Abstract
The chemokines CCL2 and CCL7 are upregulated in the brain during several neurodegenerative and acute diseases associated with infiltration of peripheral leukocytes. Astrocytes can respond to inflammatory cytokines like IL-1β and TNF-α by producing chemokines. This study aims to test the ability of IL-1β and TNF-α to stimulate CCL2 and CCL7 protein production in rat astrocyte cultures, and to elucidate signaling pathways involved in the cytokine-stimulated chemokine upregulation. Astrocytes were stimulated with IL-1β or TNF-α, and CCL2 and CCL7 levels determined by ELISA. Our results show that IL-1β and TNF-α each stimulate production of the chemokines CCL2 and CCL7 in astrocytes in a concentration- and time-dependent manner, with CCL2 showing a more rapid and robust response to the cytokine treatment than CCL7. As a first step to determine the signaling pathways involved in CCL2 and CCL7 upregulation, we stimulated astrocytes with IL-1β or TNF-α in the presence of selective inhibitors of MAPK pathways (SB203580 and SB202190 for p38, SP600125 for JNK, and U0126 for ERK) or NFκB pathways (MG-132 and SC-514). We found that NFκB pathways are important for the cytokine-stimulated CCL2 and CCL7 production, whereas MAPK pathways involving p38 and JNK, but not ERK, may also contribute but to a lesser extent. These data document for the first time that CCL7 protein production can be stimulated in astrocytes by cytokines, and that the upregulation may involve NFκB- and p38/JNK-regulated pathways. In addition, our results suggest that CCL2 and CCL7 share similarities in the signaling pathways necessary for their upregulation.
Keywords: CCL2, CCL7, astrocytes, inflammatory cytokines, NFκB, signal transduction
1. Introduction
Chemokines or “chemotactic cytokines” are critical for directing cell migration during many diverse processes such as development, angiogenesis, infections, and inflammation . During inflammation, chemokines act in concert with selectins and integrins to cause adhesion and directed migration of specific sets of leukocytes to sites of inflammation . In the brain, several cell types are capable of producing chemokines including astrocytes. When activated by a variety of stimuli, astrocytes can upregulate adhesion and co-stimulatory molecules, express pattern recognition receptors such as toll like receptors, and produce inflammatory mediators like reactive oxygen species, cytokines and chemokines . The chemokine monocyte chemoattractant protein-1 (MCP-1/CCL2) is a potent stimulator of monocyte chemotaxis and has been implicated in the progression of several peripheral and central nervous system (CNS) diseases associated with increased mononuclear cell infiltrates . Astrocytes are a major source of CCL2 in several experimental models such as experimental allergic encephalomyelitis, brain mechanical injury, entorhinodentate lesions, middle cerebral artery occlusion (MCAO), and endotoxemia as well as in the human diseases multiple sclerosis (MS), amyotrophic lateral sclerosis, and Alzheimer’s disease .
Another member of the MCP family is CCL7 (MCP-3), a chemokine that attracts several types of leukocytes including monocytes, lymphocytes, granulocytes, NK cells, and dendritic cells . CCL7 is upregulated in the CNS in several disorders associated with increased inflammatory infiltrates such as demyelinating diseases, simian virus induced acquired immune deficiency syndrome (AIDS) encephalitis, lymphocytic choriomeningitis, and MCAO . CCL7 has been localized to astrocytes during MS , and CCL7 mRNA is increased in astrocytes infected with the hepatitis virus or Theiler’s murine encephalomyelitis virus (Palma and Kim, 2004).
Because overexpression of CCL7 and CCL2 can lead to increased leukocyte infiltration into the CNS during disease states and subsequent inflammation and tissue damage, it is critical to understand the mechanisms by which production of these chemokines is regulated. The pro-inflammatory cytokines interleukin (IL)-1β and tumor necrosis factor (TNF)-α are increased in several neurodegenerative and acute inflammatory diseases of the CNS , and stimulation with either cytokine increases CCL2 and CCL7 mRNA levels in a variety of cell types . However, regulation of CCL2 and CCL7 protein production in astrocytes has not been explored in detail in response to IL-1β and TNF-α. Therefore, the goal of this study was to test the ability of IL-1β and TNF-α to stimulate CCL2 and CCL7 protein production in rat astrocyte cultures, and to elucidate signaling pathways involved in the cytokine-stimulated chemokine upregulation. We focused on the mitogen activated protein kinase (MAPK) and nuclear factor kappa B (NFκB) pathways because of their important role in upregulation of various inflammatory mediators and their known activation in response to IL-1β and TNF-α (Gosselin and Rivest, 2007; McCoy and Tansey, 2008; O'neill, 2006).
We report here that IL-1β and TNF-α each stimulate increased levels of CCL2 and CCL7 in rat astrocytes in a concentration- and time-dependent manner, although the response of CCL7 to the cytokine stimulation is quantitatively less and delayed compared to CCL2. Using selective inhibitors of the p38, extracellular signal-regulated kinase 1/2 (ERK1/2), and c-Jun N-terminal kinase 1/2 (JNK1/2) MAPKs and NFκB pathways, we found for both CCL2 and CCL7 that the NFκB pathway appears to play an important role in cytokine-induced chemokine production. In contrast, the p38 and JNK1/2 pathway inhibitors only partially blocked CCL2 and CCL7 production, and the ERK pathway inhibitor had no effect. These data suggest that IL-1β and TNF-α regulate CCL2 and CCL7 protein production through similar signaling pathways in astrocytes.
2. Results
2.1 Cytokine-stimulated upregulation of the chemokines CCL2 and CCL7 in rat astrocytes
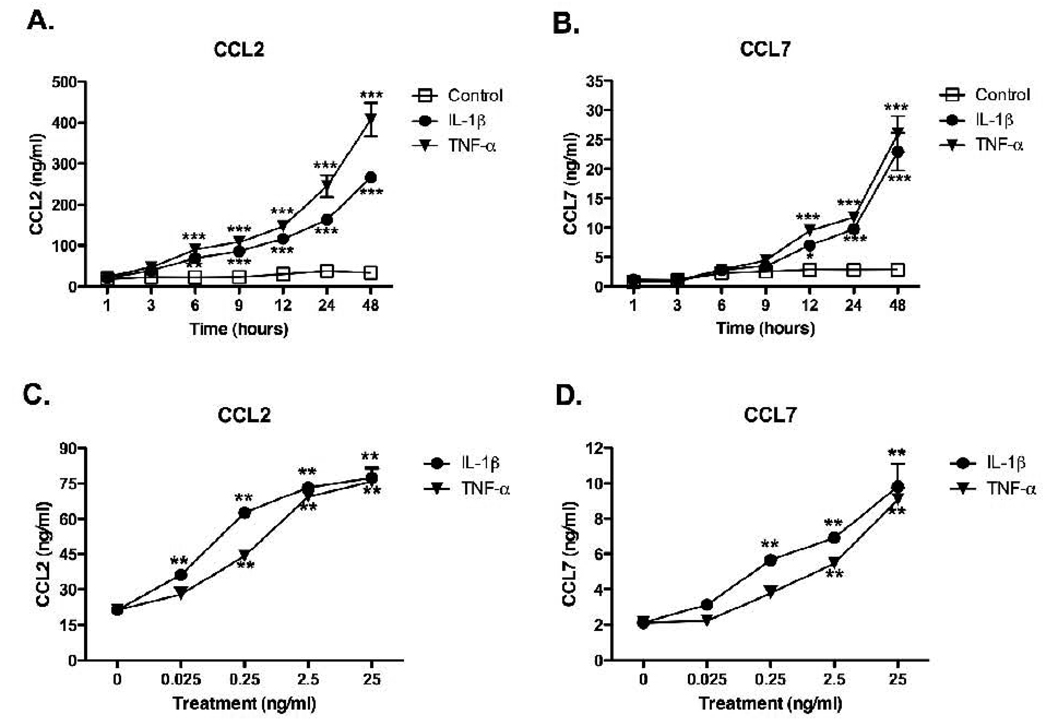
To examine the time- and concentration-dependent effects of inflammatory cytokines on CCL2 and CCL7 production, we treated rat astrocyte cultures with IL-1β or TNF-α for different lengths of time up to 48 hrs (Fig. 1A,B) or with increasing concentrations of IL-1β or TNF-α (0.025 – 25 ng/ml) for 6 hr (Fig. 1C) or 24 hr (Fig. 1D). CCL2 (Fig. 1A,C) and CCL7 (Fig. 1B,D) protein levels in conditioned media were measured by ELISA. IL-1β and TNF-α induced significant increases in CCL2 levels (Fig. 1A) above control beginning by 6 hrs and continuing until at least 48 hrs, whereas significant increases in CCL7 protein were not seen until 12 hrs after cytokine addition (Fig. 1B). Both cytokines induced significant concentration-dependent increases in CCL2 (Fig. 1C) and CCL7 (Fig. 1D) protein levels, with ~10-fold higher concentrations of TNF-α required compared to IL-1β to reach the same level of chemokine induction until this difference leveled out at higher concentrations of the cytokines. In addition, ~10-fold higher concentrations of IL-1β and TNF-α were required to induce a significant increase in CCL7 compared to CCL2, and ~10-fold less CCL7 protein was produced at all time points and all concentrations of cytokines. For subsequent experiments, we harvested conditioned media at 6 hr for CCL2 and at 24 hr for CCL7 because these represent times at which we found a 3–4 fold increase in stimulated protein levels over basal protein levels.
Figure 1. IL-1β and TNF-α stimulate CCL2 and CCL7 in rat astrocytes in a concentration-and time- dependent manner.
Rat astrocytes were stimulated with serum-free media (Control) (□), 2.5 ng/ml IL-1β (●) or 25 ng/ml TNF-α (▼) for increasing amounts of time over 48 hrs (A,B), or were stimulated with increasing concentrations of IL-1β (●) or TNF-α (▼) for 6 hr (C) or 24 hr (D). Conditioned media was analyzed by ELISA for CCL2 (A,C) or CCL7 (B,D) as described in Methods. Data are mean ± SEM from triplicate determinations and show a representative 1 of 3 independent experiments. Data were analyzed with two way ANOVA followed by Bonferroni post test (A,B) or with one way ANOVA followed by Dunnett’s multiple comparison test (C,D). *p<0.05, **p<0.01, ***p<0.001, vs. controls.
2.2 Activation of MAPK and NFκB pathways by IL-1β and TNF-α
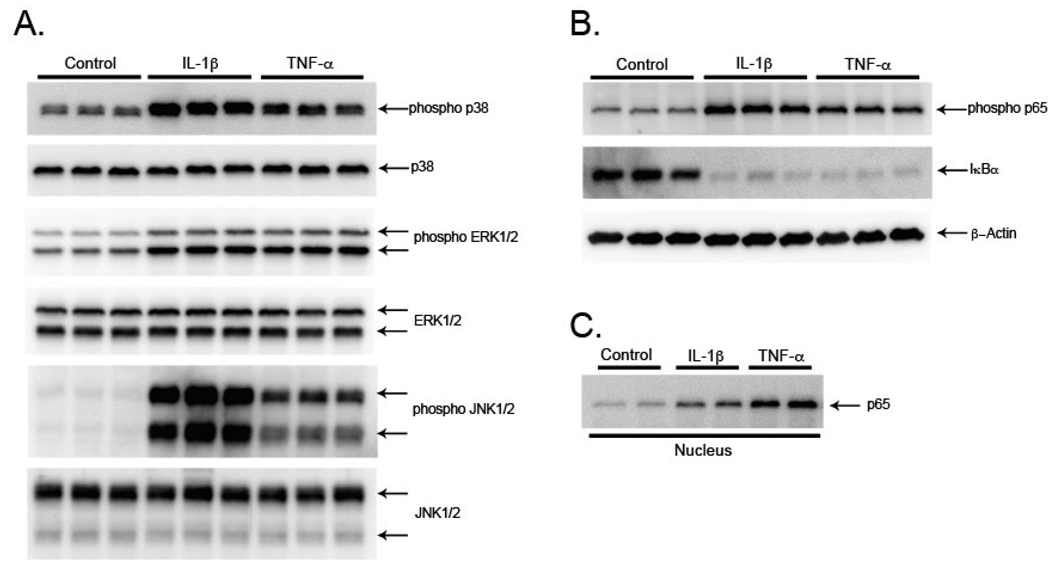
Both IL-1β and TNF-α activate the MAPKs and NFκB (McCoy and Tansey, 2008; Gosselin and Rivest, 2007), and these pathways are important in production of many inflammatory proteins including chemokines (O’neill, 2006). To confirm activation of the MAPK and NFκB pathways in our rat astrocyte cultures, we studied the activation state of these pathways 30 min after IL-1β and TNF-α treatment. The activation of p38, ERK1/2, and JNK1/2 was analyzed in cell lysates by using antibodies that selectively recognize the phosphorylated (activated) forms, as well as total protein forms of each MAPK. Both IL-1β and TNF-α consistently produced an increase in the phosphorylated forms of each MAPK, while the total protein levels of each MAPK were not changed (Fig. 2A). To verify activation of the NFκB pathway, cell lysates were analyzed for changes in levels of phosphorylated p65 and total IκBa, because phosphorylation of the p65 NFκB subunit and degradation of IκBα are associated with activation of the NFκB pathway (Hayden and Ghosh, 2008). IL-1β and TNF-α induced an increase in phosphorylated p65 and a decrease in protein levels of IκBα (Fig. 2B). To further examine activation of NFκB, we studied translocation of p65 from the cytosol to the nucleus, and found increased levels of p65 in nuclear extracts of rat astrocytes 2 hr after IL-1β and TNF-α stimulation (Fig. 2C). These data demonstrate that IL-1β and TNF-α stimulation of rat astrocytes activates the MAPK and NFκB pathways and that this activation occurs before an increase in CCL2 and CCL7 protein levels.
Figure 2. IL-1β and TNF-α activate MAPK and NFκB pathways in rat astrocytes.
Rat astrocytes were stimulated for 30 min (A,B) with serum-free media (Control), 2.5 ng/ml IL-1β or 25 ng/ml TNF-α. The levels of the activated MAPKs (phospho p38, phospho ERK1/2, and phospho JNK1/2), and total MAPKs (p38, ERK1/2, and JNK1/2) were measured by Western blots of cell lysates with antibodies against the phosphorylated and total forms of the MAPKs (A). Activation of the NFκB pathway (phospho p65 and IκBα) was measured by Western blots of cell lysates with antibodies against phosphorylated p65 and total IκBα and β-Actin loading control (B). Translocation of p65 to the nucleus was measured by Western blot of nuclear extracts with an antibody against total p65 (C). Data show one representative experiment of 3 independent experiments.
2.3 Suppression of cytokine-stimulated CCL2 and CCL7 production by MAPK inhibitors
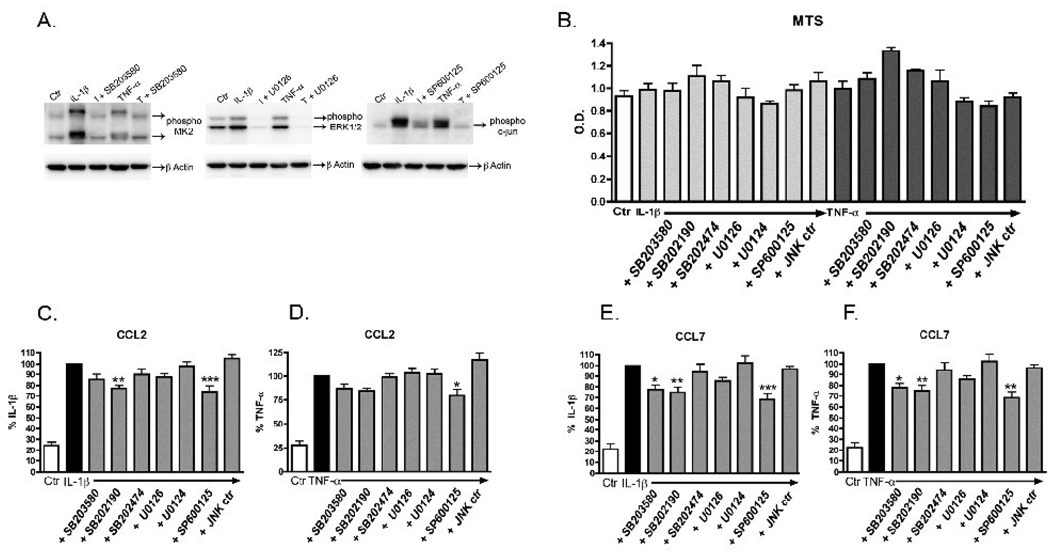
To validate our experimental system, we first confirmed that each MAPK inhibitor would block activation of its respective pathway in cytokine-stimulated rat astrocytes. We stimulated cells with IL-1β or TNF-α in the presence of one of the MAPK inhibitors and determined if activation (phosphorylation) of a substrate downstream of the inhibitor target was blocked. We analyzed by Western blots the levels of phospho MK2 for the p38 inhibitor SB203580, phospho ERK1/2 for the MEK1/2 inhibitor U0126, and phospho c-jun for the JNK inhibitor SP600125. In each case, phosphorylation of the downstream substrate was blocked by its respective pathway inhibitor at 10 µM (Fig. 3A), confirming that the MAPK inhibitors are functional in our in vitro system. Next, we confirmed with a MTS assay that there was no decrease in cell viability after 24 hr with the concentrations of inhibitors used (Fig. 3B). Further, no obvious phenotypic changes were seen by visual examination of the rat astrocytes after treatments.
Figure 3. Inhibition of CCL2 and CCL7 production by single MAPK inhibitors.
Rat astrocytes were pre-treated with 10 µM of selective inhibitors of MAPK pathways (SB203580 and SB202190 for p38, U0126 for ERK1/2, or SP600125 for JNK1/2) or control compounds (SB202474, U0124, or JNK ctr- B-F only) for 20 min before addition of 2.5 ng/ml IL-1β or 25 ng/ml TNF-α for 30 min (A), 24 hr (B,E,F) or 6 hr (C,D). Inhibition of specific MAPK pathways was analyzed by Western blots of cell lysates with antibodies against the activated (phosphorylated) form of a substrate downstream of the inhibitor target: phospho MK2 (for p38 inhibitor SB203580), phospho ERK1/2 (for MEK1/2 inhibitor U0126), phospho c-jun (for JNK1/2 inhibitor SP600125), and β-Actin loading control (A). Cell viability was measured by MTS assay (B). After stimulation of cells with IL-1β or TNF-α for 24 hr, MTS reagent was added into each well, cultures were incubated for an additional hr, and the absorbance at 490 nm was measured as described in Methods. Levels of CCL2 (C,D) or CCL7 (E,F) in conditioned media from astrocytes stimulated with IL-1β (C,E) and TNF-α (D,F) were determined by ELISA as described in Methods. Data are expressed as % IL-1β alone or % TNF-α alone. Data are mean ± SEM from triplicate determinations and show a representative 1 of 3 independent experiments (A,B), or are the mean ± SEM of 9–11 independent experiments of triplicate determinations (C–F). Data were analyzed by one way ANOVA followed by Dunnett’s multiple comparison test, *p<0.05, **p<0.01, ***p<0.001 vs. IL-1β alone or vs. TNF-α alone.
To test whether MAPK pathways are important in CCL2 and CCL7 production, we stimulated rat astrocytes in the presence of selective inhibitors of the MAPK pathways. Astrocytes were pre-treated for 20 min with each inhibitor and then stimulated with IL-1β or TNF-α for 6 hr (CCL2) or 24 hr (CCL7). Levels of CCL2 (Fig. 3C,D) and CCL7 (Fig. 3E,F) in conditioned media were measured by ELISA. Inhibition of the ERK pathway with U0126 or treatment with U0124, a negative control compound, did not result in any inhibition of CCL2 or CCL7 (Fig. 3C–F). Inhibition of the JNK pathway with SP600125 resulted in a partial yet significant inhibition (20–30%) for both IL-1β or TNF-α stimulated CCL2 and CCL7, while the negative control compound (JNK ctr) did not inhibit chemokine production (Fig. 3C–F). Similarly, inhibition of the p38 pathway with either SB203580 or SB202190 partially blocked IL-1β or TNF-α stimulated CCL7 production, whereas the negative control compound SB202474 had no effect (Fig. 3C–F). The p38 inhibitors had less effect on CCL2 levels, with only the SB202190 compound showing significant inhibition of IL-1β induced CCL2 (Fig. 3C), although there was a trend toward inhibition of TNF-α induced CCL2 (Fig. 3D).
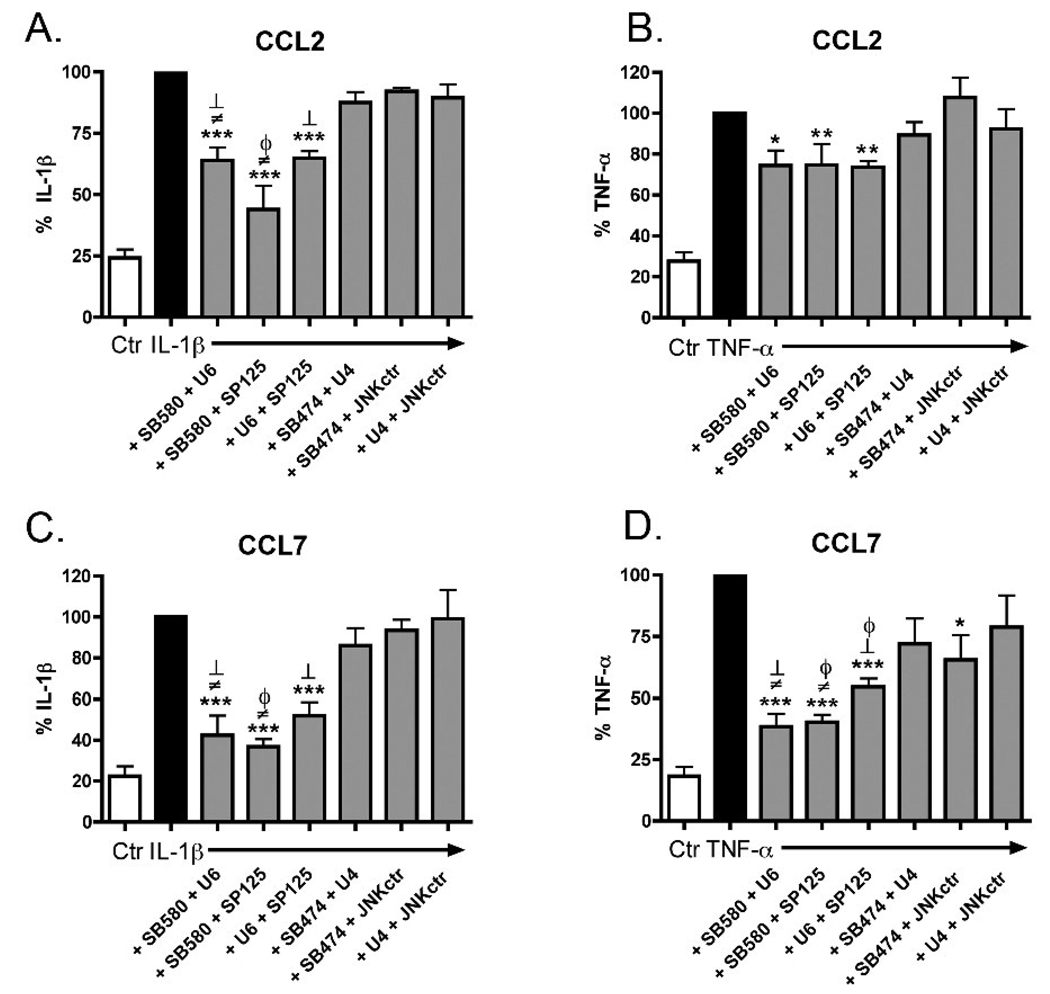
These data suggest that inhibition of a single MAPK pathway is not sufficient to completely suppress cytokine-induced CCL2 and CCL7 upregulation. To determine if multiple MAPK pathways are important for CCL2 and CCL7 production, we stimulated rat astrocytes in the presence of different combinations of MAPK inhibitors or the negative control compounds at 10 µM (Fig. 4). A combination of SB203580 + U0126 or SB203580 + SP600125 resulted in a significant decrease in IL-1β stimulated CCL2 or CCL7 production (Fig. 4A,C) compared to each inhibitor alone (see Fig. 3C,E), suggesting an additive effect of inhibiting the p38 and ERK, or p38 and JNK pathways. When SB203580 was added at a lower concentration, 0.625 µM, to 10 µM of U0126 there was also a significant reduction in IL-1β stimulated CCL2 or CCL7 protein compared to U0126 alone (data not shown). These data suggest that the additive effect of inhibiting both p38 and ERK is due mostly to inhibition of p38, and less to inhibition of ERK. Additionally, a combination of U0126 + SP600125 resulted in a significant decrease in IL-1β stimulated CCL2 or CCL7 levels (Fig. 4A,C) compared to U0126 alone, but not to SP600125 alone (see Fig. 3C,E), suggesting that inhibition of both the ERK and JNK pathways results in no further effect than inhibition of the JNK pathway alone. The chemokine responses to TNF-α and inhibitor combinations were slightly different. SB203580 + U0126, SB203580 + SP600125, and U0126 + SP600125 all resulted in a significant decrease in TNF-α stimulated CCL2, however this inhibition was not significantly different from any of the inhibitors added alone (Fig 4B, compare to Fig. 3D). In contrast, all three combinations of inhibitors significantly inhibited TNF-α stimulated CCL7 production compared to each inhibitor alone (Fig. 4D; compare to Fig. 3F). Again, a lower concentration of SB203580 (0.625 µM) plus U0126 or plus SP600125 was able to significantly inhibit CCL7 production compared to U0126 or SP600125 alone (data not shown). Similarly, SP600125 (0.625 µM) plus U0126 was able to significantly inhibit TNF-α stimulated CCL7 compared to U0126 alone (data not shown). None of the combinations of negative controls compounds at 10 µM caused inhibition of CCL7 or CCL2, except TNF-α plus SB202474 + JNK ctr at 24 hr. This was not due to a loss in cell viability because none of the treatments decreased cell viability in the MTS assay (data not shown).
Figure 4. Inhibition of CCL2 and CCL7 production by combinations of MAPK inhibitors.
Rat astrocytes were pre-treated with 10 µM of either SB203580 (SB580) + U0126 (U6), SB203580 + SP600125 (SP125), U0126 + SP600125, SB202474 (SB474) + U0124 (U4), SB202474 + JNK ctr, or U0124 + JNK ctr for 20 min before addition of 2.5 ng/ml IL-1β or 25 ng/ml TNF-α for 6 hr (A,B) or 24 hr (C,D), and conditioned media was analyzed by ELISA for CCL2 (A,B) or CCL7 (C,D). Data are expressed as % IL-1β alone or % TNF-α alone. Data are means ± SEM of 3 independent experiments of triplicate determinations. Data were analyzed by one way ANOVA followed by Dunnett’s multiple comparison test for treatments versus stimulus alone, *p<0.05, **p<0.01, ***p<0.001, or by student’s t-test for treatments versus inhibitors alone (Fig 3), ϕ =versus SB203580, ≠ =versus U0126, and ┴=versus SP600125, p<0.05.
2.4 Suppression of cytokine-stimulated CCL2 and CCL7 production by NFκB inhibitors
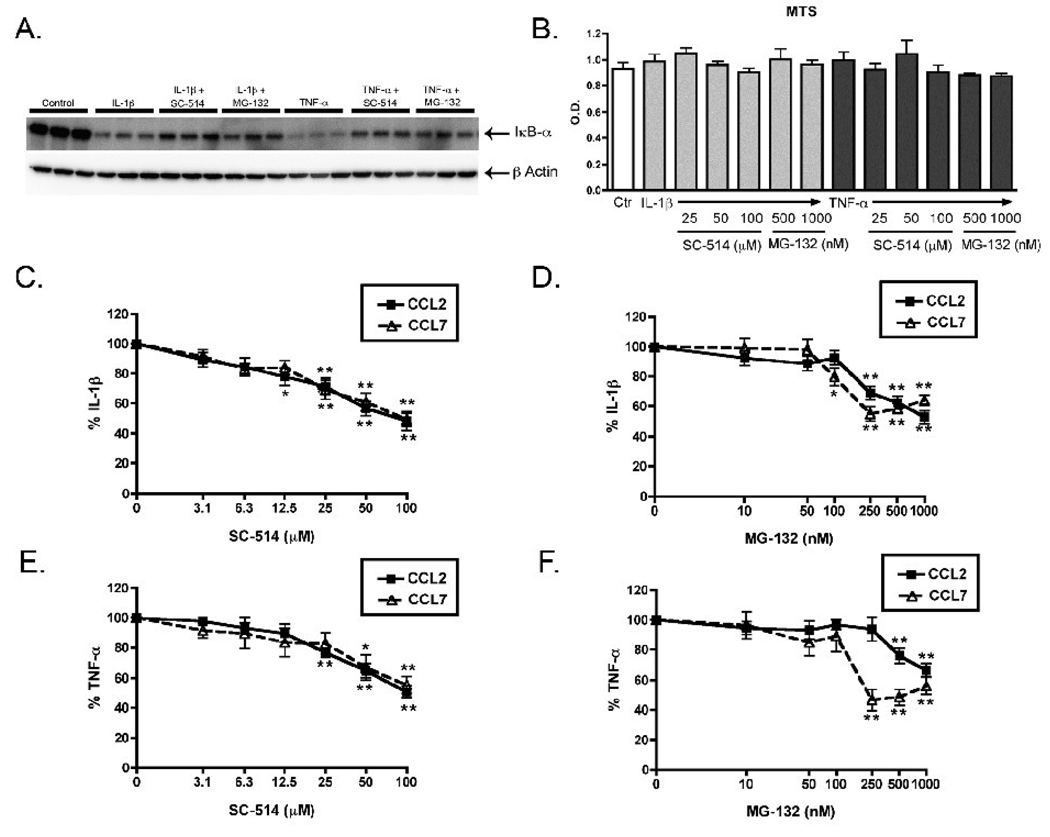
To evaluate whether inhibition of the NFκB pathway blocked CCL2 and CCL7 production in IL-1β or TNF-α stimulated rat astrocytes, we used two different inhibitors of the NFκB pathway, MG-132 and SC-514. We first confirmed that both inhibitors blocked NFκB pathway activation by showing that the inhibitors prevented the IL-1β or TNF-α induced reduction in IκB-α levels (Fig. 5A). We also showed by MTS assay that the inhibitors did not cause a decrease in cell viability at any of the concentrations used (Fig. 5B). To test whether the NFκB pathway is important for CCL2 and CCL7 production, we stimulated astrocytes in the presence of selective inhibitors of the NFκB pathway. Astrocytes were pre-treated for 20 min with increasing concentrations of MG-132 or SC-514, and then stimulated with IL-1β or TNF-α for 6 hr (CCL2) or 24 hr (CCL7). Treatment of astrocytes with SC-514 resulted in a very similar concentration-dependent inhibition of IL-1β or TNF-α stimulated CCL2 and CCL7 production (Fig. 5C,E). At the highest concentration of SC-514 (100 µM), both chemokines were inhibited about 50% after stimulation with either IL-1β or TNF-α (Fig. 5C,E). A second NFκB inhibitor, MG-132 also concentration-dependently inhibited IL-1β or TNF-α stimulated CCL2 or CCL7 production (Fig. 5D,F). Higher concentrations (5 and 10 µM) of MG-132 did not result in any further inhibition of CCL2 or CCL7 protein production, or any further restoration of IκB-α levels (data not shown). CCL2 was inhibited concentration-dependently by MG-132 through 1000 nM, while CCL7 reached its maximal inhibition at 250 nM, and leveled off at higher doses of MG-132 (Fig. 5 D, F). The maximal inhibition seen with MG-132 for both chemokines was about 50%, the same level of inhibition seen with SC-514.
Figure 5. Inhibition of CCL2 and CCL7 production by NFκB inhibitors.
Rat astrocytes were pre-treated with 100 µM of SC-514 or 1 µM of MG-132 (NFκB inhibitors) for 20 min before addition of serum-free media (Control), 2.5 ng/ml IL-1β or 25 ng/ml TNF-α for 15 min (A). Inhibition of the NFκB pathway was analyzed by Western blots of cell lysates with antibodies against IκB-α and β-Actin loading control (A). Rat astrocytes were pre-treated with increasing concentrations of SC-514 or MG-132 for 20 min before addition of IL-1β or TNF-α, and after 24 hr MTS reagent was added into each well and cultures were incubated for an additional hr. The absorbance at 490 nm in each well was then measured as described in Methods (B). Rat astrocytes were pre-treated with increasing concentrations of SC-514 or MG-132 for 20 min before addition of IL-1β or TNF-α, and media was collected at 6 hr (for CCL2) and 24 hr (for CCL7) and analyzed by ELISA (C–F). Data are expressed as % IL-1β alone or % TNF-α alone (C–F). Data are mean ± SEM of triplicate determinations of 1 of 3 independent experiments (A,B) or are the mean ± SEM of 6 independent experiments of triplicate determinations (C–F). Data were analyzed by one way ANOVA followed by Dunnett’s multiple comparison test, *p<0.05, **p<0.01, vs. IL-1β or vs. TNF-α.
3. Discussion
We have demonstrated here that IL-1β and TNF-α each stimulate production of the chemokines CCL2 and CCL7 in rat astrocyte cultures, with CCL2 showing a more rapid and robust response to the cytokine treatment compared to CCL7. In addition, we have shown that NFκB pathways are important for the cytokine-stimulated CCL2 and CCL7 production, whereas MAPK pathways involving p38 and JNK, but not ERK, may also contribute though to a lesser extent. Our results provide several new findings related to the mechanisms of chemokine production. First, although CCL2 upregulation had been studied in certain other cell types, information about the regulation of CCL2 production in astrocytes in response to IL-1β or TNFα was lacking. We demonstrate that IL-1β- or TNFα- stimulated CCL2 protein production may involve NFκB- and p38/JNK-regulated pathways. Second, our findings showing upregulation of CCL7 protein production in cytokine-stimulated astrocytes and the signaling pathways involved are amongst the first data related to regulation of the protein levels of this chemokine in any cell type. Finally, our results suggest that even though CCL2 and CCL7 show differences in their responses to IL-1β or TNF-α in terms of the temporal onset and protein levels produced, the chemokines share similarities in the signaling pathways necessary for their upregulation.
IL-1β or TNF-α stimulation of rat astrocytes produced significant concentration- and time- dependent production of CCL2 and CCL7. We found several differences in the protein production of the two chemokines: 1) approximately 10-fold more CCL2 protein than CCL7 was produced both in unstimulated cells and in response to all concentrations of IL-1β or TNF-α, 2) 10-fold higher concentrations of IL-1β and TNF-α were required to produce significant amounts of CCL7 compared to CCL2, and 3) significant increases in CCL7 protein were not seen until 12 hr after cytokine addition compared to 6 hr for CCL2. These data confirm previous reports documenting differences in production of CCL2 and CCL7 in other systems. For example, considerably lower amounts of CCL7 than CCL2 were produced in cytokine-stimulated human osteosarcoma cells, monocytes, and endothelial cells , and in the brains of mice with lymphocytic choriomeningitis (Asensio and Campbell, 1997). Further, CCL7 but not CCL2 was found upregulated in simian virus induced AIDS encephalitis .
Our data suggest that both IL-1β and TNF-α signal through the same converging pathways to induce protein production of CCL2 and CCL7, because we found comparable results with the inhibitors blocking either the IL-1β or TNF-α induced chemokines. Both NFκB inhibitors blocked protein production of the chemokines to about 50% of stimulus alone in response to either IL-1β or TNF-α. Similarly, the p38 and JNK, but not ERK MAPKs may also be important to a lesser extent in the CCL2 and CCL7 response to IL-1β or TNF-α. Although the receptors and upstream post-receptor signaling are distinct for IL-1β or TNF-α, both cytokines activate the NFκB and MAPK pathways in many different cell types including astrocytes. Once activated, these two pathways can result in production of many pro-inflammatory genes (O'neill, 2006).
The regulation of CCL2 in response to IL-1β and TNF-α has been studied previously but is variable, and the contributions of the MAPK and NFκB pathways may differ depending on the cell type and stimulus conditions. Several studies have reported participation of the NFκB pathway in TNF-α and IL-1β stimulated CCL2 protein production . However, the contributions of the MAPK pathways to IL-1β or TNF-α stimulated CCL2 production are more uncertain. For example, some studies have shown that p38 MAPK is important for IL-1β or TNF-α stimulated CCL2 , while other studies have not found any dependence on p38 . Likewise, JNK MAPK was required for IL-1β or TNF-α stimulated CCL2 under certain conditions but not others. Consistent with our findings with astrocytes, most previous studies also find only a partial inhibition of CCL2 protein production when blocking either p38 or JNK. Since we did not see total inhibition of stimulated CCL2 or CCL7 with any of the inhibitors alone or in any of the combinations, this suggests that several converging signaling pathways are necessary for CCL2 and CCL7 production. In particular, p38 MAPK has numerous direct and indirect interactions with NFκB , so an obvious direction for future studies will be to examine the interactions of the MAPK and NFκB pathway and their roles in CCL2 and CCL7 protein production.
In contrast to an extensive literature on CCL2 regulation, much less is known about the regulation of CCL7 protein in any cell type. However, there is some evidence implicating the MAPK and NFκB pathways in CCL7 mRNA production. Inhibition of p38 or NFκB blocked CCL7 mRNA in IL-1β stimulated human airway smooth muscle cells , inhibition of ERK blocked amyloid β stimulated CCL7 mRNA in microglia , and inhibition of the NFκB or JNK pathways inhibited free fatty acid induced CCL7 mRNA in adipocytes . Additionally, inhibition of transforming growth factor β-activated protein kinase 1 (TAK1), (a MAPKKK upstream of MAPKs) strongly suppressed TNF-α stimulated CCL7 mRNA in mouse fibroblasts . Further, in vivo administration of a NFκB inhibitor to LPS treated apoE−/− mice blocked upregulation of plasma CCL7 levels . The data reported here are consistent with the importance of signaling pathways involving NFκB, p38 and JNK in the regulation of IL-1β- or TNF-α- stimulated CCL7 production in astrocytes.
Astrocytes are the most numerous cell type in the brain, comprise part of the BBB, and become activated in response to a variety of stimuli which all can affect infiltration of circulating leukocytes from the periphery into the CNS. The chemokines CCL2 and CCL7 are upregulated in several neurodegenerative disorders that are associated with increased leukocyte infiltration. Although the functions of the two chemokines overlap (recruitment of monocytes/macrophages), the in vivo specificities of the two chemokines are likely to be distinct. CCL7 attracts a wider range of leukocytes and interacts with more chemokine receptors than CCL2 . Further, distinct actions of each chemokine can result from differences in cellular localization, the amount of each chemokine produced in response to the same stimuli, and the effect of each chemokine on a responding leukocyte. Our data are consistent with another potential regulatory mechanism that could influence chemokine action. Specifically, our results suggest that multiple, distinct signaling pathways can converge on the same disease-relevant biological endpoints (chemokine upregulation), but that the relative quantitative contribution of each pathway to the mechanisms of chemokine production may differ. More detailed analyses must be done in future studies to address the complex interactions and cross-talk among contributing signaling pathways that culminate in CCL2 and CCL7 upregulation in response to cytokines. These kinds of studies are required as a foundation to evaluate the possibility that components of these signaling pathways may represent points of future drug discovery efforts that target inflammatory responses of activated astrocytes.
4. Experimental Procedure
4.1 Preparation of Reagents
IL-1β and TNF-α (R&D Systems, Minneapolis, MN) were prepared in sterile phosphate buffered saline (PBS) at 20 µg/ml and 50 µg/ml respectively, and used at the concentrations indicated. Inhibitors of MAPK pathways, SB203580 and SB202190 (p38 MAPK inhibitors), U0126 (MEK inhibitor), SP600125 (JNK inhibitor) and control compounds SB202474 (negative control for p38 MAPK inhibitor), U0124 (negative control for MEK inhibitor), and JNK Inhibitor II negative control (JNK ctr) (all from Calbiochem, La Jolla, CA) were prepared as 30 mM stocks in dimethysulfoxide (DMSO; Sigma, cell culture grade) and used at the concentrations indicated. Inhibitors of the NFκB pathway, SC-514 and MG-132 (Calbiochem) were prepared as 100 mM and 30 mM stocks respectively in DMSO, and were used at the concentrations indicated.
4.2 Cell Culture
Mixed glial cultures were prepared from the cerebral cortex and hippocampus of neonatal Sprague-Dawley rats as described previously and maintained in complete α-MEM (media supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 2 mM glutamine and antibiotics [100 units/ml penicillin/100 µg/ml streptomycin (Mediatech, Inc., Manassas, VA)]) and incubated at 37°C with 5% carbon dioxide. To maximize astrocyte purity and reduce contamination of other cell types (especially microglia) in our cultures, we utilized the following steps: 1) Glial cultures were fed twice weekly, and after 10–14 days in culture, primary cultures of glial cultures were trypsinized and passaged to secondary cultures (1:4). Cells were again grown for at least 10 days before being used as tertiary passages. Subculturing has been shown to reduce the proportion of other contaminating cell types, and therefore enriches the astrocyte population (Saura, 2007). 2) The glia cultures were subjected to a shaking protocol that resulted in “shaking off” of less adherent cells like microglia and oligodendrocytes. The flasks were shaken for 2 hours on an orbital shaker at 250 rpm at 37°C, the media containing loosely adherent cells was aspirated, and the flasks were shaken for an additional 22 hours. The remaining adherent cells were trypsinized and plated as described below. This procedure resulted in both type 1 and type 2 astroglia . To confirm astrocyte purity, we performed GFAP (an astrocyte specific intermediate filament) staining as described previously . Cultures were routinely >97% GFAP positive, while in contrast staining for OX-42, a rat microglial marker, was <1.5%.
4.3 Cell Stimulation
Rat astrocytes were plated at 4 × 104 cells in 48 well plates (for ELISAs), 1 × 105 cells in 24 well plates (for Western blot experiments), and 1 × 106 cells in 60-mm dishes (for nuclear extractions) in complete α-MEM and allowed to grow for 24 hours. Twenty-four hours before treatment, media was removed and replaced with serum-free α-MEM containing N2 supplements (Invitrogen, Carlsbad, CA). Cells were treated with either diluent, IL-1β, or TNF-α in the absence or presence of a MAPK inhibitor or NFκB inhibitor for various times. When inhibitors were used, they were added to the cells 20 min before IL-1β or TNF-α. The concentration of DMSO never exceeded 0.1% in any cell treatments.
4.4 ELISA and Western blots
The levels of CCL2 and CCL7 released into the conditioned media were measured by ELISA (CCL2 BD OptEIA, BD Biosciences, San Jose, CA and CCL7 Construction Kit, Antigenix, Huntington Station, NY) according to the manufacturer’s instructions. Cell lysates were harvested for Western blot analysis using lysis buffer (40 mM Tris-HCL, pH 6.8, 2% SDS, 10% glycerol, 0.02% NaN3, 1 µg/ml aprotinin, 1 mM phenylmethylsulphonylfluoride, 1 µg/ml leupeptin, and 2 mM NaVO3). Analysis of cell lysates was performed as described previously (Fuller and Van Eldik, 2008) and visualized using SuperSignal West Pico or West Femto Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL). Images of blots were acquired for quantification using Kodak CF440 digital imager and analyzed with Kodak Molecular Imaging Software (Kodak, Rochester, NY). The primary antibodies used were (at 1:1000, unless noted) mouse monoclonal anti-phospho p65, rabbit polyclonal anti-p65, rabbit polyclonal anti-phospho p38 MAPK, rabbit polyclonal anti-p38 MAPK (1:2500), mouse monoclonal anti-phospho JNK p46/54, rabbit polyclonal anti-JNK p46/54, mouse monoclonal anti-phospho ERK p42/44 MAPK (1:20,000), rabbit polyclonal anti-ERK p42/44 MAPK (1:5000), rabbit polyclonal anti-IκB-α, rabbit polyclonal anti-phospho c-jun, rabbit polyclonal anti-phospho MK2 (all from Cell Signaling, Beverly, MA) and mouse monoclonal anti-β-Actin (Sigma-Aldrich, St. Louis, MO). The secondary antibodies used were horseradish peroxidase-conjugated goat anti-mouse (1:2000) and goat anti-rabbit (1:5000) (Jackson Immuno Research, West Grove, PA).
4.5 Nuclear Extractions
Rat astrocytes were treated in 60-mm dishes for 2 hr and nuclear extractions were prepared as described previously .
4.6 Cell Viability MTS Assay
The number of viable cells was determined using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI) according to manufacturer instructions. This assay, known as the MTS assay, utilizes the chemical reduction of a tetrazolium substrate by the NADPH or NADH present in living cells. Therefore, a reduction in the number of living cells in a sample results in less color product formation at 490 nm.
4.7 Statistical Analysis
Data were analyzed by one way ANOVA followed by Dunnett’s multiple comparison test, with two way ANOVA followed by Bonferroni post test, or with the student’s t-test using a statistical software package (GraphPad Prism, version 4.0; Graphpad Software, San Diego, CA). Statistical significance was established when P<0.05.
Acknowledgements
These studies were supported in part by NIH grant R37 AG013939 (LVE). WT was a predoctoral trainee on NIH T32 AG000260 and is currently supported by NIH F31 NS055471 predoctoral fellowship. We thank Dr. Lucia de Almeida for preparation of the primary glial cultures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed RA, Murao K, Imachi H, Yoshida K, Dobashi H, Hosomi N, Ishida T. c-Jun N-terminal kinases inhibitor suppresses the TNF-alpha induced MCP-1 expression in human umbilical vein endothelial cells. Endocrine. 2008 doi: 10.1007/s12020-008-9136-0. in press. [DOI] [PubMed] [Google Scholar]
- Akama KT, Albanese C, Pestell RG, Van Eldik LJ. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- Asensio VC, Campbell IL. Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J. Virol. 1997;71:7832–7840. doi: 10.1128/jvi.71.10.7832-7840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J. Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron P, Bussini S, Cardin V, Corbo M, Conti G, Galimberti D, Scarpini E, Bresolin N, Wharton SB, Shaw PJ, Silani V. Production of monocyte chemoattractant protein-1 in amyotrophic lateral sclerosis. Muscle Nerve. 2005;32:541–544. doi: 10.1002/mus.20376. [DOI] [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J. Neurosci. Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Bian ZM, Elner SG, Yoshida A, Kunkel SL, Su J, Elner VM. Activation of p38, ERK1/2 and NIK pathways is required for IL-1beta and TNF-alpha-induced chemokine expression in human retinal pigment epithelial cells. Exp. Eye Res. 2001;73:111–121. doi: 10.1006/exer.2001.1019. [DOI] [PubMed] [Google Scholar]
- Boutin H, Kimber I, Rothwell NJ, Pinteaux E. The expanding interleukin-1 family and its receptors: do alternative IL-1 receptor/signaling pathways exist in the brain? Mol. Neurobiol. 2003;27:239–248. doi: 10.1385/MN:27:3:239. [DOI] [PubMed] [Google Scholar]
- Carpentier P, Begolka W, Olson J, Elhofy A, Karpus W, Miller S. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2004;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem. 1999;274:30858–30863. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- Chen YM, Chiang WC, Lin SL, Wu KD, Tsai TJ, Hsieh BS. Dual regulation of tumor necrosis factor-alpha-induced CCL2/monocyte chemoattractant protein-1 expression in vascular smooth muscle cells by nuclear factor-kappaB and activator protein-1: modulation by type III phosphodiesterase inhibition. J. Pharmacol. Exp. Ther. 2004;309:978–986. doi: 10.1124/jpet.103.062620. [DOI] [PubMed] [Google Scholar]
- Cuaz-Pérolin C, Billiet L, Baugé E, Copin C, Scott-Algara D, Genze F, Büchele B, Syrovets T, Simmet T, Rouis M. Antiinflammatory and antiatherogenic effects of the NF-kappaB inhibitor acetyl-11-keto-beta-boswellic acid in LPS-challenged ApoE–/– mice. Arterioscler. Thromb. Vasc. Biol. 2008;28:272–277. doi: 10.1161/ATVBAHA.107.155606. [DOI] [PubMed] [Google Scholar]
- Daly C, Rollins B. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. 2003;10:247–257. doi: 10.1038/sj.mn.7800190. [DOI] [PubMed] [Google Scholar]
- De Keulenaer GW, Ushio-Fukai M, Yin Q, Chung AB, Lyons PR, Ishizaka N, Rengarajan K, Taylor WR, Alexander RW, Griendling KK. Convergence of redox-sensitive and mitogen-activated protein kinase signaling pathways in tumor necrosis factor-alpha-mediated monocyte chemoattractant protein-1 induction in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2000;20:385–391. doi: 10.1161/01.atv.20.2.385. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fuller AD, Van Eldik LJ. MFG-E8 regulates microglial phagocytosis of apoptotic neurons. J. Neuroimmune Pharmacol. 2008;3:246–256. doi: 10.1007/s11481-008-9118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J. Immunol. 1996;156:4363–4368. [PubMed] [Google Scholar]
- Godiska R, Chantry D, Dietsch GN, Gray PW. Chemokine expression in murine experimental allergic encephalomyelitis. J. Neuroimmunol. 1995;58:167–176. doi: 10.1016/0165-5728(95)00008-p. [DOI] [PubMed] [Google Scholar]
- Goebeler M, Kilian K, Gillitzer R, Kunz M, Yoshimura T, Bröcker EB, Rapp UR, Ludwig S. The MKK6/p38 stress kinase cascade is critical for tumor necrosis factor-alpha-induced expression of monocyte-chemoattractant protein-1 in endothelial cells. Blood. 1999;93:857–865. [PubMed] [Google Scholar]
- Gosselin D, Rivest S. Role of IL-1 and TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr. Hyde duality of the innate immune system. Brain. Behav. Immun. 2007;21:281–289. doi: 10.1016/j.bbi.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Gourmala NG, Buttini M, Limonta S, Sauter A, Boddeke HW. Differential and time-dependent expression of monocyte chemoattractant protein-1 mRNA by astrocytes and macrophages in rat brain: effects of ischemia and peripheral lipopolysaccharide administration. J. Neuroimmunol. 1997;74:35–44. doi: 10.1016/s0165-5728(96)00203-2. [DOI] [PubMed] [Google Scholar]
- Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S, Palmer SS. Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Mol. Pharmacol. 2008;73:1394–1404. doi: 10.1124/mol.107.042176. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Holzberg D, Knight CG, Dittrich-Breiholz O, Schneider H, Dörrie A, Hoffmann E, Resch K, Kracht M. Disruption of the c-JUN-JNK complex by a cell-permeable peptide containing the c-JUN delta domain induces apoptosis and affects a distinct set of interleukin-1-induced inflammatory genes. J. Biol. Chem. 2003;278:40213–40223. doi: 10.1074/jbc.M304058200. [DOI] [PubMed] [Google Scholar]
- Hu J, Castets F, Guevara JL, Van Eldik LJ. S100 beta stimulates inducible nitric oxide synthase activity and mRNA levels in rat cortical astrocytes. J. Biol. Chem. 1996;271:2543–2547. doi: 10.1074/jbc.271.5.2543. [DOI] [PubMed] [Google Scholar]
- Ito S, Sawada M, Haneda M, Ishida Y, Isobe K. Amyloid-beta peptides induce several chemokine mRNA expressions in the primary microglia and Ra2 cell line via the PI3K/Akt and/or ERK pathway. Neurosci. Res. 2006;56:294–299. doi: 10.1016/j.neures.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Jiao P, Chen Q, Shah S, Du J, Tao B, Tzameli I, Yan W, Xu H. Obesity-related Upregulation of Monocyte Chemotactic Factors in Adipocytes: Involvement of NF-{kappa} B and JNK Pathways. Diabetes. 2009;58:104–115. doi: 10.2337/db07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TE, Asensio VC, Yu N, Paoletti AD, Campbell IL, Buchmeier MJ. Dynamic regulation of alpha- and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 1998;160:970–978. [PubMed] [Google Scholar]
- Levinson SW, McCarthy KD. Astroglia in Culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. Cambridge: MIT Press; 1991. pp. 309–336. [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Martin T, Cardarelli PM, Parry GC, Felts KA, Cobb RR. Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-kappa B and AP-1. Eur. J. Immunol. 1997;27:1091–1097. doi: 10.1002/eji.1830270508. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J. Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF. MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J. Neuroimmunol. 1998;86:20–29. doi: 10.1016/s0165-5728(98)00002-2. [DOI] [PubMed] [Google Scholar]
- Menten P, Wuyts A, Van Damme J. Monocyte chemotactic protein-3. Eur. Cytokine Netw. 2001;12:554–560. [PubMed] [Google Scholar]
- O'neill L. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat. Rev. Drug Discov. 2006;5:549–563. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- Palma JP, Kim BS. The scope and activation mechanisms of chemokine gene expression in primary astrocytes following infection with Theiler's virus. J. Neuroimmunol. 2004;149:121–129. doi: 10.1016/j.jneuroim.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Ping D, Boekhoudt G, Boss JM. trans-Retinoic acid blocks platelet-derived growth factor-BB-induced expression of the murine monocyte chemoattractant-1 gene by blocking the assembly of a promoter proximal Sp1 binding site. J. Biol. Chem. 1999;274:31909–31916. doi: 10.1074/jbc.274.45.31909. [DOI] [PubMed] [Google Scholar]
- Polentarutti N, Introna M, Sozzani S, Mancinelli R, Mantovani G, Mantovani A. Expression of monocyte chemotactic protein-3 in human monocytes and endothelial cells. Eur. Cytokine Netw. 1997;8:271–274. [PubMed] [Google Scholar]
- Proost P, Wuyts A, Van Damme J. Human monocyte chemotactic proteins-2 and −3: structural and functional comparison with MCP-1. J. Leukoc. Biol. 1996;59:67–74. doi: 10.1002/jlb.59.1.67. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- Rovin BH, Wilmer WA, Danne M, Dickerson JA, Dixon CL, Lu L. The mitogen-activated protein kinase p38 is necesssary for interleukin 1beta-induced monocyte chemoattractant protein 1 expression by human mesangial cells. Cytokine. 1999;11:118–126. doi: 10.1006/cyto.1998.0409. [DOI] [PubMed] [Google Scholar]
- Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat. Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- Sasseville VG, Smith MM, Mackay CR, Pauley DR, Mansfield KG, Ringler DJ, Lackner AA. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am. J. Pathol. 1996;149:1459–1467. [PMC free article] [PubMed] [Google Scholar]
- Saura J. Microglial cells in astroglial cultures: a cautionary note. J. Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina N, Jia T, Hohl TM, Pamer E. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng WS, Hu S, Ni HT, Rowen TN, Lokensgard JR, Peterson PK. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J. Leukoc. Biol. 2005;78:1233–1241. doi: 10.1189/jlb.0405221. [DOI] [PubMed] [Google Scholar]
- Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J. Neuroimmunol. 1998;84:238–249. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte Chemoattractant Protein-1 Plays a Dominant Role in the Chronic Inflammation Observed in Alzheimer's Disease. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00188.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teferedegne B, Green MR, Guo Z, Boss JM. Mechanism of action of a distal NF-kappaB-dependent enhancer. Mol. Cell Biol. 2006;26:5759–5770. doi: 10.1128/MCB.00271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiefes A, Wolter S, Mushinski JF, Hoffmann E, Dittrich-Breiholz O, Graue N, Dörrie A, Schneider H, Wirth D, Luckow B, Resch K, Kracht M. Simultaneous blockade of NFkappaB, JNK, and p38 MAPK by a kinase-inactive mutant of the protein kinase TAK1 sensitizes cells to apoptosis and affects a distinct spectrum of tumor necrosis factor target genes. J. Biol. Chem. 2005;280:27728–27741. doi: 10.1074/jbc.M411657200. [DOI] [PubMed] [Google Scholar]
- Uddin J, Garcia HH, Gilman RH, Gonzalez AE, Friedland JS. Monocyte-astrocyte networks and the regulation of chemokine secretion in neurocysticercosis. J. Immunol. 2005;175:3273–3281. doi: 10.4049/jimmunol.175.5.3273. [DOI] [PubMed] [Google Scholar]
- Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J. Immunol. 1994;153:2052–2063. [PubMed] [Google Scholar]
- Wang X, Li X, Yaish-Ohad S, Sarau HM, Barone FC, Feuerstein GZ. Molecular cloning and expression of the rat monocyte chemotactic protein-3 gene: a possible role in stroke. Brain Res. Mol. Brain Res. 1999;71:304–312. doi: 10.1016/s0169-328x(99)00203-x. [DOI] [PubMed] [Google Scholar]
- Wong C, Tsang C, Ip W, Lam C. Molecular mechanisms for the release of chemokines from human leukemic mast cell line (HMC)-1 cells activated by SCF and TNF-alpha: roles of ERK, p38 MAPK, and NF-kappaB. Allergy. 2006;61:289–297. doi: 10.1111/j.1398-9995.2006.00972.x. [DOI] [PubMed] [Google Scholar]
- Wuyts WA, Vanaudenaerde BM, Dupont LJ, Demedts MG, Verleden GM. Involvement of p38 MAPK, JNK, p42/p44 ERK and NF-kappaB in IL-1beta-induced chemokine release in human airway smooth muscle cells. Respir. med. 2003;97:811–817. doi: 10.1016/s0954-6111(03)00036-2. [DOI] [PubMed] [Google Scholar]