Abstract
Objective
Increased neuronal density in prefrontal, parietal and temporal white matter of schizophrenia subjects is thought to reflect disordered neurodevelopment; however, it is not known if this cellular alteration affects the cingulate cortex and whether similar changes exist in bipolar disorder.
Method
82 postmortem specimens (bipolar 15, schizophrenia 22, control 45) were included in this clinical study. Densities for two neuronal markers, neuron-specific nuclear protein (NeuN) and neuregulin 1 alpha (NRG), were determined in white matter up to 2.5 mm beneath the anterior cingulate cortex; NeuN+ density was also determined for a subset of cases in prefrontal cortex. Changes during normal development were monitored in a separate cohort of 14 brains.
Results
Both the schizophrenia and bipolar cohorts demonstrated a two-fold increase in NeuN+ density in cingulate white matter; this effect could be attributed to ~25% of cases that exceeded the second standard deviation from controls. Similar changes were observed in prefrontal cortex. In contrast, NRG+ neuronal density was unaltered. Cases with increased NeuN+ densities in 2-dimensional (2D) counts also showed a pronounced, > 5-fold elevation in NeuN+ nuclei/mm3. Additionally, the developmental cohort demonstrated a 75% decline in NeuN+ neuronal density during the first postnatal year, but was stable thereafter.
Conclusions
Increased neuronal density in white matter of cingulate cortex in schizophrenia provides further evidence that this alteration occurs in multiple cortical areas. Similar changes in some cases with bipolar illness suggests that the two disorders may share a common underlying defect in late prenatal or early postnatal neurodevelopment.
INTRODUCTION
Alterations of subcortical white matter in schizophrenia are thought to reflect the neurodevelopmental origins of this disorder. For example, recent neuroimaging studies describe changes in morphology and integrity of white matter tracts that are present from the earliest stages of these disorders (1, 2). There is also progressive reduction of frontal lobe white matter (3) and—in striking contrast to overlying cortex—a relative glucose hypermetabolism in unmedicated schizophrenia subjects (4). Of note, the 22q11.2 deletion syndrome (22qDS)—one of the strongest genetic risk factors for bipolar disorder and schizophrenia (5)—was associated with heterotopias and numerous ectopic neurons scattered throughout the white matter of the frontal lobe (6). While heterotopias and other markers of more severe migration defects are not known to be characteristic of schizophrenia, these changes observed in some cases may be indicative of a more subtle developmental defect that is present in a larger proportion of the patient population.
In support of this idea, increased densities, or altered distribution, of white matter neurons have been reported in 11 out of 13 studies on schizophrenia postmortem brain (7–20). Some of these cells are thought to be derived from the embryonic subplate, a transient structure during pre- and early postnatal development that plays a pivotal role in orderly development of cortical connectivity (21, 22). The majority of studies reported either a generalized increase of neurons (7, 9, 10, 13–16, 20), or redistribution towards deeper white matter space (9, 19). See also the Supplemental Table for an overview. However, there is little consensus as to which regions of subcortical white matter are definitively affected. This lack of agreement is not unexpected due to methodological differences between studies—even those studies which focused on the same brain region vary in terms of sulcogyral position sampled, neuronal markers employed, tissue processing, and characteristics of the clinical cohort. Furthermore, there is evidence that a robust increase in white matter neuron density is not a uniform feature in psychosis, but limited to approximately one third of cases diagnosed with schizophrenia (8). Therefore, while it is difficult to reach a firm conclusion regarding white matter neuron changes in schizophrenia, the studies—when taken together—strongly suggest that this cell population is affected and may reflect defective brain development during the second half of gestation, when neurons are migrating through the interstitial zone, or, alternatively, during early postnatal life, when the majority of these cells undergo apoptosis (23).
The goal of the present study was: (1) to determine whether or not white matter neuron abnormalities extend beyond the diagnosis of schizophrenia and also occur in bipolar disorder; (2) to estimate the proportion of schizophrenia or bipolar cases affected by this alteration; (3) to find out whether these alterations affect widespread portions of the frontal lobe, including the white matter space beneath the cingulate cortex, a region strongly implicated in the pathophysiology of both bipolar disorder and schizophrenia (24, 25); (4) to monitor white matter neuron densities during the course of normal development and aging. We chose NeuN (neuron-specific nuclear antigen) (26), because neurons immunoreactive for this protein were previously reported to be elevated in frontal lobe white matter in schizophrenia (13, 14), and the alpha splice form of neuregulin 1, a schizophrenia risk gene implicated in neuronal migration (27–29). (5) Finally, because microglial activation appears to be associated with increased NeuN-immunoreactive neurons in white matter in multiple sclerosis, we also assessed immunoreactivity for Iba1 (ionized calcium-binding adaptor molecule 1), a ubiquitous marker for microglia that is upregulated upon activation (30, 31).
METHODS
Brain Tissue
82 postmortem brain specimens (22 schizophrenia, 15 bipolar, and 45 controls) (Table 1) were obtained from the Harvard Brain Tissue Resource Center (HBTRC, Belmont, MA). A prospective design was chosen to ensure that each specimen that newly arrived at the brain bank would be processed in a similar manner. Tissue blocks (~1.5 – 2 cm3) were resected from each case from cingulum at an anterior-posterior level immediately rostral to the central sulcus, corresponding to the caudal sector of Brodmann area (BA) 24 (Fig. 1A). Care was taken to include portions of the adjacent corpus callosum and cingulate gyrus in each resected block. Immediately after resection, blocks were immersion-fixed in 10% phosphate-buffered formalin for a period of 4 days, then cryoprotected for 1 week in phosphate-buffered 30% sucrose; subsequently, tissue was frozen and 25 μm coronal sections cut on a cryostat. In addition, for a select group of clinical cases with elevated white matter neuron densities in cingulate (and controls matched for age, gender and autolysis time), additional sections were obtained from paraffin-embedded superior frontal gyrus (BA 9) (Table 1). Furthermore, fresh frozen cingulate tissue was also obtained for such cases for counting nuclei (see below) (Table 1).
Table 1.
| Cingulate cortex (BA 33) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | N | Age | PMI | Gender | Hemisphere | APD | MS | ADD |
| Years | Hours | M / F | L / R | % | % | % | ||
| Bipolar | 15 | 74.3±2.29 | 22.5±2.40 | 4 / 11 | 9 / 6 | 80% (4/5) | 60% (3/5) | 40% (2/5) |
| Schizophrenia | 22 | 67.8±2.94 | 20.6±1.71 | 9 / 13 | 8 / 14 | 64% (7/11) | 36% (4/11) | 36% (4/11) |
| Control | 45 | 69.8±1.87 | 23.2±0.85 | 24 / 21 | 21 / 24 | 0 | 0 | 0 |
| Cingulate cortex for nuclei counts | ||||||||
|---|---|---|---|---|---|---|---|---|
| Years | Hours | M / F | L / R | % | % | % | ||
| Schizophrenia | 6 | 69.5±6.53 | 24.0±2.44 | 2 / 4 | 3 / 3 | 80% (4/5) | 40% (2/5) | 20% (1/5) |
| Control | 5 | 71.8±6.68 | 21.5±4.10 | 2 / 3 | 2 / 3 | 0 | 0 | 0 |
| Dorsolateral prefrontal cortex (BA 9) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Years | Hours | M / F | L / R | % | % | % | ||
| Schizophrenia | 6 | 69.2±6.60 | 21.5±2.52 | 3 / 3 | 4 / 2 | 100% (4/4) | 50% (2/4) | 25% (1/4) |
| Control | 6 | 66.7±6.10 | 25.4±2.67 | 3 / 3 | 3 / 3 | 0 | 0 | 0 |
| Prefrontal cortex (BA 10) | |||||
|---|---|---|---|---|---|
| Age Range | Hours | M / F | L / R | ||
| Perinatal / infant | 4 | 40 ewg - 9 months | 15±6.58 | 2 / 2 | 4 / 0 |
| Child & adult | 10 | 2 – 51 years | 15.7±6.06 | 6 / 4 | 9 / 1 |
PMI = postmortem interval
ewg = estimated weeks gestation
APD = antipsychotic drugs (typical and atypical)
MS = mood stabilizers (lithium, depakote)
ADD = antidepressant drugs (Zoloft, Paxil, Wellbutrin, Effexor, trazodone, remeron)
Age and PMI expressed as mean ± standard error.
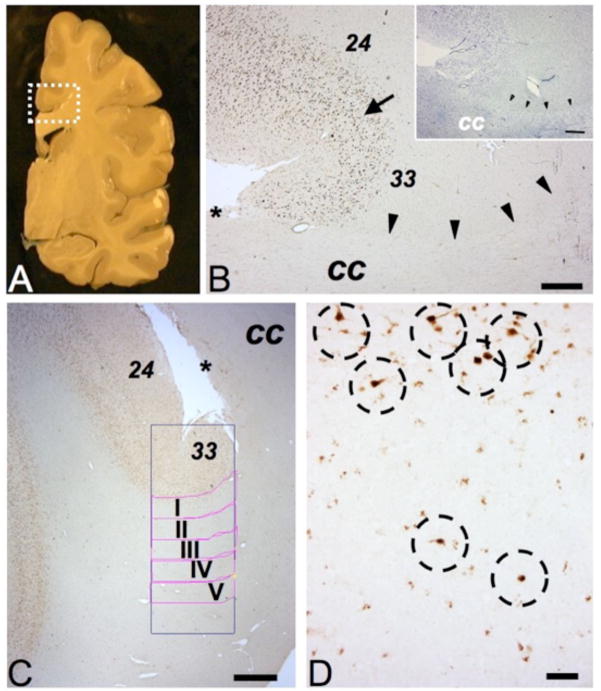
Figure 1. Gray and white matter structures surrounding cingulate cortex.
(A) Representative coronal slab of a hemisphere (postmortem), rectangle outlines approximate position and size of tissue block destined for present study. (Image courtesy of Mr. L. Fernandez, Harvard Brain and Tissue Resource Center.) (B) Parallel sections stained for NeuN immunoreactivity or Nissl (insert) showing cingulate cortex with dorsal BA24 and ventral BA33, transition marked by arrow. See text for details. The border between the cingulate white matter (including cingulum bundle) and the fiber tracts of bordering corpus callosum (cc) is demarcated by arrowheads. (C) Digitized image from region-of-interest, including the position of counting frame and white matter compartments I-V each 500 micron deep and 2000 microns wide (see Methods). * in B, C marks dorsal line of corpus callosum with induseum griseum. (D) Higher resolution image from superficial white matter bordering cingulate cortex, showing several NeuN+ neurons (marked by dotted circles), including two cells completely surrounded by WM tissue (bottom). Size bars (in μm), (B) 500, (C) 1000 and (D) 100.
For the developmental study, unfixed frozen tissue blocks from the pole of the frontal lobe were obtained for 14 additional subjects ranging in age from 40 weeks of gestation to 51 years of age. None of these subjects had been diagnosed with neurological or psychiatric disease. We chose the pole of the frontal lobe, instead of the anterior cingulate, as this region can be identified unambiguously in both young and old brain. These samples were obtained from the Brain and Tissue Bank for Developmental Disorders, University of Maryland (National Institute of Child Health and Human Development Contract NO1-HD-8-3284) and the University of California, Davis (Center for Neuroscience) (Table 1). Tissue blocks were fixed in 4% formaldehyde for 20 hours, after which 25 μm coronal sections were cut and directly mounted on glass slides; subsequently, tissue was fixed again for 5 minutes in 100% acetone and re-hydrated prior to immunostaining. This additional acetone-based fixation was necessary due to the fragility of some of the younger samples.
See Supplementary Methods for details on immunohistochemistry, microscopy (including both 2D and 3D-like counting procedures), and statistics.
RESULTS
In all cases and controls, the massive callosal fiber tracts were readily discernable from bordering cingulum bundle and subcortical white matter (Fig. 1A, B). The ventral portion of the cingulate cortex in cases and controls showed the expected bipartition with the six-layered cortex of BA24, bordering upon the less laminated BA33, which is defined by “merging” of prominent pyramidal cell laminae of lower layer III and layer V (32) (Fig. 1B, C).
NeuN+ neuronal density is increased in cingulate white matter in schizophrenia and bipolar disorder
In sections immunostained for NeuN, the gray/white matter border was readily discernable, particularly at low magnification (Fig. 1B, C). The NeuN+ neurons were defined by robust immunoreactivity in the nucleus and more lightly stained cytoplasm and processes, which were overall more readily discernable in gray matter than in white matter (Fig. 1D). In the gray matter, a large majority of neurons were NeuN+, resulting in a Nissl-type staining patterns (Fig. 1B, C).
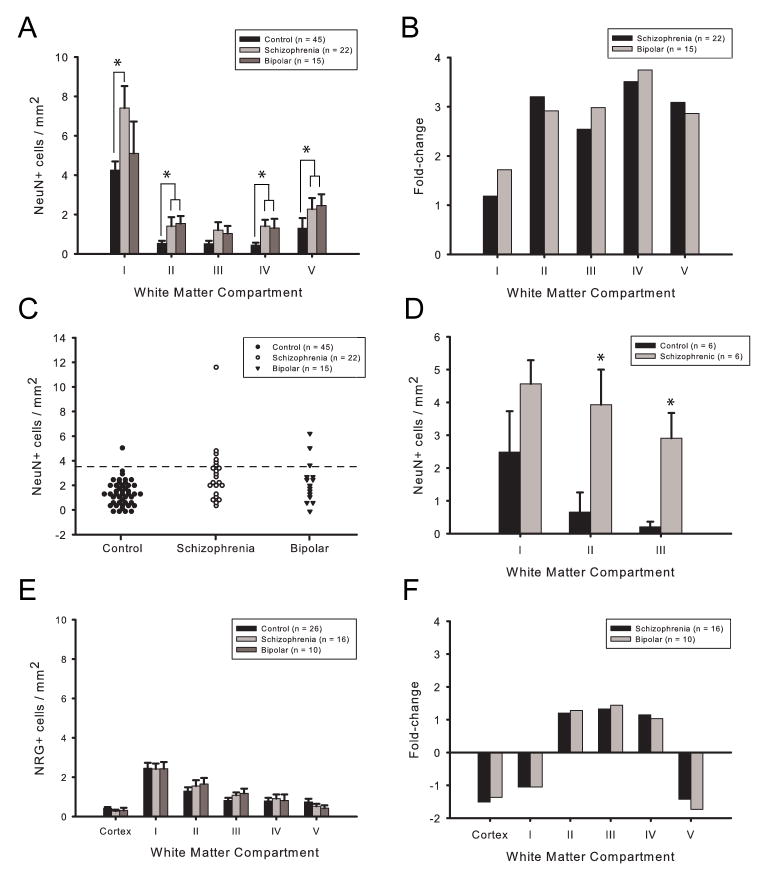
In controls, NeuN+ neuronal density was highest in the most superficial white matter (4.25 cells/mm2), and subsequently declined in the deeper white matter to densities of 0.794 cells/mm2 (Fig. 3A). Both schizophrenia and bipolar groups were observed to have greater overall mean NeuN+ neuronal densities compared to controls (2.87 ± 0.50 and 2.29 ± 0.44 cells/mm2 versus 1.28 ± 0.13 cells/mm2; mean ± standard error). Diagnosis had a significant effect on overall mean NeuN+ density (K = 14.0; p = 0.001). The increased NeuN+ densities observed in schizophrenia and bipolar groups were both significantly different from control density (Q = 13.6, p < 0.01 and Q = 7.24, p < 0.01), but the two patient groups were not significantly different from one another (Q = − 2.51, p > 0.05). When each compartment was analyzed individually, diagnosis was found to have a significant effect on NeuN+ density in all five compartments (K = 6.32, p = 0.042; K = 10.5, p = 0.005; K = 6.83, p = 0.033; K = 11.8, p = 0.003; K = 12.4, p = 0.002), with fold increases ranging from 1.17 to 3.75 (Fig. 3A, B). The increased NeuN+ densities observed in schizophrenia were significantly different from controls in all compartments (Q = 8.83, p < 0.01; Q 7.93, p < 0.01; Q = 8.43, p < 0.01; Q = 10.8, p < 0.01; and Q = 9.90, p < 0.01). NeuN+ densities in the bipolar cohort were significantly different from controls in compartments II–V (Q = 8.90, p < 0.01; Q = 4.42, p < 0.01; Q = 6.87, p < 0.01; and Q = 9.66, p < 0.01).
Figure 3. Increased NeuN+ neuronal density in white matter in schizophrenia and bipolar disorder.
(A) NeuN+ neuronal density (presented as cells/mm2, y-axis) in white matter compartments I–V (x-axis) beneath cingulate cortex of controls (N = 45, black), schizophrenia (N = 22, light gray), and bipolar disorder (N = 15, dark gray). Notice decline in NeuN+ neuronal density in white matter > 500 micron apart from overlying cortex (compartments II–V). Also notice higher overall densities in the two disease groups, as compared to controls. (B) Fold-change in NeuN+ neuronal densities in white matter compartments of schizophrenia (black) and bipolar subjects (gray), relative to control cohort. A -2-fold change is equivalent to a 50% decrease. (C) Frequency distribution of NeuN+ cell densities in cingulate white matter of the 3 cohorts, as indicated. Dotted line demarcates second standard deviations above the mean from controls. (D) NeuN+ neuronal density in the superior frontal gyrus white matter of 6 schizophrenia and 6 control subjects; the 6 clinical cases also showed increased NeuN+ density in the cingulate (see Results). (E) NRG+ cell densities, including (F) fold-change from controls, in cingulate cortex and white matter beneath BA33 of schizophrenia and bipolar subjects, as indicated (control N = 26; schizophrenia N = 16; bipolar N = 10). Notice that fold-changes in NRG+ neuronal densities in the clinical cohorts are < 2 from controls.
Notably, 7 out of 22 schizophrenia subjects and 3 of 15 bipolar subjects demonstrated overall NeuN+ densities more than two standard deviations above the control mean (Fig. 3C); this increased proportion of cases exceeding the 95th percentile of control mean was significant for both schizophrenia (χ2 = 12.3, p < 0.0001) and bipolar groups (χ2 = 5.7, p = 0.017). We conclude that approximately 25% of cases diagnosed with bipolar disorder or schizophrenia show a more robust increase in cingulate white matter neuron densities. Importantly, when these 10 clinical cases were removed from the analyses, differences between the remaining schizophrenia, bipolar and the control subjects for each of the five compartments were not significant when corrected for multiple comparisons. No demographic or other factor was identifiable that could distinguish between the 10 cases with white matter neuron density above the 95th percentile of controls and the remaining patients. For example, 3/7 (4/7) affected schizophrenia subjects were male (female), while all 3 affected bipolar subjects were female. Approximately one half of these cases were from the left and from the right hemisphere, and ages were not significantly different from the cohort means.
Also of note, NeuN+ neurons were present in the deeper white matter in only 24/45 of control subjects, but were found in 12/15 of bipolar subjects and in 20/22 schizophrenia subjects. These differences in distribution were significant for both schizophrenia (χ2 = 10.3, p = 0.001) and bipolar groups (χ2 = 8.4, p = 0.004). These findings also resonate with several previous reports suggesting elevated neuronal numbers in deeper white matter (8, 19, 20). Among the 4 cases that were not on antipsychotic medication prior to death, 3 cases (75%) were found to harbor NeuN+ neurons in white compartments III–V. Therefore, the much higher proportion of subjects with NeuN+ neurons in deeper white matter in the two disease cohorts is unlikely to be due to antipsychotic medication. No association was observed between NeuN+ densities and age (r = 0.050) or PMI (r = −0.029), and there was no robust correlation with age-of-onset (r = −0.36, R2 = 0.14), albeit information about this clinical parameter was available only for a subset of cases. Additionally, there was no significant difference in NeuN+ density between left and right hemispheres (U = 154.5; p = 0.480). Furthermore, there were no significant differences between genders within the 3 diagnostic categories (control: U = 65.5, p = 0.552; schizophrenia: U = 36.0, p = 0.335; bipolar: U = 18.0, p = 0.087). Finally, among 5 factors tested by ANCOVA (diagnosis, hemisphere, PMI, age, gender) only diagnosis had a significant effect on overall NeuN density (F = 8, p = 0.0007). Therefore, the observed increases in white matter NeuN+ neuronal density in the clinical samples of our cohort are most likely related to the disease itself and not due to other factors.
NRG+ neuronal density is unaltered in cingulate white matter in schizophrenia and bipolar disorder
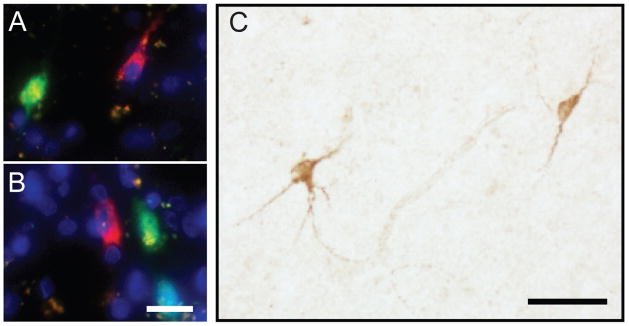
Next, we wanted to study additional white matter neuron populations. To this end, we noticed that while immunostaining with the anti-Neuregulin α (NRG) antibody (see Methods) resulted in robust labeling of a subpopulation of bi- or multipolar neurons residing in cortex and white matter (Fig. 2C), none of the NeuN+ neurons in white matter (Fig. 2A, B) expressed NRG. Therefore, the white matter neuronal population expressing NeuN does not overlap with NRG+ cells, which is consistent with previous reports that NeuN is expressed in some—but not all—subpopulations of neurons (33, 34).
Figure 2. NeuN and NRG1α(NRG) immunoreactivity define two non-overlapping neuronal subpopulations.
(A, B) Digitized images from cingulate white matter sections triple-stained with the nucleophilic dye, DAPI (blue), and anti-NeuN (green) and anti-NRG (red) antibodies, showing both NeuN+ and NRG + neurons, but no double-labeled cells. (C) Representative image from white matter of section processed for NRG immunoreactivity with DAB/peroxidase labeling (brown), showing neurons with robust staining of cytoplasm and dendrites. Size bars: (A, B) 20 μm, and (C) 50 μm.
We determined the density of NRG+ neurons in both gray and white matter in a subset of 10 bipolar, 16 schizophrenia, and 26 control subjects. (We had previously observed increased NeuN+ white matter neurons in these clinical cases, similar to the ones reported above for the larger cohorts (data not shown).) For each of the 3 diagnostic groups, NRG+ density was lower in the cortical gray matter of cingulate as compared to the white matter (Fig. 3E), which is consistent with a previous report demonstrating that NRG+ neurons are less common in gray matter compared to white in adult human brain (27). In white matter, both schizophrenia and bipolar groups demonstrated similar mean NRG+ densities compared to controls (1.34 ± 0.099 and 1.31 ± 0.18 cells/mm2 versus 1.26 ± 0.13 cells/mm2), and there was no significant effect of diagnosis on overall, or compartment-specific, NRG+ density (K = 1.8, p = 0.406) (Fig. 3E, F). These findings further confirm that the observed increase in NeuN+ density in cingulate white matter bipolar disorder and schizophrenia is specific for that neuronal population.
NeuN+ neuronal density in cingulate gray matter is unaltered in schizophrenia and bipolar disorder
NeuN+ densities in gray matter were typically 50–150 fold higher than in white matter, but there was no significant correlation between the two (bipolar r = 0.0005; schizophrenia r = 0.004; control r = 0.0319; all subjects r = 0.0013) (Supplemental Figure 1). We conclude that the variation in neuronal densities in cingulate white matter is dissociated from any density changes of the overlying cortex.
Size of NeuN+ neurons in cingulate white matter is unaltered in schizophrenia and bipolar disorder
To rule out that the observed increase in NeuN+ density in bipolar disorder and schizophrenia could be an artifact due to a larger size of the NeuN-immunoreactive cells, we determined the average area of these cells in bipolar, schizophrenia, and control subjects, and no significant differences between diagnostic groups were found (0.092 ± 0.006; 0.099 ± 0.003; and 0.101 ± 0.003 mm2) (F = 1.09; p = 0.367).
NeuN+ neuronal density is also increased in dorsolateral prefrontal cortex (BA9) white matter in schizophrenia
In the control subjects of the current study, the average density of NeuN+ neurons in cingulate white matter near the sulcal bottom ranged from 0.79 to 4.31 cells/mm2. This contrasts with a previous study conducted on the middle banks of dorsolateral prefrontal cortex that reported NeuN+ densities of up to 40 cells/mm2 in sections of similar thickness (14). These differences are not unexpected, because the number of interstitial white matter neurons tend to decline towards sulcal fundi and, moreover, the thickness of the neuronal layer in the embryonic interstitial zone (future white matter), is thinner underneath the cingulate in comparison to the lateral cortex (23).
Therefore, we wanted to determine whether neuronal densities show regional differences in frontal lobe white matter and whether increased NeuN+ densities in the cingulate could be extrapolated to other areas. Indeed, NeuN+ densities in white matter beneath the sulcal bottom of the superior frontal gyrus (BA9) of 5 μm thick sections (see Methods) ranged from 0.21–2.48 cells/mm2 in control subjects. If adjusted for differences in section thickness, NeuN+ neuron density in BA9 is 5-fold higher when compared to the cingulate. NeuN+ densities in the 6 schizophrenia cases showed a significant increase in BA9, compared to controls matched for age, gender and autolysis time (Fig. 3D); these same 6 subjects also demonstrated increased NeuN+ densities in cingulate cortex as compared to the 6 controls (mean = 4.01 cells/mm2 versus 1.28 cells/mm2). Therefore, altered NeuN+ densities in psychosis potentially affect widespread portions of the frontal lobes.
NeuN+ nuclei are increased in cingulate white matter in schizophrenia
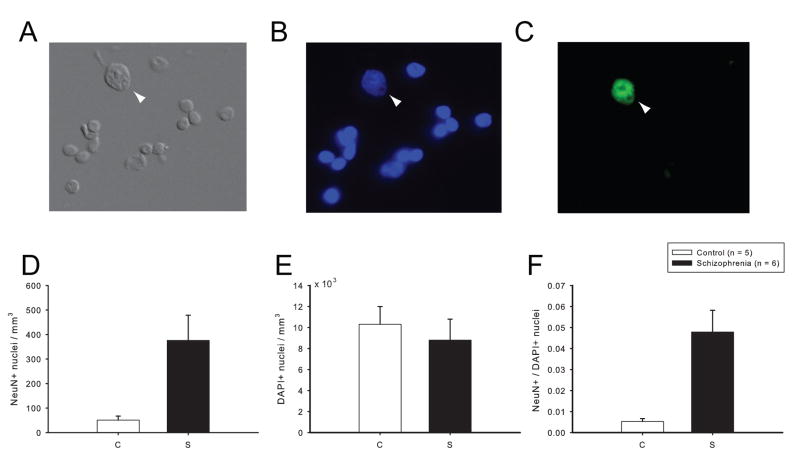
Next, we wanted to confirm that the elevated two-dimensional (2D) NeuN+ cell density can be replicated with a three-dimensional counting method. However, optical dissectors are not ideal for white matter neuron quantifications because neuronal numbers are low overall and unevenly distributed (35). To bypass these limitations, we calculated the total number of NeuN+ and NeuN− nuclei extracted from a standardized column of white matter tissue from frozen, unfixed anterior cingulate (see Methods) (Fig. 4A–C). These additional studies were conducted on 6 schizophrenia cases that—in comparison to 5 matched controls—showed a 6.6-fold increase in overall NeuN+ densities as determined by the 2D approach (schizophrenia: 3.59 ± 0.70 cells/mm2; controls: 0.55 ± 0.41 cells/mm2, Mann-Whitney U = 31, p < 0.05). As shown in Fig. 4D, these 6 schizophrenia cases also showed a significant, 7.4-fold increase in NeuN+ nuclei per mm3 white matter tissue (schizophrenia: 376 ± 253 nuclei/mm3; controls: 51 ± 38 cells/mm3; Mann-Whitney U = 0, p = 0.006). Across the 11 subjects, there was a good linear correlation between the 2D and 3D counts (R = + 0.72). In contrast to the observed increase in NeuN+ nuclei, the schizophrenia group did not show increased numbers of overall nuclei (neuronal and non-neuronal) (Fig. 4B, E). This increase in NeuN+ nuclei—in the absence of an increase in DAPI+ nuclei—makes sense given that less than 4% of the white matter nuclei population is neuronal (Fig. 4D–F), and, thus, an increase in neuronal nuclei will not necessarily be reflected by an increase in all nuclei. From these findings, we draw two conclusions: First, increased numbers and densities of NeuN+ white matter neurons in our clinical cases are apparent when employing two different methodologies. Second, this alteration is highly specific, because overall numbers of cells in the white matter remained unaltered.
Figure 4. Isolation and quantification of nuclei from cells residing in white matter.
(A–C) Representative digitized photomicographs from purified nuclei extracted from cingulate white matter (see Methods). (A) Differential Interference Contrast (DIC), (B) DAPI nucleophilic dye counterstain, and (C) NeuN immunoreactivity. Arrow demarcates nucleus of NeuN+ white matter neuron, notice comparatively larger size and the prominent nucleolus. Bar in (C) = 20 μm. (D–F) Bar graphs showing numbers of (D) NeuN+ and (E) DAPI stained nuclei per mm3 white matter, and (F) proportion of NeuN+ nuclei in 6 schizophrenia and 5 control subjects (see Results). Data expressed as mean ± S.E.M.
Iba1 immunoreactivity is unaltered in cingulate white matter in schizophrenia and bipolar disorder
Next, we wanted to explore whether the observed alterations in NeuN+ density in white matter were related to a change in the microglia population, because a recent study in multiple sclerosis suggests a potential link between microglial activation—a hallmark of the inflammatory response in brain—and increased white matter neuron densities (30). To this end, we graded immunoreactivity for the microglial marker, Iba1 (see Methods), on a scale of 0–4 in all schizophrenia and bipolar subjects, together with a subset of controls (Supplemental Figure 2A). We found no significant difference in Iba1 immunoreactivity between groups, nor was it associated with NeuN+ density in white matter (Supplementary Figure 2B, C). We conclude that increased presence of NeuN+ neurons in white matter of a subset of schizophrenia and bipolar subjects is not associated with microglial activation.
NeuN+ neuronal density in white matter decreases during development
Interstitial white matter neurons of adult brain are thought to be vestiges of the subplate, a transient structure of the developing brain that shows a progressive involution starting in the 3rd trimester (36). Previous studies utilized Nissl, Golgi and acetylcholinesterase staining to determine developmental changes in subplate neuronal densities subjacent to visual, somatosensory, motor, and prefrontal cortices (23, 37). While there is general consensus that neuronal density in subcortical white matter declines during the first year of postnatal life, the specific timing of this event varied depending upon the cell markers used.
Presently, it is not known whether NeuN-immunoreactive white matter neurons undergo changes in numbers or densities during development. Therefore, we monitored the temporal course of NeuN+ neuronal density in postmortem specimens of a new postmortem cohort ranging in age from 40 weeks of gestation to 51 years of age (Table 1). To avoid variability of frontal lobe anatomy as potential confound, we focused on white matter space underneath the lobe’s rostral pole, which is unambiguously identifiable in both young and old brain. There was a steep decline in NeuN+ densities during the first year of life, after which the density plateaued and remained relatively constant throughout childhood, adolescence, and adulthood (Fig. 5). The mean NeuN+ density for the 10 subjects > 1 year (14.6 ± 6.42 cells/mm2) demonstrated a 76.7% decrease as compared to the mean density of 4 subjects < 1 year (62.7 ± 29.6 cells/mm2); this difference was significant (U = 0, p = 0.005; 1 degree of freedom).
Figure 5. White matter NeuN+ neuronal density demonstrates an age-associated decline during development.
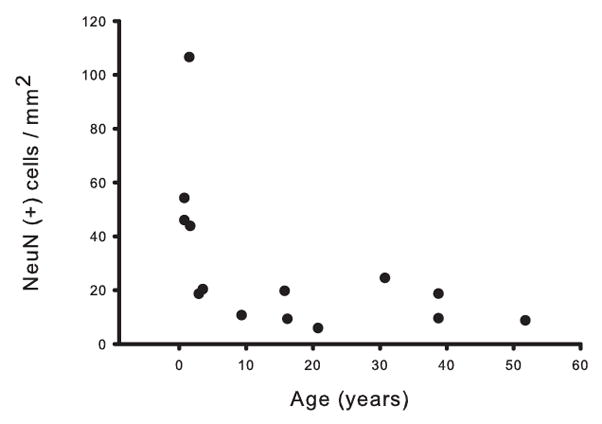
Age (in years) was plotted against overall NeuN+ density (cells/mm2) for each of the 14 subjects in the developmental cohort. Notice the steep decline in density during the first postnatal year.
DISCUSSION
We report that approximately 25% of subjects diagnosed with bipolar disorder or schizophrenia show an elevated density of NeuN-immunoreactive cells in cingulate white matter. These changes were observed both by 2- and 3-dimensional counting techniques. The alteration was specific, because (i) no association between NeuN+ densities in gray and white matter was observed and (ii) the density of a different population of white matter neurons, immunoreactive for NRG, in the disease cohorts was not significantly different from controls. Increased NeuN+ density in cingulate white matter was paralleled by a similar increase in dorsolateral prefrontal white matter, suggesting that these cellular alterations affect widespread portions of the frontal lobe. Additionally, the density of NeuN+ neurons in white matter during normal development was found to decline during the first year of postnatal life, but remained stable thereafter, suggesting that the observed alterations in the schizophrenia and bipolar cohorts may reflect a disturbance during the late prenatal or early postnatal period. In multiple sclerosis, chronic lesions within subcortical white matter harbor increased numbers of neurons (30) in conjunction with increased microglia, but, in the present study, there was no association between these two white matter cell populations.
The fact that NeuN+ neuronal density was increased in cingulate white matter not only in schizophrenia, but also in bipolar disorder, was unexpected because two earlier studies on the dorsolateral prefrontal cortex had reported negative findings (11, 17). However, the overall smaller sample sizes in those studies may have precluded the recognition of a subgroup of approximately 20–30% of affected subjects that, according to the present study, show a robust elevation in white matter neuron numbers. In either case, the findings presented here add to the emerging evidence that neurodevelopmental mechanisms play a role in some cases with bipolar illness (38) and, moreover, that such mechanisms may represent a common underlying pathophysiology of psychotic spectrum disorders.
The mechanism by which this increase in white matter neurons in psychosis occurs is unclear, but failure of neuronal migration or decreased cell death could be involved (7–20); the latter hypothesis adds to the notion of faulty apoptosis in some cases with psychosis (39). It is unlikely that the increase in NeuN+ cells in white matter of clinical cases is due to newly generated neurons later in life, because—thus far—evidence for neurogenesis in postnatal human cerebral cortex is lacking (40).
Strikingly, a number of the emerging susceptibility genes and pathways for schizophrenia and bipolar disorder—including Disrupted-in-schizophrenia 1 (DISC1) and the Neuregulin 1 (NRG1)-erbB4 tyrosine kinase—are important regulators of migration, positioning, and formation of functional circuits of immature neurons during the development of cerebral cortex and other forebrain structures (41, 42). As mentioned above, the 22q11.2 deletion syndrome (22qDS), a genetic cause for bipolar disorder and schizophrenia (5)—is associated with heterotopias and numerous ectopic neurons scattered throughout the white matter of the frontal lobe (6). Therefore, further study of these risk genes and genetic syndromes may provide important insights into the mechanisms underlying white matter abnormalities in psychotic disorders.
The pathophysiological consequences of increased white matter neuron density in cingulate and prefrontal cortices remain unknown. Notably, white matter neurons surviving preprogrammed cell death during development are known to become functionally integrated with the circuitry of the mature cortex (43) and, furthermore, remain interconnected with the thalamus (44), suggesting that any excess of white matter neurons in schizophrenia and bipolar disorder could indeed impair cortical and subcortical circuitries. It is intriguing to speculate that elevated neuronal numbers in adult white matter reflect a defect present from the prenatal period onwards, because a subset of the neurons residing beneath the developing cortical plate in the fetal brain are essential for orderly development of the GABAergic circuitry in the overlying cortex (45). Therefore, the dysfunction of inhibitory interneurons—a core component in the pathophysiology of some types of psychosis (46, 47)—ultimately may have its roots in a neurodevelopmental defect of the future white matter.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. F.M. Benes, Mr. George Tejada, and Mr. Louis Fernandez from the Harvard Brain Tissue Resource Center at McLean Hospital, Belmont MA; Dr. E.G. Jones, Dr. W.E. Bunney Jr, and Mr. Phong Nguyen from the Center for Neuroscience at the University of California at Davis; Dr. R. Zielke and Mr. R. Johnson of the Brain and Tissue Bank for Developmental Disorders, University of Maryland, for providing brain tissues, and Dr. A. Chang and Dr. B.D. Trapp for generously providing us with Iba1 antibody. Supported by grants from the National Institute of Mental Health.
Footnotes
Financial Disclosures The author reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler CM, Adams J, DelBello MP, Holland SK, Schmithorst V, Levine A, Jarvis K, Strakowski SM. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry. 2006;163(2):322–4. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- 2.Szeszko PR, Ardekani BA, Ashtari M, Kumra S, Robinson DG, Sevy S, Gunduz-Bruce H, Malhotra AK, Kane JM, Bilder RM, Lim KO. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162(3):602–5. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- 3.Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60(6):585–94. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 4.Buchsbaum MS, Buchsbaum BR, Hazlett EA, Haznedar MM, Newmark R, Tang CY, Hof PR. Relative glucose metabolic rate higher in white matter in patients with schizophrenia. Am J Psychiatry. 2007;164(7):1072–81. doi: 10.1176/ajp.2007.164.7.1072. [DOI] [PubMed] [Google Scholar]
- 5.Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Dev Disabil Res Rev. 2008;14(1):3–10. doi: 10.1002/ddrr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiehl TR, Chow EW, Mikulis DJ, George SR, Bassett AS. Neuropathologic Features in Adults with 22q11.2 Deletion Syndrome. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn066. [DOI] [PubMed] [Google Scholar]
- 7.Akbarian S, Bunney WE, Jr, Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry. 1993;50(3):169–77. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- 8.Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr, Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53(5):425–36. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- 9.Akbarian S, Vinuela A, Kim JJ, Potkin SG, Bunney WE, Jr, Jones EG. Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry. 1993;50(3):178–87. doi: 10.1001/archpsyc.1993.01820150016002. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SA, Volk DW, Lewis DA. Increased density of microtubule associated protein 2-immunoreactive neurons in the prefrontal white matter of schizophrenic subjects. Schizophr Res. 1996;19(2–3):111–9. doi: 10.1016/0920-9964(96)88521-5. [DOI] [PubMed] [Google Scholar]
- 11.Beasley CL, Cotter DR, Everall IP. Density and distribution of white matter neurons in schizophrenia, bipolar disorder and major depressive disorder: no evidence for abnormalities of neuronal migration. Mol Psychiatry. 2002;7(6):564–70. doi: 10.1038/sj.mp.4001038. [DOI] [PubMed] [Google Scholar]
- 12.Bertram I, Bernstein HG, Lendeckel U, Bukowska A, Dobrowolny H, Keilhoff G, Kanakis D, Mawrin C, Bielau H, Falkai P, Bogerts B. Immunohistochemical evidence for impaired neuregulin-1 signaling in the prefrontal cortex in schizophrenia and in unipolar depression. Ann N Y Acad Sci. 2007;1096:147–56. doi: 10.1196/annals.1397.080. [DOI] [PubMed] [Google Scholar]
- 13.Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry. 2003;8(9):769, 821–31. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- 14.Eastwood SL, Harrison PJ. Interstitial white matter neuron density in the dorsolateral prefrontal cortex and parahippocampal gyrus in schizophrenia. Schizophr Res. 2005;79(2–3):181–8. doi: 10.1016/j.schres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick B, Conley RC, Kakoyannis A, Reep RL, Roberts RC. Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: An unbiased cell-counting study. Synapse. 1999;34(2):95–102. doi: 10.1002/(SICI)1098-2396(199911)34:2<95::AID-SYN2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Kirkpatrick B, Messias NC, Conley RR, Roberts RC. Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. J Nerv Ment Dis. 2003;191(9):563–7. doi: 10.1097/01.nmd.0000087181.61164.e1. [DOI] [PubMed] [Google Scholar]
- 17.Molnar M, Potkin SG, Bunney WE, Jones EG. MRNA expression patterns and distribution of white matter neurons in dorsolateral prefrontal cortex of depressed patients differ from those in schizophrenia patients. Biol Psychiatry. 2003;53(1):39–47. doi: 10.1016/s0006-3223(02)01456-7. [DOI] [PubMed] [Google Scholar]
- 18.Nobuhara K, Okugawa G, Minami T, Takase K, Yoshida T, Yagyu T, Tajika A, Sugimoto T, Tamagaki C, Ikeda K, Sawada S, Kinoshita T. Effects of electroconvulsive therapy on frontal white matter in late-life depression: a diffusion tensor imaging study. Neuropsychobiology. 2004;50(1):48–53. doi: 10.1159/000077941. [DOI] [PubMed] [Google Scholar]
- 19.Rioux L, Nissanov J, Lauber K, Bilker WB, Arnold SE. Distribution of microtubule-associated protein MAP2-immunoreactive interstitial neurons in the parahippocampal white matter in subjects with schizophrenia. Am J Psychiatry. 2003;160(1):149–55. doi: 10.1176/appi.ajp.160.1.149. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K, Ikeda K, Iritani S, Ueno H, Niizato K. Distribution of neuropeptide Y interneurons in the dorsal prefrontal cortex of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(2):379–83. doi: 10.1016/j.pnpbp.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Kostovic I, Judas M. Transient patterns of cortical lamination during prenatal life: do they have implications for treatment? Neurosci Biobehav Rev. 2007;31(8):1157–68. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Kanold PO. Transient microcircuits formed by subplate neurons and their role in functional development of thalamocortical connections. Neuroreport. 2004;15(14):2149–53. doi: 10.1097/00001756-200410050-00001. [DOI] [PubMed] [Google Scholar]
- 23.Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297(3):441–70. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- 24.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290–5. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61(7):649–57. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 26.Sarnat HB, Nochlin D, Born DE. Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev. 1998;20(2):88–94. doi: 10.1016/s0387-7604(97)00111-3. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein HG, Lendeckel U, Bertram I, Bukowska A, Kanakis D, Dobrowolny H, Stauch R, Krell D, Mawrin C, Budinger E, Keilhoff G, Bogerts B. Localization of neuregulin-1alpha (heregulin-alpha) and one of its receptors, ErbB-4 tyrosine kinase, in developing and adult human brain. Brain Res Bull. 2006;69(5):546–59. doi: 10.1016/j.brainresbull.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Sei Y, Ren-Patterson R, Li Z, Tunbridge EM, Egan MF, Kolachana BS, Weinberger DR. Neuregulin1-induced cell migration is impaired in schizophrenia: association with neuregulin1 and catechol-o-methyltransferase gene polymorphisms. Mol Psychiatry. 2007;12(10):946–57. doi: 10.1038/sj.mp.4001994. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125(1):127–42. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang A, Smith MC, Yin X, Fox RJ, Staugaitis SM, Trapp BD. Neurogenesis in the chronic lesions of multiple sclerosis. Brain. 2008;131(Pt 9):2366–75. doi: 10.1093/brain/awn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57(1):1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 32.Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359(3):490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- 33.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116(1):201–11. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 34.Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blumcke I. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44(10):1167–71. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- 35.Benes FM, Lange N. Two-dimensional versus three-dimensional cell counting: a practical perspective. Trends Neurosci. 2001;24(1):11–7. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- 36.Samuelsen GB, Larsen KB, Bogdanovic N, Laursen H, Graem N, Larsen JF, Pakkenberg B. The changing number of cells in the human fetal forebrain and its subdivisions: a stereological analysis. Cereb Cortex. 2003;13(2):115–22. doi: 10.1093/cercor/13.2.115. [DOI] [PubMed] [Google Scholar]
- 37.Meyer G, Wahle P, Castaneyra-Perdomo A, Ferres-Torres R. Morphology of neurons in the white matter of the adult human neocortex. Exp Brain Res. 1992;88(1):204–12. doi: 10.1007/BF02259143. [DOI] [PubMed] [Google Scholar]
- 38.Sanches M, Keshavan MS, Brambilla P, Soares JC. Neurodevelopmental basis of bipolar disorder: A critical appraisal. Prog Neuropsychopharmacol Biol Psychiatry. 2008 doi: 10.1016/j.pnpbp.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Jarskog LF, Glantz LA, Gilmore JH, Lieberman JA. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):846–58. doi: 10.1016/j.pnpbp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122(1):133–43. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 41.Kamiya A, Tan PL, Kubo K, Engelhard C, Ishizuka K, Kubo A, Tsukita S, Pulver AE, Nakajima K, Cascella NG, Katsanis N, Sawa A. Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: a candidate for psychiatric illnesses. Arch Gen Psychiatry. 2008;65(9):996–1006. doi: 10.1001/archpsyc.65.9.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7(6):575–80. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 43.Torres-Reveron J, Friedlander MJ. Properties of persistent postnatal cortical subplate neurons. J Neurosci. 2007;27(37):9962–74. doi: 10.1523/JNEUROSCI.1536-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277(2):195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- 45.Kanold PO, Shatz CJ. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 2006;51(5):627–38. doi: 10.1016/j.neuron.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Berretta S, Munno DW, Benes FM. Amygdalar activation alters the hippocampal GABA system: “partial” modelling for postmortem changes in schizophrenia. J Comp Neurol. 2001;431(2):129–38. doi: 10.1002/1096-9861(20010305)431:2<129::aid-cne1060>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34(5):944–61. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.