carcinogens.
The three genetic studies identified specific chromosomal variants associated with an increased risk for developing lung cancer, located on an area of chromosome 15 that contains several genes encoding portions of nicotinic acetylcholine receptors. Two of the studies found that the relationship between the genetic variants and the lung cancer appears to be through an increased susceptibility to lung cancer, possibly via one of the mechanisms of nicotine action listed below (Amos et al., 2008; Hung et al., 2008). The third study, however, suggested a correlation between the genetic variant and both smoking quantity and nicotine dependence, which may in turn increase smokers’ risk for disease, including lung cancer (Thorgeirsson et al., 2008). Nonetheless, given that the vast majority of animal studies, including long-term nicotine exposure studies, do not indicate a carcinogenic effect of nicotine, studies suggesting theoretical mechanisms by which nicotine might produce a carcinogenic effect should be considered preliminary pending further evidence.
Another rationale for the current research is based on the presence of significant misperceptions regarding nicotine replacement therapy among smokers. In a survey of more than 1,000 adult cigarette smokers, researchers discovered that while more than half believe that nicotine is a cause of cancer, only one in three respondents surveyed believe that nicotine patches are safer than cigarettes (Bansal, Cummings, Hyland, & Giovino, 2004; Cummings et al., 2004). The scientific literature, apart from the above genetic studies, indicates predominantly that nicotine is safe (Benowitz, 1998; Fiore et al., 2008). Given this discrepancy between scientific and public beliefs regarding the risks of cigarette smoking among current smokers, understanding the cancer experience among the only available long-term cohort of nicotine replacement therapy users may have considerable implications for treatment and influences on cessation behavior.
There is no doubt that tobacco smoking causes cancer (Anthonisen et al., 2005; Samet, 2005). Tobacco smoke contains many chemicals other than nicotine that have been clearly shown to be the major etiologic agents in smoking-induced cancers (Hoffmann & Hoffmann, 1997). In tobacco, nicotine can be nitrosated to form nitrosamines, a group of potent carcinogens (Hecht, 1998; Hoffmann & Hoffmann). In animal studies, however, nicotine itself has repeatedly failed to show carcinogenic effects (Levy & Martin, 1989). In one animal study, rats breathed in a chamber with nicotine at a concentration twice that found in the plasma concentration of heavy smokers (Waldum et al., 1996). Nicotine was given for 20 hr a day, 5 days a week over a 2-year period. The authors found no increase in mortality or frequency of tumors in these rats compared with controls. Specifically, there were neither microscopic nor macroscopic lung tumors nor any increase in pulmonary neuroendocrine cells. Thus, even long-term exposure to inhaled nicotine at relatively high doses does not appear to have a carcinogenic effect.
Research has suggested a number of mechanisms by which nicotine might theoretically induce or promote carcinogenesis or tumor development under certain conditions. These include activation of Akt signaling pathways via nicotinic acetylcholine receptors in bronchial epithelial cells by nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (West et al., 2003), suppression of apoptosis (Mai, May, Gao, Zhaohui, & Deng, 2003; Maneckjee & Minna, 1994; Sugano, Minegishi, Kawamoto, & Ito, 2001), and promotion of angiogenesis (Heeschen et al., 2001; Natori et al., 2004).
Furthermore, a federally funded clinical trial of about 6,000 smokers found that nicotine gum can be used safely for up to 5 years without any cardiovascular illnesses or other serious side effects (Murray et al., 1996). The 2008 U.S. Public Health Service panel on treating tobacco use and dependence also concluded that the risks of nicotine replacement therapy are theoretical at this point and likely to be small if present at all—particularly when compared with the alternative risk of continuing to smoke cigarettes (Fiore et al., 2008). The panel confirmed that each of the nicotine replacement therapies approximately doubles a smoker’s chance of success in quitting.
It has been difficult to evaluate the effect of nicotine itself or nicotine replacement therapy in human carcinogenesis because human nicotine exposure usually comes from tobacco, which also delivers dozens of known carcinogens. One exception is the Lung Health Study, which studied smokers with subclinical chronic obstructive pulmonary disease, and provided nicotine gum over a period of 5 years in some participants (Murray et al., 1996). The large sample size, encouragement to use nicotine replacement therapy over several years, and long follow-up period make the Lung Health Study a uniquely valuable dataset for providing insight into the potential effects of prolonged use of nicotine gum.
This analysis examines the risk of cancer in relation to long-term use of nicotine replacement therapy in the Lung Health Study sample. We recognize the complexity of the confound with the cancer risk of smoking, although in the Lung Health Study, participants who were abstinent for the first 5 years were likely still abstinent for the next 6 years as well (Murray, Connett, Rand, Pan, & Anthonisen, 2002).
Methods
Sample
The original Lung Health Study enrolled 5,887 participants in a randomized trial to investigate the possibility of preventing emphysema. Beginning in November of 1986, two thirds of participants (n = 3,923) were randomized to a smoking intervention. For any individual participant in Lung Health Study 1, the duration of the study was 5 years. In Lung Health Study 1, participants were encouraged to use nicotine replacement therapy (Nicorette 2 mg, Marion Merrell Dow, Kansas City, MO) liberally for 6 months, although some continued to use nicotine replacement therapy well beyond that point (Murray, Nides, Istvan, & Daniels, 1998). After 2.5 years, they were encouraged to cease using nicotine replacement therapy. Lung Health Study 1 was followed by the Lung Cancer Substudy in May 1994. From May 1994 to April 1998, detailed morbidity records were obtained for cancers only. After April 1998, consenting participants were enrolled in Lung Health Study 3, in which hospitalizations and emergency room visits were monitored for all causes until December 2001. The subjects in this study are the 3,320 Lung Health Study smoking intervention participants with no diagnosis of cancer during Lung Health Study 1 who completed the fifth annual visit in Lung Health Study 1 and who were enrolled in the Lung Cancer Substudy. Our intent was to look at cancer after the onset of nicotine replacement therapy. These participants comprise 85% of the original smoking intervention group and were followed for 7.5 years after Lung Health Study 1.
Design
This is a prospective study using available Lung Health Study data. Smoking and nicotine replacement therapy exposure are estimated over the 5 years of Lung Health Study 1, since that is the interval for which detailed nicotine replacement therapy data are available. Cancer outcomes in the Lung Health Study are based on both mortality and morbidity data. Cancer mortality data are derived from the National Death Index. The Lung Health Study includes documentation of cancer morbidity events that required hospitalization, coded by cause by a trained nosologist. Analyses that follow report mortality and morbidity combined. Cancer events were reported as hospitalizations or deaths, since that is the way the data were collected.
Measures
The use of nicotine replacement therapy was measured by self-report at 4-month visits and more frequently at visits when participants appeared at the clinics for a supply of nicotine replacement therapy. The Lung Health Study data on the use of nicotine replacement therapy are considered reliable, since the study provided participants with their supply, and in most of the study years, it was not otherwise available without prescription. Self-report of amount of current nicotine replacement therapy use at 4-month visits (expressed in pieces per day) until the fifth annual visit is used in these analyses. Total nicotine replacement therapy exposure is taken as the mean of pieces per day at the fifteen 4-month visits. Missed 4-month visits are counted as visits with zero nicotine replacement therapy use, since none was dispensed at those times.
Smoking exposure during Lung Health Study 1 is calculated from current cigarettes per day reported at annual visits and recalled months with one cigarette or more during the past year, also collected at annual visits. These monthly reports of cigarettes per day are summed for the first 5 years of the study and expressed as pack-years of smoking during the study.
Surveillance for outcome events began immediately after the fifth annual visit five and continued until the end of Lung Health Study 3 in December of 2001. Diagnoses of cancer in this report are grouped into lung cancers, gastrointestinal tract cancers (including oral cancers), and all cancers. Gastrointestinal tract cancers were a main focus of the analysis based on the notion that nicotine swallowed as a result of gum use provides exposure in the stomach.
Statistical methods
Mean values, SDs, and percents of covariates are presented in tables. Cox proportional hazards methods are used to model time-to-event data. Time-to-event is defined as the time in years from a time following the individual’s fifth-year annual visit until the first occurrence of hospitalization or death due to lung cancer, gastrointestinal cancer, or cancer from all causes. Average gum pieces per day during the study and pack-years of cigarette smoking during the study predicted cancers in models adjusted by covariates: baseline age, gender, cigarettes per day, and lifetime pack-years of smoking. Separate models estimate the hazards of lung cancer, gastrointestinal cancer, and any cancer. Each table shows models with nicotine replacement therapy use, with cigarette use, and with both nicotine replacement therapy use and cigarette use.
Results
Baseline characteristics of study participants in the smoking intervention who used some nicotine replacement therapy versus no nicotine replacement therapy are shown in Table 1. Among women, a significant difference was found in age (p = .03). Women who used nicotine replacement therapy were older than those who did not use nicotine replacement therapy. Among men, significant differences were found in baseline cigarettes per day (p = .03) and in baseline pack-years (p = .03). Male nicotine replacement therapy users were heavier smokers and had a heavier history of smoking at baseline.
Table 1.
Baseline characteristics of the Lung Health Study smoking intervention participants who were enrolled in the Lung Cancer Substudy
| Used nicotine replacement therapy (n = 1,986) |
Used no nicotine replacement therapy (n = 1,329) |
|||
| Characteristics | M | SD | M | SD |
| Age, years | ||||
| Men | 48.6 | 6.81 | 48.3 | 7.10 |
| Women | 48.9 | 6.45 | 48.1* | 6.66 |
| Cigarettes per day | ||||
| Men | 33.2 | 13.1 | 31.9* | 13.5 |
| Women | 28.7 | 11.4 | 29.2 | 12.6 |
| Cigarettes, pack-years | ||||
| Men | 43.6 | 20.0 | 41.6* | 20.1 |
| Women | 36.4 | 15.7 | 35.1 | 16.7 |
Note. Means above are compared for participants who used nicotine replacement therapy vs. no nicotine replacement therapy with t tests. Percents are compared using chi-square tests.
p < .05.
Mean (SD) pieces per day of nicotine replacement therapy during Lung Health Study 1 were as follows: at 12 months, 2.17 (4.62) pieces per day; at 24 months, 1.96 (4.78); at 36 months, 1.66 (4.54); at 48 months, 1.18 (3.98); and at 60 months, 0.75 (3.20). The mean nicotine replacement therapy use over 60 months for those who had quit smoking at the 12-month point in Lung Health Study 1 and remained abstinent from smoking at 12-month follow-ups thereafter was 1.99 (3.89) pieces per day. For those participants who were smoking at some annual follow-ups and not at others, the mean was 2.89 (3.64) pieces per day. For those who were smoking at all annual follow-ups, the mean was 0.66 (1.22) pieces per day.
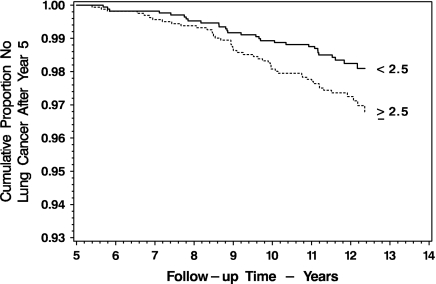
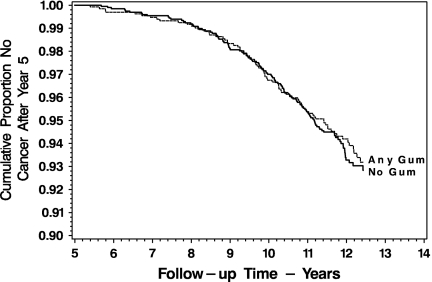
Mean nicotine replacement therapy use over the 60 months of Lung Health Study 1 was distributed as follows: 0 pieces per day, 40.1%; 0.05–1.00 pieces per day, 25.5%; 1.05–3.00 pieces per day, 16.9%; and 3.05 or more pieces per day, 17.5%. The present study identified 75 lung cancer events, 33 gastrointestinal tract cancers, and 203 cancers from all causes. Figure 1 indicates that intervention participants who are above and below the median cigarette exposure during Lung Health Study 1 differ in the incidence of lung cancers in the 7.5 years following (46 vs. 29 events, p = .03). Figure 2 compares the survival of intervention participants from any diagnosis of cancer and does not differ between users of any nicotine replacement therapy versus users of no nicotine replacement therapy (133 vs. 90 events, p = .72).
Figure 1.
Survival without any diagnosis of lung cancer following 5 years in the Lung Health Study, for participants in the Lung Cancer Substudy, by cigarette pack-years above and below the median (2.5) of pack-years of smoking in the study.
Figure 2.
Survival without any diagnosis of cancer following 5 years in the Lung Health Study, for participants in the Lung Cancer Substudy, by any versus no nicotine replacement therapy use during the initial study.
Table 2 summarizes Cox proportional hazards regression models predicting lung cancer after the fifth annual visit in Lung Health Study 1, adjusted for age, gender, baseline cigarettes per day, and lifetime pack-years of smoking. Model 1 indicates no relationship between nicotine replacement therapy use and subsequent lung cancer (p = .67). Model 2 indicates a significant relationship between cigarette use during Lung Health Study 1 and subsequent lung cancer, with an adjusted model (p = .03). Model 3 has both nicotine replacement therapy use and cigarette use. Nicotine replacement therapy use is still not significant (p = .25), and cigarette use is now clearly significant (p = .02).
Table 2.
Proportional hazards regression predicting 75 fatal and nonfatal lung cancer events among 3,295 smoking intervention participants enrolled in the Lung Cancer Substudy
| Model | Coefficient | SE | Hazard ratio | 95% CI | p value |
| 1. Mean nicotine replacement therapy use, pieces per day over 5 years | .015 | 0.035 | 1.02 | 0.95–1.09 | .67 |
| 2. Cigarette use, pack-years over 5 years | .079 | 0.037 | 1.08 | 1.01–1.16 | .03 |
| 3. Mean nicotine replacement therapy use, cigarette use during study | .042 | 0.036 | 1.04 | 0.97–1.12 | .25 |
| .093 | 0.039 | 1.10 | 1.02–1.19 | .02 |
Note. Models are adjusted by baseline age, gender, cigarettes per day, and lifetime pack-years of smoking.
The models in Table 2 were replicated including only the 820 participants who were sustained quitters of cigarettes during the Lung Health Study. There were 14 fatal and nonfatal events in this subset. The pattern of significant results was unchanged from Table 2, with the exception that in Model 3, mean nicotine replacement therapy use was marginally significant (p = .045). With the small number of events, this result was not regarded as particularly reliable.
Table 3 displays results of adjusted models with the same pattern of covariates as the models in Table 2. The difference is that the outcomes in Table 3 are occurrences of gastrointestinal cancer rather than lung cancer. Nicotine replacement therapy use alone (Model 1) is not significant (p = .61), cigarette use alone (Model 2) is not significant (p = .59), and in Model 3 where the effects of nicotine replacement therapy and smoking are examined together, neither is significant.
Table 3.
Proportional hazards regression predicting 33 fatal and nonfatal gastrointestinal cancer events among 3,304 smoking intervention participants enrolled in the Lung Cancer Substudy
| Model | Coefficient | SE | Hazard ratio | 95% CI | p value |
| 1. Mean nicotine replacement therapy use, pieces per day over 5 years | −.032 | 0.063 | 0.97 | 0.86–1.10 | .61 |
| 2. Cigarette use, pack-years over 5 years | .029 | 0.054 | 1.03 | 0.93–1.14 | .59 |
| 3. Mean nicotine replacement therapy use, cigarette use during study | −.035 | 0.084 | 0.97 | 0.82–1.14 | .68 |
| .030 | 0.065 | 1.03 | 0.91–1.17 | .64 |
Note. Models are adjusted by baseline age, gender, cigarettes per day, and lifetime pack-years of smoking.
The adjusted models for cancer from all causes are shown in Table 4. Nicotine replacement therapy use is not a significant predictor (p = .94), cigarette use is also not a significant predictor in these data (p = .17), and when both of these are included in the same model, neither is significant (p = .62 and p = .14, respectively).
Table 4.
Proportional hazards regression predicting 203 fatal and nonfatal cancer events from all causes among 3,288 smoking intervention participants enrolled in the Lung Cancer Substudy
| Model | Coefficient | SE | Hazard ratio | 95% CI | p value |
| 1. Mean nicotine replacement therapy use, pieces per day over 5 years | .002 | 0.023 | 1.00 | 0.96–1.05 | .94 |
| 2. Cigarette use, pack-years over 5 years | .033 | 0.024 | 1.03 | 0.99–1.08 | .17 |
| 3. Mean nicotine replacement therapy use, cigarette use during study | .012 | 0.024 | 1.01 | 0.97–1.06 | .62 |
| .036 | 0.025 | 1.04 | 0.99–1.09 | .14 |
Note. Models are adjusted by baseline age, gender, cigarettes per day, and lifetime pack-years of smoking.
The models in Tables 2–4 were replicated using average nicotine replacement therapy use (pieces per day) during the study instead of the nicotine replacement therapy use dichotomy (results not shown). Again, nicotine replacement therapy was not found to be a significant predictor of cancer.
Nicotine replacement therapy use and cigarette use are negatively correlated in this study (Pearson’s r = −.23, p = .0001), indicating that most participants used either nicotine replacement therapy or cigarettes, as instructed, rather than using both concurrently. Pack-years of smoking during Lung Health Study 1 are correlated with the following covariates used in the models in the regression tables: baseline cigarettes per day, r = .39, p = .0001, and lifetime pack-years, r = .18, p = .0001. Of the covariates in the models, only age was found to be significant, and it was uniformly hazardous.
Discussion
In the Cox regression models predicting lung cancer, gastrointestinal cancer, and all cancers, smoking during Lung Health Study 1 predicts lung cancer, and the use of nicotine replacement therapy does not. In fact, nicotine replacement therapy is not a significant predictor in any of the models shown except in the small-sample model including only sustained quitters.
The purpose of this paper was to demonstrate whether there are any indicators of an association between nicotine replacement therapy use and subsequent cancer incidence. The absence in general of a relation between nicotine replacement therapy and cancer across the models adds credence to our conclusion that nicotine replacement therapy does not cause cancer.
Our basic question was whether nicotine replacement therapy causes cancer to an extent comparable to that caused by smoking cigarettes. We failed to find evidence of such an effect of nicotine replacement therapy in this study, and the sample size, close monitoring of the use of nicotine replacement therapy and cigarettes, and the well-documented outcomes of this study will be difficult for future studies to match.
Although we believe that the Lung Health Study offers the most suitable existing dataset for the demonstration of whether nicotine replacement therapy is carcinogenic at the population level, this study is hampered by several significant limitations. There is evident confounding between historical smoking and current smoking and between current smoking and current nicotine replacement therapy use. We see continuing smokers having the lowest mean nicotine replacement therapy use. It is logical to assume that current smoking is related to smoking history. The demonstrated significant effect of cigarette use during the 5-year study and the hazard of lung cancer more logically represent a relationship between a history of smoking and a lung cancer outcome. Further, there could be a relation between cigarette use during the 5-year study and subsequent smoking, also influencing the hazard of lung cancer. While we can describe these relationships, we cannot entirely eliminate them from the analyses.
Whatever the exposure time necessary for smoking to result in cancer, it is usually not assumed to be as brief as 5 years. As mentioned above, we interpret that the “significant” effects of within-study pack-years in some of our models are probably not the direct effect of within-study smoke exposure but rather a result of confounding with baseline pack-years. The same reasoning, however, is not available with respect to nicotine replacement therapy use. There is no previous nicotine replacement therapy use related to Lung Health Study 1 nicotine replacement therapy use, since nicotine replacement therapy was not readily available before the Lung Health Study. This can serve as an alternate hypothesis consistent with our study findings of no effect of nicotine replacement therapy on cancer. Little is known about the effect on cancer risk of the distribution of smoking across time or of the distribution of nicotine replacement therapy exposure. It was therefore decided to express both of these as mean values for the 5 years of Lung Health Study 1, followed by surveillance of cancer events immediately after the 5-year visit.
A further argument could be made with a statistical power analysis of the effect of nicotine replacement therapy use on gastrointestinal cancer risk. Such an analysis, however, calls for awkward equating of the effect size for smoking in this study (which is shown to be sufficient to demonstrate significance but likely as a surrogate for smoking history) and the required effect size for nicotine replacement therapy use. We will therefore resort to the less satisfactory assertion that a study of sufficient power to demonstrate the risk of harm from smoking has a reasonable chance to demonstrate harm from nicotine replacement therapy, if present. However, the small number of cases and the likely low statistical power of the nicotine replacement therapy analyses provide a limit to the potential of the study to disprove such a relationship between nicotine replacement therapy and smoking, if one exists.
Nicotine gum provides low mean doses of nicotine relative to smoking. From 2 mg nicotine gum, around 1 mg is absorbed, and in our study, those participants who had quit smoking and remained abstinent used an average of around 2 pieces per day over 60 months. This is a serious limitation in a study attempting to model nicotine replacement therapy in general.
A type of administration of nicotine that provides nicotine exposure more closely corresponding to that achieved from smoking is snus (Scandinavian moist snuff). While researchers have regarded snus as a low risk alternative to smoking (Foulds, Ramstrom, Burke, & Fagerström, 2003), recent studies have reported significantly increased hazards of oral and gastrointestinal cancers among never-smoking users of snus (Roosaar, Johansson, Sandborgh-Englund, Axéll, & Nyrén, 2008; Zendehdel et al., 2007). These findings present the possibility that our failure to find harm from nicotine replacement therapy may in part be due to lower nicotine exposure associated with nicotine gum.
In a broader perspective, this type of research will be important as a contribution to the policy discussion about the range of “harm reduction” options being considered for current tobacco users (Hatsukami et al., 2007; Pisinger & Godtfredsen, 2007; Royal College of Physicians, 2007; Stratton, Shetty, Wallace, & Bondurant, 2001). The value of the research may not only be the actual results but also that these types of long-term experimental and quasi-experimental studies are possible.
Funding
This study was supported by contract N01-HR-46002 from the Division of Lung Diseases of the National Heart, Lung, and Blood Institute (NHLBI).
Declaration of Interests
L.M.Z. is an employee of Pinney Associates, Inc., a consulting firm that provides services to GlaxoSmithKline Consumer Healthcare, a marketer of nicotine replacement medications.
Supplementary Material
Acknowledgments
The authors would like to thank Saul Shiffman and Joe Gitchell for their comments on earlier drafts of this paper. The principal investigators and senior staff of the clinical and coordinating centers, the NHLBI, and members of the Safety and Data Monitoring Board are as follows: Case Western Reserve University, Cleveland, OH, M.D. Altose, M.D. (Principal Investigator), C.D. Deitz, Ph.D. (Project Coordinator); Henry Ford Hospital, Detroit, MI, M.S. Eichenhorn, M.D. (Principal Investigator), K.J. Braden, A.A.S. (Project Coordinator), P.A. Fantuz, R.N., B.S.N., R.L. Jentons, M.A.L.L.P. (Project Coordinator); Johns Hopkins University School of Medicine, Baltimore, MD, R.A. Wise, M.D. (Principal Investigator), C.S. Rand, Ph.D. (Co-Principal Investigator), K.A. Schiller (Project Coordinator); Mayo Clinic, Rochester, MN, P.D. Scanlon, M.D. (Principal Investigator), G.M. Caron (Project Coordinator), K.S. Mieras, L.C. Walters; Oregon Health Sciences University, Portland, OR, A.S. Buist, M.D. (Principal Investigator), L.R. Johnson, Ph.D. (LHS Pulmonary Function Coordinator), V.J. Bortz (Project Coordinator); University of Alabama at Birmingham, Birmingham, AL, W.C. Bailey, M.D. (Principal Investigator), L.B. Gerald, Ph.D., M.S.P.H. (Project Coordinator); University of California, Los Angeles, CA, D.P. Tashkin, M.D. (Principal Investigator), I.P. Zuniga (Project Coordinator); University of Manitoba, Winnipeg, Manitoba, Canada, N.R. Anthonisen, M.D. (Principal Investigator, Steering Committee Chair), J. Manfreda, M.D. (Co-Principal Investigator), RPM., Ph.D. (Co-Principal Investigator), S.C. Rempel-Rossum (Project Coordinator); University of Minnesota Coordinating Center, Minneapolis, MN, JEC, Ph.D. (Principal Investigator), P.G. Lindgren, M.S., M.A. Skeans, M.S., H.T. Voelker; University of Pittsburgh, Pittsburgh, PA, R.M. Rogers, M.D. (Principal Investigator), M.E. Pusateri (Project Coordinator); University of Utah, Salt Lake City, UT, R.E. Kanner, M.D. (Principal Investigator), G.M. Villegas (Project Coordinator); Data Monitoring Board, C. Furberg, M.D., Ph.D., J.R. Landis, Ph.D., E. Mauger, Ph.D., J.R. Maurer, M.D., Y. Phillips, M.D., J.K. Stoller, M.D., I. Tager, M.D., A. Thomas, Jr., M.D.; Morbidity and Mortality Review Board, R.S. Crow, M.D., T.E. Cuddy, M.D., R.S. Fontana, M.D., R.E. Hyatt, M.D., C.T. Lambrew, M.D., B.A. Mason, M.D., D.M. Mintzer, M.D., R.B. Wray, M.D.; and National Heart, Lung and Blood Institute Staff, Bethesda, MD, T. Croxton, M.D., Ph.D. (Project Officer), J.P. Kiley, Ph.D. (Director, Division of Lung Diseases), G. Weinmann, M.D. (Director, Airway Biology and Disease Program, DLD), M.C. Wu, Ph.D. (Division of Epidemiology and Clinical Applications).
References
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nature. 2008;452:1–7. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality: A randomized clinical trial. Annals of Internal Medicine. 2005;142:233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: Who uses them, who misuses them, and who is misinformed about them? Nicotine & Tobacco Research. 2004;6:S303–S310. doi: 10.1080/14622200412331320707. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Risks and benefits of nicotine. In: Benowitz N, editor. Nicotine safety and toxicity. New York: Oxford University Press; 1998. pp. 185–194. [Google Scholar]
- Cummings KM, Hyland A, Giovino GA, Hastrup JL, Bauer JE, Bansal MA. Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine & Tobacco Research. 2004;6:S333–S340. doi: 10.1080/14622200412331320734. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Humans Services. Public Health Service; 2008. [Google Scholar]
- Foulds J, Ramstrom L, Burke M, Fagerström K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco Control. 2003;12:349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Joseph AM, LeSage M, Jensen J, Murphy SE, Pentel PR, et al. Developing the science base for reducing tobacco harm. Nicotine & Tobacco Research. 2007;9:S537–S553. doi: 10.1080/14622200701679040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical Research in Toxicology. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Jang JJ, Weis M, Pathak A, Shujichiro K, Hu RS, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nature Medicine. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I. The changing cigarette, 1950-1995. Journal of Toxicology and Environmental Health. 1997;50:307–64. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Levy LS, Martin PA. Toxicology of nicotine—Its role in the aetiology of cancer due to cigarette smoking and cardiovascular disease. In: Wald N, Froggatt P, editors. Nicotine, smoking and the low tar programme. Oxford, UK: Oxford University Press; 1989. pp. 13–23. [Google Scholar]
- Mai H, May WS, Gao F, Zhaohui J, Deng X. A functional role for nicotine in bcl2 phosphorylation and suppression of apoptosis. Journal of Biological Chemistry. 2003;278:1886–1891. doi: 10.1074/jbc.M209044200. [DOI] [PubMed] [Google Scholar]
- Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell Growth & Differentiation. 1994;5:1033–1040. [PubMed] [Google Scholar]
- Murray RP, Bailey WC, Daniels K, Bjornson WM, Kurnow K, Connett JE, et al. Safety of nicotine polacrilex gum used by 3,094 participants in the Lung Health Study. Chest. 1996;109:438–45. doi: 10.1378/chest.109.2.438. [DOI] [PubMed] [Google Scholar]
- Murray RP, Connett JE, Rand CS, Pan W, Anthonisen NR. Persistence of the effect of the Lung Health Study (LHS) smoking intervention over eleven years. Preventive Medicine. 2002;35:314–319. doi: 10.1006/pmed.2002.1087. [DOI] [PubMed] [Google Scholar]
- Murray RP, Nides MA, Istvan JA, Daniels K. Levels of cotinine associated with long-term ad-libitum nicotine polacrilex use in a clinical trial. Addictive Behaviors. 1998;23:529–535. doi: 10.1016/s0306-4603(97)00073-7. [DOI] [PubMed] [Google Scholar]
- Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. Nicotine enhances neovascularization and promotes tumor growth. Molecules and Cells. 2004;16:143–146. [PubMed] [Google Scholar]
- Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine & Tobacco Research. 2007;9:631–646. doi: 10.1080/14622200701365327. [DOI] [PubMed] [Google Scholar]
- Roosaar A, Johansson ALV, Sandborgh-Englund G, Axéll T, Nyrén O. Cancer and mortality among users and nonusers of snus. International Journal of Cancer. 2008;123:168–173. doi: 10.1002/ijc.23469. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians. Harm reduction in nicotine addiction: Helping people who can’t quit. 2007. A report by the Tobacco Advisory Group of the Royal College of Physicians. London: Royal College of Physicians. [Google Scholar]
- Samet JM. Smoking kills: Experimental proof from the Lung Health Study. Annals of Internal Medicine. 2005;142:299–301. doi: 10.7326/0003-4819-142-4-200502150-00012. [DOI] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S Committee to Assess the Science Base for Tobacco Harm Reduction, Institute of Medicine. Clearing the smoke: Assessing the science base for tobacco harm reduction. Washington, DC: National Academy Press; 2001. [Google Scholar]
- Sugano N, Minegishi T, Kawamoto K, Ito K. Nicotine inhibits UV-induced activation of the apoptotic pathway. Toxicology Letters. 2001;125:61–65. doi: 10.1016/s0378-4274(01)00416-7. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldum HL, Nilsen OG, Nilsen T, Rorvik H, Syversen U, Sandvik AK, et al. Long-term effects of inhaled nicotine. Life Sciences. 1996;58:1339–1346. doi: 10.1016/0024-3205(96)00100-2. [DOI] [PubMed] [Google Scholar]
- West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. Journal of Clinical Investigation. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zendehdel K, Nyrén O, Luo J, Dickman PW, Boffetta P, Englund A, et al. Risk of gastroesophageal cancer among smokers and users of Scandanavian moist snuff. International Journal of Cancer. 2007;122:1095–1099. doi: 10.1002/ijc.23076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.