Abstract
Recent evidence has suggested a role for soluble oligomeric Aβ species in the pathology of Alzheime’s disease (AD). Fibrillar plaque deposits are present in non-demented individuals and levels of soluble Aβ correlate better with cognitive dysfunction in AD and transgenic mouse models. We have previously reported that there are at least two conformationally distinct types of Aβ oligomers: prefibrillar oligomers that are kinetic intermediates in fibril assembly reactions and are specifically recognized by A11 antibody and fibrillar oligomers that may represent fibril seeds or small pieces of fibrils and are recognized by a fibril specific antibody, OC. We have examined the levels of these two types of oligomers in the PBS soluble fraction of brain tissue from control cases, cases with senile degenerative changes (SDC) and AD patients. We found that the levels of soluble fibrillar oligomers detected by OC antibody are significantly elevated in multiple brain regions of AD patients. The elevated fibrillar oligomer levels were found not to be an artifact of tissue homogenization, nor a result of increased Aβ or APP levels. The concentration of fibrillar oligomers in adjacent brain regions of the same patient can vary widely and were not detected in post mortem cerebrospinal fluid. In contrast, the level of prefibrillar oligomers are variable in both AD and age matched controls, indicating that they are not correlated with cognitive dysfunction and suggesting that they precede dementia in AD. Significant correlations were found between the levels of fibrillar oligomers and cognitive decline (MMSE scores) as well as the neuropathological hallmarks of AD. These results indicate that fibrillar oligomers may play a key role in the pathology of AD and may be a new target for diagnostic and therapeutic development.
Keywords: Alzheimer, Aβ, amyloid oligomers, cognitive dysfunction, dementia, conformation dependent antibodies, fibrillar oligomers, prefibrillar oligomers, annular protofibrils
Introduction
The genetics of inherited forms of Alzheime’s disease (AD) provides strong evidence for a causal role of Aβ, especially the faster aggregating Aβ42, in AD (Hardy, 2006; Tanzi and Bertram, 2005). However, while some groups have reported a clear correlation between the number of Aβ plaques and dementia severity in support of this hypothesis (Blessed et al., 1968; Cummings and Cotman, 1995), others found no such correlation (Bennett et al., 1993; McKee et al., 1991; Terry et al., 1991). Additionally, individuals with normal cognitive function have been observed that have Aβ plaque loads that either meet or exceed the criteria for AD diagnosis (Katzman et al., 1988; Dickson et al., 1992). These observations suggest that fibrillar Aβ may not be the primary toxic assembly state responsible for the pathology of AD. Subsequent studies of human subjects have revealed a positive correlation between soluble Aβ levels and the severity of dementia (Lue et al., 1999; McLean et al., 1999; Wang et al., 1999), further indicating a role for soluble oligomeric species in the pathology of AD. Additional in vitro studies have reported that not only are soluble oligomers and protofibrils toxic to neuronal cell cultures (Hartley et al., 1999; Lambert et al., 1998), but that they are more toxic than fibrils (Dahlgren et al., 2002; Kayed et al., 2003).
The particular type of oligomer that is most closely associated with AD is a subject of considerable interest. Oligomers have been distinguished on the basis of size and elevated levels of approximately 56 kDa Aβ oligomers (ADDLs) have been identified in human brain (Gong et al., 2003) and a 56 kDa oligomer (Aβ*56) has been reported that is correlated with cognitive dysfunction in transgenic mouse models (Lesne et al., 2006). Soluble dimers have been purified from human AD brain that inhibit LTP and cause cognitive dysfunction in rats (Shankar et al., 2008). Another way of classifying oligomers is on the basis of their underlying structure, using conformation dependent antibodies (Barghorn et al., 2005; Kayed et al., 2007; Kayed et al., 2003; Kayed et al., 2009; Lambert et al., 2001). These antibodies are specific for aggregated forms of Aβ and show low or no immunoreactivity against monomeric Aβ. A11, OC and αAPF recognize generic epitopes that are specifically associated with prefibrillar oligomers (A11) (Kayed et al., 2003), fibrils or fibrillar oligomers (OC) (Kayed et al., 2007) and annular protofibrils or pores (αAPF) (Kayed et al., 2009). M94 and 5598 antisera show a significant preference for aggregated Aβ as compared to monomer (Barghorn et al., 2005; Lambert et al., 2001). We have previously reported the presence of prefibrillar oligomers in AD patient’s brain using the anti-oligomer antibody A-11 (Kayed et al., 2003) and M94 and 5598 detect oligomers in AD brain (Barghorn et al., 2005; Lambert et al., 2001). Recently, we have reported the development of two new polyclonal antisera that detect unique generic epitopes associated with specific amyloid assembly states. OC recognizes a conformation dependent epitope specific to amyloid fibrils as well as 100,000 × G soluble fibrillar oligomers (Kayed et. al., 2007) and αAPF recognizes annular protofibrils, which are ring-like structures that resemble membrane pores (Kayed et al., 2009). It is not yet clear how the levels of oligomers detected by A11, OC and αAPF are correlated with dementia severity in AD and how these antibodies compare to other conformation dependent antibodies, M94 and 5598 (Barghorn et al., 2005). In this report, we have analyzed levels of distinct types of oligomers that are the present in the PBS soluble extracts of human brain samples from normal control cases, cases with degenerative changes but insufficient for a diagnosis of AD and patients with AD. The results demonstrate that soluble fibrillar oligomers that are recognized by the OC antibody are significantly increased in AD patients as compared to A11 and αAPF positive oligomers that are also elevated in some age matched control brains.
Methods and Materials
Patient Brain Tissue Selection
Frozen brain tissue was obtained from the Institute for Brain Aging and Dementia and was collected from individuals enrolled in the Alzheimer Disease Research Center. Subjects enrolled in the ADRC were given the Mini Mental State Examination (MMSE) and scores from this test were used for correlative studies. As a standard protocol for ADRC autopsy cases, Braak & Braak neurofibrillary tangle and plaque staging was completed (Braak and Braak, 1991). We chose to examine tissues from the frontal cortex (Brodmann's Area's 4, 9 and 11), hippocampus, entorhinal cortex, transentorhinal cortex, cerebellum and the olfactory bulb based on the progression of β-amyloid deposition over the course of the disease as described by Thal et. al. (Thal et al., 2002). We selected cases that would represent the spectrum of Braak neurofibrillary tangle staging (Braak and Braak, 1991) seen in AD progression and Table 1 lists the clinical and pathological details of the cases used in this study. Patients were matched for age, gender and post-mortem interval (PMI). There were a total of 12 cases used, 5 were clinically normal, 1 was mildly cognitively impaired and 6 were demented. In terms of neuropathology, 4 showed little or no AD pathology, 2 showed mild AD pathology but insufficient for a diagnosis of AD (senile degenerative changes – SDC) and 5 had AD pathology.
Table 1.
Patient Demographics
| Patient Number | Clinical Diagnosis* | Neuropath. Diagnosis* | Sex | Tau Tangle Stage | Aβ Plaque Stage | Post Mortem Index (Hours) | Age at Death | MMSE Score |
|---|---|---|---|---|---|---|---|---|
| 1 | N/A | Normal | M | I | A | 4.9 | 71 | N/A |
| 2 | Control | Normal | F | I | 0 | 4.5 | 87 | N/A |
| 3 | CIND | Normal | M | II | 0 | 6.4 | 81 | 27 |
| 4 | Control | Normal | F | II | 0 | 5.0 | 88 | 29 |
| 5 | Control | SDC | F | III | A | 5.8 | 84.8 | 29 |
| 6 | MCI/AD | SDC | M | III | 0 | 6.3 | 84.5 | 22 |
| 7 | DLB | AD/DLB | F | IV | B | 5.4 | 77 | 0 |
| 8 | Possible AD | AD | M | IV | C | 4.5 | 77.5 | 19 |
| 9 | Possible AD | AD | M | V | B | 5.0 | 81 | 12 |
| 10 | Probable AD | AD | F | V | B | 4.3 | 81 | 0 |
| 11 | Possible AD | AD | F | VI | B | 6.6 | 82 | 4 |
| 12 | Possible AD | AD | M | VI | C | 3.5 | 83.2 | 0 |
CIND – cognitively impaired not demented
MCI – mild cognitive impairment
DLB – diffuse Lowy Body Disease
AD – Alzheimer disease
SDC – Senile degenerative changes
Human brain sample preparation
Frozen tissues were weighted, diced and homogenized in a 4:1 volume of freshly prepared ice-cold PBS, 0.02% NaN3, pH 7.4 with protease inhibitor cocktail (PBS volume/brain wet weight). The samples were ultracentrifuged at 100,000 × G for 1 hr at 4°C. The PBS soluble fraction was collected, aliquoted and stored at –80°C for future testing. The insoluble pellet was resuspended in 150 mM NaCl, 10 mM Tris, 2% Triton X-100, 0.02% NaN3, pH 8.0 with protease inhibitor cocktail and sonicated. Samples were then incubated on ice for 1 hr prior to being ultracentrifuged at 100,000 × G for 1 hr at 4o C. The resultant Triton X-100 soluble fraction was aliquoted and stored at –80° C for future testing. The Triton X-100 insoluble pellet was resuspended in PBS, 0.02% NaN3, pH 7.4 with protease inhibitor cocktail, sonicated and diluted with PBS to equal protein concentrations based on the BCA Protein Assay (Thermo Scientific, Rockford, IL). Finally, the Triton X-100 insoluble pellet insoluble pellet was solubilized into 8 M urea, 50 mM Tris, pH 7.6 and incubated overnight at room temperature. The total protein concentration of all samples was determined by the BCA assay.
Dot Blot Assay
PBS soluble or urea-treated Triton X-100 insoluble samples were spotted onto nitrocellulose membrane (Whatman 10 402 495), and allowed to air dry. The total protein amount spotted onto the membrane for the PBS soluble and Triton X-100 insoluble fractions was 5.2 µg and 17.0 µg, respectively. Non-reactive sites were blocked with 10% Milk in low- Tween TBS-T, (20 mM Tris, 137 mM NaCl, 0.01% Tween 20 pH 7.6) for one hour at room temperature with shaking. Following three 5-minute washes, the blot was incubated overnight at 4oC in primary antibody (OC 1:10,000, A11 1:2000, αAPF 1:5,000). After washing, the blot was incubated with goat-α-rabbit HRP conjugated secondary antibody (Jackson Immuno Research 111-035&delhyphen;003, 1:12000) for 1 hr at room temperature, washed again and detected with ECL (Amersham, RPN2106). The membrane was then exposed to film (Denville, E-3012) and the film developed by the Kodak M35A X-OMAT Processor. Additional dot blots were also performed using anti-Aβ 17–24 (Covance, 4G8 1:10000), anti-N-terminal of amyloid precursor protein (APP) (Chemicon, 22C11 1:5000) followed by incubation with goat-α-mouse HRP conjugated secondary antibody (Jackson Immuno Research 115-035–146, 1:12000) and ECL detection. As a control for brain tissue preparation, the neuronal marker α-synaptophysin was detected in the Triton X-100 soluble fractions using anti- α-synaptophysin (Calbiochem, 1:1000), followed by incubation with goat-α-mouse HRP conjugated secondary antibody (Jackson Immuno Research 115-035–146, 1:12000) and ECL detection.
Quantitation of fibrillar oligomers and statistical analysis
Films were scanned and the sample dots quantitated using the freely available Java image processing program (ImageJ). The results, expressed as ng/µl, were calculated using a reference curve based on the reactivity of synthetic Aβ prefibrillar oligomers, fibrillar oligomers and annular protofibrils prepared as previously described (Kayed et al., 2007; Kayed et al., 2003; Kayed et al., 2009), taken as standards. The detection limit is approximately 1 ng/ul of Aβ for prefibrillar oligomers fibrillar oligomers and annular protofibrils. Non reactive sites of the film were also scanned and used to subtract the background for the sample dots. All statistical analyses were performed using GraphPad Instat Version 3.0 (GraphPad Software, San Diego CA). Values were expressed as means ± standard deviation (SD) and are the result of four independent experiments. To compare oligomer immunoreactivity between AD patients and non-AD patients, one-way analysis of variance (ANOVA) followed by Bonferroni’s t-test was performed. A probability value less than 0.05 was accepted as statistically significant. Spearman correlations were calculated to determine relationships between oligomer reactivity with dementia severity, as measured by the Mini Mental Status Examination (Folstein et al., 1975), and neuropathology, as determined by Braak and Braak staging (Bancher et al., 1993; Braak and Braak, 1991; Kayed et al., 2003).
Results
Fibrillar Oligomers are Elevated in AD Brain
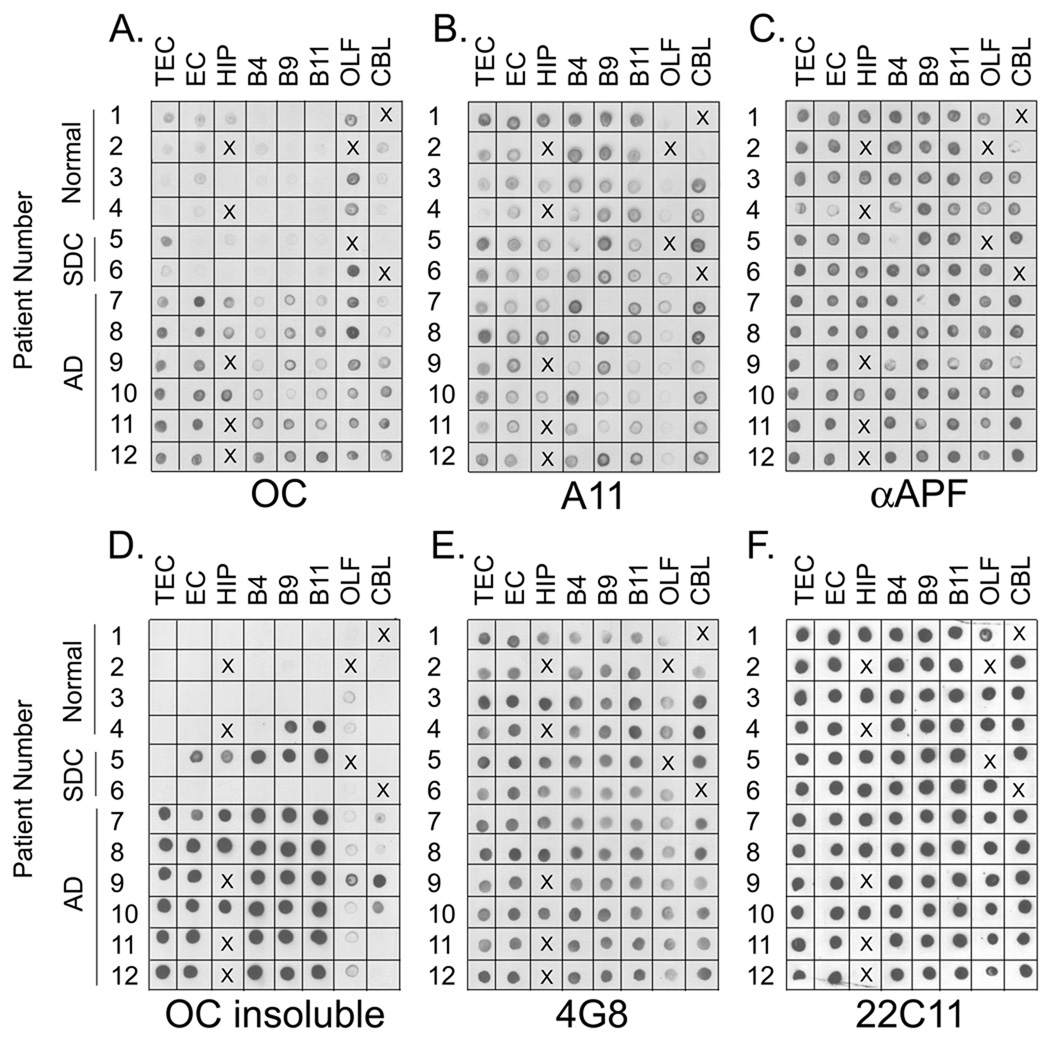
To examine the levels of soluble oligomers in human brain, PBS lysates of tissue samples from 8 different brain regions were prepared as described above and centrifuged at 100,000 × G for 1 hr to separate the soluble supernatant and insoluble pellet fractions. Samples were obtained from 12 different cases: patients with AD, cases with senile degenerative changes (SDC –i.e. AD pathology insufficient for a diagnosis of disease) and age matched controls. The clinical and neuropathological diagnoses of the cases used in the study are listed in Table I. The fractions were analyzed by dot blot and ELISA assays, but since the signal was higher for the dot blot samples (ELISA data not shown) the levels of oligomers was determined by quantitation of the dot blot images (Fig. 1). The levels of 100,000 × G soluble fibrillar oligomers detected with the fibril specific OC antibody were elevated in AD brain compared to normal controls and patients with senile degenerative changes (Figure 1a). The level of fibrillar oligomers was conspicuously higher in the olfactory bulb of normal controls and SDC samples compared to the other regions of the brain, suggesting that higher levels of fibrillar oligomers may first appear in the olfactory bulb. The levels of soluble fibrillar oligomers is also variable between different regions of the same brain, indicating that they do not diffuse far and suggesting that they may be produced locally and either retained or degraded in the same location. Fibrillar oligomer levels were below the limit of detection in AD lumbar cerebrospinal fluid samples using the dot blot assay (data not shown).
Figure 1. Soluble Oligomer levels in AD Brain.
A. Dot blot analysis of PBS soluble fraction with the anti-fibril antibody ( OC). PBS soluble fractions from the transentorhinal cortex (TEC), entorhinal cortex (EC), hippocampus (HIP), Brodmann’s Area 4 (B4), Brodmann’s Area 9 (B9), Brodmann’s Area 11 (B11), olfactory bulb (OLF) and cerebellum (CBL) were spotted onto nitrocellulose membrane and reacted with the OC antibody. The most prominent immunoreactivity was found in all brain regions of AD cases while reduced or no immunoreactivity was present in normal controls or cases with senile degenerative changes (SDC). The signal present in these cases indicates the presence of soluble fibrillar oligomers. B, C. Dot blot analysis of prefibrillar oligomers with A11 antibody and annular protofibrils with αAPF antibody, respectively. Although prefibrillar oligomer levels are low in the olfactory bulb, no differences in the levels were observed in AD, SDC and normal controls. D. The 100,000 × G insoluble fractions were solublized with 8M urea, 50 mM Tris pH 7.4 and spotted onto nitrocellulose membrane for detection with OC antibody. Prominent immunoreactivity was found in all AD cases as well as two cases (4 and 5) with mild neuropathology. The presence of immunoreactivity in the insoluble fraction of these two cases and the lack of immunoreactivity in the PBS fractions of the same cases (A) indicates that the soluble fibrillar oligomers present in Figure 1a are not an artifact of homogenization. E, F. Dot blot analysis of PBS soluble fraction with 4G8 (anti-Aβ) and 22C11 (anti-APP) antibodies, respectively. The levels of total soluble Aβ do not differ significantly between AD, SDC and normal controls. The uniform distribution of 22C11 immunoreactivity indicates that equal amounts of lysate were spotted. X indicates that this sample was not available for that case.
Brain tissue from patients with severe AD is known to contain fewer neurons and more astrocytes in areas particularly vulnerable to AD pathology. Therefore, the blots were also probed for α-synaptophysin which is a typical neuronal protein whose levels have been shown to be reduced in the brains of AD subjects (Masliah et al., 2001). As expected, soluble fibrillar oligomer levels were also found to be significantly elevated when normalized to α-synaptophysin reactivity (Fig S1).
The PBS soluble fraction was prepared by homogenizing brain tissue samples and since many samples in this group have insoluble amyloid plaques, this raises the possibility that the soluble fibrillar oligomers may be an artifact of the homogenization process by breaking up the fibrillar amyloid plaques. To exclude this possibility we compared the soluble, fibrillar oligomer levels and the insoluble fibril levels in the same tissue samples by dot blot analysis. As with the PBS soluble fraction (Figure 1a), all AD patients demonstrated strong immunoreactivity to the OC antibody in the Triton X-100 insoluble fraction (Figure 1d). However, two patients (4 and 5,) who were not demented at death with mild neuropathology demonstrated strong OC positive immunoreactivity in the insoluble fraction while no OC immunoreactivity was present in the PBS soluble fraction. The lack of fibrillar oligomers in the PBS soluble fractions of these two patients that contain large amounts of insoluble fibrils argues that the soluble fibrillar oligomers present in the AD patients of Figure 1a are not an artifact of the homogenization process. With few exceptions, the levels of insoluble fibrils are low or undetectable in the olfactory bulb and cerebellum. Detectable levels of soluble fibrillar oligomers are observed in the cerebellum of two AD samples (11, 12) that contain no detectable insoluble fibrils, which indicates that the presence of insoluble fibrils is not necessary for the observation of soluble fibrillar oligomers.
Prefibrillar oligomer and annular protofibrils levels are not elevated in AD brains
Prefibrillar oligomers and annular protofibrils are structurally and immunologically distinct from fibrillar oligomers, although the sizes of these oligomers broadly overlap (Kayed et al., 2007; Kayed et al., 2003; Kayed et al., 2009). The levels of prefibrillar oligomers and annular protofibrils were determined in the same samples using A11 and αAPF antibodies (Fig. 1b, c). Although A11 positive prefibrillar and αAPF positive annular protofibrils were detected in the samples, no difference was observed in AD brain and normal controls and SDC samples. As observed for fibrillar oligomers, the levels of prefibrillar oligomers and annular protofibrils are variable among different brain regions, indicating that they do not diffuse ideally. In particular, the levels of prefibrillar oligomers are low in the olfactory bulb compared to other brain regions. We also examined the levels of Aβ in the brain samples using 4G8, which recognizes residues 17–28 of Aβ (Fig. 1e). Unlike previous reports (Lue et al., 1999; McLean et al., 1999), we did not observe a statistically significant difference in the levels of soluble Aβ in AD, SDC and control samples although the methods and antibodies used here are different than those of the earlier reports. We used the level of soluble APP (sAPP) detected by 22C11 as a loading control to confirm equal amounts of protein were loaded in each sample (Fig. 1f).
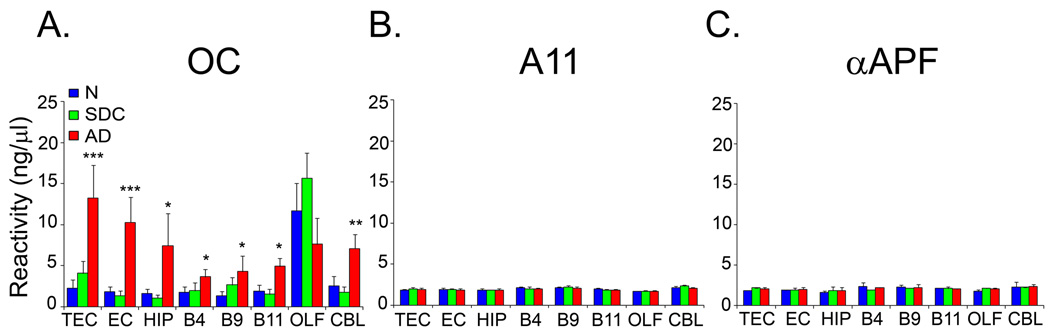
Oligomer levels were quantified from dot blots in quadruplicate, using synthetic Aβ fibrillar oligomers, prefibrillar oligomers and annular protofibrils as standards. The quantitative analysis confirms that the elevation of soluble fibrillar oligomer levels in some of the brain regions is highly statistically significant (Fig. 2). Soluble fibrillar oligomer levels are elevated approximately 2–5 fold in all regions of the brain examined, except olfactory bulb. The difference between soluble fibrillar oligomer levels in AD and the other groups is highly statistically significant in the transentorhinal cortex, entorhinal cortex and hippocampus (P < 0.001), and significant (P < 0.05) in the frontal cortex. Surprisingly, soluble fibrillar oligomer levels are also elevated in the cerebellum (P < 0.01), a region that is believed to be spared until late in the disease.
Figure 2. Fibrillar oligomers are elevated in AD brain.
Quantitative analysis of the dot blot data in Figure 1, showing human brain sample reactivity with A OC, B A11, and C. αAPF antibodies. A. Fibrillar oligomer levels were significantly higher in AD cases (AD, red bars) compared to normal (N, blue bars) and SDC (SDC, green bars) subjects in all the brain regions examined except OLF. B., C. No significant difference in A11 and αAPF reactivity between normal, SDC and AD samples were found. Human brain sample reactivity is expressed as ng/µl on the basis of a standard curve obtained using synthetic fibrillar oligomer, prefibrillar oligomer and annular protofibrillar preparations. Values are the mean ± SD of four independent experiments (* p ≤ 0.05; ** p ≤ 0.01, *** p≤ 0.001).
Soluble Fibrillar Oligomers Correlate with Cognitive Dysfunction and Pathology
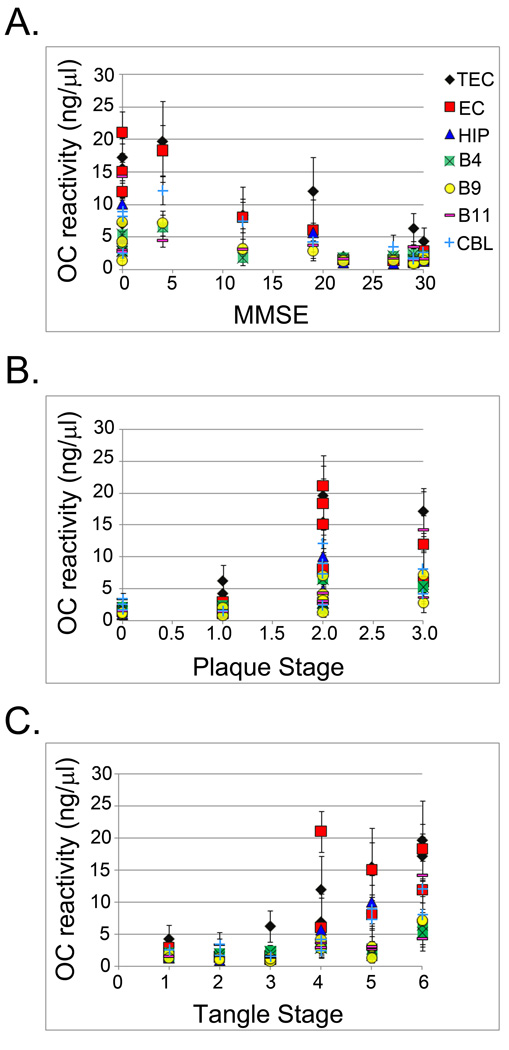
We examined whether the amounts of soluble fibrillar oligomers correlated with quantitative measures of cognitive dysfunction as measured by the MMSE (Folstein et. al., 1975) and neuropathological indices of amyloid plaque and tau tangle staging (Fig. 3a). We found that oligomer levels are positively correlated with cognitive decline. As shown in Table 2, Spearman correlation analysis revealed significant correlations between MMSE score and soluble fibrillar oligomer levels in the transentorhinal cortex, entorhinal cortex, Brodmann’s Area 4, Brodmann’s Area 9, Brodmann’s Area 11, and cerebellum. Levels of soluble fibrillar oligomers hippocampus (r = −0.61, p = 0.11) neared, but did not reach, significance, whereas no significant difference in the olfactory bulb was found (r = 0.20, p = 0.58). Significant correlations were present between soluble fibrillar oligomer levels and plaque stage (Fig. 3b and Table 2) in the transentorhinal cortex, entorhinal cortex, hippocampus, Brodmann’s Area 4 and Brodmann’s Area 9, and Brodmann’s Area 11. The cerebellum (r = 0.57, p = 0.08) neared, but did not reach significance, while the olfactory bulb (r = −0.14, p = 0.70) did not demonstrate significant correlations with extent of soluble fibrillar oligomers. Finally, significant correlations were also observed between soluble fibrillar oligomers and tangle stage (Figure 3c and Table 2) in all brain regions except olfactory bulb that failed to reach significance (r = −0.46, p = 0.18). This may indicate that soluble fibrillar oligomers are involved with initiating tau pathology, as has been suggested recently by Oddo et. al. (2007). Together, these data indicate that increased levels of soluble fibrillar oligomers are associated with increased levels of dementia and pathological hallmarks of AD, thus suggesting a potential role for the involvement of soluble fibrillar oligomers in the pathogenesis of AD.
Figure 3. Soluble fibrillar oligomer levels in AD brain correlate with cognitive decline and neuropathology.
A. OC immunoreactivity was compared to the MMSE, a measure of cognitive dysfunction. Significant inverse correlations were determined for TEC (black diamond), EC (red square), HIP (blue triangle), B4 (green square), B9 (yellow circle) and CBL (light blue cross).
B. OC immunoreactivity was compared to the plaque stage and significant correlations between fibrillar oligomer levels and amyloid pathology were found in TEC, EC, HIP, B4 and B9.
C. OC immunoreactivity was compared to the tangle stage and significant correlations between fibrillar oligomer levels and tau pathology were found in TEC, EC, HIP, B4, B9, B11 (purple dash) and CBL. The r and p values are reported in Table 2.
Table 2.
Soluble Fibrillar Oligomers Correlate with Cognitive Decline and Pathology
| MMSE | Plaque Stage | Tangle Stage | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | r2 | p | r | r2 | p | r | r2 | p | N | |
| TEC | −0.72 | 0.52 | 0.01 | 0.90 | 0.80 | 0.00 | 0.86 | 0.74 | 0.00 | 12 |
| EC | −0.78 | 0.61 | 0.00 | 0.72 | 0.52 | 0.01 | 0.69 | 0.48 | 0.01 | 12 |
| HIP | −0.61 | 0.38 | 0.11 | 0.92 | 0.84 | 0.00 | 0.76 | 0.58 | 0.05 | 7 |
| B4 | −0.67 | 0.45 | 0.02 | 0.70 | 0.49 | 0.02 | 0.71 | 0.51 | 0.01 | 11 |
| B9 | −0.58 | 0.34 | 0.02 | 0.70 | 0.49 | 0.01 | 0.59 | 0.35 | 0.04 | 12 |
| B11 | −0.65 | 0.43 | 0.02 | 0.66 | 0.43 | 0.02 | 0.72 | 0.52 | 0.01 | 12 |
| OLF | 0.20 | 0.04 | 0.58 | −0.14 | 0.02 | 0.70 | −0.46 | 0.21 | 0.18 | 10 |
| CBL | −0.63 | 0.39 | 0.05 | 0.57 | 0.33 | 0.08 | 0.78 | 0.61 | 0.01 | 10 |
Correlation coefficient (Spearman r), coefficient of determination (r2) and significant values (p) are reported; significant values are highlighted in grey.
Discussion
The availability of specific antibodies that can recognize prefibrillar oligomers (A11), fibrils and fibrillar oligomers (OC) and annular protofibrils (aAPF) has allowed a detailed analysis of the levels and distributions of these distinct types of soluble oligomers in AD in individuals with plaque and tangle pathology but without dementia and nondemented brains with low or undetectable levels of pathology. We found significantly elevated levels of soluble fibrillar oligomers in AD patients compared to SDC and controls. Elevated soluble fibrillar oligomers were present in all frontal cortex regions tested, as well as the hippocampus, entorhinal cortex, transentorhinal cortex and cerebellum. With the exception of the cerebellum, each of these regions is known to develop neuropathology over the course of AD progression. We determined that the elevated levels of fibrillar oligomers were not an artifact created by homogenization of plaque-containing tissue because regions of control and SDC tissue that have high levels of insoluble fibrillar plaques did not have correspondingly high level of soluble fibrillar oligomers. Further, the soluble fibrillar oligomers were found not to be a product of increased expression of APP or Aβ. Having ruled these possibilities out, we can be confident that the fibrillar oligomers reported here are indeed a distinct structure and aggregation state present primarily in AD brain.
The only brain region examined that did not exhibit elevated levels of soluble fibrillar oligomers in AD was the olfactory bulb. The olfactory bulb fibrillar oligomer levels are elevated in SDC and age matched normal control brain, compared to other regions of the same brain. Neurons in the olfactory bulb are known to degenerate in AD and this is associated with the focal accumulation of Aβ (Struble and Clark, 1992). The elevation of fibrillar oligomers in the olfactory bulb is potentially interesting for two reasons. The first is that olfactory function declines with age overall (Schiffman, 1997), and overall high levels of soluble fibrillar oligomers may represent an aging phenomenon. Thus, one extension of the current study would be to examine younger individuals with the prediction that levels of oligomers would be low or undetectable. Second, there is evidence that a loss of smell may be an early indicator of incipient AD and is detectable in individuals that go on to develop mild cognitive impairments (Gilbert and Murphy, 2004; Wilson et al., 2007). The potential correlation between olfactory bulb fibrillar oligomer levels and olfactory deficits warrants further investigation.
The finding that soluble fibrillar oligomer levels are elevated in cerebellum is interesting because this brain region is not known to be functionally impaired in AD. Amyloid plaques have been reported in the cerebellum of AD patients, although this region does not exhibit neurofibrillary tangle pathology (Braak et al., 1989). Lacor et al. have also reported elevated levels of Aβ oligomers in AD brain, but the levels were not statistically significantly elevated compared to controls (Lacor et al., 2004). The reasons for the discrepancy between the significantly elevated levels we observe are not entirely clear, but could be due to differences in the selectivity of the antibodies used. If soluble fibrillar oligomers are causally related to neuronal dysfunction, the fact that their levels are elevated in the cerebellum suggests that cerebellar neurons may be less susceptible to their toxic activity. This is consistent with previous reports of the resistance of cerebellar neurons to aggregated Aβ in vitro (Ueda et al., 1994).
We also found an inverse correlation between soluble fibrillar oligomers and MMSE score. With increasing levels of fibrillar oligomers in brain regions associated with intact memory function, decreased cognitive function was evident. We also found significant correlations between fibrillar oligomer levels and the neuropathological hallmarks of AD, amyloid plaques and tau tangles. Considering the variable reports of both a lack of correlation between insoluble fibrillar aggregates or a high correlation with cognitive dysfunction, the correlation of soluble fibrillar oligomers we observe in the current study to the clinical and neuropathological progression of AD points to this species as being potentially a key factor involved with neurodegeneration and thus a potential therapeutic target.
In contrast, we found that prefibrillar oligomer and annular protofibril levels do not correlate with cognitive dysfunction in AD brain. We previously reported that A11 positive prefibrillar oligomers are detected in human AD brain (Kayed et al., 2003), but the quantitative analysis conducted here indicate that they are also found in age-matched non demented control brain. Prefibrillar oligomers are toxic in vitro (Demuro et al., 2005; Kayed et al., 2003), so if they intrinsically toxic, this suggests that something that is present in brains of non-demented individuals may modulate their toxicity. Since prefibrillar oligomers are kinetic intermediates in fibril formation (Kayed et al., 2003), it is possible that their conformational conversion to fibrillar oligomers is important for toxicity and AD pathogenesis. Prefibrillar oligomers appear to be precursors for annular protofibril levels. Since prefibrillar oligomer levels are elevated in age matched control brain, it is perhaps not surprising that annular protofibril levels are also not correlated with AD.
In summary, soluble fibrillar oligomers are specifically correlated with pathology and cognitive dysfunction in AD brain. Soluble fibrillar oligomers may represent a target for the development of immunological reagents or small molecules that specifically inhibit their formation or toxicity.
Supplementary Material
Densitometric analysis of OC reactivity after normalization to the neuronal marker α-synaptophysin. Fibrillar oligomer levels were significantly higher in AD cases (AD, red bars) compared to normal (N, blue bars) and SDC (SDC, green bars) subjects in all the brain regions examined. Olfactory bulb is omitted because the sample availability was not enough to perform a statistically significant number of experiments. Values are the mean ± SD of three independent experiments (* p≤0.05; ** p ≤ 0.01, *** p ≤ 0.001).
Acknowledgement
This work was supported by a grant from the NIH AG00538 and grants from the Cure Alzheimer Fund and The Larry L. Hillblom Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bancher C, Braak H, Fischer P, Jellinger KA. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheime's and Parkinson’s disease patients. Neurosci Lett. 1993;162:179–182. doi: 10.1016/0304-3940(93)90590-h. [DOI] [PubMed] [Google Scholar]
- Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, Bahr M, Schmidt M, Bitner RS, Harlan J, et al. Globular amyloid beta-peptide oligomer - a homogenous and stable neuropathological protein in Alzheime's disease. J Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Cochran EJ, Saper CB, Leverenz JB, Gilley DW, Wilson RS. Pathological changes in frontal cortex from biopsy to autopsy in Alzheime's disease. Neurobiol Aging. 1993;14:589–596. doi: 10.1016/0197-4580(93)90043-b. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J, Lang W. Alzheime's disease: amyloid plaques in the cerebellum. J Neurol Sci. 1989;93:277–287. doi: 10.1016/0022-510x(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Cotman CW. Image analysis of beta-amyloid load in Alzheime's disease and relation to dementia severity. Lancet. 1995;346:1524–1528. doi: 10.1016/s0140-6736(95)92053-6. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. Differences between recognition memory and remote memory for olfactory and visual stimuli in nondemented elderly individuals genetically at risk for Alzheime's disease. Exp Gerontol. 2004;39:433–441. doi: 10.1016/j.exger.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheime's disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci U S A. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. Has the amyloid cascade hypothesis for Alzheime's disease been proved? Curr Alzheimer Res. 2006;3:71–73. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. Journal of Neuroscience. 1999;19:8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol Neurodegener. 2007;2:1–11. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kayed R, Pensalfini A, Margol L, Sokolov Y, Sarsoza F, Head E, Hall JE, Glabe C. Annular protofibrils are a structurally and functionally distinct type of amyloid oligomer. J Biol Chem. 2009 doi: 10.1074/jbc.M808591200. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, et al. Synaptic targeting by Alzheime's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Viola KL, Chromy BA, Chang L, Morgan TE, Yu J, Venton DL, Krafft GA, Finch CE, Klein WL. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J Neurochem. 2001;79:595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheime's disease. American Journal of Pathology. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheime's disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- McKee AC, Kosik KS, Kowall NW. Neuritic pathology and dementia in Alzheime's disease. Ann Neurol. 1991;30:156–165. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheime's disease. Annals of Neurology. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Taste and smell losses in normal aging and disease. JAMA. 1997;278:1357–1362. [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheime's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struble RG, Clark HB. Olfactory bulb lesions in Alzheime's disease. Neurobiol Aging. 1992;13:469–473. doi: 10.1016/0197-4580(92)90074-8. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheime's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheime's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Ueda K, Fukui Y, Kageyama H. Amyloid beta protein-induced neuronal cell death: neurotoxic properties of aggregated amyloid beta protein. Brain Res. 1994;639:240–244. doi: 10.1016/0006-8993(94)91736-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheime's disease from normal and pathologic aging. Experimental Neurology. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Densitometric analysis of OC reactivity after normalization to the neuronal marker α-synaptophysin. Fibrillar oligomer levels were significantly higher in AD cases (AD, red bars) compared to normal (N, blue bars) and SDC (SDC, green bars) subjects in all the brain regions examined. Olfactory bulb is omitted because the sample availability was not enough to perform a statistically significant number of experiments. Values are the mean ± SD of three independent experiments (* p≤0.05; ** p ≤ 0.01, *** p ≤ 0.001).