Translation of recent advances
The most profound consequences of immune senescence with respect to human health are the increased susceptibility to infectious diseases and decreased vaccine efficacy. Changes in both innate and adaptive immune function converge in the reduced response to vaccination and protection against infection and related diseases. The decline in thymic output of naïve T cells diminishes responses to novel antigens, such as West Nile Virus, while clonal expansions leading to defects in the T cell repertoire are associated with blunted responses of memory T cells to conserved epitopes of the influenza virus. Recent studies on how immunologic mechanisms of protection change during aging have led to novel strategies for improving vaccine responsiveness and outcomes of infectious diseases in older adults.
Introduction
Aging of the immune system results in a loss of adaptive immune function with relative preservation of innate immunity. There is a decline in the absolute number of B cells and helper (CD4+) and cytotoxic (CD8+) T lymphocytes with a relative increase in natural killer (NK) cells, such that the overall lymphocyte count does not change with aging. Thymic involution and a decline in naïve T cell output with increasing age, together with a lifetime of exposure to a variety of pathogens, leads to a dramatic reduction in the naïve T cell pool and a relative increase the proportion of memory T cells. Within the total memory pool, arguably, the most dramatic functional changes occur in the CD8+ T cell subset, where progressive exhaustion of this compartment leads to the loss of costimulatory molecules (CD28), shortening of telomeres, and terminal differentiation to end stage cells that are resistant to the usual apoptotic mechanisms that control the size of memory T cell clones responding to a particular pathogen [1]. These changes are associated with an increase in levels of inflammatory cytokines, or “inflammaging”, which may also contribute to the dysregulation of the cell-mediated immune response [2]. This review will focus on strategies that could promote more effective adaptive immune responses to infectious agents and to prophylactic vaccines, and will also suggest possible methods to measure these responses in the older adult population.
Drivers of Immunosenescence: Role of Latent Infections
Early studies showed that human somatic cells have a finite number of replicative cycles [3] and more recently, these observations have been extended to T lymphocytes under conditions of repetitive stimulation and proliferation in long term culture (reviewed in [4]). The term replicative sensescence is used to describe the stage at which telomeres are shortened to a critical length such that a lymphocyte proliferative response can no longer be elicited and CD8+ T cells show permanently suppressed expression of the co-stimulatory molecule, CD28. Subsequent in vivo studies documented an association between increased proportions of CD8+CD28- T cells and poor antibody responses to influenza vaccination [5,6] and seropositivity for cytomegalovirus (CMV)[7]. Indeed, it has been shown that most of these CD8+CD28- memory T cells are part of large clonal expansions that are specific for persistent viruses, mainly cytomegalovirus (CMV), but also Epstein-Barr virus (EBV) and varicella zoster virus (VZV) [4]. Although these viruses typically establish asymptomatic latent infection with intermittent subclinical episodes of reactivation, suppression of disease activity is related to CD8+ T lymphocyte presence and function. By old age, excessive accumulation of these virus-specific CD8+ T lymphocytes eventually overgrows the T lymphocyte pool, compromising immune function and restricting the overall immune repertoire [8]. Restrictions in the T cell repertoire related to clonal expansions have also been demonstrated in naïve CD8+ T cells in aged mice [9,10]. A similar situation occurs in young persons infected with another virus that establishes latency, namely HIV-1. Indeed, the accumulation of clonally expanded populations of CD8+CD28- T cells occurs decades earlier in HIV-infected persons. Moreover, reminiscent of longitudinal studies in the elderly [11], the increased proportion of these cells early during the infection is actually predictive of more rapid progression to AIDS [12].
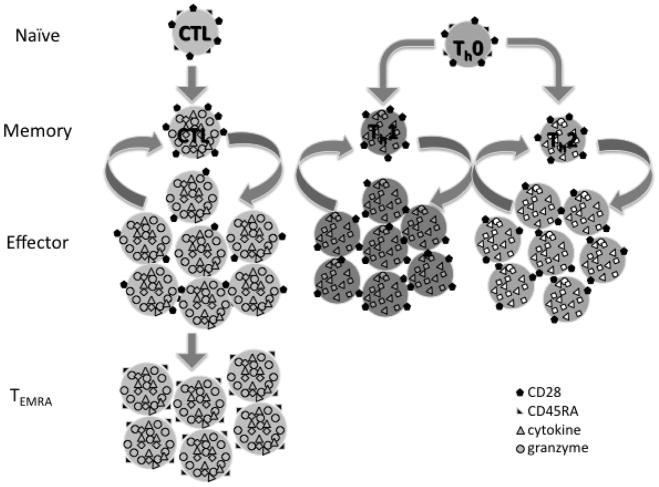
Chronic CMV infection has been suggested as the main stimulus driving the in vivo process of replicative senescence, which in many studies is associated with clonal expansion of CD8+ T cells, an inverted CD4:CD8 ratio (i.e., <1), and increased numbers of CD8+CD28- T cells [13]. Other studies showed that CMV-specific T cells are largely terminally differentiated effector memory T cells (Figure 1) expressing CD45RA (TEMRA)[14]. Although there is, in fact, direct evidence that clonally expanded CD8+ T cells are CMV-specific, it is curious that those older individuals with the so-called immune risk phenotype (CD4:CD8 ratio <1) and increased mortality actually had fewer numbers of expanded CMV-specific clones [11]. Moreover, several recent studies have questioned whether chronic CMV infection is the major driver of age-related changes in CD8+ T cells [15], and some have shown that clonally expanded CD8+ T cells may have divergent properties [16,17]. Thus, the direct mechanistic link between these changes in CD8+ T cells and the dramatic increase with age in the risk for complicated viral illnesses such as influenza, respiratory syncytial virus, and reactivation of herpes zoster to cause shingles and post-herpetic neuralgia, has yet to be made. Similarly, while these changes in CD8+ T cells have been linked to poor antibody responses to influenza vaccination, only a limited number of studies measuring CD8+ T-cell responses as correlates of protection against influenza following vaccination in older adults have been published (Reviewed in [18]).
Figure 1.
Differentiation from naïve (CD28+CD45RA+) to memory T cells (CD28+CD45RA-), which after several rounds of expansion and contraction of CTL (CD8+ T cells) become terminally differentiated (TEMRA, CD28-CD45RA+).
Efforts to develop strategies to reverse or retard the process of CD8+ T cell replicative senescence are viewed as critical, since the presence of senescent CD8+ T cells in the peripheral blood of elderly persons is associated with a variety of deleterious health effects. In addition to the reduced responses to influenza vaccinations mentioned above, high proportions of CD8+ T lymphocytes with surface phenotypes suggestive of replicative senescence, are associated with osteoporotic fractures [19]. In patients with head and neck tumors, the CD8+CD28- T lymphocyte population undergoes expansion during the period of tumor growth, but is reduced following tumor resection [20], consistent with the putative role of chronic antigenic stimulation in driving CD8+ T cell senescence. CD8+CD28- T lymphocytes have also been shown to exhibit suppressor cell functions and may alter antigen presentation [21], possibly contributing to the well-documented changes in dendritic cell function during aging [22], a change that might underlie the association between these cells and reduced vaccine efficacy. Cultures of senescent CD8+ T lymphocytes also produce high levels of certain pro-inflammatory cytokines, such as TNFα and IL-6 [23], cytokines that are associated with frailty.
In contrast to what has been observed within the CD8+ T cell population during aging, CD4+ T cells are relatively less affected by replicative senescence. Indeed, CD28 expression is preserved within this subset during aging [15]. With regard to influenza vaccination, a normal CD4+ T cell response is observed in older adults, but over the long-term, the memory CD4+ T cell response is impaired [24]. Nevertheless, these long-term memory changes do not appear to affect the duration of the serum antibody response to influenza vaccination [25]. In fact, the reported decrease in antibody titer in response to influenza vaccination in older adults, rather than correlating with changes in the helper (CD4+) T cells, which are required for antibody production, is actually associated with increased proportions of CD8+CD28- T cells [5,6]. Further, differences in antibody responses to influenza vaccination in young and older adults, instead of being directly linked to age, are actually more closely related to the number of prior vaccinations, older adults tending to be more highly vaccinated [26]. While these data may suggest that changes in CD4+ T helper activity do not affect antibody responses to influenza vaccination, measures of antibody titers and avidity in serum [27] may fail to detect the more subtle changes in antibody maturation and affinity that have been documented in the mouse model [28]. Interestingly, these changes can be corrected with the addition of a cocktail of inflammatory cytokines (TNF-α, IL-1, IL-6) [29] or poly I:C [15] to the inoculum, a strategy that may be worth pursuing to improve influenza vaccine efficacy in older adults.
Translation of age-related changes in T cells to outcomes of vaccination in older adults
A multitude of changes in the immune system occur with aging (Table 1) but the specific mechanisms that increase risk for influenza illness and limit the protective effects of vaccination are poorly understood. While serum antibody titers against influenza (by the hemagglutination inhibition assay) have been used to predict influenza vaccine efficacy and have been correlated with age-related changes in T cells, mechanistic links have not been made. This may be due to the fact that antibody-mediated protection prevents infection or “sterilizing immunity” while T-cell mediated immunity is responsible for clearance of the virus once infection occurs, thus providing “clinical protection” against disease (Box 1). The increasing recognition of the importance of T cell immunity in influenza has highlighted the limitations of antibody titers as a sole measure of vaccine efficacy in older adults, and has underscored the importance of including cellular immune measures in studying the impact of immune senescence on vaccine responsiveness [30].
Table 1. Immune changes that may impact vaccine efficacy in older adults.
| CHANGE | REFERENCE # |
|---|---|
| Constricted T cell repertoire | 7,8,9,10 |
| Increased proportion of CD8+CD28- T cells | 4,6,23 |
| Altered Th function | 24,29 |
| Decreased thymic output/reduced naïve T cell number | 36,37,38 |
| Increased inflammatory cytokines | 1,2 |
| Qualitative antibody changes | 25,26,27,28 |
| Altered dendritic cell function | 22 |
BOX 1. Immune Response to Influenza
Vaccination: Role of Dendritic Cells, Helper (Th) and Cytotoxic T Lymphocytes (CTL)
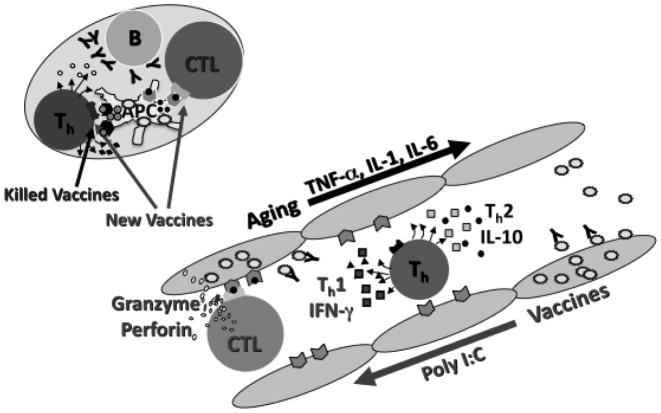
Vaccine containing influenza antigen is taken up by dendritic cells (DC) at the site of injection, the injection stimulates the “danger signal” with an inflammatory response that enhances DC activation
Viral antigen is taken by DCs to adjacent lymph nodes and peptides respectively presented on MHC I and II, to CTL and Th.
Influenza split (killed) virus vaccines generate memory Th but are a weak stimulus to CTL; new vaccines containing adjuvants such as poly I:C can activate DCs and thus provide a stronger stimulus to generate memory CTL.
Antibody responses to vaccination are strain-specific while T cell responses to conserved epitopes within the subtypes and types of influenza, are much more cross-reactive across the strains of influenza.
Influenza Infection and Viral Clearance from the Lungs
Antibody titers are a measure of the ability to prevention infection or “sterilizing immunity”.
Influenza infection occurs when there is inadequate antibody to neutralize the virus and prevent entry into the host cells.
Inside the cell, the virus sets up factories for viral replication and cellular immune defense mechanisms are required to clear the virus.
Th cells activated in the local lymph nodes direct a Th1 vs. Th2 response
With aging, the tendency toward a Th2 cytokine response in an environment of chronically elevated inflammatory cytokines (TNFa, IL-1, IL-6) may stimulate antibody production but CTL are poorly activated.
Poly I:C when added to a vaccine enhances the “danger signal” such that the recall response to influenza infection activates both Th1 and CTL to clear the virus and provide “clinical protection” against influenza illness.
CTL bind to the viral peptide-MHC I complex and granzymes and perforin released from CTL cross the intercellular space to induce apoptosis of the virus-infected cell
Vaccination studies comparing healthy young and healthy older adults show no difference in antibody response or affinity to A/H3N2 strains [27] even though strains of this influenza subtype disproportionately affect older as compared to young adults [31]. Further, our work has shown that serum antibody titers measured by the influenza hemagglutination inhibition assay do not distinguish between older individuals who subsequently develop influenza illness from those who do not [32,33]. Developing novel correlates of protection based on the cell-mediated immune response are being actively pursued but will need to overcome the challenges of technical practicality and inter-assay variability for application in large clinical studies of older populations in whom influenza outcomes can be monitored.
Given that age-related changes in immune function mainly impact on T-cell function, assays of this arm of the immune response are needed to evaluate influenza vaccine efficacy in the older population. In studying T cell function, an important consideration is that, rather than the absolute concentration of a particular cytokine at one time point, the overall regulation of the cytokine response appears to be most important for clinical protection [34]. For example, we have found that the ratio of IFNγ:IL-10 in the supernatants of ex vivo influenza-stimulated PBMC, which reflects the balance of cytokines produced by Th1 and Th2 or regulatory T cells (Treg) in the supernatants of ex vivo influenza-stimulated PBMC shows a significant correlation with protection against influenza illness in older adults [32]. Further, granzyme B, a key cytolytic mediator of the T-cell response to influenza in the lung [35], also appears to correlate with protection against influenza in vaccinated older adults [32,33] (Figure 2; Box 1). Based on these and other similar observations, the importance of eliciting robust cellular immunity is being increasingly recognized in a variety of vaccine development studies, including those directed at HIV.
Figure 2.
Generation of antibodies and memory T helper (Th) and cytotoxic T lymphocytes (CTL) in the lymph node in response to influenza vaccination (upper left). Outcome of influenza infection in cells of the respiratory tract (lower right) depends on Th1 vs. Th2 responses to vaccination and activation of CTL-mediated killing of virus-infected cells (Box 1).
Targeted interventions to improve CD8+ T cell responses
Increasing Thymic Output
Strategies to improve CD8+ T cells responses to vaccination range from those targeted to the general stabilization of different T cell subsets, to those that provide enhanced stimulation of an antigen-specific response. Since thymic involution appears to be one of the key elements of changes that occur in the immune system with aging, thymic rejuvenation techniques have been sought over many years and are now reaching the early stages of clinical trials. While a variety of growth factors, cytokines and hormonal therapies have been tested for the preservation of thymic structure and function, the most promising of these interventions appear to be keratinocyte growth factor (KGF), IL-7, and ghrelin (Reviewed in [36]). The action of KGF is to enhance IL-7 production in the thymus, by binding to the KGF receptors on thymic epithelial cells [37,38], suggesting it might have multiple beneficial effects, given the critical role of IL-7 in the development and maintenance of T cells, including long-lived memory cells, following vaccination [39]. IL-7 treatment has been shown to increase thymic output and numbers of central memory T cells (CD4+ and CD8+) and improve the antibody response to influenza vaccination in aged rhesus macaques [40]. The administration of KGF may be a strategy to locally increase IL-7 levels, thereby avoiding potential side effects of systemic IL-7 treatment. Ghrelin, a peptide hormone that binds to the receptors for growth hormone secretagogues is another evolving treatment strategy to promote thymic output in older animals [41] and reduce pro-inflammatory cytokine levels [42].
Telomerase-based enhancement of CD8+ T cell anti-viral activity
As noted above, in HIV disease, high proportions of CD8+CD28- T lymphocytes early in the infection are predictive of subsequent more rapid progression to AIDS [12], reminiscent of the predictive value of these cells as part of the so-called immune risk phenotype associated with early mortality in the very old [13]. One of the approaches to prevent or retard the generation of senescent CD8+ T cells is based on the well-documented link between telomere shortening and overall replicative potential and function of T lymhocytes [23,43]. Although telomerase is capable of elongating telomeres and is upregulated in concert with T cell activation, the activity of this enzyme is completely turned off in CD8+ T cells that are chronically stimulated in cell culture [44]. The key role of telomerase in the replicative senescence program of CD8 T cells has been documented in gene transduction studies [45] as well as more recent experiments, using a the so-called ‘TAT-2’ small molecule telomerase activator [46]. Exposure of CD8+ T cells from HIV-infected persons to TAT-2 not only increased telomerase activity and replicative potential, but also significantly enhanced a variety of anti-viral effector functions, such as antigen-specific cyotoxicity and production of IFNγ. The anti-viral functions are totally abrogated in the presence of a potent and highly specific telomerase inhibitor, demonstrating that they are mediated by telomerase itself. These observations raise the possibility that vaccines aimed at eliciting strong cellular immunity might be similarly enhanced by the incorporation of adjuvants that increase telomerase activity [30].
Adjuvanted Vaccine Formulations
Adjuvanted vaccine formulations currently approved or in clinical trials are being tested for their ability to improve the antibody response to influenza vaccination. The mechanism by which these adjuvants act on antigen-presenting cells is poorly understood, and it is not known whether they can alter the CD8+ T cell response to influenza virus. Toll-like receptor (TLR) agonists offer an alternate strategy for improving the cell-mediated immune response to influenza vaccine. In the mouse model, poly I:C (a TLR3 agonist) appears to possess a unique mechanism among other TLR agonists in its ability to enhance cognate CD4+ T cell help in aged animals [47]. As a model for pre-clinical testing of vaccine-adjuvant combinations, we have shown a dose-response improvement in the T-cell response to influenza challenge (IFNγ:IL-10 ratio and granzyme B activity) in PBMC from older adults when poly I:C was added to split-virus vaccine (SVV) (McElhaney, unpublished observations). This response was associated with an increase in IL-1, IL-6, and TNF-α levels in the supernatants of SVV/poly I:C-stimulated PBMC. However, a cocktail of these inflammatory cytokines added to SVV was found to suppress, rather than enhance, the T cell response in older adults. Thus, while inflammatory cytokines alone may be effective for reversing age-related changes in cognate CD4+ T cell function, the regulation of the release of these cytokines and the related activation of antigen-presenting dendritic cells appears to be more important for activation of CD8+ T cells.
Conclusions
Recent studies on immunosenescence have provided a more detailed understanding of cellular changes and how they might mediate reduced responses to infectious agents and to vaccines. Translating these new findings to the elderly population will require novel strategies that specifically address particular facets of cellular immune function that undergo changes with age, both within the naïve and memory populations. With the increasing age of the population, enhancing immune responses to new and previously encountered pathogens, and preventing infection via successful vaccination is critical for the healthspan of older adults. Based on some of the recent findings discussed in this review, it seems that immunologists are now well-positioned to address these challenges.
Acknowledgments
We thank Laura Haynes from the Trudeau Institute for her review of the manuscript and for sharing her experience with vaccine adjuvants in aged mouse models. Janet E. McElhaney's work is supported by National Institutes of Health Grants R01 AI068265 and U01 AI074449. Rita B. Effros' work is supported by National Institutes of Health Grant RO1 AG 023720 and by Geron Corporation and TA Therapeutics Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 4.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Is immunosenescence infectious? Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, et al. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 7.Almanzar G, Schwaiger S, Jenewein B, Keller M, Herndler-Brandstetter D, Wurzner R, Schonitzer D, Grubeck-Loebenstein B. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Chronic CMV infection drives the contraction of the T cell repertoire and dysregulated cytokine production with aging.
- 8.Ouyang Q, Wagner WM, Voehringer D, Wikby A, Klatt T, Walter S, Muller CA, Pircher H, Pawelec G. Age-associated accumulation of CMV-specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor G1 (KLRG1) Exp Gerontol. 2003;38:911–920. doi: 10.1016/s0531-5565(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 9.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Loss of T cell repertoire diversity in the aged mouse also occurs in the naïve CD8 T cell population and impairs the immune response to influenza.
- 10.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J Immunol. 2009;182:784–792. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]; *As in [9] and clonal expansions within the naïve CD8+ T cell population associated with loss of diversity.
- 11.Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Lofgren S, Ernerudh J, Pawelec G, Ferguson F, Johansson B. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]; ** Longitudinal study confirming results from other studies but importantly shows that the immune risk phenotype predicts functional outcomes.
- 12.Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, Detels R. Premature aging of T cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr. 2009;50:137–147. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–453. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 14.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 15.Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Chronic CMV infection may not explain all of the changes in CD8+ T cells that occur with aging.
- 16.Clambey ET, Kappler JW, Marrack P. CD8 T cell clonal expansions & aging: a heterogeneous phenomenon with a common outcome. Exp Gerontol. 2007;42:407–411. doi: 10.1016/j.exger.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clambey ET, White J, Kappler JW, Marrack P. Identification of two major types of age-associated CD8 clonal expansions with highly divergent properties. Proc Natl Acad Sci U S A. 2008;105:12997–13002. doi: 10.1073/pnas.0805465105. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Further evidence that chronic CMV infection may be a bystander to other mechanisms that drive CD8 T cell senescence.
- 18.McElhaney JE. Influenza vaccination in the eldery: seeking new correlates of protection and improved vaccines. Aging Health. 2008;4:603–613. doi: 10.2217/1745509X.4.6.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietschmann P, Grisar J, Thien R, Willheim M, Kerschan-Schindl K, Preisinger E, Peterlik M. Immune phenotype and intracellular cytokine production of peripheral blood mononuclear cells from postmenopausal patients with osteoporotic fractures. Exp Gerontol. 2001;36:1749–1759. doi: 10.1016/s0531-5565(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 20.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(-) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortesini R, LeMaoult J, Ciubotariu R, Cortesini NS. CD8+CD28- T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity. Immunol Rev. 2001;182:201–206. doi: 10.1034/j.1600-065x.2001.1820116.x. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal A, Agrawal S, Tay J, Gupta S. Biology of dendritic cells in aging. J Clin Immunol. 2008;28:14–20. doi: 10.1007/s10875-007-9127-6. [DOI] [PubMed] [Google Scholar]
- 23.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 24.Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- 25.Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis. 2008;197:490–502. doi: 10.1086/524146. [DOI] [PubMed] [Google Scholar]
- 26.de Bruijn IA, Remarque EJ, Beyer WE, le Cessie S, Masurel N, Ligthart GJ. Annually repeated influenza vaccination improves humoral responses to several influenza virus strains in healthy elderly. Vaccine. 1997;15:1323–1329. doi: 10.1016/s0264-410x(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 27.de Bruijn IA, Remarque EJ, Jol-van der Zijde CM, van Tol MJ, Westendorp RG, Knook DL. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J Infect Dis. 1999;179:31–36. doi: 10.1086/314540. [DOI] [PubMed] [Google Scholar]
- 28.Alter-Wolf S, Blomberg BB, Riley RL. Deviation of the B cell pathway in senescent mice is associated with reduced surrogate light chain expression and altered immature B cell generation, phenotype, and light chain expression. J Immunol. 2009;182:138–147. doi: 10.4049/jimmunol.182.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Primary defects in B cell function occur with aging in the mouse model and are independent of age-related changes in helper T cells function.
- 29.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]; *A cocktail of inflammatory cytokines that have been shown to increase with aging, enhanced rather than suppressed helper T cell responses.
- 30.Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25:599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 31.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. Jama. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 32.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]; *Granzyme B and the IFN-γ:IL-10 ratio correlate with protection against influenza following vaccination.
- 33.McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, Barry MB, Kleppinger A, Wang Y, Bleackley RC. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009;27:2418–2425. doi: 10.1016/j.vaccine.2009.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Highlights the importance of regulation of the cytokine response, thus, the kinetics of production of different cytokines should be considered in the development of correlates of protection.
- 35.Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005;174:5332–5340. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- 36.Aspinall R, Mitchell W. Reversal of age-associated thymic atrophy: treatments, delivery, and side effects. Exp Gerontol. 2008;43:700–705. doi: 10.1016/j.exger.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Min D, Panoskaltsis-Mortari A, Kuro OM, Hollander GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109:2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]; *KGF provides an alternative strategy to systemic IL-7 therapy to sustain thymic output with aging.
- 38.Min D, Taylor PA, Panoskaltsis-Mortari A, Chung B, Danilenko DM, Farrell C, Lacey DL, Blazar BR, Weinberg KI. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]; *As in [37] and demonstrates feasibility in people.
- 39.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 40.Aspinall R, Pido-Lopez J, Imami N, Henson SM, Ngom PT, Morre M, Niphuis H, Remarque E, Rosenwirth B, Heeney JL. Old rhesus macaques treated with interleukin-7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res. 2007;10:5–17. doi: 10.1089/rej.2006.9098. [DOI] [PubMed] [Google Scholar]
- 41.Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117:2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Ghrelin appears to be another strategy to sustain thymic output and has other benefits in the aging process.
- 42.Dixit VD, Yang H, Cooper-Jenkins A, Giri BB, Patel K, Taub DD. Reduction of T cell-derived ghrelin enhances proinflammatory cytokine expression: implications for age-associated increases in inflammation. Blood. 2009 doi: 10.1182/blood-2008-09-181255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouyang Q, Wagner WM, Wikby A, Walter S, Aubert G, Dodi AI, Travers P, Pawelec G. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol. 2003;23:247–257. doi: 10.1023/a:1024580531705. [DOI] [PubMed] [Google Scholar]
- 44.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8 T cells following repeated encounters with the same antigenic stimulus. Clin Immunol. 2002;105:117–125. doi: 10.1006/clim.2002.5271. [DOI] [PubMed] [Google Scholar]
- 45.Dagarag M, Evazyan T, Rao N, Effros RB. Genetic manipulation of telomerase in HIV-specific CD8+ T cells: enhanced antiviral functions accompany the increased proliferative potential and telomere length stabilization. J Immunol. 2004;173:6303–6311. doi: 10.4049/jimmunol.173.10.6303. [DOI] [PubMed] [Google Scholar]
- 46.Fauce SR, Jamieson BD, Chin AC, Mitsuyasu RT, Parish ST, Ng HL, Kitchen CM, Yang OO, Harley CB, Effros RB. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Telomerase treatment can restore telomere length and antiviral responses in humans.
- 47.Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol. doi: 10.4049/jimmunol.0804226. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Poly I:C appears to have an advantage over other TLR agonists for improving helper T cell function required for antibody production in aged mice.