Abstract
DHEA and DHEAS are steroids synthesized in human adrenals, but their function is unclear. In addition to adrenal synthesis, evidence also indicates that DHEA and DHEAS are synthesized in the brain, further suggesting a role of these hormones in brain function and development. Despite intensifying research into the biology of DHEA and DHEAS, many questions concerning their mechanisms of action and their potential involvement in neuropsychiatric illnesses remain unanswered. We review and distill the preclinical and clinical data on DHEA and DHEAS, focusing on (i) biological actions and putative mechanisms of action, (ii) differences in endogenous circulating concentrations in normal subjects and patients with neuropsychiatric diseases, and (iii) the therapeutic potential of DHEA in treating these conditions. Biological actions of DHEA and DHEAS include neuroprotection, neurite growth, and antagonistic effects on oxidants and glucocorticoids. Accumulating data suggest abnormal DHEA and/or DHEAS concentrations in several neuropsychiatric conditions. The evidence that DHEA and DHEAS may be fruitful targets for pharmacotherapy in some conditions is reviewed.
Keywords: Dehydroepiandrosterone, DHEA, DHEAS, neuroprotection, neurogenesis, apoptosis, depression, schizophrenia, dementia, cortisol
1. Introduction
Dehydroepiandrosterone (DHEA) and its sulfate ester, DHEAS, together represent the most abundant steroid hormones in the human body. Nonetheless, their physiological significance, their mechanisms of action and their possible roles in human disease are not well understood. Highlighting the potential health significance of DHEA and DHEAS, concentrations of these hormones in humans typically decrease steadily with age, approaching a nadir at about the time many diseases of aging become markedly more prevalent. Observations such as these, coupled with basic and preclinical demonstrations of DHEA’s biological effects, fostered hope that restoring DHEA to youthful levels might, conservatively, increase well-being and, optimistically, extend life, protect the brain, and retard the ravages of aging. Almost from the time of their initial discovery and synthesis, DHEA and DHEAS were evaluated in the treatment of neuropsychiatric disorders, with published reports appearing as early as 1952 [272; 298]. Large-scale enthusiasm for DHEA as a potential neuropsychiatric therapy languished until the late 1980s through the mid-1990s, when an expanding body of preclinical data plus the first adequately controlled clinical trial [206] renewed hopes for therapeutic potential.
The field of inquiry into the neurobiological actions of DHEA and DHEAS (jointly referred in this article as “DHEA(S)”) is rapidly growing. The goal of the present article is to review (1) the basic and preclinical studies of DHEA(S)’ biological actions in the brain and their purported mechanisms of action, (2) differences in endogenous circulating concentrations in normal individuals and patients with certain neuropsychiatric illnesses (depression, anxiety, schizophrenia and dementia), and (3) the therapeutic potential of DHEA(S) in treating these neuropsychiatric conditions.
2. DHEA(S) Secretion Changes Across the Lifespan
During human gestation, high concentrations of DHEAS are secreted by the fetal zone of the adrenal gland [199]. After birth, DHEA(S) concentrations decline over the first six months and remain low until adrenarche starts at six to eight years in both boys and girls, at which point DHEA(S) is synthesized and secreted from the zona reticularis layer of the adrenal cortex and circulating concentrations begin to rise [120; 234]. Adult humans secrete both DHEA and DHEAS from the zona reticularis of the adrenal cortex and also DHEA from the ovary and testis [224]. Circulating concentrations (in both plasma and cerebrospinal fluid) peak in the mid-20’s and then progressively decline with age in both men and women, approaching a nadir (approximately 20% of peak concentrations) at approximately 65 to 70 years, the age at which the incidence of many age-related illnesses steeply increases [18; 115; 254]. In men, plasma DHEAS concentrations decrease by an average of 1% to 4% per year between the ages of 40 and 80 years [214; 313] and 2% per year in women [313]. The majority of people exhibit decreases in concentrations of these hormones with aging, although one study suggested that 15% of women and 5% of men show true increases in DHEAS over a 10 to 14 year follow-up period [313].
3. Biosynthesis of DHEA(S)
Dehydroepiandrosterone, 5-androsten-3 beta-ol-17-one, is a 19 carbon steroid that is synthesized from cholesterol by two steroid metabolizing enzymes (see Figure 1; for more details about the biochemistry of steroid synthesis of DHEA see [16; 201]). The first, rate-limiting, and hormonally regulated step in the synthesis of all steroid hormones is the conversion of cholesterol into pregnenolone by the mitochondrial enzyme cholesterol side chain cleavage P450scc. Pregnenolone is converted into DHEA by the enzyme cytochrome P450c17; this single enzyme catalyzes both the 17α-hydroxylation reaction converting pregnenolone to 17-OH pregnenolone and the 17,20-lyase reaction converting 17-OH pregnenolone to DHEA [16; 201] (Figure 1). The sulfation of DHEA into its more stable sulfate ester DHEAS is catalyzed by the enzyme hydroxysteroid sulfotransferase (HST, SULT2A1), commonly known as DHEA sulfotransferase. DHEAS can be converted back into DHEA by steroid sulfatase (STS).
Figure 1.
The Δ5 and Δ4 pathways of steroid hormone synthesis. The chemical names of the enzymes are shown for each reaction. P450scc, cholesterol side chain cleavage; 3βHSD, 3β-hydroxysteroid dehydrogenase; P450c17, 17α-hydroxylase/c17,20-lyase. The dotted arrow refers to the 17,20-lyase reaction that does not occur in human beings.
People with 17α-hydroxylase deficiency are characterized by sexual infantilism in phenotypic females (due to lack of sex steroid precursors), 46,XY disorder of sexual development (lack of masculinization – female infantile external genitalia, no uterus), hypertension, and hyperkalemia [70; 264]. P450c17 is encoded by a single gene (cyp17) and mutations can cause either 17α-hydroxylase deficiency or 17,20-lyase deficiency or both [70; 264]. In addition to its expression in human adrenals and gonads, P450c17 is also expressed in the brain [66; 78; 127], where it may synthesize DHEA from pregnenolone [78; 127] (further discussion of DHEA(S) as a neurosteroid is in section 6). There are no reported neurological problems in people with P450c17 gene mutations, perhaps because they obtain sufficient quantities of 17-hydroxylated steroids from their mothers during prenatal development. Adults with P450c17 gene mutations are not well studied and may be an interesting group to examine with regard to neuropsychiatric illness, although this could be complicated with the possible psychological effects of sexual infantilism. Mouse studies knocking out this gene were uninformative, as the P450c17−/− mice died by embryonic day 7 before gastrulation, and the cause of this early lethality is unknown [19].
4. Relative DHEA(S) Concentrations in Brain vs. Plasma vs. CSF in Humans
Higher concentrations of DHEA are found in the brain compared to plasma. In a study of ten postmortem human brains, DHEA concentrations were 29.4 nmol/kg in prefrontal lobe, 16.3 nmol/kg in parietal lobe, 13.1 nmol/kg in temporal cortex, 16.9 nmol/kg in cerebellum, and 18.7 nmol/kg in corpus callosum [164]. These data were derived from nine women and one main (76–93 years old), and it is worth noting that large individual differences in DHEA brain concentrations were observed, with prefrontal lobe DHEA concentrations ranging from 9.8 to 470 nmol/kg [164]. Mean DHEA concentrations were 1.83 nM in plasma of living human subjects of similar ages, which results in a brain-to-plasma ratio of ∼6.5 [164]. Although human brain concentrations of DHEA are higher than plasma concentrations, cerebrospinal fluid (CSF) concentrations of DHEA are lower than plasma concentrations. DHEA concentrations in CSF were ∼5% of those found in the plasma of humans [115].
The validity of reported measurements of DHEAS and pregnenolone sulfate in the brain has recently been questioned [176; 277]. Many studies have relied on identification of parent compounds after separation of steroid sulfates from free steroids by organic:aqueous solvent extraction followed by a chemical reaction (solvolysis) to remove the sulfate. Analyses of sulfated steroids after extraction and solvolysis have found high concentrations of DHEAS and pregnenolone sulfate in rodent and human brains [68; 69; 165; 327]. Recent studies that measure intact sulfated compounds without deconjugation [113; 124; 125; 126; 179; 180; 203] or a protocol incorporating a solid-phase extraction column purification step and simultaneous hydrolysis/derivatization with heptafluorobutyric anhydride [176; 218] have found neither DHEAS nor pregnenolone sulfate present in abundant quantities in rodent brains. For example, DHEAS was not detected in the brains of either Sprague-Dawley rats or Swiss mice (less than 0.3 ng/g) [176; 180]. However, high DHEAS concentrations were found in two samples of human brain tissue using the new sample preparation method described above and gas chromatography-mass spectrometry (GC-MS) analysis [176]. Hence, humans may indeed have high concentrations of brain DHEAS and older studies may turn out to be correct once verified using these newer analytic protocols [165; 327]. Studies relying solely on organic:aqueous extractions and solvolysis to measure DHEAS remain questionable and need to be reassessed.
5. Species Differences - Humans vs. Rodents
Humans and rodents (rats and mice) differ in the pathways through which sex steroids are synthesized. Whereas the Δ4 pathway predominates with rodents, the 17,20-lyase activity of the human P450c17 enzyme strongly prefers the Δ5 pathway [95] (see Figure 1). Subsequent conversion of DHEA into androstenedione by 3β-hydroxysteroid dehydrogenase (3βHSD) is the only pathway by which humans produce androstenedione [67]. In rodents, conversion of cholesterol to androstenedione can occur through two pathways – the Δ5 pathway described above and the Δ4 pathway which involves the conversion of pregnenolone into progesterone (by 3βHSD) and progesterone conversion into androstenedione through the 17-OH-progesterone intermediary. Thus, humans make DHEA (Δ5 pathway) prior to downstream conversion into androstenedione and further metabolism into other sex steroids, whereas rodents go through the Δ4 pathway (predominantly) or Δ5 pathway. The species difference in predominant steroid pathways may partly explain species differences in peripheral circulating concentrations. Whereas DHEAS is the most abundant circulating steroid hormone in the human body [181], rats and mice (the species typically studied) have low circulating concentrations of DHEA(S) in the periphery [71; 323]. Unlike humans who secrete DHEA(S) from their adrenal glands and gonads, rats and mice can only synthesize and secrete DHEA(S) from their gonads, as their adrenal glands lack P450c17 [169; 237; 323].
Like humans, rats and mice have higher concentrations of DHEA in the brain compared to the plasma [68]. For example, Sprague-Dawley rats had mean DHEA concentrations of 0.08 ng/ml (0.28 nM) in plasma, while brain concentrations of DHEA were 0.42 ng/g (1.46 nmol/kg) in anterior brain and 0.12 ng/g (0.42 nmol/kg) in posterior brain [68]. These data are consistent with the hypothesis that in rodents, brain DHEA is derived mainly if not solely from local synthesis and not from peripheral synthesis. In human beings, brain DHEA may be derived from both local synthesis and peripheral synthesis. Thus, since DHEA is found in appreciable concentrations in brains of both human beings and rodents, rodents may indeed be a good model for studying the function of DHEA in the brain, but may not be an appropriate model for studying peripheral effects of these steroids.
6. DHEA(S) as a Neurosteroid
Important actions in the central nervous system (CNS) were initially inferred from observations that DHEA and DHEAS were synthesized de novo in brain, as brain concentrations were higher than plasma concentrations and brain concentrations remained high after adrenalectomy and gonadectomy of rats [68; 69]. Indeed, they have been termed “neurosteroids” for this reason [27; 28]. DHEA and DHEAS were among the first neurosteroids identified in rat brains [68; 69]. Cytochrome P450c17 was found in a subset of neurons of embryonic rodent brains [66]. P450c17 expression was mainly neuronal; its expression was found as early as embryonic day 9.5, and persisted in the CNS during embryonic development. In one study, P450c17 was not detected in the CNS in adult rats and mice by immunocytochemistry, raising the possibility that this enzyme, and its neurosteroid products, function mainly during development [66]. However, another study found P450c17 in adult male rat hippocampi by immunohistochemical staining [127]. In the hippocampus, P450c17 was localized to pyramidal neurons in the CA1-CA3 region and to granule cells of the dentate gyrus. In these cells, P450c17 was localized in pre- and post-synaptic locations and in the endoplasmic reticulum by immunoelectron microscope analysis [127]. While P450c17 protein was readily detected in the brain, the abundance of P450c17 mRNA transcripts in the embryonic mouse brain [66] or hippocampus of adult male rats was low, and was approximated to be 1/200th of the expression in the testis [127].
DHEA can be synthesized in vivo in rat and frog brains. Rat brains were capable of converting pregnenolone into DHEA and this may be activity-dependent [127]. Basal P450c17 steroidogenic enzyme activity was low in the hippocampus, but could be enhanced by exposing neurons to N-methyl-D-aspartate (NMDA) [127]. Similar findings have been reported for NMDA stimulation of pregnenolone synthesis from cholesterol in the hippocampus [151], suggesting that both P450scc and P450c17 are regulated by neurotransmitters. Frog brains also were found to synthesize DHEA from pregnenolone, and this enzymatic activity was reduced in a concentration-dependent manner by ketoconazole, an inhibitor of P450c17 [78]. P450c17 enzymatic activity and protein expression were co-localized, further indicating that the enzymatic activity was due to P450c17.
P450c17 expression has also been found in adult rat spinal cord [147]. Immunohistochemical studies localized P450c17 in both neurons and glial cells in the spinal cord. Slices of spinal cord tissue containing P450c17 protein converted [3H]pregnenolone into [3H]DHEA, and this conversion was reduced by ketoconazole. Thus, the spinal cord is one region in the CNS of rodents that expresses P450c17 and can synthesize DHEA endogenously from a precursor [147].
DHEAS may be synthesized in the brain from DHEA [154; 155]. Sulfation of DHEA has been observed in the brains of rhesus monkeys in vivo and in human fetal brain slices in vitro [155]. Conversion of [3H]DHEA into [3H]DHEAS was also found in incubations of brain homogenates from pons, hypothalamus, olfactory bulb, cortex, and striatum/hippocampus of fetal and adult Sprague-Dawley rats [246] and from thalamus, frontal cortex, basal ganglia, olfactory bulb, hippocampus, brainstem, midbrain, occipital cortex and cerebellum of adult Wistar rats [3]. In addition to mammals, DHEAS synthesis from DHEA has been observed in brain homogenates from hypothalamus and telencephalon but not rhombencephalon of adult European green frogs [29]. In frogs, DHEAS synthesis from DHEA could be inhibited by the hydroxysteroid transferase (HST) inhibitor 2,4-dichloro-6-nitrophenol (DCNP) [29] as well as by the neurotransmitter neuropeptide Y [30].
Hydroxysteroid sulfotransferase (HST) or SULT2A1, also commonly referred to as DHEA sulfotransferase, is an enzyme that sulfonates DHEA (in addition to pregnenolone) [277; 299]. Western blotting and immunohistochemistry (with an antibody directed against partially purified rat liver HST) showed protein expression of an HST in adult Wistar rat brain [3]. However, the characterization of this HST was not fully addressed, and hence its identity was uncertain. Other studies using different antibodies to purified or well-characterized proteins have confirmed the finding of HST in the brains of rats [151; 282] and frogs [29]. SULT2A1 mRNA expression has been shown in rat brains [282], thereby definitively demonstrating the presense of SULT2A1 in the brain. Future research on the activity and localization of newly discovered sulfotransferases, such as SULT2B and SULT4, may further our understanding of DHEA sulfonation in the brains of humans, rats and mice in the future [277; 283; 299].
It is unlikely that brain DHEAS comes from the periphery because sulfated steroids are hydrophilic and do not readily cross the blood-brain barrier, as evidenced by low recovery (0.03%) of radioactively labeled DHEAS in the brains of Sprague-Dawley rats following intracardiac injection [154]. Although, one study has found increased pregnenolone sulfate in the brains of Sprague-Dawley rats after i.v. injection via the tail vein [325]. What little steroid sulfates do enter the brain may occur through organic anion transporting peptides (OATP), which may work to transport DHEAS in both directions [13]. However, steroid sulfates may egress from the brain more readily than they enter. The efflux clearance of [3H]DHEAS across the blood-brain barrier was determined to be tenfold greater than its influx (118 (µl/min-g efflux vs. 11.4 µl/min-g influx) [13]. Hence, DHEAS is predominately transported out of the brain across the blood-brain barrier, further suggesting that DHEAS found in the brain is most likely due to local synthesis.
7. Mechanisms of Action for DHEA(S)
Steroid hormones affect gene transcription by binding to specific cytoplasmic receptors, and then translocating into the nucleus, or binding to receptors that are resident in the nucleus, where they bind to steroid responsive elements on DNA. To date, no nuclear steroid receptor with high affinity for either DHEA or DHEAS has been found [326; 328]. The mechanisms by which DHEA(S) operate are not fully understood [326]. DHEA(S) may mediate some of its actions through conversion into more potent sex steroids and activation of androgen or estrogen receptors in tissue (i.e. skin, liver, brain) [162]. In addition to DHEA(S) having effects through its sex steroid metabolites (i.e. estradiol and testosterone), DHEA(S) may also have effects through its more immediate metabolites, such as 7α-hydroxy-DHEA [56]. Although no unique DHEA or DHEAS nuclear steroid receptor has been found, DHEA and DHEAS have been found to affect receptors and to show affinity for some binding sites [178; 326].
In the brain, DHEA(S) modulates actions of the γ-aminobutyric acid type A (GABAA) receptor, the NMDA receptor, and the sigma subtype 1 (σ1) receptor [26; 27; 32; 65; 188; 193; 197] among others [236; 266; 268]. DHEA and DHEAS generally act as noncompetitive antagonists at the GABAA receptor, with DHEAS having more potent antagonistic effects than DHEA [132; 188; 196; 290] (see Figure 2). DHEA(S) generally acts as a positive allosteric modulator of the NMDA receptor, although the binding of DHEA(S) with an interaction site on the NMDA receptor is not well documented [27; 65]. DHEA(S) can potentiate NMDA receptor function through its actions as a σ1 receptor agonist (see Figure 2). However, in non-hippocampal brain regions DHEA(S) may inhibit glutamate neurotransmission through σ receptors, since σ receptor agonists were shown to reduce NMDA-induced dopamine release in the striatum [108]. In an electrophysiological study with Sprague-Dawley rats, intravenous (i.v.) administration of DHEA (100–500 µg/kg) potentiated the NMDA neuronal response of CA3 rat hippocampus pyramidal neurons in a dose-dependent manner [32]. The addition of σ receptor antagonist haloperidol or σ1 receptor antagonist N-dipropyl-2-(4-methoxy-3-(2-phenylethoxy)phenyl)-ethylamine monohydrochloride (NE-100), but not saline or spiperone (which has low affinity for σ receptors), inhibited the potentiating effect of DHEA, suggesting that DHEA can modulate the NMDA response through σ1 receptors [32]. DHEAS potentiated the NMDA evoked release of [3H]norepinephrine from preloaded hippocampal slices, while the addition of σ receptor antagonists haloperidol or 1-[2-(3,4-dichlorophenyl)-ethyl]-4-methylpiperazine (BD1063) blocked the potentiating effect of DHEAS [205]. Thus, DHEA(S) can modulate NMDA neurons and receptor activity by acting at the σ1 receptor (that is coupled to Gi/o proteins) in both in vivo and in vitro studies [32; 193; 205].
Figure 2.
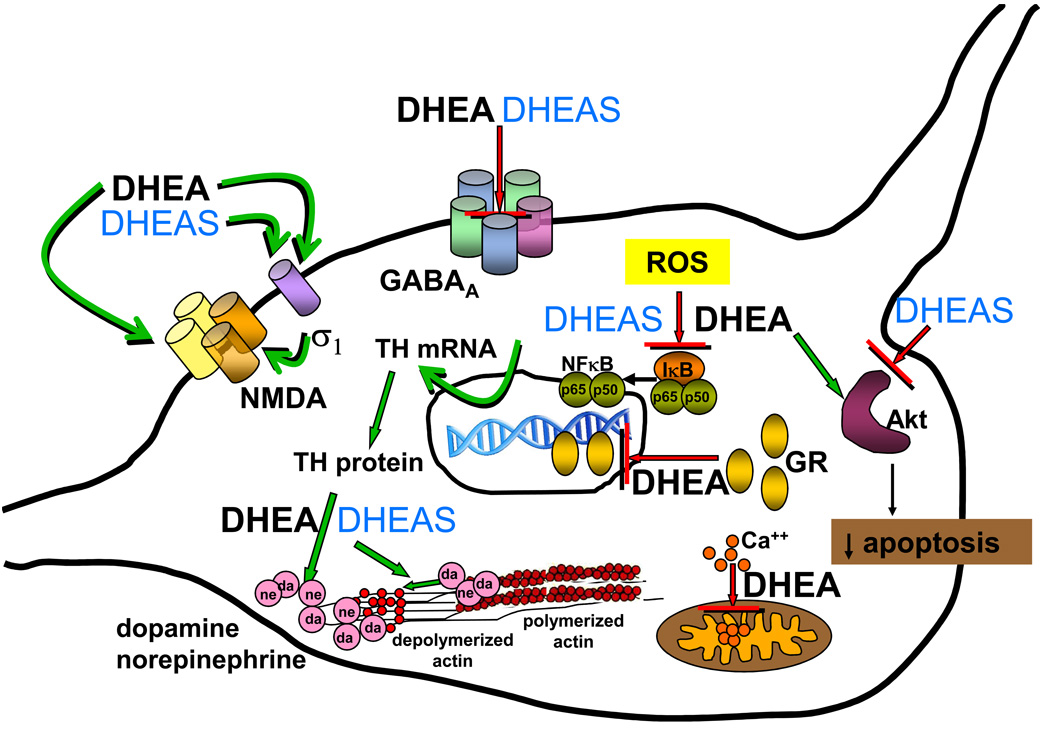
Mechanisms of action of DHEA and DHEAS in neurons. This cartoon summarizes many of the actions of DHEA and DHEAS described in detail in the text. DHEA and DHEAS have inhibitory effects (red blocking arrow) at the GABAA receptor (section 6 and 7.1). DHEA and DHEAS act as agonists (green arrow) at the σ1 receptor (section 6 and 7.1), which subsequently may activate the NMDA receptor. DHEA inhibits Ca2+ influx (red blocking arrow) into the mitochondria (section 7.1). DHEA influences embryonic neurite growth through stimulation (green arrow) of the NMDA receptor (section 7.2). DHEA increases (green arrow) kinase activity of Akt and decreases apoptosis, while DHEAS decreases (red blocking arrow) Akt and increases apoptosis (section 7.4). DHEAS increases (green arrows) TH mRNA and TH protein abundance (section 7.5) leading to increased catecholamine synthesis. DHEA and DHEAS stimulate (green arrows) actin depolymerization and submembrane actin filament disassembly and (green arrows), increasing secretion of catecholamines (“da” and “ne”) from secretory vesicles (section 7.5). DHEA and DHEAS inhibit (red blocking arrow) reactive oxygen species (ROS) activation of transcription mediated by NF-κB (section 7.6 and 7.7). DHEA inhibits (red blocking arrow) nuclear translocation of the glucocorticoid receptor (GR) (section 7.8). Mechanisms of action not pictured in this graph are: alterations of brain derived neurotrophic factor (BDNF) synthesis, inhibition of stress-activated protein kinase 3 (SAPK3) translocation, and inhibition of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSDl) activity. Abbreviations: σ1, sigma 1 receptor; Akt, serine-threonine protein kinase Akt; Ca2+, calcium; da, dopamine; GABAA, γ-aminobutyric acid type A receptor; GR, glucocorticoid receptor; ne, norepinephrine; NF-κB, nuclear factor kappa B; NMDA, N-methyl-D-aspartate receptor; ROS, reactive oxygen species; TH, tyrosine hydroxylase.
DHEAS, but not DHEA, augments cholinergic function in several animal models [101]. Intraperitoneal (i.p.) administration of DHEAS (25–250 µmol/kg) increased acetylcholine (ACh) release from hippocampal neurons in rats [256]. This effect has behavioral relevance in vivo, since DHEAS prevented (in a dose-dependent manner) the memory impairment induced by the ACh receptor antagonist scopolamine in mice [318]. The σ1 receptor antagonist NE-100 blocked the ameliorating effects of DHEAS in this model, suggesting that the modulation of the cholinergic system by DHEAS involves interaction with σ1 receptors [318]. Long term administration (15 days) of the STS inhibitor p-O-(sulfamoyl)-N-tetradecanoyl tyramine (DU-14) (which inhibits the conversion of DHEAS to DHEA) to rats increased plasma DHEAS concentrations, decreased DHEA concentrations, increased hippocampal ACh release, and blocked scopolamine-induced amnesia [257].
Additional intracellular sites where DHEA may act have also been described. DHEA may interact directly with certain cytoskeleton components or novel membrane receptors. DHEA was found to bind to microtubule-associated protein (MAP)2C with strong affinity [168]. MAP2C, which is expressed at early development stages, was found in adult retina and olfactory bulb, which are tissues in which neurogenesis persists in the adult [168]. Intriguing leads are emerging for possible DHEA receptor sites in the periphery that may also exist in the central nervous system. A DHEA receptor was found on endothelial cell plasma membranes and it was coupled to endothelial nitric-oxide synthase (eNOS) activity through Gi/o proteins Gαi2 and Gαi3 [178]. DHEA(S) may also have actions at other receptors, including the peroxisome proliferator-activated receptor α (PPARα), pregnane X receptor, constitutive androstanol receptor, and estrogen receptor β [156; 312; 326]. Additional discussions of DHEA(S)’ mechanisms are also detailed elsewhere [26; 65; 193; 197; 198; 236; 266; 267; 268; 308; 328; 329; 333; 337;353].
8. Neurobiological Actions of DHEA(S)
In this section, we focus on reported neurobiological actions of DHEA(S) and their proposed mechanisms of action, which may or may not be mediated by some of the receptors discussed above. The focus of this review is on the possible mechanisms of action of DHEAS, DHEA and its more immediate metabolites (e.g., 7α-hydroxy-DHEA) in the brain, rather than the possible effects due to conversion of DHEA(S) into sex steroids (e.g., estradiol and testosterone). Neurobiological actions of estradiol and testosterone are well established [134; 135; 136; 137; 161; 211; 212; 266; 281; 317; 329]. In this review we have focused specifically on actions attributable directly to DHEA and DHEAS. Table 1 includes studies of the biological functions of DHEA and DHEAS and their proposed mechanisms of action. While most reviews about mechanisms of action of DHEA(S) are organized around actions at specific receptors, our review of the mechanisms is organized around the major biological actions of DHEA(S) in the brain. These major biological actions of DHEA(S) involve neuroprotection, neurite growth, neurogenesis and neuronal survival, apoptosis, catecholamine synthesis and secretion, as well as anti-oxidant, anti-inflammatory and anti-glucocorticoid effects. Each of these actions is reviewed in subsequent sections below.
Table 1.
Functions of DHEA and DHEAS
| Steroid | Receptor/Mechanism | Biological Response | Dose or Concentration | in vivo/ in vitro | Tissue/Model | Function | Refs. |
|---|---|---|---|---|---|---|---|
| Neuroprotection (NP) | |||||||
| DHEA | |||||||
| (mechanism unknown) | improves recovery of motor behavior after spinal cord injury; increases area of white matter spared at epicenter of lesion and reduces area of reactive gliosis | 10−10 M patch, 10−6 M (0.02 mg/kg/day) i.p. for 12 days, and 10−6 M DHEA in drinking water for 42 days | in vivo | CD-1 female mice | NP | [93] | |
| (mechanism unknown) | decreases ischemia-induced neuronal injury in hippocampal CA1 region | 100 mg DHEA pellet implanted s.c. 12 days prior to ischemia | in vivo | male Wistar rats | NP | [174] | |
| (mechanism unknown) | protects against NMDA toxic effects on pyramidal neurons in hippocampus | 120–150 mg DHEA pellet implanted s.c. (pharmacological for rats) | in vivo | male Lister hooded rats | NP | [149] | |
| (mechanism unknown) | protects against amyloid β protein-induced neuronal cell death | 50 nM - 10 mM (optimal 5 µM) | in vitro | HT-22 (subclone of HT4 mouse hippocampal cell line) | NP | [53] | |
| inhibits NMDA-induced NO production and calcium sensitive NOS activity | protects against NMDA-induced neurotoxicity | 10 µM | in vitro | primary hippocampal cultures from E19 Wistar rat fetuses | NP | [159] | |
| inhibits Ca2+ influx into the mitochondrial matrix | protects mitochondria against intracellular Ca2+ overload | 0.3% DHEA solution; maximal response at 100 µM, EC50 = 15 µM | in vitro | primary cultures of cerebellar granule cells from 8 day old Wistar rats | NP | [139] | |
| (mechanism unknown) | increases neuronal survival following anoxia | 10−10, 10−8, and 10−6 M; effective at 10−8 and 10−6M | in vitro | embryonic Sprague-Dawley rat (E18) cerebral cortical culture | NP | [190] | |
| (mechanism unknown) | protects against NMDA-, AMPA-, and kainate-induced toxicity | 10 nM for NMDA; l00 nM for AMPA or kainic acid | in vitro | primary hippocampal cultures from E18 Sprague-Dawley rat fetuses | NP | [149] | |
| DHEAS | |||||||
| GABAA receptor involved | increases tolerance to ischemic stroke; improves mobility, tactile sensation, and use of hindlimbs after ischemic stroke | 50 mg/kg i.v. (1 dose 5 min after onset of ischemia) | in vivo | male New Zealand white rabbits | NP | [166] | |
| σ1 receptor | protects against NMDA-induced toxicity | 100 nM | in vitro | primary hippocampal cultures from E19 Wistar rat fetuses | NP | [159] | |
| GABAA receptor involved; (mechanism unclear) | protects against oxygen-glucose deprivation induced neuronal damage; protects against MPP+, colchicine, NMDA, and glutamate toxicity | 0.1 –10 µM (maximal at 10 µM) | in vitro | Wistar rat (8 day old) cerebellar granule cell culture | NP | [138] | |
| (mechanism unknown) | increases neuronal survival following anoxia | 10−10, 10−8, and 10−6 M; effective at 10−6 M | in vitro | embryonic Sprague-Dawley rat (El8) cerebral cortical culture | NP | [190] | |
| (mechanism unknown) | protects against NMDA-induced toxicity | 100 nM | in vitro | primary hippocampal cultures from E18 Sprague-Dawley rat fetuses | NP | [149] | |
| Neurotoxicity (NT) | |||||||
| DHEA | |||||||
| (mechanism unknown) | increases neuronal loss in primary motor cortex and hippocampus; increases motor impairment | diet containing 0.6% DHEA (14 mg/day) for 10 wks, then 4 wks normal diet | in vivo | male Balb/c mice | NT | [269] | |
| inhibits mitochondrial respiration by acting on complex I of the respiratory chain | neurotoxic to mesencephalic neurons (24 h) and cerebellar neurons (72 h; (effects are more pronounced under hypoglycemic conditions) | 100 µM | in vitro | Wistar rat (8 day old) cerebellar granule cell cultures; rat mesencephalic (E15) cells; primary culture rat cortical (E17) cells | NT | [269] | |
| (mechanism unknown) | decreases cell viability | 1 nM – 1 µM | in vitro | human neuroblastoma cell line (type SK-N-SH) | NT | [103] | |
| (mechanism unknown) | decreases viability of primary neuronal cells after 24 or 72 hour incubation | 1 nM - 10 µM | in vitro | primary brain cultures from E14–15 ICR mice | NT | [103] | |
| (mechanism unknown) | decreases neuronal survival | 500 nM DHEA (alone) | in vitro | Sprague-Dawley fetal rat (E18) hippocampal tissue cultures | NT | [150] | |
| Neurite Growth (NG) | |||||||
| DHEA | |||||||
| (effects mediated through brain aromatization to estradiol) | increases spine synapse density in CA1 field of the hippocampus | 1 mg/day s.c. for 2 days | in vivo | ovariectomized female Sprague-Dawley rats | NG | [116] | |
| NMDA receptor involved (not GABAA receptor) | increases length of neurites containing axonal marker Tau-1 and incidence of varicosities and basket-like process formations | 10−9 M (physiological) | in vitro | embryonic mouse (E16.5) neocortical cell cultures | NG | [64] | |
| (mechanism unknown) | increases number of neurons and astrocytes; increases extension of astrocyte processes | 10−8 - 10−4 M (optimal 10−7 M) | in vitro | embryonic mouse (E14) whole brain cultures | NG,NP | [41] | |
| DHEAS | |||||||
| (mechanism unknown) | increases length of neurites containing dendritic marker MAP2 | 10−9 M (physiological) | in vitro | embryonic mouse (E16.5) neocortical cell cultures | NG | [64] | |
| (mechanism unknown) | increases number of neurons and astrocytes; increases extension of astrocyte processes | 10−8 - 10−4 M (optimal 10−8 M) | in vitro | embryonic mouse (E14) whole brain cultures | NG,NP | [41] | |
| Neurogenesis and Neuronal Survival (NS) | |||||||
| DHEA | |||||||
| (mechanism unknown) | increases number of newly formed cells in dentate gyrus | 200–250 mg DHEA pellet implant; daily s.c. injections 10, 20, 40 mg/kg for 16 days | in vivo | male Lister Hooded rats | NS | [145] | |
| NMDA receptor signaling after σ1 receptor activation (not GABAA receptor) | in presence of EGF and LIF, increases proliferation and number of neural stem cells | medium concentration of 1 µM | in vitro | long-term human neural stem cells derived from fetal cortex (lfNSCCTX) | NS | [305] | |
| DHEAS | |||||||
| (mechanism unknown) | promotes survival of neurofilament positive, neuron-like cells; addition with FGF2 is synergistic for cell survival | 10 µg/ml | in vitro | adult human cortical brain tissue culture | NS | [45] | |
| Apoptosis (A) | |||||||
| DHEA | |||||||
| (mechanism unknown) | reduces apoptotic cell death rate due to NMDA neurotoxicity | 1 – 20 µM (effective at 10 µM) | in vitro | P19-N neuronal cells (murine pluripotent embryonic carcinoma cell line) | A;NP | [342] | |
| anti-apoptotic Bcl-2 proteins (and upstream effectors CREB and NF-κB) | protects against serum deprivation induced apoptosis | 10−7 M | in vitro | rat pheochromocytoma PC12 and rat chromaffin adrenal medulla cells | A | [57] | |
| Akt signaling pathway | increases kinase activity of Akt in neural precursor culture, and decreases apoptosis (opposite of DHEAS) | 50, 100 nM | in vitro | embryonic Sprague-Dawley rat (E13) neuroepithelium | A;NP | [350] | |
| DHEAS | |||||||
| anti-apoptotic Bcl-2 proteins (and upstream effectors CREB and NF-κB) | protects against serum deprivation induced apoptosis | 10−7 M | in vitro | rat pheochromocytoma PC12 and rat chromaffin adrenal medulla cells | A | [57] | |
| decreases serine-threonine protein kinase Akt- induced inhibition of cell apoptosis | decreases kinase activity of Akt in neural precursor culture, and increases apoptosis (opposite of DHEA) | 50, 100 nM | in vitro | embryonic Sprague-Dawley rat (E13) neuroepithelium | A | [350] | |
| Catecholamine Synthesis and Secretion (C) | |||||||
| DHEA | |||||||
| (mechanism unknown) | increases NE in lateral hypothalamus, and decreases NE and EPI in PVN (in lean rats) | chow containing 0.6% DHEA for 28 days | in vivo | lean and obese female Zucker rats | C | [309] | |
| (mechanism unknown) | decreases NE and EPI in PVN; increases DA, 5HT, and 5-HIAA in PVN | 200 mg/kg i.p. | in vivo | obese female Zucker rats | C | [307] | |
| ERK1/2 phosphorylation | decreases NGF-induced cell survival; shifts cells towards a neuroendocrine phenotype (in presence of NGF); stimulates dopamine release | 10−6 M | in vitro | rat pheochromocytoma cell line PC 12 | C | [351; 352] | |
| stimulates actin depolymerization and actin filament disassembly | increases secretion of NE and DA (faster than DHEAS) | stimulation nM range | in vitro | rat pheochromocytoma cell line PC 12 | C | [58] | |
| (mechanism unknown) | decreases IGF-II-induced cell proliferation | 10−7 - 10−5 M | in vitro | bovine chromaffin cells (from young animals) | C | [285] | |
| (mechanism unknown, but did not involve androgen or estrogen receptor, or GABAA receptor) | decreases LIF-induced cell proliferation | 10−7 - 10−5 M | in vitro | bovine chromaffin cells (from young animals) | C | [286] | |
| (mechanism unknown, but did not involve androgen or estrogen receptor, or GABAA receptor) | increases EGF-induced cell proliferation | 10−5 M | in vitro | bovine chromaffin cells (from adult animals) | C,NS | [286] | |
| DHEAS | |||||||
| stimulates actin depolymerization and actin filament disassembly; induces TH expression | increases secretion of NE and DA (slower than DHEA); stimulates catecholamine production | stimulation nM range | in vitro | rat pheochromocytoma cell line PC 12 | C | [58] | |
| (mechanism unknown) | increases secretion of DA | 10−8 and 10−6 M | in vitro | Primary rat (E18) hypothalamic cell cultures | C | [217] | |
| Antioxidant (AO) | |||||||
| DHEA | |||||||
| inhibits NF-κB activation | decreases H2O2 and HNE; increases GSH, GSH-peroxidase and catalase; decreases activation of NF-kB in hippocampus of diabetic rats | 4 mg/day for 7, 14 or 21 days by gastric intubation | in vivo | normoglycemic and streptozotocin-diabetic Male Wistar rats | AO | [7] | |
| (mechanism unknown) | decreases lipid peroxidation; prevents increases in expression, protein levels, and activity of BACE induced by oxidative stress | 0.1 –1 µM | in vitro | NT2 neurons | AO | [311] | |
| (mechanism unknown) | protects against H2O2 toxicity; protects against SNP toxicity | 10–100 µM for H2O2 (max at 100 µM); 100 µM for SNP | in vitro | glial/neuronal mixed hippocampal cell cultures from E19 Sprague-Dawley rat fetuses | AO,NP | [24] | |
| (mechanism unknown) | inhibits lipid oxidation stimulated by H2O2/FeSO4 | 10–100 µM (max at 100 µM) | in vitro | human hippocampal tissue from Alzheimer’s disease patients and age-matched controls | AO | [24] | |
| DHEAS and DHEA | PPARα receptor | decreases tissue lipid peroxidation, decreases activity of NF-κB, and decreases pro-inflammatory cytokine production | 100 µg/ml DHEAS in drinking water (resulting in dose of 300–500 µg/day) and chow containing 0.5% DHEA (25 mg/day) | in vivo | wild and homozygous C57BL/6 PPARα knockout mice | AO,AI | [244] |
| Anti-Inflammatory (AI) | |||||||
| DHEA | |||||||
| (mechanism unknown) | decreases serum concentrations of TNFα | chow containing 0.4% DHEA for 10 days | in vivo | female obese Zucker rats | AI | [152] | |
| inhibits NF-κB activation | inhibits basal and TNFα-stimulated NF-κB activation | 1 nM - 10 µM | in vitro | HuH7 human hepatocyte cell cultures | AI,AO | [133] | |
| (mechanism unknown) | inhibits mycoplasma- and LPS-induced increases in TNFα and IL-6 | 5 −50 µg/ml | in vitro | glial cell cultures from fetal (E20–21) rats | AI | [153] | |
| (mechanism unknown) | decreases IL-6 production | 5×l0−9−5×l0−6nmol/l; max at 5×l0−8 nmol/1 | in vitro | human peripheral blood mononuclear cells (PBMC) | AI | [296] | |
| DHEAS | |||||||
| inhibits NF-κB activation | inhibits basal and TNFα-stimulated NF-κB activation; inhibits H2O2-induced NF-κB activity; inhibits AP1; fos/jun-mediated transcription | 10 nM - 100 µM | in vitro | HuH7 human hepatocyte cell culture | AI,AO | [133] | |
| Anti-Glucocorticoid (AGC) | |||||||
| DHEA | |||||||
| (mechanism unknown) | antagonizes negative effects of corticosterone on neurogenesis | 200–250 mg DHEA pellet implant; daily s.c. injections 10, 20, 40 mg/kg (40 mg effective) | in vivo | male Lister Hooded rats | AGC | [145] | |
| (mechanism unknown) | decreases 11β-HSD1 mRNA expression in liver and white adipose tissue | diet containing 0.2% DHEA for 12 days | in vivo | male C57BL/6J mice | AGC | [6] | |
| (mechanism unknown) | increases 11β-HSD2 mRNA expression in kidney; increases renal 11β-HSD2 enzyme activity | diet containing 0.2% DHEA for 12 days | in vivo | C57BL/6J mice | AGC | [20] | |
| (mechanism unknown) | decreases 11β-HSDl mRNA expression in liver, white adipose tissue, and kidney; increases 11β-HSD2 mRNA expression in kidney; increases renal 11β-HSD2 enzyme activity | diet containing 0.2% DHEA for 13 days | in vivo | Sprague-Dawley rats | AGC | [20] | |
| decreases glucocorticoid receptor (GR) nuclear localization | protects against glutamate-induced neuronal cell death | 50 nM - 10 mM (optimal 5 µM) | in vitro | HT-22 (subclone of HT4 mouse hippocampal cell line) | AGC; NP | [53] | |
| attenuates corticosterone-induced stress activated protein kinase 3 (SAPK3) nuclear translocation | prevents neurotoxic actions of corticosterone | 20–500 nM (100 nM for SAPK3) | in vitro | Sprague-Dawley fetal rat (E18) hippocampal tissue cultures | AGC; NP | [150] | |
| CCAAT/enhancer-binding proteins (C/EBP) involved | decreases 11β-HSDl mRNA expression; decreases 11β-HSDl enzyme activity | 12.5–50 µM (mRNA expression); 25–100 µM (enzyme activity) | in vitro | murine 3T3-L1 adipocyte cell line | AGC | [6] | |
| Akt kinase and C/EBP involved | increases 11β-HSD2 mRNA levels; increases 11β-HSD2 reductase activity | 25 µM | in vitro | rat renal cortical collecting duct (RCCD2) cells | AGC | [20] | |
| 7α-OH-DHEA, 7β-OH-DHEA | competes with cortisone for use of 11β-HSD1 as a substrate | competes with cortisone for use of 11β-HSDl as a substrate, which results in less Cortisol | 0.5 µM | in vitro | human skin tissue samples | AGC | [122] |
8.1. Neuroprotection
A major biological action of DHEA(S) is neuroprotection. After contusive spinal cord injury (SCI), CD-1 female mice treated with DHEA had better locomotor recovery, left-right coordination, and fine motor control than control animals with SCI treated with dimethyl sulfoxide (DMSO) vehicle [93]. Mice treated with DHEA also had significantly more white matter spared at the epicenter of the injury and reduced area of reactive gliosis surrounding the lesion. DHEA treatment was intensive and consisted of three different modes of administration: a DHEA Matrigel patch (10−10 M) applied to the spinal cord before closure of the wound, followed by 12 days of i.p. injections of saline containing DHEA (10−6 M or 0.02 mg/kg/day) after SCI, and DHEA (10−6 M) in the drinking water for 42 days [93].
Male Wistar rats implanted with 100 mg DHEA pellets subcutaneously (s.c.) 12 days prior to forebrain ischemia had reduced neuronal injury in the hippocampal CA1 region compared to controls implanted with placebo pellets [174]. Similarly, rabbits treated with DHEAS intravenously at a dose of 50 mg/kg five minutes after ischemic stroke had prolonged tolerance to ischemia compared to the vehicle-treated control group [166]. Although DHEAS acts as a noncompetitive GABAA receptor antagonist [188], co-administration of the GABAA receptor antagonist bicuculline with DHEAS ameliorated the neuroprotective effect of DHEAS [166] (see Figure 2), suggesting that DHEAS mediated its effects through GABAA receptors. Although not examined in this study, the effects may be due to the metabolism of DHEAS into a GABAA receptor agonist in vivo, such as androstanediol or androsterone [143].
DHEA and DHEAS are also neuroprotective in vitro. When rat cerebral cortical cultures were subjected to anoxia for two hours in an anaerobic chamber and pretreated with DHEA (10−8 and 10−6 M) or DHEAS (10−6 M), there was increased neuronal survival [190].This increase in neuronal survival was not due to metabolism of DHEA(S) into estradiol, since concentrations of 17β-estradiol were not detectable in culture media [190]. In an in vitro model of brain ischemia, DHEAS was neuroprotective against mild to medium oxygen-glucose deprivation (OGD) (10–20% cell death) in rat cerebellar granule cell cultures [138]. OGD was induced by replacing the culture media with deoxygenated, glucose-free balanced salt solution. DHEAS was neuroprotective in a dose-dependent manner with 0.5 µM providing 50% of maximal neuroprotection and 10 µM providing almost complete neuroprotection [138]. DHEAS was ineffective against medium to severe OGD (greater than 40–50% of cell death). The addition of either the GABAA receptor agonist pentobarbital (100 µM) or GABAA receptor antagonist picrotoxin (200 µM) blocked the protective effect of DHEAS; thus, GABAA receptor mediated neuronal excitation as well as inhibition reduced the neuroprotective effects of DHEAS [138]. The finding that pentobarbital and picrotoxin had similar results in blocking the neuroprotective effects of DHEAS does not make intuitive sense. Given our current understanding that DHEAS acts as a GABAA receptor antagonist, it is unclear how blocking the GABAA receptor channel blocked the neuroprotective effect of DHEAS. It is possible that different GABAA receptor modulators are acting at different sites of the GABAA receptor [233] or perhaps at different sub-populations of GABAA receptors.
DHEA(S) may also be neuroprotective via blockade of excitotoxicity. The neurotoxic effects of ischemia could be due to the release of excessive amounts of excitatory amino acids. DHEAS protected cultured rat cerebellar granule cells against the toxic effects of glutamate, NMDA, 1-methyl-4-phenylpyridinum (MPP+) and colchicine [138]. DHEA was neuroprotective against glutamate and amyloid β protein (Aβ) toxicity in HT-22 cells in a dose-dependent manner [53]. Both DHEA and DHEAS protect against NMDA toxicity in fetal rat hippocampal cultures [149]. DHEA was also protective against α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainic acid toxicity in vitro. Male Lister hooded rats implanted with DHEA pellets subcutaneously had reduced hippocampal lesions in response to intracerebral infusion of NMDA compared to controls implanted with paraffin pellets [149]. The finding that DHEA(S) is neuroprotective against NMDA toxicity highlights another conceptual difficulty. Because DHEA and DHEAS lead to stimulation of the NMDA receptor in the hippocampus, we would predict that DHEA(S) would worsen the toxicity of NMDA instead of being neuroprotective against it. However, DHEA(S) may be neuroprotective against NMDA toxicity through an alternative pathway, such as the σ1 receptor [193] or protecting the mitochondria against intracellular Ca2+ overload [139]. In this regard, it has been found that DHEA reduced cytoplasmic Ca2+ overload-induced loss of mitochondrial membrane potential by preventing Ca2+ influx into the mitochondrial matrix in primary cerebellar granule cell culture [139] (see Figure 2).
DHEA and DHEAS may act through different mechanisms. The neuroprotective effect of DHEA against NMDA-induced cytoxicity may be mediated by the NMDA receptor through modulation of the calcium/nitric oxide (NO) signaling pathway. DHEA, but not DHEAS, inhibited NMDA-induced NO production and NO synthase (NOS) activity in hippocampal cell culture [159]. The neuroprotective effect of DHEAS against NMDA-induced cytoxicity may be mediated via the σ1 receptor (see Figure 2). The σ1 receptor antagonists rimcazole and BD1063 partially, but significantly, reversed the protective effect of DHEAS against NMDA-induced neurotoxicity [159]. These data suggest that DHEA and DHEAS have distinct and different mechanisms by which they may be neuroprotective.
While low concentrations of DHEA(S) can be neuroprotective, high concentrations of DHEA can be ineffective or neurotoxic. In mouse embryonic neuronal culture, DHEA (10−8 and 10−7 M) treatment increased neuronal survival, with higher concentrations (10−6, 10−5, and 10−4 M) being less effective in a dose-dependent manner [41]. The administration of 500 nM DHEA (the highest concentration examined) was neurotoxic to rat hippocampal cultures [150]. High concentrations of DHEA (micromolar concentrations) have also been neurotoxic in vitro, with effects mediated through inhibition of complex I of the mitochondrial respiratory chain [269]. Neurotoxic effects have also been demonstrated in vivo. Male Balb/c mice fed a diet containing 0.6% DHEA for 10 weeks (average dose = 14 mg/day) and then a normal diet for 4 weeks had lower neuronal density in primary motor cortex and in hippocampal CA1 region compared to mice fed a standard diet [269]. In mouse primary neuronal cultures derived from whole brain, incubation with DHEA at micromolar concentrations for 24 hours inhibited viability of neurons, and incubation with DHEA at 10 nM to micromolar concentrations for 72 hours reduced viability of neurons [103]. Although DHEA had neurotoxic effects on pure neuronal cultures, incubations of DHEA with whole brain cultures containing mixed populations of neurons and glia had no detrimental effect on cell viability. Unlike DHEA, DHEAS had no effect on cell viability in either pure neuronal cultures or a mixed neuron and glia cultures. Similar results were obtained using SK-N-SH human neuroblastoma cells, with DHEA decreasing cell viability and DHEAS having no effect [103]. When neuroblastoma cells were incubated with both DHEA and DHEAS, DHEAS completely antagonized the neurotoxic effect of DHEA [103]. These data further support the conclusion that DHEA and DHEAS have distinct and different, and perhaps opposing functions.
8.2. Neurite Growth
DHEA and DHEAS have dramatic and different effects on growth of embryonic rodent cortical neuronal [64] and glial [41] neurites. In neocortical neurons [64], DHEA at low nanomolar concentrations increased the length of Tau-immunopositive neurites. These neurites were identified as axons. DHEA had much less effect on microtubule-associated protein-2 (MAP-2) immunopositive neurites (dendrites). By contrast, DHEAS at low nanomolar concentrations had no effect on axonal growth, but stimulated dendritic growth. DHEA stimulation of embryonic cortical neurons caused a dose-dependent increase in calcium entry into cells. This effect was blocked by the NMDA receptor antagonists (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate (MK801) and D-2-amino-5-phosphonopentanoic acid (D-AP5), suggesting that DHEA’s effects involved NMDA receptors (see Figure 2). These data, together with the data suggesting activity-dependent neurosteroid synthesis [127], suggest that DHEA may be synthesized and act locally to cause axonal growth in cortical embryonic neurons [64]. Similar studies were not done in adult neuronal cultures, so it remains unknown if DHEA’s effects on axonal growth are limited to embryos. DHEA was reported to have effects on synapse formation in adult rat hippocampal neurons [116]. Treatment of ovariectomized rats with subcutaneous injections of 1 mg DHEA per day for two days increased CA1 spine synapse density more than 50% compared to the vehicle-treated control group. However, this effect of DHEA was likely mediated through local aromatization to estradiol as the aromatase inhibitor letrozole inhibited the effect of DHEA [116].
8.3. Neurogenesis and Neuronal Survival
DHEA(S) promotes neurogenesis and neuronal survival. Male Lister hooded rats implanted with 200–250 mg DHEA pellets had increased neurogenesis in the dentate gyrus compared to animals who received paraffin pellets [145]. A possible mechanism by which DHEA(S) could promote neurogenesis and neuronal survival is by affecting concentrations of brain-derived neurotrophic factor (BDNF), a member of the nerve growth factor family that plays a role in central nervous system development and plasticity. Single i.p. injections of either DHEA (25 mg/kg) or DHEAS (50 mg/kg) into adult male Sprague-Dawley rats changed regional brain concentrations of BDNF during the 300 minutes of the experiment [220]. Rats that received DHEA had decreased BDNF content in the hippocampus, no change in BDNF content in the amygdala, and increased BDNF in the hypothalamus compared to sham rats that received sesame oil. Rats that received DHEAS had decreased BDNF 30 minutes after injection and increased BDNF 180 min after injection in the hippocampus, biphasic increases in BDNF in the amygdala, and decreased BDNF in the hypothalamus [220]. Although these data suggest that DHEA and DHEAS alter BDNF concentrations in the brain, it is not known how these changes relate to neurogenesis and neuronal survival since this was not examined in the study. Further, these data show that DHEA and DHEAS have different effects and suggest that they may operate through different mechanisms.
DHEAS promoted survival of adult human cortical brain tissue in vitro [45]. In enriched neuronal cultures from three adult participants, DHEAS (10 (µg/ml) was as effective as human recombinant fibroblast growth factor (FGF2) in promoting survival of neurofilament positive, neuron-like cells. DHEAS and FGF2 were synergistic in increasing cell survival [45]. In another study, DHEA increased neurogenesis in addition to neuronal survival in cultures of human neural stem cells derived from fetal cortex [305]. Both epidermal growth factor (EGF) and leukemia inhibitory factor (LIF) were required to elicit the proliferative effect of DHEA. When other steroids were tested, neither DHEA’s precursor pregnenolone nor DHEA’s metabolites (7α-hydroxy-DHEA, 7β-hydroxy-DHEA, 7-oxo-DHEA, nor androstenediol) had the same effect on proliferation as DHEA. The proliferative effects of DHEA could be blocked by the NMDA receptor antagonist MK801 and the σ1 receptor antagonists BD1063 and haloperidol, whereas the GABAA receptor antagonist bicuculline had no effect [305]. This suggests that DHEA’s NMDA and σ1-mediated effects are more consequential than GABAA receptor effects on neurogenesis and neuronal survival.
8.4. Apoptosis
DHEA and DHEAS influence apoptosis. Using cultured neural precursors from rat embryonic forebrains, DHEA (50 and 100 nM) activated the serine-threonine protein kinase Akt, which is widely implicated in cell survival signaling [350] (see Figure 2). Activation of Akt has been shown to enhance neuronal survival through inhibition of apoptosis [79]. Pretreatment with the estrogen receptor blocker tamoxifen and the androgen receptor blocker flutamide did not affect the DHEA-induced Akt increase, suggesting that DHEA’s effects were not mediated through its conversion to estrogens or androgens. Interestingly, DHEAS (50 and 100 nM) had the opposite effect of DHEA; DHEAS decreased Akt and increased apoptosis [350] (see Figure 2). Similarly, in cultured P19-N neurons, DHEA, but not DHEAS, decreased the rate of apoptotic cell death due to NMDA neurotoxicity [342]. Thus, once again, DHEA and DHEAS have different effects on neural survival, suggesting that the balance between these two neurosteroids may play a critical role in nervous system development and maintenance.
In rat chromaffin cells and the sympathoadrenal pheochromocytoma PC12 cells, both DHEA and DHEAS protected against apoptosis induced by serum deprivation, through mechanisms independent of NMDA and NOS inhibition [57]. The effects of DHEA and DHEAS were time- and dose-dependent, with half maximal effective concentration (EC50) in the low nanomolar range. The prosurvival effect involved the antiapoptotic Bcl-2 proteins, and the activation of transcription factors cAMP response element binding protein (CREB) and nuclear factor-kappa B (NF-κB), upstream effectors of the antiapoptotic Bcl-2 protein expression, as well as antiapoptotic protein kinase C (PKC)α/β, a posttranslational activator of Bcl-2 [57]. The prosurvival effect of DHEA and DHEAS appeared to be mediated by G-protein-coupled-specific membrane binding sites in PC12 cells, and did not involve NMDA, GABAA or σ1 receptors [59]. This G-protein-coupled binding site may be similar to the DHEA receptor found on endothelial cell plasma membranes that is coupled to endothelial nitric-oxide synthase (eNOS) activity through Gi/o proteins Gαi2 and Gαi3 [178]. It is worth noting that PC12 cells do not have functional GABAA or NMDA receptors, further suggesting that the effects of DHEA and DHEAS on apoptosis are not mediated by these receptors.
8.5. Catecholamine Synthesis and Secretion
DHEA(S) influence catecholamine synthesis and secretion [236]. DHEAS was protective against the neurotoxin MPP+ (which inhibits catecholamine synthesis and triggers cell death) in rat cerebellar granule cell cultures [138]. In an in vivo study of long-term DHEA treatment, obese and lean female Zucker rats were fed chow containing 0.6% DHEA for 28 days [309]. Lean rats had higher norepinephrine (NE) in the lateral hypothalamus but lower NE in the paraventricular nucleus (PVN) of the hypothalamus compared to lean rats fed the control diet [309]. There were no differences among obese Zucker rats in NE, serotonin (5HT), and serotonin metabolite 5-hydroxyindole-3-acetic acid (5-HIAA) in the hypothalamus [309]. In another study, obese female Zucker rats treated with a large, acute i.p. injection of DHEA (200 mg/kg) had increased concentrations of dopamine (DA), 5HT, and 5-HIAA, and decreased concentrations of NE and epinephrine (EPI) in the paraventricular nucleus compared to controls that received oil vehicle [307]. DHEA treatment had no effect on neurotransmitter concentrations in the lateral hypothalamus or ventromedial hypothalamus [307]. These in vivo studies suggest that the duration and/or dose of DHEA treatment may be important and that DHEA may have different effects on different parts of the hypothalamus. In vitro, DHEAS (10−8 M and 10−6 M) has been found to stimulate dopamine release from rat hypothalamic cells in primary cultures [217].
DHEA(S) affects proliferation of catecholamine-producing adrenomedullary chromaffin cells. Although DHEA and DHEAS do not induce proliferation by themselves, they may modulate proliferation induced by growth factors and do so in an age-dependent manner. In bovine chromaffin cells from young animals, DHEA decreased cell proliferation induced by insulin-like growth factor-II (IGF-II), but had no effect on proliferation induced by basic fibroblast growth factor (bFGF) [285]. In another study, DHEA decreased cell proliferation induced by leukemia inhibiting factor (LIF) in bovine chromaffin cells from young animals [286]. In bovine chromaffin cells from adult animals, DHEA decreased cell proliferation induced by epidermal growth factor (EGF) [286]. DHEAS had no effect on LIF-induced proliferation of cells from young animals, but high micromolar concentrations of DHEAS enhanced EGF-induced proliferation of cells from adults [286]. The effects of DHEA and DHEAS were not due to downstream metabolism into sex steroids since neither the estrogen receptor antagonist ICI 182,780 nor the androgen receptor antagonist flutamide affected chromaffin cell proliferation [286]. Thus, local production of DHEA and DHEAS in the adrenal cortex can influence proliferation of chromaffin cells, and may have similar effects on catecholamine-producing neurons in the brain.
DHEA and DHEAS stimulate secretion of catecholamines from rat pheochromocytoma PC12 cells and are involved in inhibition of neuronal proliferation and promotion of differentiation of adrenal medullary cells to a more neuroendocrine phenotype. Administration of nerve growth factor (NGF) induced PC12 cells to differentiate into a neuronal phenotype, while administration of DHEA alone had no effect [351]. PC12 cells incubated with both NGF and DHEA had lower survival and less neurite outgrowth than cells incubated with NGF alone in serum-free medium [351]. NGF dose-dependently induced phosphorylation of extracellular signal-regulated kinases (ERK)1/2, which distinguished proliferation from differentiation processes, and this ERK1/2 phosphorylation was inhibited by DHEA [351; 352]. Furthermore, DHEA stimulated dopamine release from NGF-treated cells, while neither NGF nor DHEA alone had an effect on dopamine release [352]. Another study compared the stimulatory effects of DHEA and DHEAS on catecholamine synthesis. The effect of DHEA was faster than that of DHEAS; whereas the effect of DHEA peaked at 10 min, the effect of DHEAS peaked at 30 min [58]. In addition to stimulating secretion, DHEAS (but not DHEA) also stimulated catecholamine production. DHEAS increased tyrosine hydroxylase (TH) protein abundance in PC12 cells after four hours of stimulation, and also increased TH mRNA abundance in PC12 cells after only two hours of stimulation [58] (see Figure 2). These data suggest that DHEA and DHEAS function differently, and that DHEAS may directly affect TH gene transcription. Experiments to determine if DHEA increases TH gene transcription directly have not yet been reported.
DHEA and DHEAS have non-transcriptional effects on catecholamine secretion. DHEA and DHEAS have been found to stimulate actin depolymerization and submembrane actin filament disassembly, a fast-response cellular system regulating trafficking of catecholamine vesicles [58]. An actin meshwork inhibits catecholamine secretory vesicles from reaching exocytosis sites. By decreasing this actin meshwork, DHEA and DHEAS increase the ability of catecholamines to be secreted from secretory vesicles (see Figure 2). Addition of DHEA and DHEAS to PC12 cells induced actin depolymerization, as measured by the ratio of G-monomeric to total cellular actin, an established marker of actin cytoskeleton dynamics [58]. When PC12 cells were exposed to phallacidin, an actin filament stabilizer, the stimulatory effect of DHEA and DHEAS on both dopamine and norepinephrine secretion was prevented [58]. These studies show that DHEA and DHEAS exert a direct effect on PC-12 cells (a model of chromaffin cells), and thus, provide in vitro evidence of how the zona reticularis and the adrenal medulla may be interacting in vivo. These findings also raise the possibility that DHEA and DHEAS could increase catecholamine production and release in the brain.
8.6. Antioxidant
DHEA and DHEAS have antioxidant effects. Oxidative stress is associated with increased tissue levels of highly reactive and toxic substances. In rat primary hippocampal cell cultures, DHEA pre-treatment protected against the toxicity induced by the oxidants hydrogen peroxide and sodium nitroprusside [24]. The neuroprotective effect of DHEA was not attributable to neurotrophic action. In human tissue, DHEA was able to prevent lipid oxidation stimulated by hydrogen peroxide/ferrous sulfate (H2O2/FeSO4). This was seen in hippocampal tissue from both patients with Alzheimer’s disease and their age-matched controls [24]. DHEA concentrations may have implications for Alzheimer’s disease since DHEA was also neuroprotective against amyloid β protein (Aβ) toxicity in vitro [53]; this neuroprotective effect could be due to DHEA’s antioxidant effects. DHEA administration reduced the rate of lipid peroxidation in NT2 neurons stimulated by the prooxidant H2O2/FeSO4 [311]. Exposure of NT2 neurons to prooxidants increased mRNA and protein levels of β-site Aβ precursor protein-cleaving enzyme (BACE) [311]. BACE is the enzyme that initiates the production of Aβ, which is a major component of senile plaques found in brain tissue of patients with Alzheimer’s disease. Pretreatment of NT2 neurons with DHEA prevented increases in expression, protein levels, and activity of BACE induced by oxidative stress [311]. It is possible that DHEA administration may decrease Aβ accumulation via inhibition of BACE.
Hyperglycemia causes an imbalance in the oxidative state of tissue and can be induced in male Wistar rats by a single injection of streptozotocin (STZ), which is toxic to the insulin-producing beta cells of the pancreas. STZ-diabetic rats treated with DHEA by gastric intubation for 21 days showed decreased hydrogen peroxide and the toxic aldehyde 4-hydroxynonenal (HNE) levels, higher levels of the antioxidant glutathione (GSH), and higher levels of the antioxidant enzymes GSH-peroxydase and catalase in the hippocampus compared to STZ-diabetic control rats treated with vehicle [7]. A major target of reactive oxygen species is the transcription factor NF-κB, which is involved in the activation of genes relevant to inflammation, cytokines, cell proliferation and cell survival. STZ-diabetic rats treated with DHEA showed reduced NF-κB activation over time, as measured by DNA binding activity [7] (see Figure 2). The time course of DHEA’s inhibitory effects on NF-κB activation paralleled DHEA’s effects on oxidative balance [7]. The peroxisome proliferator-activated receptor α (PPARα) has been implicated in antioxidant effects due to its role in inhibiting NF-κB. Aged wild-type mice (PPARα+/+) treated with both DHEA (chow containing 0.5% DHEA or 25 mg/day) and DHEAS (100 µg/ml in drinking water) had lower tissue lipid peroxidation, decreased NF-κB activity in the spleen, and lower pro-inflammatory cytokine production compared to homozygous knockout (PPARα−/−) mice treated with both DHEA and DHEAS, suggesting that DHEA(S)’ influences on these effects are mediated through PPARα [244]. However, no specific binding of DHEA or DHEAS to PPARα has been demonstrated in vitro [240]. Although PPARα receptors are mainly found in the liver, there is increasing evidence for a role of PPAR receptors in oxidative stress and inflammation in the brain [294].
8.7. Anti-inflammatory
DHEA and DHEAS have anti-inflammatory and immunomodulatory effects [61; 140]. DHEA decreases pro-inflammatory cytokine production both in vivo and in vitro. Zucker rats fed a diet with DHEA (0.4%) for 10 days had lower serum concentrations of the proinflammatory cytokine tumor necrosis factor (TNF)α compared to rats fed a control diet [152]. Human peripheral blood mononuclear cells (PBMC) incubated with DHEA (maximal effective concentration = 5 × 10−8 nM) had decreased production of pro-inflammatory cytokine interleukin (IL)-6 [296]. DHEA’s anti-inflammatory effects have also been observed in vitro in brain tissue cultures. There was a dose-dependent inhibitory effect of DHEA on mycoplasma- and lipopolysaccharide (LPS)-induced production of TNFα and IL-6 in rat glial cell cultures [153].
In human hepatocyte cell cultures transfected with an NF-κB-luciferase expression vector, DHEA and DHEAS inhibited both basal and TNFα-stimulated NF-κB-dependent luciferase transcription in a time- and dose-dependent manner [133]. DHEA and DHEAS had stronger inhibitory effects on TNFα-stimulated NF-κB transcription than basal transcription, with DHEAS having a stronger effect than DHEA. Estradiol and dihydrotestosterone did not have these inhibitory effects on NF-κB, so the effects of DHEA(S) were not due to conversion into these steroids [133].
DHEAS also inhibited TNFα-stimulated NF-κB binding to DNA but did not inhibit NF-κB p65 enhanced transcription, suggesting that DHEAS exerts its inhibitory effect on NF-κB activation [133]. DHEAS inhibited hydrogen peroxide-induced NF-κB activity and activator protein-1 (AP1; fos/jun)-mediated transcription, which is known to be a radical-sensitive transcription factor [133]. Together, this evidence suggests that DHEAS acts on NF-κB indirectly through a cytokine-induced signaling pathway involving reactive oxygen species (ROS) [133] (see Figure 2). By contrast, TNFα inhibits the transactivation of P450c17 gene transcription by nuclear receptor steroidogenic factor 1 (SF-1) via induction of NF-κB [128]. This effect is due to competition of NF-κB (p65) with SF-1 for binding to the P450c17 promoter. Thus, inflammatory cytokines inhibit P450c17 and most likely DHEA production, while DHEA(S) inhibits TNFα- NF-κB stimulated transcription.
8.8. Anti-glucocorticoid
DHEA has anti-glucocorticoid effects [130; 140]. DHEA is protective against the neurotoxic effects of corticosterone both in vivo and in vitro. Corticosterone treatment decreased neurogenesis in the dentate gyrus of male Lister Hooded rats while co-treatment with DHEA suppressed the effects of corticosterone [145]. In another group of rats, animals were treated with daily s.c. injections of either DHEA (10, 20, or 40 mg/kg/day), pregnenolone (40 mg/kg/day), androstenediol (40 mg/kg/day) or vehicle (oil) for 16 days and then starting on day 10, all animals received daily s.c. injections of corticosterone (40 mg/kg/day). Only animals that received the highest dose of DHEA (40 mg/kg/day) showed antagonism of the negative effect of corticosterone on neurogenesis in the dentate gyrus [145]. This effect appeared to be specific for DHEA, since neither pregnenolone nor androstenediol had this anti-glucocorticoid effect. Similarly, in vitro, DHEA prevented hippocampal neurotoxicity induced by corticosterone in primary rat tissue cultures [150]. DHEA attenuated the corticosterone-induced nuclear translocation of stress-activated protein kinase 3 (SAPK3), which might be important in the sequence of events leading to either neuronal death or survival [150]. While DHEA is associated with attenuation of SAPK3 translocation, it is unclear if this is the mechanism responsible for DHEA’s anti-glucocorticoid effects. In another in vitro study, DHEA was neuroprotective against glutamate toxicity in HT-22 cells in a dose-dependent manner [53]. HT-22 neuronal cells treated with glutamate for 20 hours showed high nuclear localization of glucocorticoid receptor (GR), while cells treated with DHEA for 24 hours and then treated with glutamate for 20 hours had suppressed nuclear localization of GR as assessed by immunocytochemical staining with GR antibody [53]. Thus, inhibition of GR translocation into the nucleus is a possible mechanism of DHEA’s anti-glucocorticoid effects (see Figure 2).
While DHEA is widely considered to have anti-glucocorticoid effects, the mechanisms underlying these effects are unclear. DHEA may affect local glucocorticoid metabolism through its effects on 11β-hydroxysteroid dehydrogenase (11β-HSD) type 1 and type 2, both at the level of enzyme inhibition and at the level of mRNA expression. 11β-HSD1 catalyzes the conversion of cortisone (an inactive glucocorticoid) to cortisol (an active glucocorticoid), and 11β-HSD2 catalyzes the reverse reaction. Sprague-Dawley rats and C57Bl/6J mice fed chow containg 0.2% DHEA for 12–13 days had lower 11β-HSD1 mRNA expression in liver,white adipose tissue, and kidney [6; 20] and higher 11β-HSD2 mRNA expression in kidney compared to control animals fed a standard diet [20]. DHEA-treated rats and mice also had higher renal 11β-HSD2 enzyme activity than controls [20].
DHEA’s effects on 11β-HSD type 1 and type 2 mRNA expression (and hence gene transcription) may be via regulation of CCAAT/enhancer-binding protein (C/EBP) expression by DHEA. C/EBP-α induces 11β-HSD1 transcription, and DHEA inhibits C/EBP-α expression. In vitro, DHEA inhibited 11β-HSD1 mRNA expression and 11β-HSD1 activity in murine 3T3-L1 adipocytes by reducing C/EBP-α expression [6]. C/EBP-β induces 11β-HSD2 transcription, and DHEA stimulates C/EBP-β mRNA expression. In another in vitro experiment, DHEA increased 11β-HSD2 mRNA expression and 11β-HSD2 reductase activity in rat renal cortical collecting duct (RCCD2) cells [20]. These effects were not due to downstream metabolism of DHEA into 17β-estradiol or testosterone since neither the estrogen receptor antagonist tamoxifen nor the androgen receptor antagonist flutamide affected the DHEA-mediated upregulation of 11β-HSD2 activity in RCCD2 cells [20].
Some of DHEA’s effects may be mediated by its metabolites 7α-hydroxy-DHEA or 7β-hydroxy-DHEA. In the brain, DHEA can be converted into 7α-hydroxy-DHEA by cytochrome P4507b1. 7α-hydroxy-DHEA is a substrate for 11β-HSD1, which has an additional enzymatic activity and interconverts 7α-hydroxy-DHEA and 7β-hydroxy-DHEA through a 7-oxo-intermediary [213]. 7α-hydroxy-DHEA and 7β-hydroxy-DHEA can act as anti-glucocorticoids through competitive inhibition of 11β-HSD1 and limit the amount of cortisone binding, which reduces the amount of cortisone that can be converted into cortisol [122]. These effects can be shown in vitro in human skin tissue samples [122], and further studies need to be done to determine the extent to which this anti-glucocorticoid effect occurs in vivo.
9. Methodological Issues
Some of the contradictory findings between studies may be due to methodological differences. As more studies demonstrating the effects of DHEA(S) on neuroprotection are published, we will gain a better understanding of the important factors involved in the process. In reviewing the previously published studies, it has become clear that three of the important methodological issues that account for some of the variability in results between studies are: (1) the timing of DHEA(S) administration, (2) the dose of DHEA(S) used, and (3) the presence of extant factors. These three issues will be discussed in more detail below, but other potentially important issues (not discussed here) include in vivo vs. in vitro studies, species studied, sex of the subject, and acute vs. chronic dosing.
9.1. Timing of Administration
The timing of DHEA(S) administration is an important factor in whether DHEA(S) are neuroprotective both in vivo and in vitro. For example, in the in vivo study on ischemia in New Zealand white rabbits, i.v. administration of DHEAS (50 mg/kg) showed neuroprotective effects when given five minutes after the onset of ischemia, but was not protective when given 30 minutes after ischemia onset [166]. In one in vitro study using hippocampal cultures, both DHEA (60 µM) and DHEAS (100 nM) were neuroprotective when co-administered with NMDA, but neither were protective when they were administered prior to NMDA [159]. However, in another in vitro study on hippocampal cultures this was not shown to be the case. Three conditions were tested for DHEA (100 nM) and DHEAS (100 nM): administration prior to NMDA, co-administration with NMDA, or administration one hour after NMDA [149]. DHEAS was found to be protective only when given prior to NMDA. DHEA was most protective when given prior to NMDA, but was also protective when co-administered with NMDA and given one hour after NMDA [149]. A comparison of these two studies suggest that the actual timing of the “pre-treatment” is important since pre-treatment occurred 24 hours prior to NMDA administration in the study by Kurata and colleagues [159] and 6 hours prior to NMDA administration in the study by Kimonides and colleagues [149].
9.2. DHEA(S) Dose
The dose administered is an important factor in whether DHEA(S) is neuroprotective both in vivo and in vitro. In the Li et al (2001) study [174], the 100 mg DHEA pellets implanted s.c. were effective in reducing neuronal injury prior to ischemia but the lower doses of 25 and 50 mg DHEA pellets were no different from placebo. In mouse embryonic neuronal culture, DHEA (10−8 and 10−7 M) treatment increased neuronal survival, with higher concentrations (10−6, 10−5, and 10−4 M) being less effective in a dose-dependent manner [41]. DHEA and DHEAS demonstrated dose-dependent inverted U-shaped effects on memory retention in mice given T-maze footshock avoidance training, with the lowest doses of DHEA or DHEAS showing no significant effect on memory, intermediate doses improving memory retention, and the highest doses showing no effect [94]. In another in vitro study, pretreatment with DHEA (10−8 and 10−6 M) or DHEAS (10−6 M) increased neuronal survival in rat cerebral cortical cultures subjected to anoxia for two hours in an anaerobic chamber [190]. However, lower concentrations of DHEA (10−10 M) and DHEAS (10−8 M) were not neuroprotective, and the lowest tested concentration of DHEAS (10−10 M) had a statistically significant negative effect on survival [190]. Thus, the concentrations of DHEA(S) that were neuroprotective followed an inverted U-shaped dose-response curve, where low and high concentrations were less effective at neuroprotection than moderate concentrations.
9.3. DHEA(S) and Extant Factors
Factors such as neurotoxins or other hormones affect whether DHEA(S) is neuroprotective, ineffective or neurotoxic. While neuroprotective effects of DHEA(S) are observed in response to physical, chemical or neurotoxic injury, incubation of healthy neurons with DHEA alone can be ineffective or neurotoxic. For example, pretreatment with DHEA or DHEAS had no effect on neuronal survival in rat cerebral cortical cultures if cultures were not subjected to anoxia [190]. In another experiment on mouse primary neuronal cultures, incubation with DHEA alone at micromolar concentrations for 24 hours inhibited viability of neurons, and incubation with DHEA at 10 nM to micromolar concentrations for 72 hours reduced viability of neurons [103]. Similar results were obtained using SK-N-SH human neuroblastoma cells, with DHEA decreasing cell viability [103]. Unlike the neurotoxic effect of DHEA, DHEAS had no effect on cell viability. However, when neuroblastoma cells were incubated with both DHEA and DHEAS, DHEAS completely antagonized the neurotoxic effect of DHEA [103]. The administration of 500 nM DHEA was neurotoxic to rat hippocampal cultures when given alone, but was neuroprotective against the toxic effects of corticosterone when co-administered with 100 nM corticosterone [150]. These studies suggest that whether a dose of DHEA is neuroprotective or neurotoxic depends not only on the concentration of DHEA, but also on what other hormones or factors are affecting the tissue (e.g. such as concentrations of glucocorticoids) and the physiological state of the organism (e.g. is the organism under stress?).
10. Implications of DHEA(S) Mechanisms and Actions for Health and Neuropsychiatric Illnesses
Preclinical findings such as those reviewed above were largely influential in kindling interest in human applications of DHEA(S) for neuropsychiatric indications. In the remainder of this paper, we review data in humans showing relationships between circulating endogenous DHEA(S) concentrations and neuropsychiatric illness and function, as well as DHEA treatment data derived from double-blind, controlled clinical trials.
In humans, DHEA(S) concentrations in blood, urine, saliva, and cerebrospinal fluid (CSF) decline with aging as well as with chronic and sub-chronic stress [107; 115; 232], inflammation [61] and many medical illnesses [80; 225; 232; 235; 293]. It is not known whether these age- and illness-related declines are pathophysiologically related to the manifestations of aging and of illness. In addition to being linked with morbidity, DHEA(S) concentrations may predict mortality. In two studies, low serum DHEA(S) concentrations were associated with increased 2 to 4 year mortality risk [35; 104], although another study [172] did not find this relationship. In one of the longest follow-up studies published (27 years), lower baseline DHEAS concentrations in men and women significantly predicted shorter longevity [83]. Longevity was independent of other risk factors, such as age, blood pressure and fasting glucose.
10.1. DHEA(S)-to-Cortisol Ratios
Unlike DHEA(S) concentrations that decline under conditions of chronic stress and medical illness, cortisol concentrations generally either rise or do not change, and subsequently result in a decrease in DHEA(S)-to-cortisol ratios [89; 109; 115; 121; 170; 171; 195; 225; 228; 232; 235; 255; 336]. As described in section 8.8, DHEA(S) and cortisol have different and often antagonistic effects on each other. The significance of considering DHEA(S)-to-cortisol ratios is exemplified by the concept of “anabolic balance,” which considers the ratio of anabolic to catabolic hormones and may indicate susceptibility to diseases of stress and aging [84; 335]. Hormones co-regulate each other, and together, co-elevations or imbalances determine net effects on tissues. Therefore, it may be important to consider the ratio of both steroids in addition to their absolute concentrations. For example, greater cognitive deterioration was observed in elderly men and women who showed larger decreases in plasma DHEAS-to-cortisol ratios over a two-year period, although changes in DHEAS concentrations alone were not significantly correlated with cognitive change [183]. Frail, institutionalized elderly people did not differ from independent community-dwelling controls in serum concentrations of cortisol or DHEAS, but did have significantly lower DHEAS-to-cortisol ratios [54].
Both DHEAS and cortisol are considered in the calculation of allostatic load, which is a measure of the cumulative physiological burden to the body of accommodating multiple stressors over time [194]. Allostatic load scores are based on ten biological parameters, including DHEAS, cortisol, epinephrine, norepinephrine, high density lipoproteins (HDL), total cholesterol, waist-to-hip ratio, glycosylated hemoglobin (HbA1C), systolic blood pressure, and diastolic blood pressure [279]. High cortisol concentrations and low DHEAS concentrations contribute to increases in allostatic load score. The hormonal profile contained within the measure of allostatic load by itself serves as a stronger predictor of cardiovascular disease than the traditional cardiovascular disease risk factors alone [146; 279]. Although the focus of this review is on DHEA(S), the DHEA(S)-to-cortisol ratios are also clinically important and are included when available.
11. DHEA(S) Concentrations and Neuropsychiatric Illnesses
Correlational studies have suggested a relationship between endogenous concentrations of DHEA(S) and depression, anxiety spectrum disorders, post-traumatic stress disorder (PTSD), schizophrenia, and dementia as well as mood, memory, and functional abilities in healthy aging individuals. However, numerous caveats (outlined more fully elsewhere [337]) are important to consider before ascribing causality in these relationships. For example, DHEA(S) concentrations often decrease non-specifically with chronic illness and this may confound studies examining differences in DHEA(S) concentrations in clinical populations, since the lowered hormone concentrations may reflect chronic medical illness, rather than having diagnostic specificity or direct pathophysiologic significance [157]. Also, correlational or cross-sectional studies in the elderly may suffer from the usual selection biases in aging studies, since fewer of the less healthy individuals may have survived to the age being studied [348].
11.1. Depression
According to current theories of the biology of depression [334; 335], DHEA(S)’ ability to modulate many neurobiological actions, including glutamate and σ1 receptors, catecholamines, neurogenesis, neuroprotection, anti-glucocorticoid, anti-inflammatory and anti-oxidant properties could all underlie relationships between endogenous and/or exogenously-supplemented DHEA(S) concentrations and depression [81; 82; 142; 220; 253; 267; 273; 300; 332; 334; 339]. An assessment of depression ratings in relation to plasma concentrations of several steroid hormones (estradiol, testosterone, estrone, androstenedione, cortisol, DHEA, and DHEAS) in 699 non-estrogen using, community dwelling, postmenopausal women (aged 50–90 years) [23] found that only DHEAS concentrations were negatively correlated with ratings of depressed mood. Specifically, higher DHEAS concentrations were associated with less depression, and this association was independent of age, physical activity and weight change. Furthermore, women with categorical diagnoses of depression had significantly lower plasma DHEAS concentrations compared to age-matched non-depressed women [23]. Similarly, in a large-scale study of 2,855 well-functioning elderly men and women, serum DHEAS concentrations were inversely correlated with depressive symptoms [210]. In a study looking at both DHEA and DHEAS in plasma, depressed patients had low DHEAS concentrations but normal DHEA concentrations [278]. Women whose first onset of major or minor depression occurred during peri-menopause showed low morning plasma DHEA and DHEAS concentrations [274]. Lower plasma DHEA concentrations during pregnancy and during the postpartum period were associated with higher postpartum ratings of depression [50]. Dysthymic patients have also been shown to have low serum DHEAS concentrations [189].
The remaining literature examining plasma and serum DHEA(S) concentrations in depression is inconsistent, with the decreased DHEA(S) findings described above, and with reports of either increased [117; 123; 310] or unaltered [86; 89; 105; 129; 255; 284] DHEA(S) concentrations in depressed patients. A study examining diurnal salivary concentrations of DHEAS and cortisol in a small group of medicated but still depressed patients with unipolar depression, found that depressed patients had elevated DHEAS concentrations compared to controls [14]. In a particularly intensive study, 24-hour plasma DHEA concentrations were assessed in un-medicated, severely depressed patients and healthy controls every 30 minutes [123]. Patients with depression had increased diurnal minimum and mean DHEA plasma concentrations. There was no difference in the diurnal maximum plasma concentrations and the diurnal amplitude of DHEA concentrations. Interestingly, the elevations in plasma DHEA concentrations paralleled elevations in plasma cortisol [123].
Several groups have found that DHEA-to-cortisol ratios in serum and saliva, rather than concentrations of either hormone alone, more accurately discriminate depressed from non-depressed individuals [14; 200; 231] with lower morning ratios seen in depression [200; 231]. The molar DHEA-to-cortisol ratio was significantly lower in the un-medicated depressed patients than in controls, and the evening salivary DHEA-to-cortisol ratio ratios were inversely correlated with the lengths of current depressive episodes [349]. Morning salivary DHEA hyposecretion as well as evening cortisol hypersecretion were significantly and independently associated with major depression in 8- to 16-year-olds [111]. Patients who remained depressed several months after the initial assessment had lower salivary DHEA-to-cortisol ratios at baseline [109; 110]. Elevated DHEA(S) concentrations, relative to cortisol, may blunt the negative effects of high cortisol concentrations on depression [109; 142]. This explanation is consistent with the anti-glucocorticoid effects of DHEA reviewed above.
The relationship between DHEA(S) concentrations and depression is complex. There is no parsimonious way of reconciling the diverse findings, but age of the subjects studied, demographic variables, comorbid psychiatric and medical diagnoses, acute vs. chronic stress, medication status and timing of the sample collection, are likely relevant. Gender may also have a significant impact. In a prospective study of a nationally representative sample, men with initially lower serum concentrations of DHEAS had greater increases in depression ratings over a three-year period [106]; this relationship in men was not observed in women. Among African-American women (but not men), lower serum DHEAS concentrations were associated with higher depression ratings [118]. In another study, men with recurrent unipolar depression had low 24-hour urinary DHEA concentrations but normal cortisol concentrations, while women had normal DHEA concentrations but elevated cortisol concentrations [243]. However, in both cases, the DHEA-to-cortisol ratios appeared to be low, suggesting that the anabolic balance may be a helpful way of identifying common states of general hormonal imbalances in certain clinical conditions.
11.2. Anxiety Spectrum Disorders and PTSD
As reviewed above, DHEA(S) has prominent effects on GABAA receptor activity; these effects could be involved in the relationship between DHEA(S) and anxiety disorders [87; 188; 196]. Increased plasma concentrations of DHEA were observed in male patients with panic disorder [43] but not in females [42], and increased serum DHEAS-to-cortisol ratios were reported in a sample of patients of both genders who had panic disorder [89]. Social anxiety disorder (also called social phobia), was not found to be associated with alterations in plasma DHEA(S) concentrations [167].
The anxiety disorder that has received the greatest attention with regard to plasma, serum, and salivary DHEA(S) concentrations is post-traumatic stress disorder (PTSD) [248; 347]. Studies have uniformly identified elevated DHEA and/or DHEAS concentrations in PTSD, as well as increases in the DHEA(S)-to-cortisol ratio [51; 241; 249; 250; 292; 347]. Untreated men with combat-related PTSD were found to have increased plasma DHEA and DHEAS concentrations [292]. Another study found that PTSD patients who attempted suicide had increased plasma DHEA concentrations [51]. Female victims of intimate partner violence with PTSD had increased evening salivary cortisol concentrations and increased morning and evening salivary DHEA concentrations compared to non-abused women [241]. However, salivary DHEA concentrations were not significantly correlated with PTSD ratings [241]. In a study of recently resettled refugees in Sweden, those with PTSD (but without depression) had elevated plasma DHEAS concentrations compared to refugees with neither PTSD nor depression [289]. Over nine months of follow-up in these refugees, increases in PTSD symptoms were associated with increases in plasma DHEAS concentrations [289].
Despite the uniformity of studies showing elevations in DHEA or DHEAS in PTSD, researchers have suggested that the increase in DHEA(S) is salutary rather than pathophysiologic. Pre-menopausal women with chronic PTSD had increased plasma DHEA responses to ACTH stimulation compared to healthy, non-traumatized participants [250]. In the women with PTSD, the peak change in plasma DHEA (in response to ACTH) was negatively correlated with PTSD symptoms, suggesting that increased capacity of adrenal DHEA release may mitigate the severity of PTSD symptoms [250]. Consistent with that interpretation, another group found that, although plasma DHEA and DHEAS concentrations were elevated in male veteran PTSD patients, concentrations of both hormones were directly correlated with symptom improvement and better coping [347]. In another study, PTSD patients who responded to psychotherapy with a decrease in PTSD symptoms had an increase in DHEA concentrations in plasma, while patients who did not respond to psychotherapy had decreases in DHEA concentrations (after controlling for depressive symptoms) [230].
Increased DHEAS concentrations under conditions of stress may indicate a salutary process. Although not in PTSD patients per se, a study of 19 men undergoing stressful military training including captivity exercises showed significant increases in both cortisol and DHEAS in saliva during the acute stress of training [314]. Performance during a low intensity captivity challenge (but not during a high intensity one) was positively correlated with salivary DHEAS concentrations. In another study of 25 elite special operations soldiers exposed to prolonged and extreme training stress, soldiers experiencing fewer symptoms of dissociation and showing superior military performance had significantly higher ratios of plasma DHEAS-to-salivary cortisol [208]. In light of such data, DHEA(S) has been proposed to play a role in resilience and in successful adaptation to stress [60; 208; 346; 347].
11.3. Schizophrenia
Schizophrenia is linked to alterations in DHEA(S) in many studies, but with findings in both directions – elevations and abnormally low concentrations. For example, there are reports of patients with schizophrenia having low serum concentrations of DHEA [85; 229; 316], or elevated serum DHEAS concentrations [227]. In a small study comparing 13 acutely exacerbated paranoid schizophrenics with matched controls, the schizophrenic group had lower serum DHEA and higher serum DHEAS concentrations, although these differences were not statistically significant [48].
Several recent studies have found elevated plasma and serum DHEA concentrations in medicated schizophrenic patients [100; 258; 259]. In addition to elevated DHEA concentrations, one study also found decreased serum concentrations of DHEAS in medicated schizophrenic patients [259]. In a study of chronic, medicated schizophrenic patients who were institutionalized, higher morning serum DHEA concentrations and/or higher serum DHEA-to-cortisol ratios were correlated with better performance on aspects of memory performance and lower ratings of psychosis and parkinsonian movements (after controlling for age) [119].
In a study of first-episode un-medicated patients with schizophrenia, schizophrenic patients had higher serum concentrations of both DHEA and DHEAS compared to matched controls [302]. It was postulated that in the first episode of schizophrenic psychosis, increased DHEA(S) concentrations serve as a protective or compensatory factor, and that DHEA(S) concentrations diminish later in the course of chronic illness [302]. Indeed, in a follow-up study, more chronic patients were observed to have decreased serum DHEA-to-cortisol and DHEAS-to-cortisol ratios, and these ratios were negatively correlated with the duration of illness [260]. However, duration of illness may be confounded with age at the time of participation in the study, and these ratios would be expected (irrespective of the schizophrenia diagnosis) to decrease with age. In opposition to the hypothesis of decreasing DHEA(S), medicated chronically ill (average duration of illness = 12.8 years) patients with schizophrenia had elevated plasma concentrations of DHEA compared to controls [74]. In one of the only studies to examine tissue concentrations of DHEA in post-mortem brain specimens, patients with schizophrenia had higher concentrations of DHEA in the posterior cingulate and parietal cortex compared to matched controls [191].
Schizophrenia is a heterogeneous disease with multiple symptom profiles and comorbidities. Within schizophrenic samples, correlations were found between lower serum DHEA(S) concentrations and the presence of negative symptoms [112], movement disorders [119], greater duration of illness and age of onset of illness [260], length of hospitalization and severity of illness [302], cognitive impairment [119; 287], depression, anger and hostility [260], and anxiety [258]. These correlations suggest that the variability in clinical features may help explain the discrepant results reported between studies of schizophrenic patients.
11.4. Dementia
The effects of DHEA(S) on σ1 [318] and cholinergic [101; 256] neurotransmission, and amyloid β (Aβ) protein [311] may underlie their possible relationship with dementia. DHEA(S) concentrations are not clearly associated with cognition in dementia; however, it may be important to compare relative concentrations of DHEA-to-DHEAS and other metabolites of DHEA. Patients with Alzheimer’s disease and/or multi-infarct dementia have been reported to exhibit decreased [17; 34; 62; 91; 216; 223; 288; 304; 306; 344], as well as increased or unchanged [38; 40; 92; 172; 276; 291] plasma, serum and CSF DHEA(S) concentrations. In several instances, plasma and serum DHEA(S)-to-cortisol ratios distinguish demented participants from controls better than DHEA(S) or cortisol concentrations alone [12; 73; 170; 171; 192]. Although low plasma DHEAS concentrations and DHEAS-to-cortisol ratios were reported in a study of Alzheimer’s disease, these measures were not significantly correlated with dementia severity as rated by Mini-Mental Status Examination (MMSE) performance [12]. In a study of demented patients, DHEAS-to-cortisol molar ratios in blood were decreased compared to controls [186]. Decreases in the DHEAS-to-cortisol ratio were linked with cognitive impairment and correlated with smaller hippocampal volume, as measured by magnetic resonance imaging (MRI) [186]. Another study found that serum DHEA-to-cortisol ratios were positively correlated with cognitive performance in Alzheimer’s disease [192].
Further insights into the relationship between DHEA(S) and dementia have been gained by studies directly examining the central nervous system. One study found decreased DHEAS concentrations but increased DHEA concentrations in the CSF of patients with Alzheimer’s disease (as well as in patients with vascular dementia) compared to controls [148]. This yielded a significantly lower DHEAS-to-DHEA ratio in demented patients compared to controls. In CSF, there were no differences between controls and patients with Alzheimer’s disease (or vascular dementia) in the DHEA metabolites examined (including 7α-hydroxy-DHEA and 7β-hydroxy-DHEA) [148]. It was speculated that the elevated CSF DHEA concentrations arose from either an overproduction via an alternate synthetic pathway [49] or decreased production of metabolites due to deficient sulfation and hydroxylation [148].
The possible importance of diminished CNS DHEAS concentrations in the pathophysiology of Alzheimer’s disease is highlighted by findings that hippocampal volume [187] and perfusion [215] in Alzheimer’s disease were correlated with serum DHEAS concentrations. Bilateral hippocampal perfusion on single photon emission computed tomography (SPECT) scan was positively correlated with plasma DHEAS concentrations and was positively correlated with plasma DHEAS-to-cortisol ratios [215]. This relationship was statistically accounted for by a direct relationship of perfusion with DHEAS concentrations, rather than by a relationship with cortisol concentrations [215]. In an investigation of DHEA and DHEAS concentrations in postmortem human brains, tissue concentrations of DHEAS in striatum, cerebellum, and hypothalamus were lower in brains of people with Alzheimer’s disease compared to non-demented controls [327]. In the hypothalamus, DHEAS concentrations were negatively correlated with concentrations of pathologic phosphorylated tau proteins, suggesting a possible neuroprotective role of DHEAS [327]. In contrast to the finding of lower brain DHEAS concentrations in Alzheimer’s disease, another study found increased DHEA concentrations in the CSF as well as in the hippocampus, hypothalamus and frontal cortex from Alzheimer’s disease patients compared to age-matched controls [49]. The increases in DHEA concentrations were especially prominent in the hippocampus [49]. The suggestion that cognitive decline is more strongly associated with decreases in DHEAS concentrations (or decreases in the DHEAS-to-DHEA ratio) rather than DHEA concentrations fits nicely with preclinical data showing that the steroid sulfatase inhibitor DU-14, which increases DHEAS concentrations and decreases DHEA concentrations by blocking the conversion of DHEAS to DHEA, increased hippocampal ACh concentrations and blocked scopolamine-induced amnesia in rats [175; 257]. In an in vitro model, incubation with DHEA alone decreased cell viability of neurons, while incubation with DHEAS alone had no effect [103]. However, when neuroblastoma cells were incubated with both DHEA and DHEAS, DHEAS completely antagonized the neurotoxic effect of DHEA [103]. These data suggest that the DHEA-to-DHEAS ratio rather than the absolute concentrations of either steroid may be important.
Some investigators have hypothesized that the degree of metabolism of DHEA to 7α-hydroxy-DHEA is related to the pathology of Alzheimer’s disease [15; 37; 148; 345]. 7α-hydroxy-DHEA may have more potent bioactivity and stronger neuroprotective and anti-glucocorticoid effects than DHEA itself [207]. One study found that cytochrome P4507b (which converts DHEA into 7α-hydroxy-DHEA) gene expression was significantly decreased in dentate neurons from patients with Alzheimer’s disease compared to controls [345]. In line with this, another study found lower plasma 7α-hydroxy-DHEA concentrations in patients with Alzheimer’s disease compared to controls [37]. In that study, plasma DHEAS concentrations and, to a lesser extent, 7α-hydroxy-DHEA concentrations were positively related to dementia severity as rated by MMSE scores [37].
11.5. Normal Cognition, Functional Abilities and Quality of Life
Apart from studies examining circulating concentrations of DHEA(S) in patients with neurological or psychiatric illness, a number of studies have evaluated neuropsychiatric correlates with hormone concentrations in healthy individuals. Several studies have found positive relationships between serum DHEA(S) concentrations or DHEA(S)-to-cortisol ratios and cognitive functioning in normal aging individuals. In a study of 295 healthy women (aged 21–77 years) in the community, serum DHEAS concentrations (independent of age) were positively associated with executive function, working memory and concentration [72]. In the Massachusetts Male Aging Study, DHEA and DHEAS concentrations in blood were related to higher functioning in at least one cognitive domain [96]. In another study in older men, high morning salivary DHEA was associated with lower confusion, and higher morning salivary cortisol-to-DHEA ratios were associated with more confusion and poorer visuo-spatial memory performance [320].
In contrast to these positive findings, other studies have found no relationship or negative relationships between DHEA(S) concentrations and cognitive function. The Baltimore Longitudinal Study of Aging, which examined 883 community-dwelling men (aged 22–91 years), found that neither rates of decline in serum DHEAS concentrations nor mean DHEAS concentrations within individuals were related to cognitive status or cognitive decline [204]. A comparison between participants in the highest and lowest DHEAS quartiles also revealed no cognitive differences [204]. Similarly, in a study of 394 community-dwelling women (aged 65+ years), serum DHEAS declined with age but there was no relationship between DHEAS concentrations and cognitive function or change in cognitive performance over time [343]. In a prospective study of 270 men and 167 women, there was no significant relationship in age-adjusted plasma DHEAS concentrations between individuals with categorically defined cognitive impairment and those without [22]. Finally, some studies have found negative relationships between DHEA(S) concentrations and cognition. In a study of frail elderly patients in a nursing home, high DHEAS concentrations in blood were associated with cognitive impairment in women (but not men) [209]. Similarly, another study of elderly female nursing home residents found that blood concentrations of DHEA were inversely related to cognitive test scores [44].
Many, but not all, studies have reported lowered serum concentrations of DHEA(S) in patients with poor life satisfaction, psychosocial stress and functional limitations [1; 2; 23; 33; 35; 55; 99; 118; 141; 160; 173; 189; 209; 221; 251; 252; 255; 265; 278; 315; 343; 349]. Low plasma and serum concentrations of DHEAS have been associated with higher ratings of perceived stress [160], trait anxiety [75], as well as Type A behavior, cynicism, and hostility [88; 90; 177]. Higher plasma and serum DHEAS concentrations have been associated with higher levels of functioning [33], higher likelihood of living independently and a lower likelihood of organic brain syndrome in men [265]. Higher plasma and serum concentrations of DHEAS have also been associated with greater amount, frequency, and enjoyment of leisure activities [90], sexual gratification and frequency of masturbation (in women) [239; 319], healthier psychological profiles [90], more expansive personality ratings [319], and greater sensation-seeking and monotony-avoidance attributes [2]. Most of these studies examined concentrations of DHEAS rather than DHEA, and many assessed female rather than male populations, so the generalizability of these findings is uncertain. In some studies, the relationships were gender-specific (e.g., [209]). Menopausal status may also matter. Blood DHEAS concentrations were unrelated to well-being in post-menopausal women but were positively related to ratings of vitality in pre-menopausal women [31].
12. DHEA(S) Treatment Effects
Whether or not endogenous concentrations of DHEA(S) are abnormal in various neuropsychiatric illnesses, it is possible that exogenous DHEA supplementation could have therapeutic benefits. Several studies have examined the possibility that pharmacotherapy with DHEA might have beneficial effects, although the majority were either small-scale or short-term, so definitive conclusions are lacking. These studies will be examined in the next section.
12.1. Depression
Although clinical trials of DHEA treatment for depression are few in number, they consistently suggest beneficial effects. In an initial small-scale, open-label pilot study, DHEA treatment resulted in significant antidepressant effects in un-medicated middle-aged to elderly patients with major depression [340]. The doses of DHEA were individually adjusted between 30 and 90 mg per day for four weeks to achieve circulating DHEA and DHEAS concentrations in the mid-to-high normal physiologic range for healthy young adults. Subjects demonstrated highly significant improvements in Hamilton Depression Ratings and Symptom Checklist-90 ratings. Mood improvements were significantly related to increases in the circulating concentrations of DHEA and DHEAS and to their ratios with cortisol; changes in cortisol concentrations alone were not correlated with behavioral changes [340]. This small open-label study was followed by a double-blind, placebo-controlled trial in which 22 depressed patients received either DHEA (60 to 90 mg per day) or a placebo for 6 weeks [339]. Some patients were medication-free at the time of entering the study; others remained depressed despite being on pre-stabilized (for a minimum of 6 weeks) antidepressant medication. In the former group, DHEA or the placebo was used alone; in the latter group, DHEA or the placebo was added to the stabilized antidepressant regimen. DHEA, compared to the placebo, was associated with significant antidepressant responses. Five of 11 DHEA-treated patients showed greater than 50% improvement in depression ratings and had endpoint Hamilton Depression Rating Scale ratings of less than 10, suggesting that they had responded to treatment. None of the 11 placebo-treated patients achieved these milestones [339]. These results raised the possibility that DHEA, used alone or as an antidepressant adjunct in refractory patients, has significant antidepressant effects in some patients. Subsequently, another research group conducted a 12-week, double-blind, placebo-controlled study in un-medicated patients with mid-life dysthymia (one subject also had concurrent major depression) [39]. Subjects received, in randomized order, DHEA (90 mg per day for 3 weeks, followed by 450 mg per day for 3 weeks) or the placebo for 6 weeks. DHEA (compared to the placebo) produced a robust antidepressant response at both doses [39]. No changes in cognitive function were noted.
There were four important parallel findings in these two controlled studies, even though different types and severities of depressive disorders were studied [39; 339]. First, the response rate (defined as a greater than or equal to 50% improvement in symptoms; adjusted for placebo response) was approximately 40- 45% in both studies. Second, the psychological symptoms of depression improved in both studies to a greater extent than the neurovegetative symptoms (e.g., sleep and appetite disturbances). Third, baseline serum DHEA concentrations did not predict antidepressant response, suggesting that DHEA supplementation was not simply correcting a DHEA deficiency in these patients (in which case, it would be expected to work only in those with low DHEA at baseline). Finally, responders to DHEA in both studies achieved higher serum DHEA concentrations following treatment than did non-responders, and antidepressant effects were directly correlated with changes in serum DHEA concentrations. This concordance across two separate studies in different populations strengthens the argument that the DHEA treatment itself is related to the antidepressant responses.
In another double-blind placebo-controlled trial, DHEA monotherapy (90 mg per day for 3 weeks followed by 450 mg per day for 3 additional weeks) was associated with significant antidepressant effects in patients with both major and minor depression [275]. DHEA treatment was associated with a 45% response rate (defined as greater than 50% improvement in depression ratings), compared to only 12.5% with placebo. In this study, in contrast to those reviewed above, favorable responses were not related to end-point serum DHEA concentrations or to the treatment-associated changes in DHEA concentrations [275]. Finally, in one additional study, patients with human immunodeficiency virus (HIV) who had non-major but persistent depressive symptoms showed significant antidepressant responses to DHEA compared to placebo [245].
Thus, to date, every controlled trial of DHEA in depression has reported significant antidepressant effects. Although these data are encouraging, more large-scale studies will be required to establish the place, if any, of DHEA in the management of patients with depression. For example, there have been no head-to-head trials comparing DHEA to standard antidepressants, although in at least one trial, antidepressant non-responders did respond to DHEA augmentation [339]. The risks and benefits of long-term DHEA administration also remain to be further clarified.
12.2. Post-Traumatic Stress Disorder (PTSD)
Consistent with the notion that higher endogenous DHEA concentrations in PTSD patients and in severely stressed individuals are related to enhanced resiliency (see above), DHEA treatment may be helpful. Recently, five cases of “remarkable benefits” have been reported after extremely treatment-resistant patients with PTSD were administered 7-keto DHEA (25–150 mg per day) in an open-label manner [270]. All five of these patients had baseline serum DHEAS concentrations in the lowest quartile of the normal range. The responses in the patients were rapid, occurring within days, and were both subjectively and objectively discernible. 7-keto DHEA was chosen instead of the parent compound DHEA because the 7-keto moiety is not aromatized to testosterone or estrogen and because it may have superior anti-glucocorticoid properties [270]. Given the extreme refractoriness of these PTSD cases to multiple prior treatment attempts, DHEA (or 7-keto DHEA) treatment in PTSD seems deserving of larger-scale, blinded, placebo-controlled trials.
12.3. Schizophrenia
In a placebo-controlled study, DHEA (up to 100 mg per day for 6 weeks) significantly decreased negative symptoms, anxiety and depression in patients with schizophrenia [301]. The improvements in negative symptoms (e.g., loss of interest, loss of energy, loss of warmth, loss of humor, decreased sociality and volition) were independent of improvements in depression. Positive symptoms of schizophrenia (e.g., hallucinations and delusions) were not affected. Treatment-associated increases in serum DHEA and DHEAS concentrations were significantly correlated with the improvements in negative symptoms, but not anxiety or depression. In another study by the same group, patients with schizophrenia who were pre-stabilized on the atypical antipsychotic olanzapine, received concurrent DHEA (up to 150 mg per day) or placebo for 12 weeks [303]. Again, DHEA treatment significantly improved negative symptoms, but not positive symptoms. Some improvement was also seen in extrapyramidal motor symptoms (EPS) [303]. A potential role for DHEA in the treatment of EPS has also been suggested in another study. In antipsychotic-treated patients, double-blind DHEA (100 mg per day) significantly decreased parkinsonian symptoms but not akathisia [219]. Changes in blood concentrations of DHEA were negatively associated with changes in parkinsonian symptoms, such that higher increases in DHEA concentrations were associated with greater decreases in parkinsonian symptoms and EPS ratings [219]. The utility of DHEA in treating EPS is consistent with the findings (reviewed above) that endogenous serum concentrations of DHEA are inversely correlated with parkinsonian symptoms in patients with schizophrenia [119]. However, another study found that DHEA (200 mg per day for 6 weeks) was not superior to placebo in treating negative or positive symptoms of schizophrenia or EPS [261]. One possible explanation offered by these authors for the failure to replicate the findings of others [219; 301; 303] is that baseline DHEA concentrations in their patients were higher than those in the other studies [261].
12.4. Dementia
A small number of studies assessed whether DHEA treatment might improve memory in conditions that cause dementia. In preclinical studies, DHEAS has been found to enhance brain cholinergic function and to block scopolamine-induced amnesia in mice [257; 318]. DHEA could play a beneficial role in Alzheimer’s disease since DHEA has been found to be neuroprotective against amyloid β protein (Aβ) toxicity [53], to decrease lipid peroxidation in human brain tissue from patients with Alzheimer’s disease [24], and to decrease the BACE enzyme that initiates Aβ production [311]. In one study, open-label DHEAS administration (200 mg/day, intravenously, for 4 weeks) improved psychometric test performance in 4 of 7 patients with multi-infarct dementia [17]. In three of these cases, the improvements were judged clinically significant, and in two cases, electroencephalogram (EEG) patterns showed improvement with DHEAS treatment [17]. In a study investigating the efficacy and tolerability of DHEA vs. placebo in the treatment of Alzheimer’s disease, 58 un-medicated Alzheimer’s disease patients were randomized to treatment with DHEA alone (50 mg twice a day) or placebo alone for six months [341]. Thirty-three subjects finished the trial. DHEA treatment was well tolerated by the subjects, and interestingly, there were fewer dropouts in the DHEA group than the placebo group. After three months of treatment, DHEA tended to be superior to placebo on structured cognitive ratings (viz., specifically the Alzheimer’s Disease Assessment Scale-cognitive subscale [ADAS-Cog]) (p= 0.014), although this narrowly missed the Bonferroni-corrected level of statistical significance of 0.0125. There was no significant effect of DHEA treatment at the 6 month point, although there was, again, a trend for DHEA superiority (p= 0.10) [341]. The negative results of this pilot study must be interpreted cautiously, due to the small sample size and the attendant low power to detect statistically significant effects.
12.5. Addison’s Disease, Hypopituitarism and Exogenous Glucocorticoid Therapy
Although not strictly “neuropsychiatric disorders,” certain primary or iatrogenic endocrinopathies may show relief of some neuropsychiatric symptoms and improved overall well-being with DHEA treatment. Evidence of such an improvement comes from a study that utilized well-validated psychological measures in women with adrenal insufficiency secondary to Addison’s disease [11]. Patients were treated daily with DHEA (50 mg orally) or placebo for four months in a double-blind, cross-over study. Treatment with DHEA, but not placebo, resulted in significant improvements in well-being, mood, anxiety, depression, obsessive-compulsive traits, hostility and exhaustion [10]. These improvements were seen after four months of treatment, but not after one month, which supports the assertion that the psychological benefits of DHEA may take several months to develop [25; 242]. In a similar study, men and women with Addison’s disease showed significant improvements in self-esteem, mood and fatigue, but not in cognitive function, with three months of DHEA treatment [131]. However, another study of DHEA treatment (25 mg per day for 9 months) of women with adrenal failure found no significant beneficial effects of DHEA on any subjective measure of well-being, fatigue or sexuality compared to placebo [182]. There were no apparent differences to explain the conflictual findings in these studies, and as yet, there is no clear cut indication for DHEA administration in cases of adrenal insufficiency [36], except as an empirical, “compassionate use” trial in individual patients [5].
In one related situation, patients with hypopituitarism who were already receiving growth hormone maintenance treatment showed improvements in quality of life, social functioning, self-esteem and depression (some were seen in men, others in women) with DHEA supplementation compared to placebo [47]. In another related situation, medically ill patients treated with glucocorticoid medications (e.g., prednisone, dexamethasone) develop hypothalamic-pituitary-adrenal (HPA) axis inhibition [144]. While this HPA inhibition is salutary in the case of cortisol, since the exogenous glucocorticoid medication occupies the glucocorticoid receptors, it may be disadvantageous in the case of DHEA(S) whose actions are not mimicked by the glucocorticoid medication [98; 185]. Several investigators have suggested that DHEA(S) concentrations be monitored during chronic glucocorticoid therapy, and that DHEA supplementation be considered when endogenous concentrations are too low and the patient is symptomatic [102; 263]. In such situations, DHEA augmentation may reduce some of the morbidity associated with glucocorticoid administration [263]. In particular, one condition has received considerable attention. Several studies in glucocorticoid-treated patients with systemic lupus erythematosus have noted psychological benefits, as well as a “glucocorticoid sparing” effect, in DHEA-supplemented patients [226].
12.6. Effects on General Well-Being and Effects in Healthy Individuals
In the first published clinical trials of DHEA in the 1950’s, Sands and Strauss and colleagues [271; 272; 297; 298] reported that patients with “schizophrenia, inadequate personality, or emotional immaturity” showed rapid and impressive improvements in energy, insight, self-confidence, emotionality, vitality, adjustment to the environment, and school and occupational performance, anxiety, depression, apathy, and withdrawal. Although these studies were largely uncontrolled, in several cases the improvements dissipated following single-blind crossover to the placebo and returned with single-blind crossover back to DHEA. Similar beneficial results, seen in open-label trials, were reported in patients with “phobic-obsessive psychoneuroses, neuropsychasthenia, psychopathic personality, involutive syndromes, and depressive psychoses” by early Italian investigators [280;354].
In the first double-blind, placebo-controlled clinical trial of DHEA published in 1960, eight patients who had depression, anxiety, social phobia, shyness, lack of confidence and hyposexuality (classified by the investigators as having “vulnerable personalities”) received either DHEA (5 to 20 mg per day) or a placebo for 3 weeks each in a within-subject crossover design that had a 1-week washout between treatment arms [97]. DHEA treatment was associated with slightly more global positive assessments and with fewer negative global assessments than the placebo, but this was not interpreted as clinically significant by the authors. Unfortunately, the sample size was small, the doses were low, the trial was short, and it used non-standardized ratings and did not present statistical analyses.
After a 30 to 40 year hiatus, clinical trials with DHEA resumed. Patients with multiple sclerosis and systemic lupus erythematosus showed increased energy, libido, and sense of well-being in open-label trials of DHEA administration [52; 262; 322]. Subsequently, DHEA was administered to healthy, normal middle-aged and elderly subjects in a randomized, placebo-controlled, double-blind, crossover study [206]. Participants (aged 40–70 years) received DHEA (50 mg) or placebo every evening for 3 months. This dosing schedule restored serum DHEA(S) concentrations to youthful concentrations within 2 weeks, and concentrations were sustained for the entire 3-month period. DHEA-treated subjects showed significant increases in perceived physical and psychological well-being with no change in libido. Reported improvements included increased energy, deeper sleep, improved mood, a more relaxed feeling, and having an enhanced ability to handle stressful events [206]. These results generated considerable interest in the possibility of significant behavioral effects of DHEA, but the global subjective measure used to assess behavioral change (a single visual analog scale measuring sense of well-being) was relatively crude, and these results have not always been replicated.
Post-menopausal women (aged 60–70 years) were treated with single daily percutaneous applications of a 10% DHEA cream for 12 months [76; 163]. This was preceded or was followed by 6 months of placebo cream, although the studies do not state if this was open-label, single-blind, or double-blind. Similar to previous studies [206], 80% of the women reported enhanced well-being and an increase in energy during DHEA treatment [76; 163]. However, these behavioral changes were assessed with non-standardized daily diaries. An additional double-blind study examined the effects of two weeks of treatment with DHEA (50 mg per day) compared to placebo in healthy elderly men and women [158; 331]. Only women showed a statistical trend in reporting increases in well-being (P=0.11) and mood (P=0.10). Women showed better performance in one of six cognitive tests (picture memory) after DHEA treatment. However, after post hoc correction for multiple comparisons, this difference was no longer significant. No effects were observed in the male subjects. This study used reliable neuropsychological test instruments and had an adequate sample size, but the duration of treatment may have been too short for some behavioral changes to become manifest [242]. In a double-blind placebo-controlled study, seven days of DHEA administration enhanced mood and recollection accuracy in episodic memory [4]. Curiously, this latter study with positive findings tested healthy young men, whereas the former study with negative findings tested elderly subjects, who might have been expected to show a greater benefit, owing to their lower baseline endogenous DHEA concentrations. Another well-controlled trial examined the effects of DHEA administration (75 mg per day in men and 50 mg per day in women) for two years on participants over 60 years old and below the 15th percentile in baseline serum DHEAS concentrations for young individuals [222]. There was no change in either gender on quality of life or other non-psychiatric outcomes. In general, other controlled trials in healthy populations (even aging ones) have not been positive. For example, peri-menopausal women with complaints of “altered mood and well-being” were treated with DHEA (50 mg per day) or placebo, in a blind manner for three months [21]. DHEA had no significant effects on peri-menopausal symptoms, mood, dysphoria, libido, cognition, memory or well-being.
12.7. Effects on Cognition in Healthy Individuals
In otherwise healthy individuals, the data suggest a lack of significant, consistent effects of DHEA administration on cognitive performance. A recent review of this topic by the Cochrane Database [114] identified only three adequately controlled trials addressing this issue [21; 320; 330], and concluded that the data do not support a beneficial effect of DHEA supplementation on cognitive function of healthy, non-demented, middle-aged or elderly people. Even if DHEA fails to affect normal memory under basal conditions, it remains possible that DHEA might curtail stress-induced decrements in memory, due to its anti-glucocorticoid effects. To test this hypothesis, cognitive performance was tested before and after a laboratory psychological stressor in DHEA-treated versus placebo-treated subjects [330]. DHEA treatment yielded opposing effects on memory performance as follows; DHEA decreased the post-stress recall of visual material learned prior to the stressor, but it enhanced post-stress attentional performance [330], making the results difficult to interpret.
13. Summary
Considerable ambiguity remains regarding the role of DHEA(S) in human neuropsychiatric illness and the potential therapeutic applications of DHEA. There is intriguing but conflictual support for the use of DHEA (in addition to glucocorticoids and mineralocorticoids) in treating patients with Addison’s disease or hypopituitarism. Beneficial effects of DHEA have been consistently reported in individuals with major depression, dysthymia and schizophrenia, but these findings are based on a relatively small number of subjects, and more (and larger) studies need to be conducted. Beneficial effects on negative and extrapyramidal symptoms in individuals with schizophrenia would be an exciting and welcome addition to our treatment armamentarium, if the preliminary data are confirmed. Beneficial cognitive effects in demented patients seem mild, if present at all. More research is needed in the treatment of demented populations, since existing studies had small samples sizes with low power to detect significant effects. The studies of DHEA treatment of demented patients generally employed DHEA as a solitary treatment. It would be important to see if DHEA has a more substantial effect if used as an adjunct to standard dementia medications such as cholinesterase inhibitors. Future studies in dementia should also screen for subtypes of patients, which might respond more favorably than others. For example, DHEA blocked lipid peroxidation in vitro in brain tissue samples from Alzheimer’s disease patients with at least one Apo E3 allele, but was without effect in patients with the Apo E4/4 genotype [247].
Despite early hopes, beneficial neuropsychiatric effects of DHEA have not been seen in healthy individuals, even in those of advanced age with low circulating DHEA(S) concentrations [8; 9; 238]. There remains no evidence that DHEA is the long-sought “fountain of youth” [295]. The possible benefits of DHEA treatment in certain medical or neuropsychiatric conditions, in the face of little if any benefit in healthy individuals, even those with low circulating DHEA(S) concentrations, suggests that: (1) treatment response is not solely dependent on low baseline circulating DHEA(S) concentrations; therefore (except in Addison’s disease, hypopituitarism and glucocorticoid-induced HPA axis suppression), response to DHEA is not merely the result of replenishing a deficiency syndrome [339]. Thus, it may be not be realistic to set a goal of “normalizing” DHEA(S) concentrations in otherwise healthy individuals as a means of slowing or preventing the progression of diseases of normal aging. (2) Beneficial treatment effects are more likely to be seen in medically or neuropsychiatrically ill patients than in healthy individuals. This might be consistent with preclinical data showing that DHEAS treatment has beneficial neurobehavioral effects in mice with experimental mild traumatic brain injury but is without benefit in control mice [202], and with in vitro data showing neuroprotective effects of DHEA or DHEAS under conditions of anoxia or high levels of corticosterone, but the lack of beneficial effects in cells cultured under normal, non-toxic conditions [150; 190].
In summary, despite the considerable increase in DHEA(S) research in recent years and the ongoing discovery of its biochemical mechanisms of action, its role in neuropsychiatric diseases and its place in clinical therapeutics remain uncertain. Although the clinical data are far from conclusive in establishing a therapeutic role of DHEA treatment, patients and physicians who decide to undertake a trial of DHEA should be cognizant of several precautions and caveats, as outlined in [8; 9; 321; 338].
14. Future Research Directions
In reviewing the preclinical and clinical data regarding DHEA, one is struck by the inconsistency in the clinical findings, despite preclinical findings that DHEA and DHEAS have many biological actions. Much of this incongruity undoubtedly lies in the methodological differences on which we have commented. Alternatively, the failure to replicate uniformly the benefits seen in preclinical studies in clinical studies may lie in the nature of neuropsychiatric diagnoses. Many clinical ante mortem neuropsychiatric diagnoses (e.g. depression, anxiety, schizophrenia, dementia) rely on global phenomenologic criteria rather than on specific biochemical pathologies. Further, the clinical studies reviewed here typically assessed global outcome measures (e.g., global severity of depression, anxiety and psychosis, quality of life, subjective well-being, frailty, cognitive function, etc.) rather than parsing out the DHEA(S) effects on the core biochemical abnormalities embedded within those conditions. This global phenomenological approach to assessing outcomes fails to capitalize on the basic science advances that have been made in understanding DHEA(S)’ mechanisms of action. For example, instead of assessing correlations between endogenous DHEA(S) concentrations and overall severity of these disease manifestations, it might be more productive to examine relationships with specific mechanisms and processes, e.g., measures of oxidative stress, inflammation, neuroprotection, neurogenesis, as well as neuroanatomical and neurophysiological measures (c.f. [46; 63; 77; 184; 187; 215; 324]).
The preclinical and clinical data we have reviewed here can, perhaps, be best summarized by the conclusion drawn by two of the original investigators of DHEA(S)’ neuropsychiatric effects in 1955:
“Whether diandrone [dehydroepiandrosterone] turns out to be of therapeutic value in psychiatric practice remains to be seen…. However, we appear to have at our disposal a chemical agent that can exert a direct and prolonged action on the mental state” [297].
Acknowledgements
This research was partially funded by grants from the O’Shaughnessy Foundation, the National Alliance for Research in Schizophrenia and Affective Disorders, the Stanley Foundation and the Alzheimer’s Association to OMW; by NIH Grant (NS049462) and March of Dimes Grant (07-554) to SHM; and NM was funded by an NIMH T32 training grant (MH09391).
Table of Abbreviations
- Aβ
amyloid βprotein
- ACh
acetylcholine
- ACTH
adrenocorticotropic hormone
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP1
activator protein-1
- BACE
β-site amyloid β precursor protein-cleaving enzyme
- BD1063
1-[2-(3,4-Dichlorophenyl)ethyl]-4-methylpiperazine
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- C/EBP
CCAAT/enhancer-binding protein
- CNS
central nervous system
- CREB
cAMP response element binding protein
- CSF
cerebrospinal fluid
- D-AP5
D-2-amino-5-phosphonopentanoic acid
- DA
dopamine
- DHEA
Dehydroepiandrosterone
- DHEAS
Dehydroepiandrosterone-Sulfate
- DHEA(S)
Dehydroepiandrosterone and Dehydroepiandrosterone-Sulfate
- DMSO
dimethyl sulfoxide
- DU-14
p-O-(sulfamoyl)-N-tetradecanoyl tyramine
- EEG
electroencephalogram
- EGF
epidermal growth factor
- eNOS
endothelial nitric oxide synthase
- EPI
epinephrine
- EPS
extrapyramidal symptoms
- FGF2
fibroblast growth factor
- GABAA
γ-aminobutyric acid type A receptor
- GR
glucocorticoid receptor
- GSH
glutathione
- HIV
human immunodeficiency virus
- HNE
4-hydroxynonenal
- H2O2
hydrogen peroxide
- HPA
hypothalamic-pituitary-adrenal axis
- HST
hydroxysteroid sulfotransferase
- IL-6
interleukin-6
- i.p.
intraperitoneal
- i.v.
intravenous
- LIF
leukemia inhibitory factor
- LPS
lipopolysaccharide
- MAP
microtubule-associated protein
- MK801
(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate
- MMSE
Mini-Mental Status Examination
- MPP+
1-methyl-4-phenylpyridinum
- MRI
magnetic resonance imaging
- NE
norepinephrine
- NE-100
N-dipropyl-2-(4-methoxy-3-(2-phenylethoxy)phenyl)-ethylamine monohydrochloride
- NGF
nerve growth factor
- NF-κB
nuclear factor kappa B
- NMDA
N-methyl-D-aspartate receptor
- NO
nitric oxide
- NOS
nitric oxide synthase
- OGD
oxygen-glucose deprivation
- PBMC
peripheral blood mononuclear cells
- PKC
protein kinase C
- PPARα
peroxisome proliferator-activated receptor α
- PTSD
post-traumatic stress disorder
- PVN
paraventricular nucleus
- ROS
reactive oxygen species
- SAPK3
stress-activated protein kinase 3
- s.c.
subcutaneous
- SCI
spinal cord injury
- SF-1
steroidogenic factor 1
- SNP
sodium nitroprusside
- SPECT
single photon emission computed tomography
- STS
steroid sulfatase
- STZ
streptozotocin
- SULT2A1
hydroxysteroid sulfotransferase or DHEA sulfotransferase
- TH
tyrosine hydroxylase
- TNFα
tumor necrosis factor α
- 11β-HSD1
11β-hydroxysteroid dehydrogenase type 1
- 3βHSD
3β-hydroxysteroid dehydrogenase
- 5-HIAA
5-hydroxyindole-3-acetic acid
- 5HT
serotonin
- σ1
sigma subtype 1 receptor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbasi A, Duthie EH, Jr, Sheldahl L, Wilson C, Sasse E, Rudman I, Mattson DE. Association of dehydroepiandrosterone sulfate, body composition, and physical fitness in independent community-dwelling older men and women. J Am Geriatr Soc. 1998;46:263–273. doi: 10.1111/j.1532-5415.1998.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 2.Klinteberg Baf, Hallman J, Oreland L, Wirsen A, Levander SE, Schalling D. Exploring the connections between platelet monoamine oxidase activity and behavior. II. Impulsive personality without neuropsychological signs of disinhibition in air force pilot recruits. Neuropsychobiology. 1992;26:136–145. doi: 10.1159/000118909. [DOI] [PubMed] [Google Scholar]
- 3.Aldred S, Waring RH. Localisation of dehydroepiandrosterone sulphotransferase in adult rat brain. Brain Res Bull. 1999;48:291–296. doi: 10.1016/s0361-9230(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 4.Alhaj HA, Massey AE, McAllister-Williams RH. Effects of DHEA administration on episodic memory, cortisol and mood in healthy young men: a double-blind, placebo-controlled study. Psychopharmacology (Berl) 2006;188:541–551. doi: 10.1007/s00213-005-0136-y. [DOI] [PubMed] [Google Scholar]
- 5.Allolio B, Arlt W, Hahner S. DHEA: why, when, and how much--DHEA replacement in adrenal insufficiency. Ann Endocrinol (Paris) 2007;68:268–273. doi: 10.1016/j.ando.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Apostolova G, Schweizer RA, Balazs Z, Kostadinova RM, Odermatt A. Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E957–E964. doi: 10.1152/ajpendo.00442.2004. [DOI] [PubMed] [Google Scholar]
- 7.Aragno M, Mastrocola R, Brignardello E, Catalano M, Robino G, Manti R, Parola M, Danni O, Boccuzzi G. Dehydroepiandrosterone modulates nuclear factor-kappaB activation in hippocampus of diabetic rats. Endocrinology. 2002;143:3250–3258. doi: 10.1210/en.2002-220182. [DOI] [PubMed] [Google Scholar]
- 8.Arlt W. Dehydroepiandrosterone replacement therapy. Semin Reprod Med. 2004;22:379–388. doi: 10.1055/s-2004-861554. [DOI] [PubMed] [Google Scholar]
- 9.Arlt W. Dehydroepiandrosterone and ageing. Best Pract Res Clin Endocrinol Metab. 2004;18:363–380. doi: 10.1016/j.beem.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Arlt W, Callies F, Allolio B. DHEA replacement in women with adrenal insufficiency--pharmacokinetics, bioconversion and clinical effects on well-being, sexuality and cognition. Endocr Res. 2000;26:505–511. doi: 10.3109/07435800009048561. [DOI] [PubMed] [Google Scholar]
- 11.Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency. NEJM. 1999;341:1013–1020. doi: 10.1056/NEJM199909303411401. [DOI] [PubMed] [Google Scholar]
- 12.Armanini D, Vecchio F, Basso A, Milone FF, Simoncini M, Fiore C, Mattarello MJ, Sartorato P, Karbowiak I. Alzheimer’s disease: pathophysiological implications of measurement of plasma cortisol, plasma dehydroepiandrosterone sulfate, and lymphocytic corticosteroid receptors. Endocrine. 2003;22:113–118. doi: 10.1385/ENDO:22:2:113. [DOI] [PubMed] [Google Scholar]
- 13.Asaba H, Hosoya K, Takanaga H, Ohtsuki S, Tamura E, Takizawa T, Terasaki T. Blood-brain barrier is involved in the efflux transport of a neuroactive steroid, dehydroepiandrosterone sulfate, via organic anion transporting polypeptide 2. J Neurochem. 2000;75:1907–1916. doi: 10.1046/j.1471-4159.2000.0751907.x. [DOI] [PubMed] [Google Scholar]
- 14.Assies J, Visser I, Nicolson NA, Eggelte TA, Wekking EM, Huyser J, Lieverse R, Schene AH. Elevated salivary dehydroepiandrosterone-sulfate but normal cortisol levels in medicated depressed patients: preliminary findings. Psychiatry Res. 2004;128:117–122. doi: 10.1016/j.psychres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Attal-Khemis S, Dalmeyda V, Michot JL, Roudier M, Morfin R. Increased total 7 alpha-hydroxy-dehydroepiandrosterone in serum of patients with Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 1998;53:B125–B132. doi: 10.1093/gerona/53a.2.b125. [DOI] [PubMed] [Google Scholar]
- 16.Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Semin Reprod Med. 2004;22:281–288. doi: 10.1055/s-2004-861545. [DOI] [PubMed] [Google Scholar]
- 17.Azuma T, Nagai Y, Saito T, Funauchi M, Matsubara T, Sakoda S. The effect of dehydroepiandrosterone sulfate administration to patients with multi-infarct dementia. J Neurol Sci. 1999;162:69–73. doi: 10.1016/s0022-510x(98)00295-0. [DOI] [PubMed] [Google Scholar]
- 18.Azuma T, Matsubara T, Shima Y, Haeno S, Fujimoto T, Tone K, Shibata N, Sakoda S. Neurosteroids in cerebrospinal fluid in neurologic disorders. J Neurol Sci. 1993;120:87–92. doi: 10.1016/0022-510x(93)90030-3. [DOI] [PubMed] [Google Scholar]
- 19.Bair SR, Mellon SH. Deletion of the mouse P450c17 gene causes early embryonic lethality. Mol Cell Biol. 2004;24:5383–5390. doi: 10.1128/MCB.24.12.5383-5390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balazs Z, Schweizer RA, Frey FJ, Rohner-Jeanrenaud F, Odermatt A. DHEA induces 11 -HSD2 by acting on CCAAT/enhancer-binding proteins. J Am Soc Nephrol. 2008;19:92–101. doi: 10.1681/ASN.2007030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnhart KT, Freeman E, Grisso JA, Rader DJ, Sammel M, Kapoor S, Nestler JE. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters and health-related quality of life. J Clin Endocrinol Metab. 1999;84:3896–3902. doi: 10.1210/jcem.84.11.6153. [DOI] [PubMed] [Google Scholar]
- 22.Barrett-Connor E, Edelstein SL. A prospective study of dehydroepiandrosterone sulfate and cognitive function in an older population: the Rancho Bernardo Study. J Am Geriatr Soc. 1994;42:420–423. doi: 10.1111/j.1532-5415.1994.tb07491.x. [DOI] [PubMed] [Google Scholar]
- 23.Barrett-Connor E, von Muhlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc. 1999;47:685–691. doi: 10.1111/j.1532-5415.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 24.Bastianetto S, Ramassamy C, Poirier J, Quirion R. Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Brain Res Mol Brain Res. 1999;66:35–41. doi: 10.1016/s0169-328x(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 25.Baulieu E, Thomas G, Legrain S, Lahlou N, Roger M, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: Contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000:4279–4284. doi: 10.1073/pnas.97.8.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baulieu EE. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog Horm Res. 1997;52:1–32. [PubMed] [Google Scholar]
- 27.Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- 28.Baulieu EE, Robel P. Dehydroepiandrosterone and dehydroepiandrosterone sulfate as neuroactive neurosteroids. J Endocrinol. 1996;150 Suppl:S221–S239. [PubMed] [Google Scholar]
- 29.Beaujean D, Mensah-Nyagan AG, Do-Rego JL, Luu-The V, Pelletier G, Vaudry H. Immunocytochemical localization and biological activity of hydroxysteroid sulfotransferase in the frog brain. J Neurochem. 1999;72:848–857. doi: 10.1046/j.1471-4159.1999.720848.x. [DOI] [PubMed] [Google Scholar]
- 30.Beaujean D, Do-Rego JL, Galas L, Mensah-Nyagan AG, Fredriksson R, Larhammar D, Fournier A, Luu-The V, Pelletier G, Vaudry H. Neuropeptide Y inhibits the biosynthesis of sulfated neurosteroids in the hypothalamus through activation of Y(1) receptors. Endocrinology. 2002;143:1950–1963. doi: 10.1210/endo.143.5.8765. [DOI] [PubMed] [Google Scholar]
- 31.Bell RJ, Donath S, Davison SL, Davis SR. Endogenous androgen levels and well-being: differences between premenopausal and postmenopausal women. Menopause. 2006;13:65–71. doi: 10.1097/01.gme.0000191212.58856.96. [DOI] [PubMed] [Google Scholar]
- 32.Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berkman LF, Seeman TE, Albert M, Blazer D, Kahn R, Mohs R, Finch C, Schneider E, Cotman C, McClearn G, et al. High usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol. 1993;46:1129–1140. doi: 10.1016/0895-4356(93)90112-e. [DOI] [PubMed] [Google Scholar]
- 34.Bernardi F, Lanzone A, Cento RM, Spada RS, Pezzani I, Genazzani AD, Luisi S, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone and dehydroepiandrosterone response to corticotropin-releasing factor in patients suffering from Alzheimer’s disease and vascular dementia. Eur J Endocrinol. 2000;142:466–471. doi: 10.1530/eje.0.1420466. [DOI] [PubMed] [Google Scholar]
- 35.Berr C, Lafont S, Debuire B, Dartigues JF, Baulieu EE. Baulieu, Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: a French community-based study. Proc Natl Acad Sci U S A. 1996;93:13410–13415. doi: 10.1073/pnas.93.23.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhagra S, Nippoldt TB, Nair KS. Dehydroepiandrosterone in adrenal insufficiency and ageing. Curr Opin Endocrinol Diabetes Obes. 2008;15:239–243. doi: 10.1097/MED.0b013e3282fc7049. [DOI] [PubMed] [Google Scholar]
- 37.Bicikova M, Ripova D, Hill M, Jirak R, Havlikova H, Tallova J, Hampl R. Plasma levels of 7-hydroxylated dehydroepiandrosterone (DHEA) metabolites and selected amino-thiols as discriminatory tools of Alzheimer’s disease and vascular dementia. Clin Chem Lab Med. 2004;42:518–524. doi: 10.1515/CCLM.2004.088. [DOI] [PubMed] [Google Scholar]
- 38.Birkenhager-Gillesse EG, Derksen J, Lagaay AM. Dehydroepiandrosterone sulphate (DHEAS) in the oldest old, aged 85 and over. Ann N Y Acad Sci. 1994;719:543–552. doi: 10.1111/j.1749-6632.1994.tb56858.x. [DOI] [PubMed] [Google Scholar]
- 39.Bloch M, Schmidt PJ, Danaceau MA, Adams LF, Rubinow DR. Dehydroepiandrosterone treatment of mid-life dysthymia. Biol Psychiat. 1999;45:1533–1541. doi: 10.1016/s0006-3223(99)00066-9. [DOI] [PubMed] [Google Scholar]
- 40.Bo M, Massaia M, Zannella P, Cappa G, Ferrario E, Rainero I, Arvat E, Giordano R, Molaschi M. Dehydroepiandrosterone sulfate (DHEA-S) and Alzheimer’s dementia in older subjects. Int J Geriatr Psychiatry. 2006;21:1065–1070. doi: 10.1002/gps.1608. [DOI] [PubMed] [Google Scholar]
- 41.Bologa L, Sharma J, Roberts E. Dehydroepiandrosterone and its sulfated derivative reduce neuronal death and enhance astrocytic differentiation in brain cell cultures. J Neurosci Res. 1987;17:225–234. doi: 10.1002/jnr.490170305. [DOI] [PubMed] [Google Scholar]
- 42.Brambilla F, Biggio G, Pisu MG, Bellodi L, Perna G, Bogdanovich-Djukic V, Purdy RH, Serra M. Neurosteroid secretion in panic disorder. Psychiatry Res. 2003;118:107–116. doi: 10.1016/s0165-1781(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 43.Brambilla F, Mellado C, Alciati A, Pisu MG, Purdy RH, Zanone S, Perini G, Serra M, Biggio G. Plasma concentrations of anxiolytic neuroactive steroids in men with panic disorder. Psychiatry Res. 2005;135:185–190. doi: 10.1016/j.psychres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Breuer B, Martucci C, Wallenstein S, Likourezos A, Libow LS, Peterson A, Zumoff B. Relationship of endogenous levels of sex hormones to cognition and depression in frail, elderly women. Am J Geriatr Psychiatry. 2002;10:311–320. [PubMed] [Google Scholar]
- 45.Brewer GJ, Espinosa J, McIlhaney MP, Pencek TP, Kesslak JP, Cotman C, Viel J, McManus DC. Culture and regeneration of human neurons after brain surgery. J Neurosci Methods. 2001;107:15–23. doi: 10.1016/s0165-0270(01)00342-9. [DOI] [PubMed] [Google Scholar]
- 46.Brignardello E, Runzo C, Aragno M, Catalano MG, Cassader M, Perin PC, Boccuzzi G. Dehydroepiandrosterone administration counteracts oxidative imbalance and advanced glycation end product formation in type 2 diabetic patients. Diabetes Care. 2007;30:2922–2927. doi: 10.2337/dc07-1110. [DOI] [PubMed] [Google Scholar]
- 47.Brooke AM, Kalingag LA, Miraki-Moud F, Camacho-Hubner C, Maher KT, Walker DM, Hinson JP, Monson JP. Dehydroepiandrosterone improves psychological well-being in male and female hypopituitary patients on maintenance growth hormone replacement. J Clin Endocrinol Metab. 2006;91:3773–3779. doi: 10.1210/jc.2006-0316. [DOI] [PubMed] [Google Scholar]
- 48.Brophy MH, Rush AJ, Crowley G. Cortisol, estradiol, and androgens in acutely ill paranoid schizophrenics. Biol Psychiatry. 1983;18:583–590. [PubMed] [Google Scholar]
- 49.Brown RC, Han Z, Cascio C, Papadopoulos V. Oxidative stress-mediated DHEA formation in Alzheimer’s disease pathology. Neurobiol Aging. 2003;24:57–65. doi: 10.1016/s0197-4580(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 50.Buckwalter JG, Stanczyc FZ, McCleary CA, Bluestein BW, Buckwalter DK, Rankin KP, Chang L, Goodwin TM. Pregnancy, the postpartum, and steroid hormones: effects on cognition and mood. Psychoneuroendocrinology. 1999;24:69–84. doi: 10.1016/s0306-4530(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 51.Butterfield MI, Stechuchak KM, Connor KM, Davidson JR, Wang C, MacKuen CL, Pearlstein AM, Marx CE. Neuroactive steroids and suicidality in posttraumatic stress disorder. Am J Psychiatry. 2005;162:380–382. doi: 10.1176/appi.ajp.162.2.380. [DOI] [PubMed] [Google Scholar]
- 52.Calabrese VP, Isaacs ER, Regelson W. Dehydroepiandrosterone in multiple sclerosis: positive effects on the fatigue syndrome in a non-randomized study. In: Kalimi M, Regelson W, editors. The Biologic Role of Dehydroepiandrosterone (DHEA) Berlin: W. de Gruyter; 1990. pp. 95–100. [Google Scholar]
- 53.Cardounel A, Regelson W, Kalimi M. Dehydroepiandrosterone protects hippocampal neurons against neurotoxin-induced cell death: mechanism of action. Proc Soc Exp Biol Med. 1999;222:145–149. doi: 10.1046/j.1525-1373.1999.d01-124.x. [DOI] [PubMed] [Google Scholar]
- 54.Carvalhaes-Neto N, Huayllas MK, Ramos LR, Cendoroglo MS, Kater CE. Cortisol,DHEAS and aging: resistance to cortisol suppression in frail institutionalized elderly. J Endocrinol Invest. 2003;26:17–22. doi: 10.1007/BF03345117. [DOI] [PubMed] [Google Scholar]
- 55.Cawood EH, Bancroft J. Steroid hormones, the menopause, sexuality and well-being of women. Psychol Med. 1996;26:925–936. doi: 10.1017/s0033291700035261. [DOI] [PubMed] [Google Scholar]
- 56.Chalbot S, Morfin R. Dehydroepiandrosterone metabolites and their interactions in humans. Drug Metabol Drug Interact. 2006;22:1–23. doi: 10.1515/dmdi.2006.22.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Charalampopoulos I, Tsatsanis C, Dermitzaki E, Alexaki VI, Castanas E, Margioris AN, Gravanis A. Dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic Bcl-2 proteins. Proc Natl Acad Sci U S A. 2004;101:8209–8214. doi: 10.1073/pnas.0306631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charalampopoulos I, Dermitzaki E, Vardouli L, Tsatsanis C, Stournaras C, Margioris AN, Gravanis A. Dehydroepiandrosterone sulfate and allopregnanolone directly stimulate catecholamine production via induction of tyrosine hydroxylase and secretion by affecting actin polymerization. Endocrinology. 2005;146:3309–3318. doi: 10.1210/en.2005-0263. [DOI] [PubMed] [Google Scholar]
- 59.Charalampopoulos I, Alexaki VI, Lazaridis I, Dermitzaki E, Avlonitis N, Tsatsanis C, Calogeropoulou T, Margioris AN, Castanas E, Gravanis A. G protein-associated, specific membrane binding sites mediate the neuroprotective effect of dehydroepiandrosterone. Faseb J. 2006;20:577–579. doi: 10.1096/fj.05-5078fje. [DOI] [PubMed] [Google Scholar]
- 60.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 61.Chen CC, Parker CR., Jr Adrenal androgens and the immune system. Semin Reprod Med. 2004;22:369–377. doi: 10.1055/s-2004-861553. [DOI] [PubMed] [Google Scholar]
- 62.Cho SH, Jung BH, Lee WY, Chung BC. Rapid column-switching liquid chromatography/mass spectrometric assay for DHEA-sulfate in the plasma of patients with Alzheimer’s disease. Biomed Chromatogr. 2006;20:1093–1097. doi: 10.1002/bmc.647. [DOI] [PubMed] [Google Scholar]
- 63.Coles AJ, Thompson S, Cox AL, Curran S, Gurnell EM, Chatterjee VK. Dehydroepiandrosterone replacement in patients with Addison’s disease has a bimodal effect on regulatory (CD4+CD25hi and CD4+FoxP3+) T cells. Eur J Immunol. 2005;35:3694–3703. doi: 10.1002/eji.200526128. [DOI] [PubMed] [Google Scholar]
- 64.Compagnone NA, Mellon SH. Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. Proc Natl Acad Sci U S A. 1998;95:4678–4683. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 66.Compagnone NA, Bulfone A, Rubenstein JL, Mellon SH. Steroidogenic enzyme P450c17 is expressed in the embryonic central nervous system. Endocrinology. 1995;136:5212–5223. doi: 10.1210/endo.136.11.7588260. [DOI] [PubMed] [Google Scholar]
- 67.Conley AJ, Bird IM. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–799. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- 68.Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Corpechot C, Synguelakis M, Talha S, Axelson M, Sjovall J, Vihko R, Baulieu EE, Robel P. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983;270:119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- 70.Costa-Santos M, Kater CE, Auchus RJ. Two prevalent CYP17 mutations and genotype-phenotype correlations in 24 Brazilian patients with 17-hydroxylase deficiency. J Clin Endocrinol Metab. 2004;89:49–60. doi: 10.1210/jc.2003-031021. [DOI] [PubMed] [Google Scholar]
- 71.Cutler GB, Jr, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 1978;103:2112–2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- 72.Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93:801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- 73.de Bruin VM, Vieira MC, Rocha MN, Viana GS. Cortisol and dehydroepiandosterone sulfate plasma levels and their relationship to aging, cognitive function, and dementia. Brain Cogn. 2002;50:316–323. doi: 10.1016/s0278-2626(02)00519-5. [DOI] [PubMed] [Google Scholar]
- 74.di Michele F, Caltagirone C, Bonaviri G, Romeo E, Spalletta G. Plasma dehydroepiandrosterone levels are strongly increased in schizophrenia. J Psychiatr Res. 2005;39:267–273. doi: 10.1016/j.jpsychires.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 75.Diamond P, Brisson GR, Candas B, Peronnet F. Trait anxiety, submaximal physical exercise and blood androgens. Eur. J. Appl. Physiol. 1989;58:699–704. doi: 10.1007/BF00637379. [DOI] [PubMed] [Google Scholar]
- 76.Diamond P, Cusan L, Gomez JL, Belanger A, Labrie F. Metabolic effects of 12- month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women. J Endocrinol. 1996;150 Suppl:S43–S50. [PubMed] [Google Scholar]
- 77.Dillon JS. Dehydroepiandrosterone, dehydroepiandrosterone sulfate and related steroids: their role in inflammatory, allergic and immunological disorders. Curr Drug Targets Inflamm Allergy. 2005;4:377–385. doi: 10.2174/1568010054022079. [DOI] [PubMed] [Google Scholar]
- 78.Do Rego JL, Tremblay Y, Luu-The V, Repetto E, Castel H, Vallarino M, Belanger A, Pelletier G, Vaudry H. Immunohistochemical localization and biological activity of the steroidogenic enzyme cytochrome P450 17alpha-hydroxylase/C17, 20-lyase (P450C17) in the frog brain and pituitary. J Neurochem. 2007;100:251–268. doi: 10.1111/j.1471-4159.2006.04209.x. [DOI] [PubMed] [Google Scholar]
- 79.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 80.Drucker D, McLaughlin J. Adrenocortical dysfunction in acute medical illness. Crit Care Med. 1986;14:789–791. doi: 10.1097/00003246-198609000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Dubrovsky B. Natural steroids counteracting some actions of putative depressogenic steroids on the central nervous system: potential therapeutic benefits. Med Hypotheses. 1997;49:51–55. doi: 10.1016/s0306-9877(97)90252-8. [DOI] [PubMed] [Google Scholar]
- 82.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 83.Enomoto M, Adachi H, Fukami A, Satoh M, Otsuka SI, Kumagae Y, Nanjo Y, Shigetoh Y, Imaizumi T. Serum Dehydroepiandrosterone Sulfate Levels Predict Longevity in Men: 27-Year Follow-Up Study in a Community-Based Cohort (Tanushimaru Study. J Am Geriatr Soc. 2008 doi: 10.1111/j.1532-5415.2008.01692.x. [DOI] [PubMed] [Google Scholar]
- 84.Epel ES, Burke H, Wolkowitz OM. The psychoneuroendocrinology of aging. In: Aldwin CM, Park CL, Spior A Jr, editors. Handbook of Health Psychology and Aging. New York: Guilford Publications, Inc; 2007. pp. 119–141. [Google Scholar]
- 85.Erb JL, Kadane JB, Tourney G, Mickelsen R, Trader D, Szabo R, Davis V. Discrimination between schizophrenic and control subjects by means of plasma dehydroepiandrosterone measurements. J Clin Endocrinol Metab. 1981;52:181–186. doi: 10.1210/jcem-52-2-181. [DOI] [PubMed] [Google Scholar]
- 86.Erdincler D, Bugay G, Ertan T, Eker E. Depression and sex hormones in elderly women. Arch Gerontol Geriatr. 2004;39:239–244. doi: 10.1016/j.archger.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 87.Eser D, Schule C, Romeo E, Baghai TC, di Michele F, Pasini A, Zwanzger P, Padberg F, Rupprecht R. Neuropsychopharmacological properties of neuroactive steroids in depression and anxiety disorders. Psychopharmacology (Berl) 2006;186:373–387. doi: 10.1007/s00213-005-0188-z. [DOI] [PubMed] [Google Scholar]
- 88.Fava M, Littman A, Halperin P. Neuroendocrine correlates of the type A behavior pattern: a review and new hypotheses. Int J Psychiatry Med. 1987;17:289–307. doi: 10.2190/27le-67ju-0453-jd27. [DOI] [PubMed] [Google Scholar]
- 89.Fava M, Rosenbaum JF, MacLaughlin RA, Tesar GE, Pollack MH, Cohen LS, Hirsch M. Dehydroepiandrosterone-sulfate/cortisol ratio in panic disorder. Psychiatry Res. 1989;28:345–350. doi: 10.1016/0165-1781(89)90215-1. [DOI] [PubMed] [Google Scholar]
- 90.Fava M, Littman A, Lamon-Fava S, Milani R, Shera D, MacLaughlin R, Cassem E, Leaf A, Marchio B, Bolognesi E, et al. Psychological behavioral and biochemical risk factors for coronary artery disease among American and Italian male corporate managers. Am J Cardiol. 1992;70:1412–1416. doi: 10.1016/0002-9149(92)90291-6. [DOI] [PubMed] [Google Scholar]
- 91.Ferrari E, Arcaini A, Gornati R, Pelanconi L, Cravello L, Fioravanti M, Solerte SB, Magri F. Pineal and pituitary-adrenocortical function in physiological aging and in senile dementia. Exp Gerontol. 2000;35:1239–1250. doi: 10.1016/s0531-5565(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 92.Ferrario E, Massaia M, Aimo G, Pallavicinodi Ceva A, Fabris F. Dehydroepiandrosterone sulfate serum levels: no significance in diagnosing Alzheimer’s disease. J Endocrinol Invest. 1999;22:81. [PubMed] [Google Scholar]
- 93.Fiore C, Inman DM, Hirose S, Noble LJ, Igarashi T, Compagnone NA. Treatment with the neurosteroid dehydroepiandrosterone promotes recovery of motor behavior after moderate contusive spinal cord injury in the mouse. J Neurosci Res. 2004;75:391–400. doi: 10.1002/jnr.10821. [DOI] [PubMed] [Google Scholar]
- 94.Flood JF, Smith GE, Roberts E. Dehydroepiandrosterone and its sulfate enhance memory retention in mice. Brain Res. 1988;447:269–278. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- 95.Fluck CE, Miller WL, Auchus RJ. Auchus, The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the delta5 steroidogenic pathway. J Clin Endocrinol Metab. 2003;88:3762–3766. doi: 10.1210/jc.2003-030143. [DOI] [PubMed] [Google Scholar]
- 96.Fonda SJ, Bertrand R, O’Donnell A, Longcope C, McKinlay JB. Age, hormones, and cognitive functioning among middle-aged and elderly men: cross-sectional evidence from the Massachusetts Male Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:385–390. doi: 10.1093/gerona/60.3.385. [DOI] [PubMed] [Google Scholar]
- 97.Forrest AD, Drewery J, Fotherby K, Laverty SG. A clinical trial of dehydroepiandrosterone (diandrone) J Neurol Neurosurg Psychiatry. 1960;23:52–55. doi: 10.1136/jnnp.23.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Friel PN, Alexander T, Wright JV. Suppression of adrenal function by low-dose prednisone: assessment with 24-hour urinary steroid hormone profiles--a review of five cases. Altern Med Rev. 2006;11:40–46. [PubMed] [Google Scholar]
- 99.Furuya E, Maezawa M, Nishikaze O. [17-KS sulfate as a biomarker in psychosocial stress] Rinsho Byori. 1998;46:529–537. [PubMed] [Google Scholar]
- 100.Gallagher P, Watson S, Smith MS, Young AH, Ferrier IN. Plasma cortisol-dehydroepiandrosterone (DHEA) ratios in schizophrenia and bipolar disorder. Schizophr Res. 2007;90:258–265. doi: 10.1016/j.schres.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 101.George O, Vallee M, Le Moal M, Mayo W. Neurosteroids and cholinergic systems: implications for sleep and cognitive processes and potential role of age-related changes. Psychopharmacology (Berl) 2006;186:402–413. doi: 10.1007/s00213-005-0254-6. [DOI] [PubMed] [Google Scholar]
- 102.Geracioti TD., Jr DHEA supplementation of systemic glucocorticoids for treatment of poison ivy dermatitis. Int J Dermatol. 2005;44:974–976. doi: 10.1111/j.1365-4632.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 103.Gil-ad I, Shtaif B, Eshet R, Maayan R, Rehavi M, Weizman A. Effect of dehydroepiandrosterone and its sulfate metabolite on neuronal cell viability in culture. Isr Med Assoc J. 2001;3:639–643. [PubMed] [Google Scholar]
- 104.Glei DA, Goldman N. Dehydroepiandrosterone sulfate (DHEAS) and risk for mortality among older Taiwanese. Ann Epidemiol. 2006;16:510–515. doi: 10.1016/j.annepidem.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 105.Glei DA, Goldman N, Weinstein M, Liu IW. Dehydroepiandrosterone sulfate (DHEAS) and health: does the relationship differ by sex? Exp Gerontol. 2004;39:321–331. doi: 10.1016/j.exger.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Goldman N, Glei DA. Sex differences in the relationship between DHEAS and health. Exp Gerontol. 2007;42:979–987. doi: 10.1016/j.exger.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gomez-Merino D, Chennaoui M, Burnat P, Drogou C, Guezennec CY. Immune and hormonal changes following intense military training. Mil Med. 2003;168:1034–1038. [PubMed] [Google Scholar]
- 108.Gonzalez-Alvear GM, Werling LL. Regulation of [3H]dopamine release from rat striatal slices by sigma receptor ligands. J Pharmacol Exp Ther. 1994;271:212–219. [PubMed] [Google Scholar]
- 109.Goodyer IM, Herbert J, Altham PM. Adrenal steroid secretion and major depression in 8- to 16-year-olds, III. Influence of cortisol/DHEA ratio at presentation on subsequent rates of disappointing life events and persistent major depression. Psychol Med. 1998;28:265–273. doi: 10.1017/s0033291797006314. [DOI] [PubMed] [Google Scholar]
- 110.Goodyer IM, Herbert J, Tamplin A. Psychoendocrine antecedents of persistent first-episode major depression in adolescents: a community-based longitudinal enquiry. Psychol Med. 2003;33:601–610. doi: 10.1017/s0033291702007286. [DOI] [PubMed] [Google Scholar]
- 111.Goodyer IM, Herbert J, Altham PM, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- 112.Goyal RO, Sagar R, Ammini AC, Khurana ML, Alias AG. Negative correlation between negative symptoms of schizophrenia and testosterone levels. Ann N Y Acad Sci. 2004;1032:291–294. doi: 10.1196/annals.1314.042. [DOI] [PubMed] [Google Scholar]
- 113.Griffiths WJ, Liu S, Yang Y, Purdy RH, Sjovall J. Nano-electrospray tandem mass spectrometry for the analysis of neurosteroid sulphates. Rapid Commun Mass Spectrom. 1999;13:1595–1610. doi: 10.1002/(SICI)1097-0231(19990815)13:15<1595::AID-RCM681>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 114.Grimley Evans J, Malouf R, Huppert F, van Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for congitive function in healthy elderly people. Cochrane Database of Systematic Reviews (online) 2006 doi: 10.1002/14651858.CD006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guazzo EP, Kirkpatrick PJ, Goodyer IM, Shiers HM, Herbert J. Cortisol,dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: relation to blood levels and the effects of age. J Clin Endocrinol Metab. 1996;81:3951–3960. doi: 10.1210/jcem.81.11.8923843. [DOI] [PubMed] [Google Scholar]
- 116.Hajszan T, MacLusky NJ, Leranth C. Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized female rats. Endocrinology. 2004;145:1042–1045. doi: 10.1210/en.2003-1252. [DOI] [PubMed] [Google Scholar]
- 117.Hansen CR, Jr, Kroll J, Mackenzie TB. Dehydroepiandrosterone and affective disorders. Am J Psychiatry. 1982;139:386–387. doi: 10.1176/ajp.139.3.386a. [DOI] [PubMed] [Google Scholar]
- 118.Haren MT, Malmstrom TK, Banks WA, Patrick P, Miller DK, Morley JE. Lower serum DHEAS levels are associated with a higher degree of physical disability and depressive symptoms in middle-aged to older African American women. Maturitas. 2007;57:347–360. doi: 10.1016/j.maturitas.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Harris DS, Wolkowitz OM, Reus VI. Movement disorder, memory, psychiatric symptoms and serum DHEA levels in schizophrenic and schizoaffective patients. World J Biol Psychiatry. 2001;2:99–102. doi: 10.3109/15622970109027500. [DOI] [PubMed] [Google Scholar]
- 120.Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22:337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- 121.Hechter O, Grossman A, Chatterton RT., Jr Relationship of dehydroepiandrosterone and cortisol in disease. Med Hypotheses. 1997;49:85–91. doi: 10.1016/s0306-9877(97)90258-9. [DOI] [PubMed] [Google Scholar]
- 122.Hennebert O, Chalbot S, Alran S, Morfin R. Dehydroepiandrosterone 7alphahydroxylation in human tissues: possible interference with type 1 11beta-hydroxysteroid dehydrogenase-mediated processes. J Steroid Biochem Mol Biol. 2007;104:326–333. doi: 10.1016/j.jsbmb.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 123.Heuser I, Deuschle M, Luppa P, Schweiger U, Standhardt H, Weber B. Increased diurnal plasma concentrations of dehydroepiandrosterone in depressed patients. J Clin Endocrinol Metab. 1998;83:3130–3133. doi: 10.1210/jcem.83.9.5081. [DOI] [PubMed] [Google Scholar]
- 124.Higashi T, Daifu Y, Shimada K. Studies on neurosteroids XIV. Levels of dehydroepiandrosterone sulfate in rat brain and serum determined with newly developed enzyme-linked immunosorbent assay. Steroids. 2001;66:865–874. doi: 10.1016/s0039-128x(01)00125-8. [DOI] [PubMed] [Google Scholar]
- 125.Higashi T, Sugitani H, Yagi T, Shimada K. Studies on neurosteroids XVI. Levels of pregnenolone sulfate in rat brains determined by enzyme-linked immunosorbent assay not requiring solvolysis. Biol Pharm Bull. 2003;26:709–711. doi: 10.1248/bpb.26.709. [DOI] [PubMed] [Google Scholar]
- 126.Higashi T, Daifu Y, Ikeshima T, Yagi T, Shimada K. Studies on neurosteroids XV. Development of enzyme-linked immunosorbent assay for examining whether pregnenolone sulfate is a veritable neurosteroid. J Pharm Biomed Anal. 2003;30:1907–1917. doi: 10.1016/s0731-7085(02)00534-4. [DOI] [PubMed] [Google Scholar]
- 127.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci U S A. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hong CY, Park JH, Ahn RS, Im SY, Choi HS, Soh J, Mellon SH, Lee K. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol. 2004;24:2593–2604. doi: 10.1128/MCB.24.7.2593-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hsiao CC. Positive correlation between anxiety severity and plasma levels of dehydroepiandrosterone sulfate in medication-free patients experiencing a major episode of depression. Psychiatry Clin Neurosci. 2006;60:746–750. doi: 10.1111/j.1440-1819.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- 130.Hu Y, Cardounel A, Gursoy E, Anderson P, Kalimi M. Anti-stress effects of dehydroepiandrosterone: protection of rats against repeated immobilization stress-induced weight loss, glucocorticoid receptor production, and lipid peroxidation. Biochem Pharmacol. 2000;59:753–762. doi: 10.1016/s0006-2952(99)00385-8. [DOI] [PubMed] [Google Scholar]
- 131.Hunt PJ, Gurnell EM, Huppert FA, Richards C, Prevost AT, Wass JA, Herbert J, Chatterjee VK. Improvement in mood and fatigue after dehydroepiandrosterone replacement in Addison’s disease in a randomized, double blind trial. J Clin Endocrinol Metab. 2000;85:4650–4656. doi: 10.1210/jcem.85.12.7022. [DOI] [PubMed] [Google Scholar]
- 132.Imamura M, Prasad C. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun. 1998;243:771–775. doi: 10.1006/bbrc.1998.8177. [DOI] [PubMed] [Google Scholar]
- 133.Iwasaki Y, Asai M, Yoshida M, Nigawara T, Kambayashi M, Nakashima N. Dehydroepiandrosterone-sulfate inhibits nuclear factor-kappaB-dependent transcription in hepatocytes, possibly through antioxidant effect. J Clin Endocrinol Metab. 2004;89:3449–3454. doi: 10.1210/jc.2003-031441. [DOI] [PubMed] [Google Scholar]
- 134.Jellinck PH, Kaufmann M, Gottfried-Blackmore A, McEwen BS, Jones G, Bulloch K. Selective conversion by microglia of dehydroepiandrosterone to 5-androstenediol-A steroid with inherent estrogenic properties. J Steroid Biochem Mol Biol. 2007;107:156–162. doi: 10.1016/j.jsbmb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 135.Jellinck PH, Croft G, McEwen BS, Gottfried-Blackmore A, Jones G, Byford V, Bulloch K. Metabolism of dehydroepiandrosterone by rodent brain cell lines: relationship between 7-hydroxylation and aromatization. J Steroid Biochem Mol Biol. 2005;93:81–86. doi: 10.1016/j.jsbmb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 136.Jellinck PH, Kaufmann M, Gottfried-Blackmore A, Croft G, Byford V, McEwen BS, Jones G, Bulloch K. Dehydroepiandrosterone (DHEA) metabolism in the brain: identification by liquid chromatography/mass spectrometry of the delta-4-isomer of DHEA and related steroids formed from androstenedione by mouse BV2 microglia. J Steroid Biochem Mol Biol. 2006;98:41–47. doi: 10.1016/j.jsbmb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 137.Joels M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- 138.Kaasik A, Kalda A, Jaako K, Zharkovsky A. Dehydroepiandrosterone sulphate prevents oxygen-glucose deprivation-induced injury in cerebellar granule cell culture. Neuroscience. 2001;102:427–432. doi: 10.1016/s0306-4522(00)00489-9. [DOI] [PubMed] [Google Scholar]
- 139.Kaasik A, Safiulina D, Kalda A, Zharkovsky A. Dehydroepiandrosterone with other neurosteroids preserve neuronal mitochondria from calcium overload. J Steroid Biochem Mol Biol. 2003;87:97–103. doi: 10.1016/s0960-0760(03)00389-3. [DOI] [PubMed] [Google Scholar]
- 140.Kalimi M, Shafagoj Y, Loria R, Padgett D, Regelson W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA) Mol Cell Biochem. 1994;131:99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- 141.Kalmijn S, Launer LJ, Stolk RP, Jong FHde, Pols HA, Hofman A, Breteler MM, Lamberts SW. A prospective study on cortisol, dehydroepiandrosterone sulfate, and cognitive function in the elderly. J Clin Endocrinol Metab. 1998;83:3487–3492. doi: 10.1210/jcem.83.10.5164. [DOI] [PubMed] [Google Scholar]
- 142.Kaminska M, Harris J, Gijsbers K, Dubrovsky B. Dehydroepiandrosterone sulfate (DHEAS) counteracts decremental effects of corticosterone on dentate gyrus LTP. Implications for depression. Brain Res Bull. 2000;52:229–234. doi: 10.1016/s0361-9230(00)00251-3. [DOI] [PubMed] [Google Scholar]
- 143.Kaminski RM, Marini H, Kim WJ, Rogawski MA. Anticonvulsant activity of androsterone and etiocholanolone. Epilepsia. 2005;46:819–827. doi: 10.1111/j.1528-1167.2005.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kannisto S, Laatikainen A, Taivainen A, Savolainen K, Tukiainen H, Voutilainen R. Serum dehydroepiandrosterone sulfate concentration as an indicator of adrenocortical suppression during inhaled steroid therapy in adult asthmatic patients. Eur J Endocrinol. 2004;150:687–690. doi: 10.1530/eje.0.1500687. [DOI] [PubMed] [Google Scholar]
- 145.Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 146.Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. J Clin Epidemiol. 2002;55:696–710. doi: 10.1016/s0895-4356(02)00399-2. [DOI] [PubMed] [Google Scholar]
- 147.Kibaly C, Patte-Mensah C, Mensah-Nyagan AG. Molecular and neurochemical evidence for the biosynthesis of dehydroepiandrosterone in the adult rat spinal cord. J Neurochem. 2005;93:1220–1230. doi: 10.1111/j.1471-4159.2005.03113.x. [DOI] [PubMed] [Google Scholar]
- 148.Kim SB, Hill M, Kwak YT, Hampl R, Jo DH, Morfin R. Neurosteroids: Cerebrospinal fluid levels for Alzheimer’s disease and vascular dementia diagnostics. J Clin Endocrinol Metab. 2003;88:5199–5206. doi: 10.1210/jc.2003-030646. [DOI] [PubMed] [Google Scholar]
- 149.Kimonides VG, Khatibi NH, Svendsen CN, Sofroniew MV, Herbert J. Dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) protect hippocampal neurons against excitatory amino acid-induced neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:1852–1857. doi: 10.1073/pnas.95.4.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kimonides VG, Spillantini MG, Sofroniew MV, Fawcett JW, Herbert J. Dehydroepiandrosterone antagonizes the neurotoxic effects of corticosterone and translocation of stress-activated protein kinase 3 in hippocampal primary cultures. Neuroscience. 1999;89:429–436. doi: 10.1016/s0306-4522(98)00347-9. [DOI] [PubMed] [Google Scholar]
- 151.Kimoto T, Tsurugizawa T, Ohta Y, Makino J, Tamura H, Hojo Y, Takata N, Kawato S. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- 152.Kimura M, Tanaka S, Yamada Y, Kiuchi Y, Yamakawa T, Sekihara H. Dehydroepiandrosterone decreases serum tumor necrosis factor-alpha and restores insulin sensitivity: independent effect from secondary weight reduction in genetically obese Zucker fatty rats. Endocrinology. 1998;139:3249–3253. doi: 10.1210/endo.139.7.6118. [DOI] [PubMed] [Google Scholar]
- 153.Kipper-Galperin M, Galilly R, Danenberg HD, Brenner T. Dehydroepiandrosterone selectively inhibits production of tumor necrosis factor alpha and interleukin-6 [correction of interlukin-6] in astrocytes. Int J Dev Neurosci. 1999;17:765–775. doi: 10.1016/s0736-5748(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 154.Kishimoto Y, Hoshi M. Dehydroepiandrosterone sulphate in rat brain: incorporation from blood and metabolism in vivo. J Neurochem. 1972;19:2207–2215. doi: 10.1111/j.1471-4159.1972.tb05129.x. [DOI] [PubMed] [Google Scholar]
- 155.Knapstein P, David A, Wu CH, Archer DF, Flickinger GL, Tochstone JC. Metabolism of free and sulfoconjugated DHEA in brain tissue in vivo and in vitro. Steroids. 1968;11:885–896. doi: 10.1016/s0039-128x(68)80102-3. [DOI] [PubMed] [Google Scholar]
- 156.Kohalmy K, Tamasi V, Kobori L, Sarvary E, Pascussi JM, Porrogi P, Rozman D, Prough RA, Meyer UA, Monostory K. Dehydroepiandrosterone induces human CYP2B6 through the constitutive androstane receptor. Drug Metab Dispos. 2007;35:1495–1501. doi: 10.1124/dmd.107.016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- 158.Kudielka BM, Hellhammer J, Hellhammer DH, Wolf OT, Pirke KM, Varadi E, Pilz J, Kirschbaum C. Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. J Clin Endocrinol Metab. 1998;83:1756–1761. doi: 10.1210/jcem.83.5.4758. [DOI] [PubMed] [Google Scholar]
- 159.Kurata K, Takebayashi M, Morinobu S, Yamawaki S. beta-estradiol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate protect against N-methyl-Daspartate-induced neurotoxicity in rat hippocampal neurons by different mechanisms. J Pharmacol Exp Ther. 2004;311:237–245. doi: 10.1124/jpet.104.067629. [DOI] [PubMed] [Google Scholar]
- 160.Labbate LA, Fava M, Oleshansky M, Zoltec J, Littman A, Harig P. Physical fitness and perceived stress. Relationships with coronary artery disease risk factors. Psychosomatics. 1995;36:555–560. doi: 10.1016/s0033-3182(95)71611-5. [DOI] [PubMed] [Google Scholar]
- 161.Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- 162.Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22:299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- 163.Labrie F, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B. Effect of 12- month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab. 1997;82:3498–3505. doi: 10.1210/jcem.82.10.4306. [DOI] [PubMed] [Google Scholar]
- 164.Lacroix C, Fiet J, Benais JP, Gueux B, Bonete R, Villette JM, Gourmel B, Dreux C. Simultaneous radioimmunoassay of progesterone, androst-4-enedione, pregnenolone, dehydroepiandrosterone and 17-hydroxyprogesterone in specific regions of human brain. J Steroid Biochem. 1987;28:317–325. doi: 10.1016/0022-4731(87)91025-9. [DOI] [PubMed] [Google Scholar]
- 165.Lanthier A, Patwardhan VV. Sex steroids and 5-en-3 beta-hydroxysteroids in specific regions of the human brain and cranial nerves. J Steroid Biochem. 1986;25:445–449. doi: 10.1016/0022-4731(86)90259-1. [DOI] [PubMed] [Google Scholar]
- 166.Lapchak PA, Chapman DF, Nunez SY, Zivin JA. Dehydroepiandrosterone sulfate is neuroprotective in a reversible spinal cord ischemia model: possible involvement of GABA(A) receptors. Stroke. 2000;31:1953–1956. doi: 10.1161/01.str.31.8.1953. discussion 1957. [DOI] [PubMed] [Google Scholar]
- 167.Laufer N, Maayan R, Hermesh H, Marom S, Gilad R, Strous R, Weizman A. Involvement of GABAA receptor modulating neuroactive steroids in patients with social phobia. Psychiatry Res. 2005;137:131–136. doi: 10.1016/j.psychres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 168.Laurine E, Lafitte D, Gregoire C, Seree E, Loret E, Douillard S, Michel B, Briand C, Verdier JM. Specific binding of dehydroepiandrosterone to the N terminus of the microtubule-associated protein MAP2. J Biol Chem. 1991;278:29979–29986. doi: 10.1074/jbc.M303242200. [DOI] [PubMed] [Google Scholar]
- 169.Le Goascogne C, Sananes N, Gouezou M, Takemori S, Kominami S, Baulieu EE, Robel P. Immunoreactive cytochrome P-450(17 alpha) in rat and guinea-pig gonads, adrenal glands and brain. J Reprod Fertil. 1991;93:609–622. doi: 10.1530/jrf.0.0930609. [DOI] [PubMed] [Google Scholar]
- 170.Leblhuber F, Windhager E, Neubauer C, Weber J, Reisecker F, Dienstl E. Antiglucocorticoid effects of DHEA-S in Alzheimer’s disease. Am J Psychiatry. 1992;149:1125–1126. doi: 10.1176/ajp.149.8.aj14981125. [DOI] [PubMed] [Google Scholar]
- 171.Leblhuber F, Neubauer C, Peichl M, Reisecker F, Steinparz FX, Windhager E, Dienstl E. Age and sex differences of dehydroepiandrosterone sulfate (DHEAS) and cortisol (CRT) plasma levels in normal controls and Alzheimer’s disease (AD) Psychopharmacology (Berl) 1993;111:23–26. doi: 10.1007/BF02257402. [DOI] [PubMed] [Google Scholar]
- 172.Legrain S, Berr C, Frenoy N, Gourlet V, Debuire B, Baulieu EE. Dehydroepiandrosterone sulfate in a long-term care aged population. Gerontology. 1995;41:343–351. doi: 10.1159/000213706. [DOI] [PubMed] [Google Scholar]
- 173.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 174.Li H, Klein G, Sun P, Buchan AM. Dehydroepiandrosterone (DHEA) reduces neuronal injury in a rat model of global cerebral ischemia. Brain Res. 2001;888:263–266. doi: 10.1016/s0006-8993(00)03077-8. [DOI] [PubMed] [Google Scholar]
- 175.Li PK, Rhodes ME, Burke AM, Johnson DA. Memory enhancement mediated by the steroid sulfatase inhibitor (p-O-sulfamoyl)-N-tetradecanoyl tyramine. Life Sci. 1997;60:PL45–PL51. doi: 10.1016/s0024-3205(96)00621-2. [DOI] [PubMed] [Google Scholar]
- 176.Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjovall J, Baulieu EE. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J Lipid Res. 2004;45:2287–2302. doi: 10.1194/jlr.M400244-JLR200. [DOI] [PubMed] [Google Scholar]
- 177.Littman AB, Fava M, Halperin P, Lamon-Fava S, Drews FR, Oleshansky MA, Bielenda CC, MacLaughlin RA. Physiologic benefits of a stress reduction program for healthy middle-aged Army officers. J Psychosom Res. 1993;37:345–354. doi: 10.1016/0022-3999(93)90136-4. [DOI] [PubMed] [Google Scholar]
- 178.Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3) J Biol Chem. 2002;277:21379–21388. doi: 10.1074/jbc.M200491200. [DOI] [PubMed] [Google Scholar]
- 179.Liu S, Griffiths WJ, Sjovall J. Capillary liquid chromatography/electrospray mass spectrometry for analysis of steroid sulfates in biological samples. Anal Chem. 2003;75:791–797. doi: 10.1021/ac0262154. [DOI] [PubMed] [Google Scholar]
- 180.Liu S, Sjovall J, Griffiths WJ. Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal Chem. 2003;75:5835–5846. doi: 10.1021/ac0346297. [DOI] [PubMed] [Google Scholar]
- 181.Longcope C. Dehydroepiandrosterone metabolism. J Endocrinol. 1996;150 Suppl:S125–S127. [PubMed] [Google Scholar]
- 182.Lovas K, Gebre-Medhin G, Trovik TS, Fougner KJ, Uhlving S, Nedrebo BG, Myking OL, Kampe O, Husebye ES. Husebye, Replacement of dehydroepiandrosterone in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003;88:1112–1118. doi: 10.1210/jc.2002-020769. [DOI] [PubMed] [Google Scholar]
- 183.Lupien S, Sharma S, Arcand JF, Schwartz G, Nair NPV, Meaney MJ, Hauger RL. Dehydroepiandrosterone-sulfate (DHEA-S) levels, cortisol levels and cognitive function in elderly human subjects. International Society of Psychoneuroendocrinology, ?? 1995:24. [Google Scholar]
- 184.Lupien SJ, Leon Mde, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 185.Maggio M, Ceda GP, Denti L, Rebecchi I, Manganelli P, Ablondi F, Valenti G. Decreased DHEAS secretion in patients with chronic inflammatory diseases treated with glucocorticoids. J Endocrinol Invest. 2002;25:87–88. [PubMed] [Google Scholar]
- 186.Magri F, Cravello L, Barili L, Sarra S, Cinchetti W, Salmoiraghi F, Micale G, Ferrari E. Stress and dementia: the role of the hypothalamicpituitary-adrenal axis. Aging Clin Exp Res. 2006;18:167–170. doi: 10.1007/BF03327435. [DOI] [PubMed] [Google Scholar]
- 187.Magri F, Terenzi F, Ricciardi T, Fioravanti M, Solerte SB, Stabile M, Balza G, Gandini C, Villa M, Ferrari E. Association between changes in adrenal secretion and cerebral morphometric correlates in normal aging and senile dementia. Dement Geriatr Cogn Disord. 2000;11:90–99. doi: 10.1159/000017220. [DOI] [PubMed] [Google Scholar]
- 188.Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- 189.Markianos M, Tripodianakis J, Sarantidis D, Hatzimanolis J. Plasma testosterone and dehydroepiandrosterone sulfate in male and female patients with dysthymic disorder. J Affect Disord. 2007;101:255–258. doi: 10.1016/j.jad.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 190.Marx CE, Jarskog LF, Lauder JM, Gilmore JH, Lieberman JA, Morrow AL. Neurosteroid modulation of embryonic neuronal survival in vitro following anoxia. Brain Res. 2000;871:104–112. doi: 10.1016/s0006-8993(00)02452-5. [DOI] [PubMed] [Google Scholar]
- 191.Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249–1263. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- 192.Masera RG, Prolo P, Sartori ML, Staurenghi A, Griot G, Ravizza L, Dovio A, Chiappelli F, Angeli A. Mental deterioration correlates with response of natural killer (NK) cell activity to physiological modifiers in patients with short history of Alzheimer’s disease. Psychoneuroendocrinology. 2002;27:447–461. doi: 10.1016/s0306-4530(01)00062-2. [DOI] [PubMed] [Google Scholar]
- 193.Maurice T, Gregoire C, Espallergues J. Neuro(active)steroids actions at the neuromodulatory sigma1 (sigma1) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacol Biochem Behav. 2006;84:581–597. doi: 10.1016/j.pbb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 194.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann N Y Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 195.McKenna TJ, Fearon U, Clarke D, Cunningham SK. A critical review of the origin and control of adrenal androgens. Baillieres Clin Obstet Gynaecol. 1997;11:229–248. doi: 10.1016/s0950-3552(97)80035-1. [DOI] [PubMed] [Google Scholar]
- 196.Melchior CL, Ritzmann RF. Dehydroepiandrosterone is an anxiolytic in mice on the plus maze. Pharmacol Biochem Behav. 1994;47:437–441. doi: 10.1016/0091-3057(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 197.Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 198.Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- 199.Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev. 1997;18:378–403. doi: 10.1210/edrv.18.3.0304. [DOI] [PubMed] [Google Scholar]
- 200.Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000;48:989–995. doi: 10.1016/s0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- 201.Miller WL. Androgen biosynthesis from cholesterol to DHEA. Mol Cell Endocrinol. 2002;198:7–14. doi: 10.1016/s0303-7207(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 202.Milman A, Zohar O, Maayan R, Weizman R, Pick CG. DHEAS repeated treatment improves cognitive and behavioral deficits after mild traumatic brain injury. Eur Neuropsychopharmacol. 2008;18:181–187. doi: 10.1016/j.euroneuro.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 203.Mitamura K, Yatera M, Shimada K. Quantitative determination of pregnenolone 3-sulfate in rat brains using liquid chromotography/electrospracy ionization-mass spectrometry. Anal Sci. 1999;15:951–955. [Google Scholar]
- 204.Moffat SD, Zonderman AB, Harman SM, Blackman MR, Kawas C, Resnick SM. The relationship between longitudinal declines in dehydroepiandrosterone sulfate concentrations and cognitive performance in older men. Arch Intern Med. 2000;160:2193–2198. doi: 10.1001/archinte.160.14.2193. [DOI] [PubMed] [Google Scholar]
- 205.Monnet FP, Mahe V, Robel P, Baulieu EE. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci U S A. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–1367. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- 207.Morfin R, Starka L. Neurosteroid 7-hydroxylation products in the brain. Int Rev Neurobiol. 2001;46:79–95. doi: 10.1016/s0074-7742(01)46059-4. [DOI] [PubMed] [Google Scholar]
- 208.Morgan CA, 3rd, Southwick S, Hazlett G, Rasmusson A, Hoyt G, Zimolo Z, Charney D. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch Gen Psychiatry. 2004;61:819–825. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]
- 209.Morrison MF, Katz IR, Parmelee P, Boyce AA, TenHave T. Dehydroepiandrosterone sulfate (DHEA-S) and psychiatric and laboratory measures of frailty in a residential care population. Am J Geriatr Psychiatry. 1998;6:277–284. [PubMed] [Google Scholar]
- 210.Morsink LF, Vogelzangs N, Nicklas BJ, Beekman AT, Satterfield S, Rubin SM, Yaffe K, Simonsick E, Newman AB, Kritchevsky SB, Penninx BW. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: results from the Health ABC study. Psychoneuroendocrinology. 2007;32:874–883. doi: 10.1016/j.psyneuen.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 211.Mukai H, Tsurugizawa T, Ogiue-Ikeda M, Murakami G, Hojo Y, Ishii H, Kimoto T, Kawato S. Local neurosteroid production in the hippocampus: influence on synaptic plasticity of memory. Neuroendocrinology. 2006;84:255–263. doi: 10.1159/000097747. [DOI] [PubMed] [Google Scholar]
- 212.Mukai H, Takata N, Ishii HT, Tanabe N, Hojo Y, Furukawa A, Kimoto T, Kawato S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: synaptocrinology. Neuroscience. 2006;138:757–764. doi: 10.1016/j.neuroscience.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 213.Muller C, Hennebert O, Morfin R. The native anti-glucocorticoid paradigm. J Steroid Biochem Mol Biol. 2006;100:95–105. doi: 10.1016/j.jsbmb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 214.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- 215.Murialdo G, Nobili F, Rollero A, Gianelli MV, Copello F, Rodriguez G, Polleri A. Hippocampal perfusion and pituitary-adrenal axis in Alzheimer’s disease. Neuropsychobiology. 2000;42:51–57. doi: 10.1159/000026672. [DOI] [PubMed] [Google Scholar]
- 216.Murialdo G, Barreca A, Nobili F, Rollero A, Timossi G, Gianelli MV, Copello F, Rodriguez G, Polleri A. Dexamethasone effects on cortisol secretion in Alzheimer’s disease: some clinical and hormonal features in suppressor and nonsuppressor patients. J Endocrinol Invest. 2000;23:178–186. doi: 10.1007/BF03343703. [DOI] [PubMed] [Google Scholar]
- 217.Murray HE, Gillies GE. Differential effects of neuroactive steroids on somatostatin and dopamine secretion from primary hypothalamic cell cultures. J Neuroendocrinol. 1997;9:287–295. doi: 10.1046/j.1365-2826.1997.00582.x. [DOI] [PubMed] [Google Scholar]
- 218.Murray S, Baillie TA. Direct derivatization of sulphate esters for analysis by gas chromotography mass spectrometry. Biomed Mass Spectrom. 1979;6:82–89. [Google Scholar]
- 219.Nachshoni T, Ebert T, Abramovitch Y, Assael-Amir M, Kotler M, Maayan R, Weizman A, Strous RD. Improvement of extrapyramidal symptoms following dehydroepiandrosterone (DHEA) administration in antipsychotic treated schizophrenia patients:a randomized, double-blind placebo controlled trial. Schizophr Res. 2005;79:251–256. doi: 10.1016/j.schres.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 220.Naert G, Maurice T, Tapia-Arancibia L, Givalois L. Neuroactive steroids modulate HPA axis activity and cerebral brain-derived neurotrophic factor (BDNF) protein levels in adult male rats. Psychoneuroendocrinology. 2007;32:1062–1078. doi: 10.1016/j.psyneuen.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 221.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Serum concentrations of estradiol and dehydroepiandrosterone sulfate and soy product intake in relation to psychologic well-being in peri- and postmenopausal Japanese women. Metabolism. 2000;49:1561–1564. doi: 10.1053/meta.2000.18522. [DOI] [PubMed] [Google Scholar]
- 222.Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, 3rd, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 223.Nasman B, Olsson T, Backstrom T, Eriksson S, Grankvist K, Viitanen M, Bucht G. Serum dehydroepiandrosterone sulfate in Alzheimer’s disease and in multi-infarct dementia. Biol Psychiatry. 1991;30:684–690. doi: 10.1016/0006-3223(91)90013-c. [DOI] [PubMed] [Google Scholar]
- 224.Nieschlag E, Loriaux DL, Ruder HJ, Zucker IR, Kirschner MA, Lipsett MB. The secretion of dehydroepiandrosterone and dehydroepiandrosterone sulphate in man. J Endocrinol. 1973;57:123–134. doi: 10.1677/joe.0.0570123. [DOI] [PubMed] [Google Scholar]
- 225.Nishikaze O. [17-KS sulfate as a biomarker in health and disease] Rinsho Byori. 1998;46:520–528. [PubMed] [Google Scholar]
- 226.Nordmark G, Bengtsson C, Larsson A, Karlsson FA, Sturfelt G, Ronnblom L. Effects of dehydroepiandrosterone supplement on health-related quality of life in glucocorticoid treated female patients with systemic lupus erythematosus. Autoimmunity. 2005;38:531–540. doi: 10.1080/08916930500285550. [DOI] [PubMed] [Google Scholar]
- 227.Oades RD, Schepker R. Serum gonadal steroid hormones in young schizophrenic patients. Psychoneuroendocrinology. 1994;19:373–385. doi: 10.1016/0306-4530(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 228.Oberbeck R, Benschop RJ, Jacobs R, Hosch W, Jetschmann JU, Schurmeyer TH, Schmidt RE, Schedlowski M. Endocrine mechanisms of stress-induced DHEA-secretion. J Endocrinol Invest. 1998;21:148–153. doi: 10.1007/BF03347293. [DOI] [PubMed] [Google Scholar]
- 229.Oertel GW, Benes P, Schirazi M, Holzmann H, Hoffmann G. Interaction between dehydroepiandrosterone, cyclic adenosine-3’,5’-monophosphate and glucose-6-phosphatedehydrogenase in normal and diseased subjects. Experientia. 1974;30:872–873. doi: 10.1007/BF01938331. [DOI] [PubMed] [Google Scholar]
- 230.Olff M, de Vries GJ, Guzelcan Y, Assies J, Gersons BP. Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinology. 2007;32:619–626. doi: 10.1016/j.psyneuen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 231.Osran H, Reist C, Chen CC, Lifrak ET, Chicz-DeMet A, Parker LN. Adrenal androgens and cortisol in major depression. Am J Psychiatry. 1993;150:806–809. doi: 10.1176/ajp.150.5.806. [DOI] [PubMed] [Google Scholar]
- 232.Ozasa H, Kita M, Inoue T, Mori T. Plasma dehydroepiandrosterone-to-cortisol ratios as an indicator of stress in gynecologic patients. Gynecol Oncol. 1990;37:178–182. doi: 10.1016/0090-8258(90)90330-n. [DOI] [PubMed] [Google Scholar]
- 233.Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- 234.Parker LN, Odell WD. Control of adrenal androgen secretion. Endocr Rev. 1980;1:392–410. doi: 10.1210/edrv-1-4-392. [DOI] [PubMed] [Google Scholar]
- 235.Parker LN, Levin ER, Lifrak ET. Evidence for adrenocortical adaptation to severe illness. J Clin Endocrinol Metab. 1985;60:947–952. doi: 10.1210/jcem-60-5-947. [DOI] [PubMed] [Google Scholar]
- 236.Perez-Neri I, Montes S, Ojeda-Lopez C, Ramirez-Bermudez J, Rios C. Modulation of neurotransmitter systems by dehydroepiandrosterone and dehydroepiandrosterone sulfate: Mechanism of action and relevance to psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008 doi: 10.1016/j.pnpbp.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 237.Perkins LM, Payne AH. Quantification of P450scc, P450(17) alpha, and iron sulfur protein reductase in Leydig cells and adrenals of inbred strains of mice. Endocrinology. 1988;123:2675–2682. doi: 10.1210/endo-123-6-2675. [DOI] [PubMed] [Google Scholar]
- 238.Perrini S, Laviola L, Natalicchio A, Giorgino F. Associated hormonal declines in aging: DHEAS. J Endocrinol Invest. 2005;28:85–93. [PubMed] [Google Scholar]
- 239.Persky H, Dreisbach L, Miller WR, O’Brien CP, Khan MA, Lief HI, Charney N, Strauss D. The relation of plasma androgen levels to sexual behaviors and attitudes of women. Psychosom Med. 1982;44:305–319. doi: 10.1097/00006842-198209000-00001. [DOI] [PubMed] [Google Scholar]
- 240.Peters JM, Zhou YC, Ram PA, Lee SS, Gonzalez FJ, Waxman DJ. Peroxisome proliferator-activated receptor alpha required for gene induction by dehydroepiandrosterone-3 beta-sulfate. Mol Pharmacol. 1996;50:67–74. [PubMed] [Google Scholar]
- 241.Pico-Alfonso MA, Garcia-Linares MI, Celda-Navarro N, Herbert J, Martinez M. Changes in cortisol and dehydroepiandrosterone in women victims of physical and psychological intimate partner violence. Biol Psychiatry. 2004;56:233–240. doi: 10.1016/j.biopsych.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 242.Polleri A, Gianelli MV, Murialdo G. Dehydroepiandrosterone: dream or nightmare? J Endocrinol Invest. 1998;21:544. doi: 10.1007/BF03347343. [DOI] [PubMed] [Google Scholar]
- 243.Poor V, Juricskay S, Gati A, Osvath P, Tenyi T. Urinary steroid metabolites and 11beta-hydroxysteroid dehydrogenase activity in patients with unipolar recurrent major depression. J Affect Disord. 2004;81:55–59. doi: 10.1016/S0165-0327(03)00199-X. [DOI] [PubMed] [Google Scholar]
- 244.Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. J Biol Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 245.Rabkin JG, McElhiney MC, Rabkin R, McGrath PJ, Ferrando SJ. Placebo-controlled trial of dehydroepiandrosterone (DHEA) for treatment of nonmajor depression in patients with HIV/AIDS. Am J Psychiatry. 2006;163:59–66. doi: 10.1176/appi.ajp.163.1.59. [DOI] [PubMed] [Google Scholar]
- 246.Rajkowski KM, Robel P, Baulieu EE. Hydroxysteroid sulfotransferase activity in the rat brain and liver as a function of age and sex. Steroids. 1997;62:427–436. doi: 10.1016/s0039-128x(97)00013-5. [DOI] [PubMed] [Google Scholar]
- 247.Ramassamy C, Averill D, Beffert U, Bastianetto S, Theroux L, Lussier-Cacan S, Cohn JS, Christen Y, Davignon J, Quirion R, Poirier J. Oxidative damage and protection by antioxidants in the frontal cortex of Alzheimer’s disease is related to the apolipoprotein E genotype. Free Radic Biol Med. 1999;27:544–553. doi: 10.1016/s0891-5849(99)00102-1. [DOI] [PubMed] [Google Scholar]
- 248.Rasmusson AM, Vythilingam M, Morgan CA., 3rd The neuroendocrinology of posttraumatic stress disorder: new directions. CNS Spectr. 2003;8:651–656. 665–657. doi: 10.1017/s1092852900008841. [DOI] [PubMed] [Google Scholar]
- 249.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 250.Rasmusson AM, Vasek J, Lipschitz DS, Vojvoda D, Mustone ME, Shi Q, Gudmundsen G, Morgan CA, Wolfe J, Charney DS. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29:1546–1557. doi: 10.1038/sj.npp.1300432. [DOI] [PubMed] [Google Scholar]
- 251.Ravaglia G, Forti P, Maioli F, Boschi F, Bernardi M, Pratelli L, Pizzoferrato A, Gasbarrini G. The relationship of dehydroepiandrosterone sulfate (DHEAS) to endocrine-metabolic parameters and functional status in the oldest-old. Results from an Italian study on healthy freeliving over-ninety-year-olds. J Clin Endocrinol Metab. 1996;81:1173–1178. doi: 10.1210/jcem.81.3.8772596. [DOI] [PubMed] [Google Scholar]
- 252.Ravaglia G, Forti P, Maioli F, Boschi F, Cicognani A, Bernardi M, Pratelli L, Pizzoferrato A, Porcu S, Gasbarrini G. Determinants of functional status in healthy Italian nonagenarians and centenarians: a comprehensive functional assessment by the instruments of geriatric practice. J Am Geriatr Soc. 1997;45:1196–1202. doi: 10.1111/j.1532-5415.1997.tb03769.x. [DOI] [PubMed] [Google Scholar]
- 253.Reddy DS, Kaur G, Kulkarni SK. Sigma (sigma1) receptor mediated antidepressant-like effects of neurosteroids in the Porsolt forced swim test. Neuroreport. 1998;9:3069–3073. doi: 10.1097/00001756-199809140-00028. [DOI] [PubMed] [Google Scholar]
- 254.Regelson W, Kalimi M. Dehydroepiandrosterone (DHEA)--the multifunctional steroid. II. Effects on the CNS, cell proliferation, metabolic and vascular, clinical and other effects. Ann N Y Acad Sci. 1994;719:564–575. doi: 10.1111/j.1749-6632.1994.tb56860.x. [DOI] [PubMed] [Google Scholar]
- 255.Reus VI, Wolkowitz OM, Roberts E, Chan T, Turetsky N, Manfredi F. Dehydroepiandrosterone (DHEA) and memory in depressed patients. Neuropsychopharmacology. 1993;9:66S. [Google Scholar]
- 256.Rhodes ME, Li PK, Flood JF, Johnson DA. Enhancement of hippocampal acetylcholine release by the neurosteroid dehydroepiandrosterone sulfate: an in vivo microdialysis study. Brain Res. 1996;733:284–286. doi: 10.1016/0006-8993(96)00751-2. [DOI] [PubMed] [Google Scholar]
- 257.Rhodes ME, Li PK, Burke AM, Johnson DA. Enhanced plasma DHEAS, brain acetylcholine and memory mediated by steroid sulfatase inhibition. Brain Res. 1997;773:28–32. doi: 10.1016/s0006-8993(97)00867-6. [DOI] [PubMed] [Google Scholar]
- 258.Ritsner M, Maayan R, Gibel A, Weizman A. Differences in blood pregnenolone and dehydroepiandrosterone levels between schizophrenia patients and healthy subjects. Eur Neuropsychopharmacol. 2007;17:358–365. doi: 10.1016/j.euroneuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 259.Ritsner M, Gibel A, Ram E, Maayan R, Weizman A. Alterations in DHEA metabolism in schizophrenia: two-month case-control study. Eur Neuropsychopharmacol. 2006;16:137–146. doi: 10.1016/j.euroneuro.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 260.Ritsner M, Maayan R, Gibel A, Strous RD, Modai I, Weizman A. Elevation of the cortisol/dehydroepiandrosterone ratio in schizophrenia patients. Eur Neuropsychopharmacol. 2004;14:267–273. doi: 10.1016/j.euroneuro.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 261.Ritsner MS, Gibel A, Ratner Y, Tsinovoy G, Strous RD. Improvement of sustained attention and visual and movement skills, but not clinical symptoms, after dehydroepiandrosterone augmentation in schizophrenia: a randomized, double-blind, placebocontrolled, crossover trial. J Clin Psychopharmacol. 2006;26:495–499. doi: 10.1097/01.jcp.0000237942.50270.35. [DOI] [PubMed] [Google Scholar]
- 262.Roberts E, Fauble T. Oral dehydroepiandrosterone in multiple sclerosis. Results of a phase one, open study. In: Kalimi M, Regelson W, editors. The Biologic Role of Dehydroepiandrosterone (DHEA) Berlin: W. de Gruyter; 1990. pp. 81–94. [Google Scholar]
- 263.Robinzon B, Cutolo M. Should dehydroepiandrosterone replacement therapy be provided with glucocorticoids? Rheumatology (Oxford) 1999;38:488–495. doi: 10.1093/rheumatology/38.6.488. [DOI] [PubMed] [Google Scholar]
- 264.Rosa S, Duff C, Meyer M, Lang-Muritano M, Balercia G, Boscaro M, Topaloglu AK, Mioni R, Fallo F, Zuliani L, Mantero F, Schoenle EJ, Biason-Lauber A. P450c17 deficiency: clinical and molecular characterization of six patients. J Clin Endocrinol Metab. 2007;92:1000–1007. doi: 10.1210/jc.2006-1486. [DOI] [PubMed] [Google Scholar]
- 265.Rudman D, Shetty KR, Mattson DE. Plasma dehydroepiandrosterone sulfate in nursing home men. J Am Geriatr Soc. 1990;38:421–427. doi: 10.1111/j.1532-5415.1990.tb03540.x. [DOI] [PubMed] [Google Scholar]
- 266.Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 267.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 268.Rupprecht R, Michele Fdi, Hermann B, Strohle A, Lancel M, Romeo E, Holsboer F. Neuroactive steroids: molecular mechanisms of action and implications for neuropsychopharmacology. Brain Res Brain Res Rev. 2001;37:59–67. doi: 10.1016/s0165-0173(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 269.Safiulina D, Peet N, Seppet E, Zharkovsky A, Kaasik A. Dehydroepiandrosterone inhibits complex I of the mitochondrial respiratory chain and is neurotoxic in vitro and in vivo at high concentrations. Toxicol Sci. 2006;93:348–356. doi: 10.1093/toxsci/kfl064. [DOI] [PubMed] [Google Scholar]
- 270.Sageman S, Brown RP. 3-acetyl-7-oxo-dehydroepiandrosterone for healing treatment-resistant posttraumatic stress disorder in women: 5 case reports. J Clin Psychiatry. 2006;67:493–496. doi: 10.4088/jcp.v67n0323b. [DOI] [PubMed] [Google Scholar]
- 271.Sands DE. Further studies on endocrine treatment in adolescence and early adult life. J Ment Sci. 1954;100:211–219. doi: 10.1192/bjp.100.418.211. [DOI] [PubMed] [Google Scholar]
- 272.Sands DE, Chamberlain GH. Treatment of inadequate personality in juveniles by dehydroisoandrosterone; preliminary report. Br Med J. 1952;2:66–68. doi: 10.1136/bmj.2.4775.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 274.Schmidt PJ, Murphy JH, Haq N, Danaceau MA, St Clair L. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology. 2002;27:907–920. doi: 10.1016/s0306-4530(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 275.Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- 276.Schneider LS, Hinsey M, Lyness S. Plasma dehydroepiandrosterone sulfate in Alzheimer’s disease. Biol Psychiatry. 1992;31:205–208. doi: 10.1016/0006-3223(92)90206-f. [DOI] [PubMed] [Google Scholar]
- 277.Schumacher M, Liere P, Akwa Y, Rajkowski K, Griffiths W, Bodin K, Sjovall J, Baulieu EE. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int. 2008;52:522–540. doi: 10.1016/j.neuint.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 278.Scott LV, Salahuddin F, Cooney J, Svec F, Dinan TG. Differences in adrenal steroid profile in chronic fatigue syndrome, in depression and in health. J Affect Disord. 1999;54:129–137. doi: 10.1016/s0165-0327(98)00169-4. [DOI] [PubMed] [Google Scholar]
- 279.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Serra C. [First clinical results of application of dehydroisoandrosterone in the neuropsychiatric field.] Minerva Med. 1953;44:1731–1734. [PubMed] [Google Scholar]
- 281.Shibuya K, Takata N, Hojo Y, Furukawa A, Yasumatsu N, Kimoto T, Enami T, Suzuki K, Tanabe N, Ishii H, Mukai H, Takahashi T, Hattori TA, Kawato S. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim Biophys Acta. 2003;1619:301–316. doi: 10.1016/s0304-4165(02)00489-0. [DOI] [PubMed] [Google Scholar]
- 282.Shimada M, Yoshinari K, Tanabe E, Shimakawa E, Kobashi M, Nagata K, Yamazoe Y. Identification of ST2A1 as a rat brain neurosteroid sulfotransferase mRNA. Brain Res. 2001;920:222–225. doi: 10.1016/s0006-8993(01)03061-x. [DOI] [PubMed] [Google Scholar]
- 283.Shimizu C, Fuda H, Yanai H, Strott CA. Conservation of the hydroxysteroid sulfotransferase SULT2B1 gene structure in the mouse: pre- and postnatal expression, kinetic analysis of isoforms, and comparison with prototypical SULT2A1. Endocrinology. 2003;144:1186–1193. doi: 10.1210/en.2002-221011. [DOI] [PubMed] [Google Scholar]
- 284.Shulman LH, DeRogatis L, Spielvogel R, Miller JL, Rose LI. Serum androgens and depression in women with facial hirsutism. J Am Acad Dermatol. 1992;27:178–181. doi: 10.1016/0190-9622(92)70166-d. [DOI] [PubMed] [Google Scholar]
- 285.Sicard F, Krug AW, Ziegler CG, Sperber S, Ehrhart-Bornstein M, Bornstein SR. Role of DHEA and growth factors in chromaffin cell proliferation. Ann N Y Acad Sci. 2006;1073:312–316. doi: 10.1196/annals.1353.036. [DOI] [PubMed] [Google Scholar]
- 286.Sicard F, Ehrhart-Bornstein M, Corbeil D, Sperber S, Krug AW, Ziegler CG, Rettori V, McCann SM, Bornstein SR. Bornstein, Age-dependent regulation of chromaffin cell proliferation by growth factors, dehydroepiandrosterone (DHEA), and DHEA sulfate. Proc Natl Acad Sci U S A. 2007;104:2007–2012. doi: 10.1073/pnas.0610898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Silver H, Knoll G, Isakov V, Goodman C, Finkelstein Y. Blood DHEAS concentrations correlate with cognitive function in chronic schizophrenia patients: a pilot study. J Psychiatr Res. 2005;39:569–575. doi: 10.1016/j.jpsychires.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 288.Solerte SB, Fioravanti M, Schifino N, Cuzzoni G, Fontana I, Vignati G, Govoni S, Ferrari E. Dehydroepiandrosterone sulfate decreases the interleukin-2-mediated overactivity of the natural killer cell compartment in senile dementia of the Alzheimer type. Dement Geriatr Cogn Disord. 1999;10:21–27. doi: 10.1159/000017093. [DOI] [PubMed] [Google Scholar]
- 289.Sondergaard HP, Hansson LO, Theorell T. Elevated blood levels of dehydroepiandrosterone sulphate vary with symptom load in posttraumatic stress disorder: findings from a longitudinal study of refugees in Sweden. Psychother Psychosom. 2002;71:298–303. doi: 10.1159/000064806. [DOI] [PubMed] [Google Scholar]
- 290.Sousa A, Ticku MK. Interactions of the neurosteroid dehydroepiandrosterone sulfate with the GABA(A) receptor complex reveals that it may act via the picrotoxin site. J Pharmacol Exp Ther. 1997;282:827–833. [PubMed] [Google Scholar]
- 291.Spath-Schwalbe E, Dodt C, Dittmann J, Schuttler R, Fehm HL. Dehydroepiandrosterone sulphate in Alzheimer disease. Lancet. 1990;335:1412. doi: 10.1016/0140-6736(90)91298-o. [DOI] [PubMed] [Google Scholar]
- 292.Spivak B, Maayan R, Kotler M, Mester R, Gil-Ad I, Shtaif B, Weizman A. Elevated circulatory level of GABA(A)--antagonistic neurosteroids in patients with combat-related post-traumatic stress disorder. Psychol Med. 2000;30:1227–1231. doi: 10.1017/s0033291799002731. [DOI] [PubMed] [Google Scholar]
- 293.Spratt DI, Longcope C, Cox PM, Bigos ST, Wilbur-Welling C. Differential changes in serum concentrations of androgens and estrogens (in relation with cortisol) in postmenopausal women with acute illness. J Clin Endocrinol Metab. 1993;76:1542–1547. doi: 10.1210/jcem.76.6.8501162. [DOI] [PubMed] [Google Scholar]
- 294.Stahel PF, Smith WR, Bruchis J, Rabb CH. Peroxisome proliferator-activated receptors: “key” regulators of neuroinflammation after traumatic brain injury. PPAR Res. 2008:538141. doi: 10.1155/2008/538141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Stewart PM. Aging and fountain-of-youth hormones. N Engl J Med. 2006;355:1724–1726. doi: 10.1056/NEJMe068189. [DOI] [PubMed] [Google Scholar]
- 296.Straub RH, Konecna L, Hrach S, Rothe G, Kreutz M, Scholmerich J, Falk W, Lang B. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. J Clin Endocrinol Metab. 1998;83:2012–2017. doi: 10.1210/jcem.83.6.4876. [DOI] [PubMed] [Google Scholar]
- 297.Strauss EB, Stevenson WA. Use of dehydroisoandrosterone in psychiatric practice. J Neurol Neurosurg Psychiatry. 1955;18:137–144. doi: 10.1136/jnnp.18.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Strauss EB, Sands DE, Robinson AM, Tindall WJ, Stevenson WA. Use of dehydroisoandrosterone in psychiatric treatment; a preliminary survey. Br Med J. 1952;2:64–66. doi: 10.1136/bmj.2.4775.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- 300.Strous RD, Maayan R, Weizman A. The relevance of neurosteroids to clinical psychiatry: from the laboratory to the bedside. Eur Neuropsychopharmacol. 2006;16:155–169. doi: 10.1016/j.euroneuro.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 301.Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60:133–141. doi: 10.1001/archpsyc.60.2.133. [DOI] [PubMed] [Google Scholar]
- 302.Strous RD, Maayan R, Lapidus R, Goredetsky L, Zeldich E, Kotler M, Weizman A. Increased circulatory dehydroepiandrosterone and dehydroepiandrosterone-sulphate in first-episode schizophrenia: relationship to gender, aggression and symptomatology. Schizophr Res. 2004;71:427–434. doi: 10.1016/j.schres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 303.Strous RD, Stryjer R, Maayan R, Gal G, Viglin D, Katz E, Eisner D, Weizman A. Analysis of clinical symptomatology, extrapyramidal symptoms and neurocognitive dysfunction following dehydroepiandrosterone (DHEA) administration in olanzapine treated schizophrenia patients: A randomized, double-blind placebo controlled trial. Psychoneuroendocrinology. 2007;32:96–105. doi: 10.1016/j.psyneuen.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 304.Sunderland T, Merril CR, Harrington MG, Lawlor BA, Molchan SE, Martinez R, Murphy DL. Reduced plasma dehydroepiandrosterone concentrations in Alzheimer’s disease. Lancet. 1989;2:570. doi: 10.1016/s0140-6736(89)90700-9. [DOI] [PubMed] [Google Scholar]
- 305.Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci U S A. 2004;101:3202–3207. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Svec F, Lopez A. Antiglucocorticoid actions of dehydroepiandrosterone and low concentrations in Alzheimer’s disease. Lancet. 1989;2:1335–1336. doi: 10.1016/s0140-6736(89)91940-5. [DOI] [PubMed] [Google Scholar]
- 307.Svec F, Porter J. The effect of dehydroepiandrosterone (DHEA) on Zucker rat food selection and hypothalamic neurotransmitters. Psychoneuroendocrinology. 1997;22 Suppl 1:S57–S62. doi: 10.1016/s0306-4530(97)00004-8. [DOI] [PubMed] [Google Scholar]
- 308.Svec F, Porter JR. The actions of exogenous dehydroepiandrosterone in experimental animals and humans. Proc Soc Exp Biol Med. 1998;218:174–191. doi: 10.3181/00379727-218-44285. [DOI] [PubMed] [Google Scholar]
- 309.Svec F, Hilton CW, Wright B, Browne E, Porter JR. The effect of DHEA given chronically to Zucker rats. Proc Soc Exp Biol Med. 1995;209:92–97. doi: 10.3181/00379727-209-43883. [DOI] [PubMed] [Google Scholar]
- 310.Takebayashi M, Kagaya A, Uchitomi Y, Kugaya A, Muraoka M, Yokota N, Horiguchi J, Yamawaki S. Plasma dehydroepiandrosterone sulfate in unipolar major depression. Short communication. J Neural Transm. 1998;105:537–542. doi: 10.1007/s007020050077. [DOI] [PubMed] [Google Scholar]
- 311.Tamagno E, Guglielmotto M, Bardini P, Santoro G, Davit A, Di Simone D, Danni O, Tabaton M. Dehydroepiandrosterone reduces expression and activity of BACE in NT2 neurons exposed to oxidative stress. Neurobiol Dis. 2003;14:291–301. doi: 10.1016/s0969-9961(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 312.Tamasi V, Miller KK, Ripp SL, Vila E, Geoghagen TE, Prough RA. Modulation of receptor phosphorylation contributes to activation of peroxisome proliferator activated receptor alpha by dehydroepiandrosterone and other peroxisome proliferators. Mol Pharmacol. 2008;73:968–976. doi: 10.1124/mol.107.036780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Tannenbaum C, Barrett-Connor E, Laughlin GA, Platt RW. A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: the Rancho Bernardo Study. Eur J Endocrinol. 2004;151:717–725. doi: 10.1530/eje.0.1510717. [DOI] [PubMed] [Google Scholar]
- 314.Taylor MK, Sausen KP, Potterat EG, Mujica-Parodi LR, Reis JP, Markham AE, Padilla GA, Taylor DL. Stressful military training: endocrine reactivity, performance, and psychological impact. Aviat Space Environ Med. 2007;78:1143–1149. doi: 10.3357/asem.2151.2007. [DOI] [PubMed] [Google Scholar]
- 315.Tode T, Kikuchi Y, Hirata J, Kita T, Nakata H, Nagata I. Effect of Korean red ginseng on psychological functions in patients with severe climacteric syndromes. Int J Gynaecol Obstet. 1999;67:169–174. doi: 10.1016/s0020-7292(99)00168-x. [DOI] [PubMed] [Google Scholar]
- 316.Tourney G, Erb JL. Temporal variations in androgens and stress hormones in control and schizophrenic subjects. Biol Psychiatry. 1979;14:395–404. [PubMed] [Google Scholar]
- 317.Tsutsui K. Neurosteroids in the Purkinje cell: biosynthesis, mode of action and functional significance. Mol Neurobiol. 2008;37:116–125. doi: 10.1007/s12035-008-8024-1. [DOI] [PubMed] [Google Scholar]
- 318.Urani A, Privat A, Maurice T. The modulation by neurosteroids of the scopolamine-induced learning impairment in mice involves an interaction with sigma1 (sigma1) receptors. Brain Res. 1998;799:64–77. doi: 10.1016/s0006-8993(98)00469-7. [DOI] [PubMed] [Google Scholar]
- 319.van Goozen SH, Wiegant VM, Endert E, Helmond FA, Van de Poll NE. Psychoendocrinological assessment of the menstrual cycle: the relationship between hormones, sexuality, and mood. Archives of Sexual Behavior. 1997;26:359–382. doi: 10.1023/a:1024587217927. [DOI] [PubMed] [Google Scholar]
- 320.van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26:591–612. doi: 10.1016/s0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- 321.van Vollenhoven RF. Dehydroepiandrosterone: Uses and abuses. In: Kelley WN, Harris J, Ruddy S, Sledge CB, editors. Textbook of Rheumatology. Philadelphia, PA: W. B. Saunders Co.; 1997. pp. 1–25. [Google Scholar]
- 322.Vollenhoven RFvan, Engleman EG, McGuire JL. An open study of dehydroepiandrosterone in systemic lupus erythematosus. Arthritis Rheum. 1994;37:1305–1310. doi: 10.1002/art.1780370906. [DOI] [PubMed] [Google Scholar]
- 323.van Weerden WM, Bierings HG, van Steenbrugge GJ, de Jong FH, Schroder FH. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992;50:857–861. doi: 10.1016/0024-3205(92)90204-3. [DOI] [PubMed] [Google Scholar]
- 324.von Muhlen D, Laughlin GA, Kritz-Silverstein D, Barrett-Connor E. The Dehydroepiandrosterone And WellNess (DAWN) study: research design and methods. Contemp Clin Trials. 2007;28:153–168. doi: 10.1016/j.cct.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 325.Wang MD, Wahlstrom G, Backstrom T. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J Steroid Biochem Mol Biol. 1997;62:299–306. doi: 10.1016/s0960-0760(97)00041-1. [DOI] [PubMed] [Google Scholar]
- 326.Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab Rev. 2006;38:89–116. doi: 10.1080/03602530600569877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Weill-Engerer S, David JP, Sazdovitch V, Liere P, Eychenne B, Pianos A, Schumacher M, Delacourte A, Baulieu EE, Akwa Y. Neurosteroid quantification in human brain regions: comparison between Alzheimer’s and nondemented patients. J Clin Endocrinol Metab. 2002;87:5138–5143. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- 328.Widstrom RL, Dillon JS. Is there a receptor for dehydroepiandrosterone or dehydroepiandrosterone sulfate? Semin Reprod Med. 2004;22:289–298. doi: 10.1055/s-2004-861546. [DOI] [PubMed] [Google Scholar]
- 329.Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Brain Res Rev. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 330.Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 1998;23:617–629. doi: 10.1016/s0306-4530(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 331.Wolf OT, Neumann O, Hellhammer DH, Geiben AC, Strasburger CJ, Dressendorfer RA, Pirke KM, Kirschbaum C. Effects of a two-week physiological dehydroepiandrosterone substitution on cognitive performance and well-being in healthy elderly women and men. J Clin Endocrinol Metab. 1997;82:2363–2367. doi: 10.1210/jcem.82.7.4056. [DOI] [PubMed] [Google Scholar]
- 332.Wolkowitz OM, Reus VI. Treatment of depression with antiglucocorticoid drugs. Psychosom Med. 1999;61:698–711. doi: 10.1097/00006842-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 333.Wolkowitz OM, Reus VI. Neuropsychiatric Effects of Dehydroepiandrosterone (DHEA) In: Kalimi M, Regelson W, editors. Dehydroepiandrosterone (DHEA): Biochemical, Physiological and Clinical Aspects. Berlin: Walter De Gruyter; 2000. pp. 271–298. [Google Scholar]
- 334.Wolkowitz OM, Reus VI. Neurotransmitters, neurosteroids and neurotrophins: new models of the pathophysiology and treatment of depression. World J Biol Psychiatry. 2003;4:98–102. doi: 10.1080/15622970310029901. [DOI] [PubMed] [Google Scholar]
- 335.Wolkowitz OM, Epel ES, Reus VI. Stress hormone-related psychopathology: pathophysiological and treatment implications. World J Biol Psychiatry. 2001;2:115–143. doi: 10.3109/15622970109026799. [DOI] [PubMed] [Google Scholar]
- 336.Wolkowitz OM, Reus VI, Manfredi F, Roberts E. Antiglucocorticoid effects of DHEA-S in Alzheimer’s Disease (Reply) Am J Psychiatry. 1992;149:1126. doi: 10.1176/ajp.149.8.aj14981125. [DOI] [PubMed] [Google Scholar]
- 337.Wolkowitz OM, Kroboth P, Reus VI, Fabian TJ. Dehydroepiandrosterone in aging and mental health. In: Morrison MF, editor. Hormones, Gender and the Aging Brain: The Endocrine Basis of Geriatric Psychiatry. Cambridge: Cambridge Univ. Press; 2000. pp. 144–167. [Google Scholar]
- 338.Wolkowitz OM, Reus VI, Maninger N, Mellon SH. Dehydroepiandrosterone in the Treatment of Neuropsychiatric Conditions. Philadelphia, PA: Lippincott Williams & Wilkins; in press. [Google Scholar]
- 339.Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, Roberts E. Double-blind treatment of major depression with dehydroepiandrosterone (DHEA) Am J Psychiatry. 1999;156:646–649. doi: 10.1176/ajp.156.4.646. [DOI] [PubMed] [Google Scholar]
- 340.Wolkowitz OM, Reus VI, Roberts E, Manfredi F, Chan T, Raum WJ, Ormiston S, Johnson R, Canick J, Brizendine L, Weingartner H. Dehydroepiandrosterone (DHEA) treatment of depression. Biol Psychiatry. 1997;41:311–318. doi: 10.1016/s0006-3223(96)00043-1. [DOI] [PubMed] [Google Scholar]
- 341.Wolkowitz OM, Kramer JH, Reus VI, Costa MM, Yaffe K, Walton P, Raskind M, Peskind E, Newhouse P, Sack D, Souza EDe, Sadowsky C, Roberts E. DHEA treatment of Alzheimer’s disease: a randomized, double-blind, placebo-controlled study. Neurology. 2003;60:1071–1076. doi: 10.1212/01.wnl.0000052994.54660.58. [DOI] [PubMed] [Google Scholar]
- 342.Xilouri M, Papazafiri P. Anti-apoptotic effects of allopregnanolone on P19 neurons. Eur J Neurosci. 2006;23:43–54. doi: 10.1111/j.1460-9568.2005.04548.x. [DOI] [PubMed] [Google Scholar]
- 343.Yaffe K, Ettinger B, Pressman A, Seeley D, Whooley M, Schaefer C, Cummings S. Neuropsychiatric function and dehydroepiandrosterone sulfate in elderly women: a prospective study. Biol Psychiatry. 1998;43:694–700. doi: 10.1016/s0006-3223(97)00303-x. [DOI] [PubMed] [Google Scholar]
- 344.Yanase T, Fukahori M, Taniguchi S, Nishi Y, Sakai Y, Takayanagi R, Haji M, Nawata H. Serum dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEA-S) in Alzheimer’s disease and in cerebrovascular dementia. Endocr J. 1996;43:119–123. doi: 10.1507/endocrj.43.119. [DOI] [PubMed] [Google Scholar]
- 345.Yau JL, Rasmuson S, Andrew R, Graham M, Noble J, Olsson T, Fuchs E, Lathe R, Seckl JR. Dehydroepiandrosterone 7-hydroxylase CYP7B: predominant expression in primate hippocampus and reduced expression in Alzheimer’s disease. Neuroscience. 2003;121:307–314. doi: 10.1016/s0306-4522(03)00438-x. [DOI] [PubMed] [Google Scholar]
- 346.Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- 347.Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114:187–193. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 348.Yen SS. Dehydroepiandrosterone sulfate and longevity: new clues for an old friend. Proc Natl Acad Sci U S A. 2001;98:8167–8169. doi: 10.1073/pnas.161278698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Young AH, Gallagher P, Porter RJ. Elevation of the cortisol-dehydroepiandrosterone ratio in drug-free depressed patients. Am J Psychiatry. 2002;159:1237–1239. doi: 10.1176/appi.ajp.159.7.1237. [DOI] [PubMed] [Google Scholar]
- 350.Zhang L, Li B, Ma W, Barker JL, Chang YH, Zhao W, Rubinow DR. Dehydroepiandrosterone (DHEA) and its sulfated derivative (DHEAS) regulate apoptosis during neurogenesis by triggering the Akt signaling pathway in opposing ways. Brain Res Mol Brain Res. 2002;98:58–66. doi: 10.1016/s0169-328x(01)00315-1. [DOI] [PubMed] [Google Scholar]
- 351.Ziegler CG, Sicard F, Sperber S, Ehrhart-Bornstein M, Bornstein SR, Krug AW. DHEA reduces NGF-mediated cell survival in serum-deprived PC12 cells. Ann N Y Acad Sci. 2006;1073:306–311. doi: 10.1196/annals.1353.035. [DOI] [PubMed] [Google Scholar]
- 352.Ziegler CG, Sicard F, Lattke P, Bornstein SR, Ehrhart-Bornstein M, Krug AW. Dehydroepiandrosterone induces a neuroendocrine phenotype in nerve growth factor-stimulated chromaffin pheochromocytoma PC12 cells. Endocrinology. 2008;149:320–328. doi: 10.1210/en.2007-0645. [DOI] [PubMed] [Google Scholar]
- 353.Zinder O, Dar DE. Neuroactive steroids: their mechanism of action and their function in the stress response. Acta Physiol Scand. 1999;167:181–188. doi: 10.1046/j.1365-201x.1999.00579.x. [DOI] [PubMed] [Google Scholar]
- 354.Zubiani A, Laricchia R. [Use of dehydroisoandrosterone in psychiatry.] Minerva Med. 1953;44:344–350. [PubMed] [Google Scholar]