Abstract
The aim of this cross sectional study was to delineate age-associated kinematic and kinetic gait patterns of normal walking, and to test the hypothesis that older adults exhibit gait patterns that reduce generative mechanical work expenditures (MWEs). We studied 52 adult Baltimore Longitudinal Study of Aging participants (means age 72 ± 9, from 60 to 92 years) who could walk 4-meters unaided. 3-dimensional kinematic and kinetic parameters assessed during rotation-defined gait periods were used to estimate MWEs for the rotation of lower extremities about the medial-lateral (ML) and anterior-posterior (AP) axes of proximal joints, which represent MWEs in the AP and ML sides, respectively. Relationships between gait parameters and age were examined using regression analysis with adjustments for walking speed, sex, height, and weight. Older age was associated with slower self-selected walking speed (p < 0.001), shorter stride length (p < 0.001), and greater propensity of landing flat-footed (p = 0.003). With older age, hip generative MWE for thigh rotation was lower about the AP axis (hip abduction and adduction) during stance (p = 0.010) and higher about the ML axis (hip extension and flexion) during late stance (p < 0.001). Knee absorptive MWE for shank rotation about the AP axis (knee abduction and adduction) during early stance was also lower with older age (p < 0.003). These age-related gait patterns may represent a compensatory effort to maintain balance and may also reflect mobility limitations.
Keywords: Mechanical Work Expenditure, Walking Speed, Gait Phase, Medial-lateral Stability
Introduction
Older adults exhibit typical changes in gait patterns that are generally considered compensatory to the loss of motor function that occurs with aging (Polcyn et al., 1998; Lord et al., 1996). Understanding adaptation strategies employed by older adults is important for the development of interventions capable of correcting and preventing age-associated mobility limitations.
Self-preferred walking speed declines with age and is a well-accepted marker of overall mobility performance (Teixeira-Salmela et al., 2008; Alexander, 1996; Kerrigan et al., 2001; Dingwell et al., 2006). Walking slower may also be thought of as a compensatory strategy aimed at increasing stability, avoiding falls or reducing the energetic cost of mobility (Pavol et al., 1999; Duff-Raffaele et al., 1996). However, whether the presence of sub-clinical impairments that typically affect older persons stimulates the emergence of less energetically costly walking patterns has not been fully investigated.
Different approaches have been used to study walking energetics. The simple analysis of lower extremity joint moments provides a rough approximation of the amount of muscle activity, but does not assess joint direction or speed. By studying mechanical joint moments and angular velocities together, we can estimate the contribution of muscle group activations to joint acceleration and deceleration during gait (Teixeria-Salmela et al., 2008; Chen et al. 1997; Winter, 1983). Total generated mechanical work during acceleration and deceleration has been reported to predict gait disorders (Winter et al., 1990; Siegel et al., 2004; McGibbon et al., 2001; Teixeira-Salmela et al., 2001). To study mechanical work during normal walking, Winter and colleagues (Winter et al., 1990) divided the gait cycle into periods whose boundaries were identified as the times of peak power. The present study identifies the gait phase based on rotations of lower extremities about the medial-lateral (ML) axes of proximal joints to assess gait phases that reflect well identifiable stages during walking. Dominant muscle group activities are related with the rotations of lower extremities about the ML axes of proximal joints (extension and flexion) during walking. Also, the significant age effects on the rotations of lower extremities about the AP axes of proximal joints (abduction and adduction) were previously reported (Dean et al., 2007; Siegel et al., 2004; McGibbon et al., 2001; Teixeira-Salmela et al., 2001). To understand walking mechanisms referred to as mechanical energy expenditures (MWEs), assessing kinetic gait parameters for the rotations about the ML and AP axes of proximal joints for the lower extremities are necessary. For the specified gait stages, we calculated MWEs for the rotations of lower extremities about the ML and AP axes of proximal joints. These MWEs can be interpreted as muscle group activities of lower extremities in the AP and ML sides during operationalized gait periods. Focusing on rotation-based gait phases may be more informative than estimating energy expenditure over the entire gait cycle.
Using data collected in joint kinematically-defined phases of gait during normal walking in a cohort of older adults, we examined the hypothesis that older age is associated with gait patterns that employ less generative MWE.
Methods
Study Design
The Baltimore Longitudinal Study of Aging (BLSA) is a longitudinal cohort study conducted by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. This study was approved by the Medstar Research Institution Review Board, and was conducted with informed consent obtained from all participants. The present report is based on cross-sectional data that were collected between January and April 2008. During regular study visits, all BLSA participants underwent testing in the Clinical Research Branch Gait Laboratory following a standardized protocol.
Participants
The study sample was comprised of adult participants ages 60 years and older who were able to follow instructions and safely complete a 4-meter walking trial without assistance of a person or a walking aid. All participants enrolled in this study were free of severe osteoarthritis (OA) of the hip or knee and had no history of stroke or Parkinson disease. Participants who had BMI over 40 were excluded because of technical difficulty positioning pelvic markers.
Experimental measurement
The gait analysis is based on general principles of gait testing (Winter et al., 1990; Kerrigan et al., 2005; Teixeira-Salmela et al., 2008). Participants were dressed in form-fitting, non-reflective clothing and tested in bare feet. Reflective markers were affixed to 22 anatomical landmarks: anterior and posterior superior iliac spines, iliac crests, medial and lateral knee, medial and lateral ankle, toe, and heel, and lateral wands over the mid-femur and mid-tibia. The medial markers for knee and ankle were used for identifying knee and ankle joint centers during the static trial and then removed for the dynamic trials. A Vicon 3D motion capture system with 10-cameras (Oxford Metrics Ltd., Oxford, U.K.) was used to measure the position of the markers in 3 dimensions at a sampling frequency of 60 Hz, and filtered with fourth-order zero-lag Butterworth filter using a cutoff at 6 Hz.
Ground reaction forces were measured synchronously with motion capturing using 2 staggered force platforms (Advanced Mechanical Technologies, Inc., Watertown, MA, USA; sampling rate 1080 Hz) imbedded in the walkway. After positioning the marker set, participants were asked to walk straight along the 10-m long laboratory walkway at their preferred walking speed. Participants were not informed of the presence or location of the force platforms. Trials were continued until at least 3 left and right gait cycles with complete foot landing on the force platform were obtained.
Data processing and statistical analysis
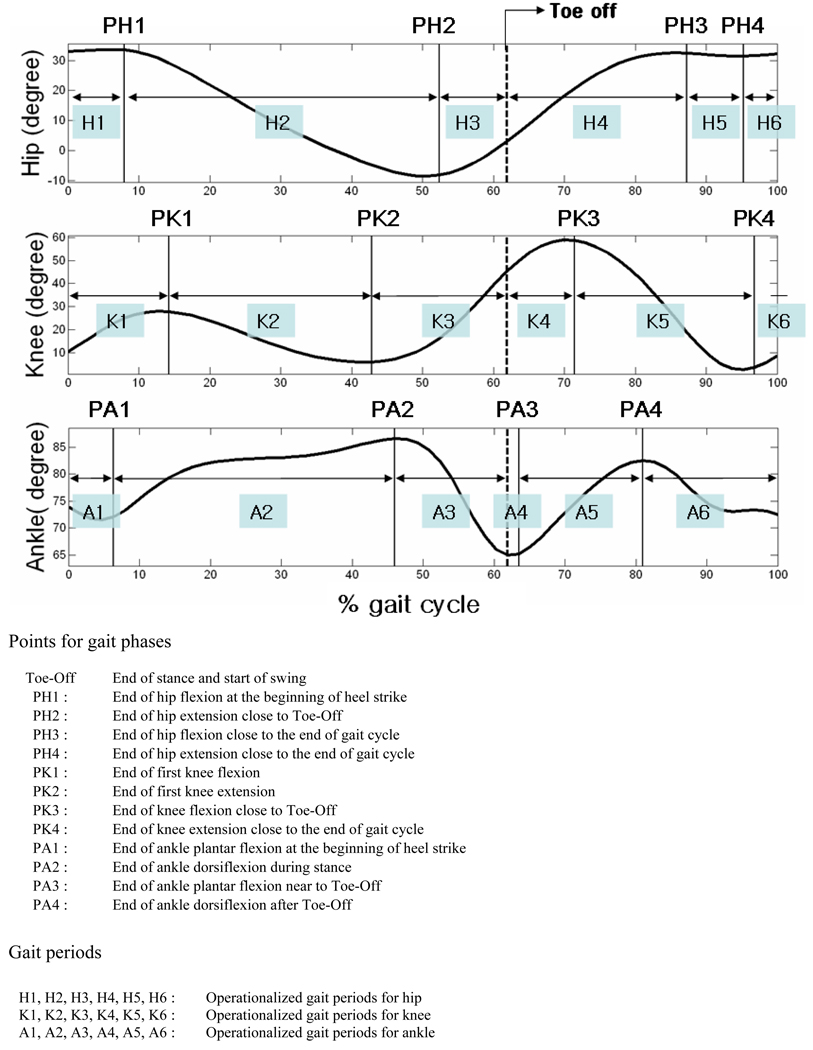
The 3D kinematic measurements for the left and right lower extremities were calculated by continuously tracking of the markers over time based on the Euler angle theorem (Ginsberg, 1995). During walking, ranges of rotational motions for the hip, knee, and ankle were measured using the peak angles between the flexion and extension and the angles between the abduction and adduction for the rotations about the ML and AP axes of proximal joints, respectively. For the calculation of kinetics, only the gait cycles (defined as heel strike to subsequent heel strike of the ipsilateral foot) with a complete foot landing on the force platform were used in the analysis. Mechanical joint moments and mechanical joint powers from each lower extremity joint were calculated by Visual3D (C-motion, Inc., Germantown, MD, USA) software using kinematic measurements, ground reaction forces, biometric measurements (weight, height, the width between ASIS) and the paradigm of inverse dynamics starting and finishing with a heel strike. Specific gait phases were defined in the gait cycle based on rotational direction changes about the ML axes of proximal joints of lower extremities (Figure 1, this figure is from one representative participant). For the hip, knee and ankle, we defined six gait periods (Figure 1), divided by 5 cut phases, within a gait cycle, namely for the hip [1) end of the first hip flexion (PH1), 2) end of the following hip extension (PH2), 3) ‘toe off’, 4) end of the subsequent hip flexion (PH3), 5) end of the following hip extension (PH4)]; for the knee [1) end of the first knee flexion (PK1), 2) end of the following knee extension (PK2), 3) ‘toe off’, 4) end of the subsequent knee flexion (PK3), 5) end of the following knee extension (PK4)]; and for the ankle [1)end of the first plantar flexion (PA1), 2) end of the following dorsiflexion (PA2), 3) ‘toe off’, 4) end of the subsequent plantar flexion (PA3), 5) end of the following dorsiflexion (PA4)]. MWEs for the hip, knee, and ankle were computed by integrating mechanical powers generated during the operationalized gait periods using custom made software written in MATLAB (The MathWorks, Inc., Natick, MA, USA). A total of 18 generative MWEs (from 6 gait periods for each of the 3 joints, the hip, knee, and ankle) and 18 absorptive MWEs were estimated for the rotations of lower extremities about the ML and AP axes.
Figure 1. Definition of the points for gait phases and periods based on rotations of lower extremities about the medial-lateral (ML) axes of proximal joints.
Cross-sectional associations between gait parameters and age were examined by a multiple regression analysis adjusted for walking speed, sex, height and weight. Statistical analyses were performed using SAS (SAS 9.1), with significance set at p < 0.05.
Results
The descriptive characteristics of the 52 subjects (19 women and 33 men) that completed the gait test are summarized in Table 1. Participants were ages 60 – 92 years, with an average body mass index of 26.1 (± 3.1 kg/m2). As summarized in Table 2, older age was significantly associated with slower walking speed, shorter stride length, and shorter stance time (p < 0.001, for all), but not with stride width. Of the gait phase parameters, older age was associated with a higher percentage of the gait cycle in the period between the heel strike and the end of first ankle plantar flexion (PA1; p = 0.003), and also with a longer stance period of ankle dorsiflexion (p = 0.017).
Table 1.
Participants Characteristics
| Variable | Mean (SD) * | Minimum | Maximum | women (N = 19) Mean (SD) * |
men (N = 33) Mean (SD) * |
|---|---|---|---|---|---|
| Age, years | 72.9 (10.1) | 60 | 92 | 71.2 (10.7) | 73.9 (9.8) |
| Height, m | 1.7 (0.1) | 1.5 | 1.9 | 1.6 (0.1) | 1.8 (0.1) |
| Weight, kg | 76.4 (14.4) | 52.1 | 120.1 | 66.7 (11.1) | 82.1 (13.2) |
| BMI, kg/m2 | 26.1 (3.1) | 19.9 | 33.4 | 25.6 (3.5) | 26.5 (2.8) |
Standard Deviation
Table 2.
Spatial-temporal gait parameters and their relationship with age
| Mean (SD) * | β ** | 95% CI | P value | |||
|---|---|---|---|---|---|---|
| Walking speed, m/sec. † | 1.10 (0.20) | −0.0125 | −0.0171, −0.0090 | <.001 | ||
| Stride length, m ‡ | 1.20 (0.18) | −0.0060 | −0.0090, −0.0030 | <.001 | ||
| Stride width, m ‡ | 0.11 (0.02) | 0.0003 | −0.0005, 0.0010 | 0.455 | ||
| Stance time, sec. ‡ | 0.67 (0.08) | −0.0031 | −0.0050, −0.0013 | <.001 | ||
| Gait phase, % gait cycle ‡ | ||||||
| Toe Off § | 63.39 (1.64) | 0.0154 | −0.0375, 0.0682 | 0.568 | ||
| Hip § | PH1 | 1.55 (2.05) | 0.0447 | −0.0241, 0.1136 | 0.203 | |
| PH2 | 52.90 (1.93) | −0.0395 | −0.0965, 0.0174 | 0.174 | ||
| PH3 | 90.81 (4.82) | −0.0728 | −0.2449, 0.0993 | 0.407 | ||
| PH4 | 98.49 (2.11) | 0.0067 | −0.0682, 0.0817 | 0.860 | ||
| Knee § | PK1 | 13.39 (1.99) | −0.0038 | −0.0671, 0.0594 | 0.906 | |
| PK2 | 40.21 (4.25) | −0.0136 | −0.1560, 0.1289 | 0.852 | ||
| PK3 | 71.84 (1.98) | 0.0087 | −0.0749, 0.0575 | 0.797 | ||
| PK4 | 92.33 (6.14) | −0.0473 | −0.2626, 0.1680 | 0.667 | ||
| Ankle § | PA1 | 5.72 (1.88) | −0.0871 | −0.1452, −0.0291 | 0.003 | |
| PA2 | 44.89 (3.46) | 0.0218 | −0.0509, 0.0945 | 0.557 | ||
| PA3 | 63.61 (4.42) | −0.0264 | −0.1504, 0.0976 | 0.676 | ||
| PA4 | 83.31 (3.04) | 0.0006 | −0.1050, 0.1061 | 0.992 | ||
Standard Deviation
Estimated coefficient for the linear model
Linear model with age after adjusted for sex, height, weight
Linear model with age after adjusted for walking speed, sex, height, weight
for the definitions of phases, see figure 1
The relationship between age with range of motion and kinetics for the rotations of the lower extremities about the ML and AP axes of proximal joints are shown in Table 3. Older age was associated with a smaller range of motion for ankle in the rotation about the ML axis (p = 0.030) and also a smaller range of motion for the hip, knee, and ankle in rotations about the AP axes (p = 0.007, p = 0.036, and p = 0.041, respectively). Older age was also associated with higher peak joint power in the rotations of the hip and knee about the ML axes (p = 0.006, and p = 0.034, respectively), and lower peak joint power in the rotation of the ankle about the AP axis (p = 0.026). With older age, total hip generative MWE was higher for thigh rotation about the ML axis (p = 0.007), while lower about the AP axis (p = 0. 014). Interestingly, higher normal walking speed was significantly associated with higher total hip generative MWE for thigh rotation about the ML axis (p <.001), but not about the AP axis. Total absorptive MWE or peak moments were similar across age.
Table 3.
Age association with gait parameters in ranges of motion and kinetics for rotations of lower extremities about the medial-lateral (ML) and anterior-posterior (AP) axes of proximal joints
| For rotations about medial-lateral (ML) axes of lower extremities (extension and flexion) |
For rotations about medial-lateral (AP) axes of lower extremities (abduction and adduction) |
||||||
|---|---|---|---|---|---|---|---|
| Mean (SD) * |
β ** (95% CI) |
P value | Mean (SD) * |
β ** (95% CI) |
P value | ||
| Range of motion, degree | |||||||
| Hip | 39.17 (5.44) |
−0.0819 (−0.2190, 0.0552) |
0.242 | 9.70 (2.55) |
−0.0990 (−0.1704, −0.0276) |
0.007 | |
| Knee | 52.63 (7.305) |
0.0169 (−0.2132, 0.2470) |
0.885 | 12.07 (4.41) |
−0.1558 (−0.3016, 0.0100) |
0.036 | |
| Ankle | 24.03 (3.99) |
−0.1403 (−0.2671, −0.0135) |
0.030 | 8.66 (2.30) |
−0.0829 (−0.1624, 0.0035) |
0.041 | |
| Peak moment, Nm/kg | |||||||
| Hip | 1.21 (0.21) |
0.0027 (−0.0027, 0.0081) |
0.329 | 0.87 (0.10) |
−0.0013 (−0.0045, 0.0020) |
0.444 | |
| Knee | 0.68 (0.18) |
0.0031 (−0.0028, 0.0091) |
0.303 | 0.46 (0.13) |
−0.0038 (−0.0081, 0.0006) |
0.090 | |
| Ankle | 1.05 (0.23) |
0.0000 (−0.0077, 0.0078) |
0.994 | 0.16 (0.05) |
0.0001 (−0.0015, 0.0013) |
0.875 | |
| Peak power, J/kg sec | |||||||
| Hip | 1.39 (0.39) |
0.0112 (0.0032, 0.0191) |
0.006 | 0.53 (0.18) |
−0.0043 (−0.0098, 0.0012) |
0.122 | |
| Knee | 1.60 (0.50) |
0.0154 (0.0012, 0.0296) |
0.034 | 0.17 (0.08) |
−0.0020 (−0.0046, 0.0007) |
0.143 | |
| Ankle | 1.66 (0.564) |
−0.0036 (−0.0192, 0.0119) |
0.649 | 0.13 (0.06) |
−0.0018 (−0.0034, 0.0002) |
0.026 | |
| Total generative MWE, J/kg | |||||||
| Hip | 0.28 (0.08) |
0.0028 (0.0008, 0.0049) |
0.007 | 0.09 (0.04) |
−0.0013 (−0.0024, −0.0003) |
0.014 | |
| Knee | 0.11 (0.00) |
0.0013 (−0.0003, 0.0028) |
0.113 | 0.01 (0.01) |
−0.0002 (−0.0005, 0.0001) |
0.246 | |
| Ankle | 0.17 (0.01) |
−0.0010 (−0.0026, 0.0005) |
0.194 | 0.02 (0.01) |
0.0000 (−0.0004, 0.0003) |
0.788 | |
| Total absorptive MWE, J/kg | |||||||
| Hip | −0.24 (0.09) |
0.0014 (−0.0013, 0.0042) |
0.299 | −0.06 (0.03) |
−0.0001 (−0.0010, 0.0008) |
0.880 | |
| Knee | −0.35 (0.09) |
−0.0019 (−0.0045, 0.0006) |
0.134 | −0.03 (0.01) |
0.0003 (−0.0001, 0.0007) |
0.155 | |
| Ankle | −0.15 (0.05) |
0.0000 (−0.0019, 0.0018) |
0.995 | −0.01 (0.01) |
−0.0000 (−0.0002, 0.0002) |
0.792 | |
Standard Deviation
Estimated coefficient for the linear model
All linear models for kinematics are adjusted for walking speed, sex, height, and weight
All linear models for kinetics (already normalized by mass) are adjusted for walking speed, sex, and height
The analysis of MWEs during the operationalized gait periods revealed significant age effects on the hip and knee. Specifically, older age was associated with higher hip generative MWE for thigh rotation about the ML axis during hip flexion (period H3; p <.001; Figure 1), and lower hip generative MWE for thigh rotation about the AP axis during hip extension (period H2; p = 0.043) and hip flexion (period H3; p = 0.032). Older age was also associated with lower knee absorptive MWE for shank rotation about the AP axis during the first knee flexion (period K1; p = 0.003).
Discussion
Understanding adaptation strategies in response to reduced energy availability which are aimed to increase safety is a prerequisite to the development of intervention strategies of mobility-disability prevention in older persons. In this cross-sectional study we observed specific kinematic and kinetic gait patterns associated with older age probably due to declining generative MWE for the rotation about the AP axis of proximal joints for the lower extremities.
Consistent with previous conclusions, we observed that older adults tend to walk slower and with shorter strides (Olney et al., 1994; Judge et al., 1996; Kerrigan et al., 2001; Dingwell et al., 2006). According to current views, preferred walking speed is selected to maximize comfort and endurance, and represents the best compromise between parsimony in energy utilization and safety. For this reason, when studying age-related kinematic and kinetic gait parameters, it is difficult to distinguish between age-related differences due to a difference in walking speed and those truly attributable to aging. To address this problem, all analyses presented in this report were adjusted for walking speed, under the assumption that the motor strategies utilized to slow down or accelerate are similar in older individuals. Interestingly, we found that age was not associated with differential and specific gait pattern in the operationalized gait phases (except for ankle planter flexion period, PA1). These findings indirectly confirm that the motor strategies used to modulate walking speed are similar in older individuals. As the only noticeable exception, older participants showed shorter period of ankle planter flexion (PA1) resulting in a longer dorsiflexion duration in stance, which can be interpreted as a tendency for flat-footed landing. This observation and interpretation is consistent with previous reports (Winter et al., 1990; Murray et al., 1969), and may also explain why reduced push-off is often reported as a typical gait feature in older individuals (DeVita et al., 2000; Judge et al., 1996).
Decreases in range of motion were observed for ankle rotation about the ML axis, and for hip, knee, and ankle rotations about the AP axes with older age. Reduced range of motion for the ankle and hip may suggest that older persons tend to use shorter stride length and lower hip MWE even when the effect of slower walking is dissected out by regression analysis. As prior studies have suggested, higher peak mechanical power of the hip and knee and higher total hip generative MWE for rotations about the ML axes in older individuals may be interpreted as a compensatory mechanism to the reduced range of rotational motion in the ankle (Lewis et al., 2008; Devita et al., 2000).
By convention, if the direction of increasing hip rotation (the sign of angular velocity) is the same as the direction of the applied joint moment, mechanical power is considered generative (concentric), while if direction of increasing rotation is opposite, the mechanical power is considered absorptive (eccentric). Since the dominant hip joint moment for thigh rotation about the AP axis is an abductor, hip generative MWE for thigh rotation about the AP axis can be thought to be generated from increasing hip abduction, which is the effort to align the leg in a medial-laterally stable position by lifting up laterally instead of collapsing medially. Since this movement is essential to the maintenance of stable kinetic balance, lower hip generative MWE for thigh rotation about the AP axis in older persons may account for the reduced ML stability often described in older individuals. Age associated declines in walking speed and total hip generative MWE for thigh rotation about the AP axis were illustrated in the Figure 2 and Figure 3, respectively. It is interesting that lower walking speed was not significantly associated with lower total hip generative MWE for thigh rotation about the AP axis (p = 0.407). These mutually independent gait parameters in walking speed and total hip generative MWE for thigh rotation about the AP axis (resulting in MWE in the ML side) are difficult to reconcile and may have different meanings and objective, such as increasing safety and limiting energy consumption, respectively.
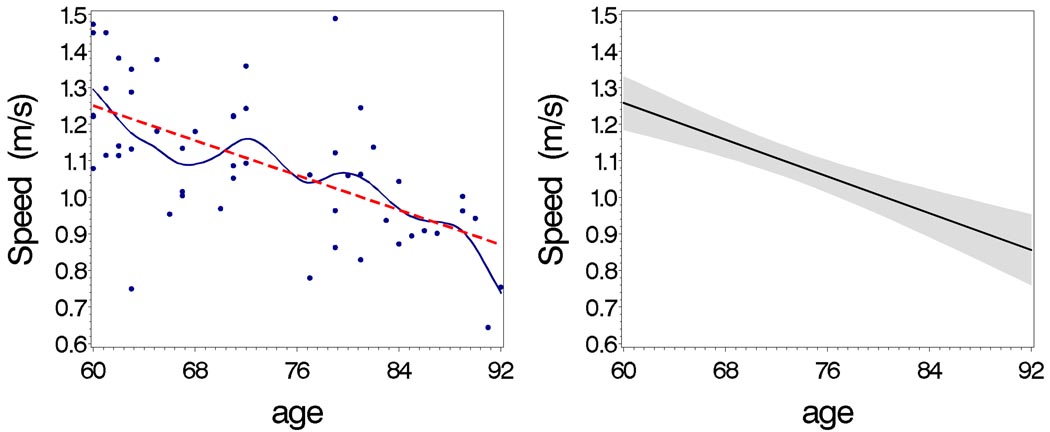
Figure 2. Age-associated decline in walking speed.
Walking speed vs. age in smoothing (—) line and predicting (---) line (left), and the estimated line (—) with confidential interval (gray area) from regression model (right)
Figure 3. Age-associated decline in total hip generative MWE for thigh rotation about the AP axis of proximal joint.
Total hip generative MWE for thigh rotation about the AP axis (results in MWE in ML side) vs. age in smoothing (—) line and predicting (---) line (left), and the estimated line (—) with confidential interval (gray area) from regression model (right)
Active hip muscle activities, which have the same direction of force and movement (concentric) showed significant age-associations, while passive hip muscle activities (with opposite force and movement; eccentric) did not. Thus, concentric hip muscle activations seem to be more sensitive to the aging process compared to eccentric hip muscle activations.
Finding no significant age-associated difference in knee joint moment, lower knee absorptive MWE for shank rotation about the AP axis during first knee flexion in stance (period K1) with older age may suggest that knee adduction rotational speed during the first knee flexion period is limited. This smaller adduction angular speed, which results in a lower knee absorptive MWE medial-laterally, may contribute to medial femoral condyle cartilage thinning which is often found in older individuals and has been reported as a preclinical stage of OA (Andriacchi et al., 2004). Higher hip generative MWE for thigh rotation about the ML axis during hip flexion in stance (period H3) with older age provides a specific period of compensatory effort from the hip during walking.
MWEs from the operationalized gait phases provide information on the combined kinematic and kinetic gait parameters generated by muscular activations during the specific gait periods. The normalized and standardized gait parameters of MWEs from the present study can be used both in the cross-sectional and longitudinal gait studies to understand the effect of age, diseases, environmental stress, and specific interventions on gait parameters.
Our study has limitations. First, these observations were collected in a relatively small sample. However, the methodological strategy developed and piloted in this study will be implemented on a much larger scale using data collected in the BLSA. Second, the analytic models assume symmetry during walking. To minimize the effect of this problem, participants with substantial mobility limitations were excluded from this study and the symmetry of gait cycle speed from the left and right sides was checked by examining the ratio of relatively slower and faster sides, which was close to 1 (0.93±0.145). However, we acknowledge that subtle asymmetry of gait dynamics which were not readily detectable in the clinical examination may be of physiologic importance. Finally, our study is observational in nature and cannot determine which of the characteristics identified as age-specific are primary as opposed to compensatory, nor can we ascertain which of these characteristics are beneficially adaptive as opposed to detrimental to aging individuals. The BLSA is currently collecting longitudinal data. The analysis of these data may overcome, at least in part, some of these limitations.
Acknowledgements
This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging. Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All the authors declare that no financial or personal relationships were conducted with other people or organizations that could inappropriately influence or bias this work.
References
- Alexander NB. Gait disorders in older adults. Journal of the American Geriatrics Society. 1996;44:343–351. doi: 10.1111/j.1532-5415.1996.tb06417.x. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Annals of Biomedical Engineering. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- Chen IH, Kuo KN, Andriacchi TP. The influence of walking speed on mechanical joint power during gait. Gait Posture. 1997;6:171–176. [Google Scholar]
- Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Transactions on Biomedical Engineering. 2007;54(11):1919–1926. doi: 10.1109/TBME.2007.901031. [DOI] [PubMed] [Google Scholar]
- DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. Journal of Applied Physiology. 2000;88(5):1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Dingwell JB, Marin LC. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. Journal of Biomechanics. 2006;39(3):444–452. doi: 10.1016/j.jbiomech.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Duff-Raffaele M, Kerrigan DC, Corcoran PJ, Saini M. The proportional work of lifting the center of mass during walking. American Journal of Physical Medicine and Rehabilitation. 1996;75:375–379. doi: 10.1097/00002060-199609000-00014. [DOI] [PubMed] [Google Scholar]
- Ginsberg JH. Advanced Engineering Dynamics. 2nd ed. Cambridge, UK: Cambridge University Press; 1995. pp. 55–73. [Google Scholar]
- Judge JO, Davis RB, 3rd, Ounpuu S. Step length reductions in advanced age: The role of ankle and hip kinetics. Journals of Gerontology, Series A. Biological Sciences and Medical Sciences. 1996;51A(6):M303–M312. doi: 10.1093/gerona/51a.6.m303. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Lee LW, Collins JJ, Riley PO, Lipsitz LA. Reduced hip extension during walking: Healthy elderly and fallers versus young adults. Archives of Physical Medicine and Rehabilitation. 2001;82(1):26–30. doi: 10.1053/apmr.2001.18584. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Johansson JL, Bryant MG, Boxer JA, Della Croce U, Riley PO. Moderate-heeled shoes and knee joint torques relevant to the development and progression of knee osteoarthritis. Archives of physical medicine and rehabilitation. 2005;86(5):871–875. doi: 10.1016/j.apmr.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Lewis CL, Ferris DP. Walking with increased ankle pushoff decreases hip muscle moments. Journal of Biomechanics. 2008;41:2082–2089. doi: 10.1016/j.jbiomech.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Lloyd DG, Li SK. Sensori-motor function, gait patterns and falls in community-dwelling women. Age Ageing. 1996;25(4):292–299. doi: 10.1093/ageing/25.4.292. [DOI] [PubMed] [Google Scholar]
- McGibbon CA, Krebs DE, Puniello MS. Mechanical energy analysis identifies compensatory strategies in disabled elders’ gait. Journal of Biomechanics. 2001;34:481–490. doi: 10.1016/s0021-9290(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Murray MP, Kory RC, Clarkson BH. Walking patterns in healthy old men. Journal of Gerontology. 1969;24(2):169–178. doi: 10.1093/geronj/24.2.169. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Griffin MP, McBride ID. Temporal, kinematic, and kinetic variables related to gait speed in subjects with hemiplegia: A regression approach. Physical Therapy. 1994;74(9):872–885. doi: 10.1093/ptj/74.9.872. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Owings TM, Foley KT, Grabiner MD. Gait characteristics as risk factors fro falling from trips induced in older adults. Journal of Gerontology: Medical Sciences. 1999;54:M583–M590. doi: 10.1093/gerona/54.11.m583. [DOI] [PubMed] [Google Scholar]
- Polcyn AF, Lipsitz LA, Kerrigan DC, Collins JJ. Age-related changes in the initiation of gait: degradation of central mechanisms for momentum generation. Archives of Physical Medicine and Rehabilitation. 1998;79(12):1582–1589. doi: 10.1016/s0003-9993(98)90425-7. [DOI] [PubMed] [Google Scholar]
- Siegel KL, Kepple TM, Stanhope SJ. Joint moment control of mechanical energy flow during normal gait. Gait Posture. 2004;19:69–75. doi: 10.1016/s0966-6362(03)00010-9. [DOI] [PubMed] [Google Scholar]
- Teixeira-Salmela LF, Nadeau S, Mcbride I, Olney SJ. Effects of muscle strengthening and physical conditioning training on temporal, kinematic and kinetic variables during gait in chronic stroke survivors. Journal of Rehabilitation Medicine. 2001;33(2):53–60. doi: 10.1080/165019701750098867. [DOI] [PubMed] [Google Scholar]
- Teixeira-Salmela LF, Nadeau S, Milot MH, Gravel D, Requião LF. Effects of cadence on energy generation and absorption at lower extremity joints during gait. Journal of Clinical Biomechanics. 2008;23(6):769–778. doi: 10.1016/j.clinbiomech.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Winter DA. Biomechanical motor patterns in normal walking. Journal of Motor Behavior. 1983;15(4):302–330. doi: 10.1080/00222895.1983.10735302. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Frank JS, Walt SE. Biomechanical walking changes in the fit and healthy elderly. Physical Therapy. 1990;70:340–347. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]