Abstract
 Novel near-infrared pyrimidine-fused pH fluorescent probes were prepared by an unusual barbiturate-mediated debenzoindolation and subsequent heteroannulation. A plausible mechanistic pathway is proposed and the final structures were further elucidated by 2D-NMR. All new compounds are highly fluorescent in the near-infrared region and possess excellent spectral sensitivities to environmental pH changes.
Novel near-infrared pyrimidine-fused pH fluorescent probes were prepared by an unusual barbiturate-mediated debenzoindolation and subsequent heteroannulation. A plausible mechanistic pathway is proposed and the final structures were further elucidated by 2D-NMR. All new compounds are highly fluorescent in the near-infrared region and possess excellent spectral sensitivities to environmental pH changes.
Near-infrared (NIR) cyanine dyes are increasingly employed as fluorescent probes for interrogating biological processes such as enzyme activity1, nitric oxide2, calcium3, 4, zinc5, and proton concentration.6, 7 The key idea is to incorporate different functionalities into a cyanine molecular framework to obtain environment-sensitive fluorophores where the spectral properties are influenced by the presence of specific compounds or ions in complex samples. This requires a precise choice of functionalities as well as a feasible synthetic methodology. A majority of these dyes are constructed via a well-established SN1 mechanistic pathway where the meso-chlorine atom of heptamethine dye is substituted with various nucleofugal functionalities.8 This route renders an array of fluorophores that contain highly functional aminoalkyl-5, 9, hydroxyalkyl-10, hydroxyaryl-11, thioalkyl-12, thioaryl-13 substituents that can be further conjugated to biomolecules or other metal ligands. Recently we reported the first C-C bond formation by Suzuki-coupling approach at the meso-position to give functionalized aryl-substituted fluorophores with reinforced chemical stability.14
In this paper, we report an unusual barbiturate-mediated synthesis of NIR fluorescent pH-sensitive probes.
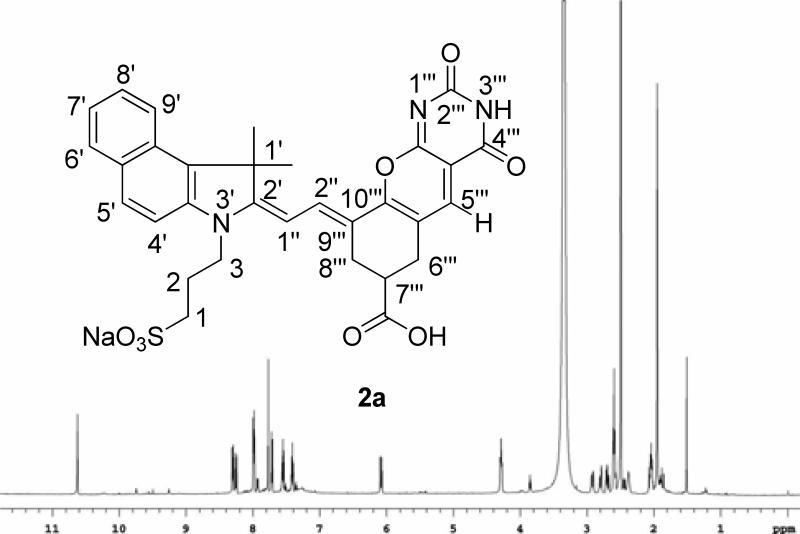
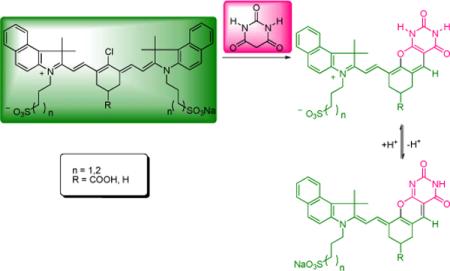
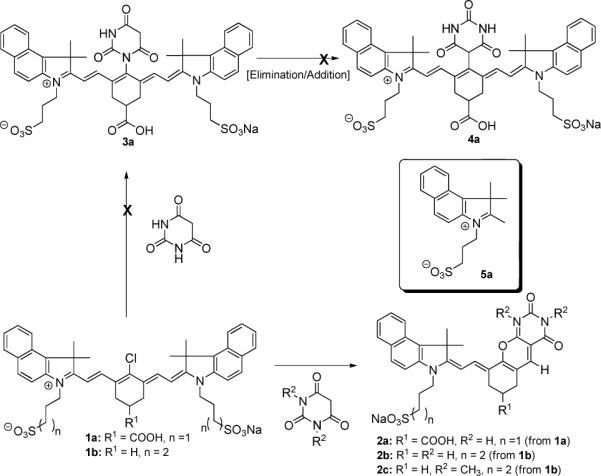
The new probes were prepared by a “one-pot” synthetic procedure (Scheme 1). Previously Nagao et. al. utilized barbituric acid and substituted chlorine atom at the meso-position of polymethine dye (IR-820) by heating at 50 °C in a 1:1 mixture of MeOH/CH2Cl2 to give the resultant dye with an absorption maxima at 795 nm with no apparent pH-responding properties.15 Surprisingly, reaction of cyanine dye 1a with barbituric acid in acetonitrile at room temperature resulted in the formation of a blue solid with absorption maxima at 690 nm and a trace amount of dye with absorption maxima at 795nm. Initially, the dye structure with absorption maxima at 690 nm was tentatively assigned as 3a as shown in Scheme 1. This assignment was based on the previous findings that N-methyl substitution at the meso-position resulted in the hypsochromic shift of approximately 200 nm from the parent dye (820 nm).8 Based on these results, it was hypothesized that 3a is first formed as a kinetic product which then subsequently converted to dye 4a via elimination/substitution process. To validate this hypothesis, dye 3a was isolated in pure form and further treated with barbituric acid under the same reaction conditions. However, the reaction failed to produce 4a from 3a. During the course of the investigation, it was also detected that the LC-MS spectrum of the reaction solution exhibited an intense peak at m/z 331 corresponding to (5a)+. However, the LC-MS analysis of the starting chloro dye 1a did not exhibit the same spectral pattern, suggesting that it is not a residue from the starting dye but rather a reaction by-product. Moreover, the tentatively assigned structure 3a did not coincide with the LC-MS and NMR data. Based on the LC-MS and 1H-NMR analysis (1D and 2D), the final structure was reassigned as 2a. The 1H-NMR spectrum exhibits two distinct singlet resonances at 7.77 ppm corresponding to H-5’’’ and 10.62 ppm corresponding to – NH proton as shown Figure 1.
Scheme 1.
Synthesis of pyrimidine-fused dyes 2a-2c.
Figure 1.
Structure and 1H-NMR spectrum of 2a.
The final structure of 2a was then further confirmed by extensive NMR experiments (COSY, NOESY, HMQC). Proton and carbon chemical shifts of 2a were assigned by analysis of COSY and HMQC and HMBC spectra. Resonances of the indole N-substituents (H1-H3), vinyl bridge (H1’’-H2’’) and H6’’’-H8’’’ chromeno fragment were identified by the through bond spin propagation. The correlated proton resonances at 4.29 and 8.25 ppm (Fig. 2a,b) were unambiguously assigned to H3 and H2’’, respectively. Proton connectivity was confirmed by the vicinal J-coupling in COSY experiment. All the connections between adjacent protons were observed and a continuous path indicates the segment of H1’’ to H2’’ at the vinyl bridge as well as H1-H3 (Fig. 2a, 2b) methylene chain in indole N-substituents. Assignments of 2a were obtained by tracing the HMQC and HMBC cross peaks to the same proton chemical shift at the COSY spectra. Connections between Figures 2a, 2c and 2b, 2d revealed that not only all aliphatic and aromatic carbon-carbon connections were observed, but also the methylene H6’’’ and H8’’’ in 2a can be assigned unambiguously. In particular, the observed simultaneous HMBC correlations of C10’’’ to 6’’’, 8’’’, 5’’’ and 2’’ protons (Fig. 2e, 2f) confirm the connection of the vinyl bridge to the chromeno-pyrimidine side. Furthermore, correlations of
Figure 2.
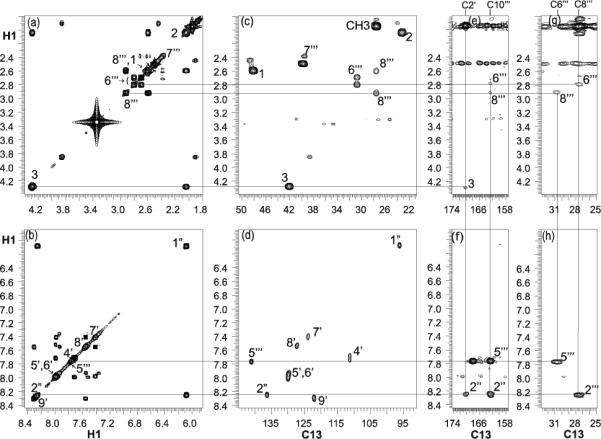
Expansion of (a) (b) COSY, (c) (d) HMQC, and (e) (f) (g) (h) HMBC of 600 MHz spectra of 2a in DMSO-d6 at 25 °C. Connections for one-bond and long range proton-carbon correlations are indicated.
H5’’’ to C10’’’, C6’’’ and cross peaks of H2’’ to C10’’’, C8’’’ in figure 2f, 2h revealed that the vinyl bridge is connected through the C9’’’-C10’’’ double bond of the chromeno segment. Likewise, multiple bond carbon-proton correlations of C2’ to 2”, 3 and 1’-methyl protons confirm the vinyl bridge-indole connections.
A plausible mechanistic pathway proposed for the synthesis of pyrimidine derivative 2a is shown in Scheme 2.
Scheme 2.
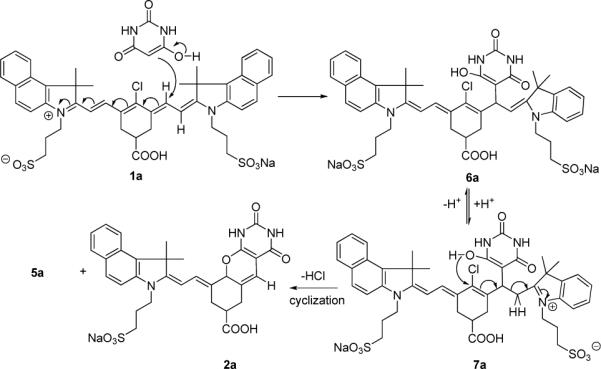
A plausible mechanistic pathway
Addition of a barbituric acid moiety at the C-2’(6’) position of heptamethine dye and subsequent enamineimine equilibrium leads to cyclization/elimination to give the final compound 2a and the by-product 5a. While nucleophilic addition at the C-2(2’’) or C-4’ position of cyanine dye is known, the addition at the C-2’(6’) has not been reported (Figure 3). The ground-state electron density of a similar heptamethine cyanine system can provide an insight into the chemical reactivity of cyanine dyes. As shown in Figure 3, the meso (C4’) carbon of the heptamethine chain was found to possess the lowest magnitude of positive character (+0.082) while the C1’ and C7’ accommodate the greatest amount of negative charge (−0.328). Accordingly, the susceptibility to nucleophilic addition at the carbons is in the decreasing order of C2(2”) > C2’(6’) > C4’.16
Figure 3.
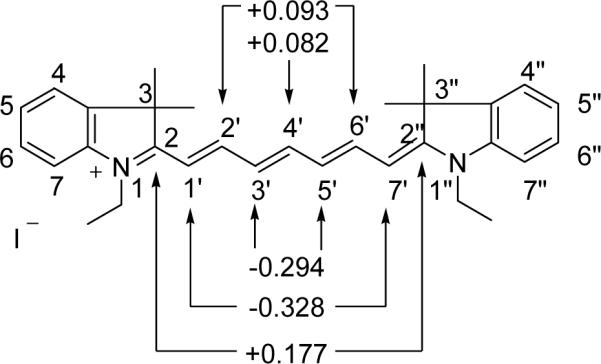
Point charges of a selected indolium heptamethine cyanine dye at selected carbon atoms obtained from ampac calculations.16
This trend is more evident in the presence of a base where the reversible addition of OH− occurs at the C-2 position.17 However, in the presence of a bulky nucleophile such as barbituric acid, the sterically hindered C-2 position facilitates the competitive addition at C-2’(6’) or C-4’ positions. In addition to the steric factor, the solvent may also play a major role on the mechanistic pathway and the resultant outcome of the reaction.
The scope of the reaction was then further exploited utilizing a commercially available dye 1b (IR-820) with barbituric acid or N,N-dimethylbarbituric acid under identical reaction conditions to give dye 2b or 2c, respectively. NMR analysis of 2b exhibited similar carbon-proton correlations as in 2a except that the H7’’’ and C7’’’ were shifted to upfield (Figure S7 in the Supporting Information) due to the absence of carboxyl group. Although the H6’’’ and H8’’’ resonances were overlapped; however, the C6’’’ and C8’’’ can be distinguished by the HMBC correlations to H5’’’ and H2’’, respectively.
Spectrophotometric analysis of 2a at various pHs revealed that both absorption and fluorescence emission are pH-dependent. The absorption spectra of 2a exhibit an intense peak at 690 nm in neutral pH with a hypsochromic shift to 605 nm at acidic pH (Fig. 4, left). The fluorescence intensity was minimal below pH 2 but increased > 6-fold as pH increased from 2 to 9 (Fig. 4, right). The pKa values of 2a determined by absorption and fluorescence titration plots (Fig. 5, left) were found to be comparable at ∼3.5, suggesting similar acidity in the ground and excited states.
Figure 4.
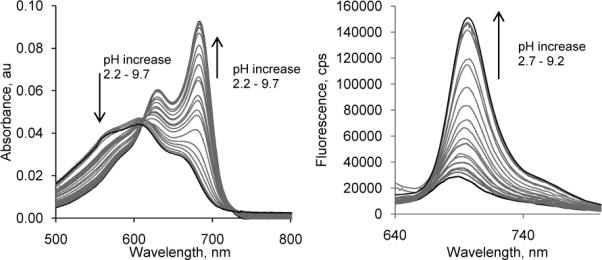
Absorption (left) and emission spectra (right) of 2a in water, excitation 605 nm.
Figure 5.
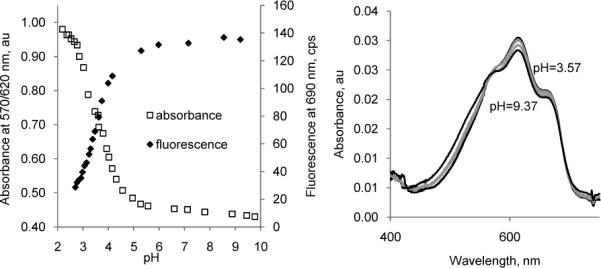
Left: Titration absorption and emission intensity diagrams of 2a in water, excitation 605 nm. From absorbance: pKa = 3.58+/− 0.00, from emission: pKa = 3.53 +/− 0.02. Right: Absorption spectra for dimethylated 2c show no change between pH 3.57 and 9.37.
A drastically low pKa (∼3.5) for N-H dissociation equilibrium of 2a compared to the typical pKa of ∼9.5 for uracile derivatives is attributed to the presence of delocalized positive charge across the cyanine molecular framework in which deprotonation results in the loss of net charge. This process is energetically favorable and thus lowers the pKa values. Deprotonation of one of the two nitrogens with the formation of uracilate and its resonant form is shown in Figure 6. Compound 2b showed a similar pH-response as 2a (Figure S8 in the Supporting Information).
Figure 6.
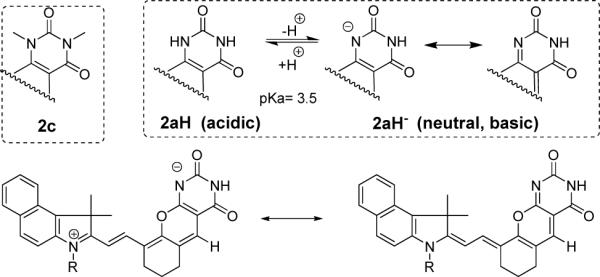
Top: Protonated and deprotonated forms of 2a from comparative analysis with 2c under acidic and basic conditions Bottom: resonance between two forms of 2a under neutral and basic conditions..
The proposed isomeric forms are more evident when compared to the optical properties of N,N-dimethylated barbituric derivative 2c. The absorption spectra of 2c in the absence of deprotonation site did not exhibit spectral sensitivities to various pHs and showed the absorption maxima at 605 nm at pH ranges from 2−9. The optical properties, including the absorption and emission spectra, as well as the quantum yield of 2c is almost identical to those of 2aH (acidic), suggesting that both 2c and 2aH have similar electronic distribution. Thus, it is evident from these studies that the presence of free -NH moiety is crucial in inducing pH-sensitivity where substitution of N-H to N-CH3 did not facilitate the spectral changes as a function of pH.
In conclusion, we demonstrated an unusual barbiturate-mediated approach to NIR fluorescent pH probes. The structural features of newly developed fluorescent pH probes suggest a novel substitution/elimination pathway. The detailed mechanistic study and its application are underway in our laboratory and will be reported in the future.
Supplementary Material
Acknowledgment
This study was supported in part by the National Institutes of Health (NIBIB R01 EB7276).
Footnotes
Supporting Information Available: Synthesis and characterization (1H NMR, 13C NMR, 1H-1H COSY, HMQC, HMBC spectra and UV-Vis and fluorescence spectra) of 2a-2c. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Weissleder R, Tung CH, Mahmood U, Bogdanov A. Nature Biotech. 1999;17(4):375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki E, Kojima H, Nishimatsu H, Urano Y, Kikuchi K, Hirata Y, Nagano T. J. Am. Chem. Soc. 2005;127(11):3684–3685. doi: 10.1021/ja042967z. [DOI] [PubMed] [Google Scholar]
- 3.Ellis AL, Mason JC, Lee HW, Strekowski L, Patonay G, Choi H, Yang JJ. Talanta. 2002;56(6):1099–1107. doi: 10.1016/s0039-9140(01)00641-5. [DOI] [PubMed] [Google Scholar]
- 4.Ozmen B, Akkaya EU. Tetrahedron Lett. 2000;41(47):9185–9188. [Google Scholar]
- 5.Kiyose K, Kojima H, Urano Y, Nagano T. J. Am. Chem. Soc. 2006;128(20):6548–6549. doi: 10.1021/ja060399c. [DOI] [PubMed] [Google Scholar]
- 6.Mason JC, Patonay G, Strekowski L. Heterocycl. Commun. 1997;3(5):409–411. [Google Scholar]
- 7.Tang B, Liu X, Xu K, Huang H, Yang G, An L. Chem. Commun. 2007;(36):3726–8. doi: 10.1039/b707173f. [DOI] [PubMed] [Google Scholar]
- 8.Strekowski L, Lipowska M, Patonay G. J. Org. Chem. 1992;57(17):4578–4580. [Google Scholar]
- 9.Peng XJ, Song FL, Lu E, Wang YN, Zhou W, Fan JL, Gao YL. J. Am. Chem. Soc. 2005;127(12):4170–4171. doi: 10.1021/ja043413z. [DOI] [PubMed] [Google Scholar]
- 10.Zhang ZR, Achilefu S. Org. Lett. 2004;6(12):2067–2070. doi: 10.1021/ol049258a. [DOI] [PubMed] [Google Scholar]
- 11.Lipowska M, Patonay G, Strekowski L. Heterocycl. Commun. 1995;1(5−6):427–430. [Google Scholar]
- 12.Bouteiller C, Clave G, Bernardin A, Chipon B, Massonneau M, Renard PY, Romieu A. Bioconjugate Chem. 2007;18(4):1303–1317. doi: 10.1021/bc0700281. [DOI] [PubMed] [Google Scholar]
- 13.Strekowski L, Mason CJ, Lee H, Gupta R, Sowell J, Patonay G. J. Heterocycl. Chem. 2003;40(5):913–916. [Google Scholar]
- 14.Lee H, Mason JC, Achilefu S. J. Org. Chem. 2006;71(20):7862–7865. doi: 10.1021/jo061284u. [DOI] [PubMed] [Google Scholar]
- 15.Nagao Y, Sakai T, Kozawa K, Urano T. Dyes Pigm. 2007;73(3):344–352. [Google Scholar]
- 16.Mason JC. Ph.D. Thesis. Georgia State University; Atlanta: 2001. [Google Scholar]
- 17.Strekowski L, Mason JC, Britton JE, Lee H, Van Aken K, Patonay G. Dyes Pigm. 2000;46(3):163–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.