Abstract
The emotional significance of objects and events depends on the context in which they occur. Using functional magnetic resonance imaging, we examined the modulation of neural responses to monetary outcomes while subjects performed a decision-making task in a positive and a negative economic context. Neural responses indicated a relative regional specialization in the neural coding of outcome valence and followed three distinct patterns. The nucleus accumbens (NAc) and orbital frontal cortex (OFC) appeared to code the most extreme outcome in each context, with a potentiated response for favorable outcomes by a positive context. The amygdala and insula appeared to also code highly salient outcomes, but showed a potentiated response to unfavorable outcomes occurring in a negative context. The medial prefrontal cortex (medPFC), on the other hand, only coded favorable responses occurring in a positive context. Moreover, the medPFC showed large inter-individual variability when responding to outcomes in a negative context, suggesting that its role in a negative context may depend on a number of individual factors. The results of this work provide evidence of complex valence-based regional dissociations that are influenced by contextual factors.
The outcomes people receive after making a decision are accompanied by an emotional response (Mellers, Schwartz, Ho, & Ritov, 1997). The emotional valence associated with these outcomes can be positive and promote future decisions and behaviors that attempt to achieve similar outcomes. On the other hand, a negative emotional valence can promote future decisions that attempt to avoid like outcomes. However, the emotional response associated with an outcome is not always consistent with the absolute value of the outcome. In most instances, the positive or negative association with an outcome will be a function of the context in which the decision is made and the outcome received. For example, receiving a monetary outcome of $0 in an upward stock market might be experienced negatively, whereas an outcome of $0 in a downward stock market might be experienced positively (Byrne, 2002; Mellers et al., 1997). In this example, the market conditions establish a context or frame of reference for a given absolute value of the outcomes, and it is the relative relationship between the absolute outcome value and the context that determines the valence associated with the outcome.
Cognitive neuroscience research has examined the functional neural circuitry underlying outcome coding and processing of the valence associated with outcomes. A majority of this work has focused on valence as it relates to manipulations of the absolute outcome value, and has focused on the response to both rewarding and punishing outcomes. These studies have lead to the identification of brain regions recruited during outcome processing, and have revealed a certain degree of valence-based regional specialization. Based on findings from a large number of studies (Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; Elliott, Newman, Longe, & Deakin, 2003; Ernst & Paulus, 2005; Harris, McClure, van den Bos, Cohen, & Fiske, 2007; Knutson, Adams, Fong, & Hommer, 2001; Knutson, Fong, Bennett, Adams, & Hommer, 2003; O'Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001; Rogers et al., 2004; Yacubian et al., 2006), regions expected to be activated during positive events included striatum, medial prefrontal cortex, and OFC, while those expected to be activated during negative events included amygdala and insula.
However, this valence-based specialization remains controversial. For example, whereas the striatum has a well established role in reward processes, and is frequently reported during receipt of rewarding outcomes (Delgado et al., 2000; Elliott et al., 2003; Ernst et al., 2005; Seymour, Daw, Dayan, Singer, & Dolan, 2007), it is also engaged in response to aversive or punishing events (Becerra, Breiter, Wise, Gonzalez, & Borsook, 2001; Jensen et al., 2003; Knutson et al., 2003; Knutson, Westdorp, Kaiser, & Hommer, 2000). Similarly, the amygdala, known for its role in the coding of negative emotion and fear responses (i.e., LeDoux, 2000), also responds to rewarding outcomes (Ernst et al., 2005; Murray, 2007; Sergerie, Chochol, & Armony, 2008). In a similar vein, the insula, which is thought to track visceral sensations as they relate to emotional experience (Craig, 2002), has been implicated in the coding of valence, particularly in response to negative outcomes or events (Elliott, Friston, & Dolan, 2000; Liu et al., 2007; Paulus, Rogalsky, Simmons, Feinstein, & Stein, 2003), but also in response to positive outcomes.
The lack of clear valence-related dissociations in neural coding might reflect limitations in the paradigms used to study the neural response to outcomes. Specifically, the inconsistent results could partially be an artifact of equating the valence associated with an outcome with the absolute value of that outcome, rather than considering valence as a function of the context in which the outcome occurs. This possibility is supported by evidence from studies that have specifically examined the effect of context on outcome coding, and have reported context-related effects (Akitsuki et al., 2003; Breiter, Aharon, Kahneman, Dale, & Shizgal, 2001; Nieuwenhuis et al., 2005). These context-related effects have been observed in regions such as the striatum and amygdala (Breiter et al., 2001; Nieuwenhuis et al., 2005) that have shown inconsistent valence-related responses when only the absolute value of outcomes is considered.
The present study was designed to address questions concerning the influence of context on the coding of outcomes and the valence associated with outcomes. Specifically, neural function was examined when outcomes of positive and negative absolute values were received in contexts that were either positive or negative. The goal of this design was to potentiate a positive valence for favorable (positive) outcomes by presenting them in a positive context, and reciprocally, to potentiate a negative valence for unfavorable (negative) outcomes by presenting them in a negative context. This strategy was expected to enhance the ability to dissociate regions implicated in the preferential coding of positive valence from those implicated in the preferential coding of negative valence. Likewise, it was expected to elucidate how the influence of context impacts outcome coding in these regions. Engagement of regions that have been implicated in response to reward, such as the striatum, the OFC, and the medial prefrontal cortex (medPFC), was expected to be stronger for favorable relative to unfavorable outcomes, with this response being enhanced when outcomes occurred in a positive context. On the other hand, regions implicated in processing negative outcomes or events, such as the amygdala and insula, were expected to be preferentially engaged for unfavorable relative to favorable outcomes, with this response being facilitated when outcomes occurred in a negative context.
Materials and Methods
Sample
Eighteen healthy adults volunteers (mean age 29.0 ± 4.8 years, mean IQ 119 ± 12.7) participated in the study. All participants were right handed, free of any acute or chronic medical illness, and had no history of psychiatric illness. Participants were recruited through local newspaper advertisements and were financially compensated for their participation. In addition to the compensation for participating in the study, participants also received the amount of money won during the study task. This study was approved by the NIMH Institutional Review Board and all participants underwent informed consent procedures.
Task and experimental design
A wheel of fortune task (WOF) was used to examine responses to favorable and unfavorable outcomes occurring in either positive or negative contexts. The WOF task used in the current study was a modified version of a computerized two-choice decision-making task that has been used in previous studies (e.g. Ernst et al., 2004; Ernst et al., 2005; Eshel, Nelson, Blair, Pine, & Ernst, 2007).
In the current study, the WOF task was administered in four runs during fMRI scanning. Two runs were dedicated to a Positive context condition, and two runs were dedicated to a Negative context condition. In the Positive context, participants could either win money or not win money depending on their decisions. In the Negative context, participants could either lose money or not lose money depending on their decisions. In the Positive context, winning money was considered a Favorable outcome, and not winning money was considered an Unfavorable outcome. In the negative context, not losing money was considered a Favorable outcome, and losing money was considered an Unfavorable outcome. To optimize task salience, the conditions were presented in a fixed Positive-Negative-Positive-Negative order (Figure 1). Participants accumulated winnings during the Positive context of the first run. These winnings then served as their asset as they played the Negative context of the second run. The third run was again a Positive context and allowed participants to make up the losses previously incurred. Participants, in turn, used the cumulative gains of the third run as their asset for the Negative context of the fourth run. Prior to the start of the task, participants were provided with a $20 endowment to offset the possibility of negative dollar amounts during the Negative context blocks.
Figure 1.
Schematic representation of the Wheel of Fortune task design. a) The three different probability wheel, and control wheel used in the task. b) Example of the three task phases. Analyses were conducted for the Outcome phase. c) The task was presented in four blocks, with each block representing either a Positive or Negative context condition. An event-related design was used within each context block (see supplemental material for full description).
Participants played 46 trials during each context block (184 trials total across all four blocks; 92 in a Positive context; 92 in a Negative context). Each trial consisted of three phases: selection (3000ms), anticipation (3500ms), and outcome (4000s) (Figure 1). During the selection phase participants selected one of two options from a wheel of fortune. During the following anticipation phase, participants rated on a five-point rating scale their level of confidence in receiving a favorable outcome. Finally, in the outcome phase, participants were shown the outcome of their decision and the cumulative dollar amount won or lost. They also rated on a five-point rating scale how they felt about the outcome of that trial. The current study focused on the outcome phase. More detail on the wheel of fortune task, and specific wheel probabilities and money amounts can be found in the supplemental materials.
Imaging
An event-related imaging design was embedded in the context-based block design of the WOF paradigm. Scanning was conducted in four runs that corresponded with the four context runs. However, within each run, an event-related scanning design was employed. The current study focused on the neural responses to outcomes.
Participants were scanned on a 3 Tesla General Electric Signa scanner. Avotec Silent Vision Glasses (Stuart, FL) were used to present the task to subjects during scanning. Gradient echo planar (EPI) images were acquired following sagittal localization and a manual shim procedure. EPI images were acquired in 23 contiguous 5mm axial slices per brain volume positioned parallel to the AC-PC line. Images were acquired using echoplanar single shot gradient echo T2 weighting. The following imaging parameters were used: matrix was 64mm × 64 mm; TR = 2000 ms; TE = 40 ms; field of view = 240 mm; voxels were 3.75mm × 3.75mm × 5 mm. After EPI acquisition, a high resolution T1 weighted anatomical image was acquired to aid with spatial normalization. A standardized magnetization prepared gradient echo sequence was used (180, 1mm sagittal slices, FOV = 256, NEX = 1, TR = 11.4 ms, TE = 4.4 ms, matrix = 256 × 256, TI = 300 ms, bandwidth = 130 Hz/pixel, 33 kHz/256 pixels).
Analysis
Behavioral analyses
Behavioral analyses were conducted on the self-reported affective ratings made when outcomes were presented. The affective ratings were scored on a five-point scale (-2= negative affective response, 0= neutral response, +2= positive affective response). The means and standard deviations of these ratings were subjected to a two Context (Positive, Negative) by two Outcome (Favorable, Unfavorable) repeated measures ANOVA.
Imaging analyses
Reconstructed functional images were analyzed with Medx software to check for excessive motion. Data from participants moving more than 1.5 mm in any plane was discarded. All subsequent analyses were conducted with SPM2 software (Welcome Department of Neurology) and additional routines written in Matlab. Data preprocessing included correction for slice sequence acquisition, motion correction, and spatial normalization to the Montreal Neurological Institute (MNI) T1-weighted template image supplied with SPM.
Neuroimaging analyses were based on the assumption that the transform of neural to fMRI signal is linear and time-invariant, with a known impulse response function (Zarahn, 2000). This assumption is acceptable for events greater than two seconds duration (Buckner, 1998). At the individual participant level, event-related response amplitudes were estimated using a General Linear Model (GLM) for four regressors of interest. These regressors of interest included: favorable outcome in a positive context; unfavorable outcome in a positive context; favorable outcome in a negative context; and unfavorable outcome in a negative context. Additionally, events from the selection and anticipation phases of the task were also modeled, but were as regressors of no-interest. The waveform used to model each type of event-related response in the GLM was a rectangular pulse of the duration of the event convolved with the synthetic hemodynamic response function provided by SPM. The implicit baseline comprised all residual activity that was not modeled.
Contrast images were generated for each participant using pairwise comparisons of the event-related BOLD responses across event types of interest. Prior to group level analysis, each contrast image was divided by the participant-specific voxel time series means. This yielded values proportional to percent fMRI signal change. These normalized contrast images were then smoothed with an isotropic gaussian kernel (FWHM = 8mm) to mitigate any non-stationarity in spatial autocorrelation structure introduced by the previous step.
While the primary analyses of this study were conducted on a priori defined regions of interests (ROIs), an initial whole brain voxel-wise exploratory analysis was performed on the contrast [favorable outcomes/positive context - unfavorable outcomes/positive context] vs. [favorable outcomes/negative context - unfavorable outcomes/negative context]. The analysis was conducted to identify regions recruited by the interaction of Context and Outcome factors, and also to verify the recruitment of the a priori selected ROIs in the processing of outcomes. Because the whole-brain analysis was exploratory in nature and a traditional threshold (e.g., p<.001) resulted in a large cluster of activation that encompassed numerous anatomical regions, a relatively high statistical threshold of puncorrected <.00001 was applied. This more stringent threshold allowed us to identify with some degree of specificity significantly recruited regions that can be followed up with future studies.
For all group-level analyses a random effects model was employed to permit population-level inferences (Holmes & Friston, 1998). The primary analyses were restricted to five a priori bilateral regions of interest (ROIs) that are consistently implicated in response to rewarding and/or punishing outcomes (Figure 2). These ROIs included (1) nucleus accumbens (NAc), (2) orbitofrontal cortex (OFC), (3) medial prefrontal cortex (medPFC), (4) amygdala, and (5) insula. ROIs were manually drawn from standard anatomical criteria (Holmes & Friston, 1998; Szeszko et al., 1999; Szeszko et al., 2002; Talairach & Tournoux, 1988) onto a single MNI template and applied to all normalized brains at the group level. Because of their large size, the OFC and medPFC ROIs were restricted to the subregions most commonly implicated in outcome studies and were centered on peak activations reported in previous work (e.g., posterior OFC, x =-10, y =16, z =-18, Rogers et al., 2004; ventral-rostral medPFC, x =-1, y =53, z =-6, Knutson et al., 2003). Consistent with the current standard (Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002; Winston, Strange, O'Doherty, & Dolan, 2002), a Gaussian random field threshold (α = .05 corrected) with small volume correction was used for all regions.
Figure 2.
Overlay of the NAc (bilateral), OFC, medPFC, Amygdala (bilateral), and Insula (bilateral) regions of interest on a MNI template.
The primary study analyses were conducted on the five a priori selected ROIs. To help clarify the significant BOLD responses observed in each ROI following the SPM analysis, the mean BOLD signal change values were extracted from each ROI. These mean BOLD values were subjected to a five Region (NAc, OFC, medPFC, amygdala, insula) by two Context (Positive, Negative) by two Outcome (Favorable, Unfavorable) repeated measures ANOVA. Significant interactions were followed by explanatory post-hoc analyses.
Results
Behavioral Performance
A Context (positive, negative) by Outcome (favorable, unfavorable) repeated measures ANOVA was conducted on the affective rating scores made in response to each outcome. This analysis revealed a main effect of Context, such that participants rated a favorable outcomes more positively in the positive than in the negative context, F(1,17)=41.9, p<0.001, and they rated an unfavorable outcome more negatively in the negative context than in the positive context (Figure 3). Additionally, a main effect of Outcome indicated an overall more positive affective response following favorable outcomes than unfavorable outcomes, F(1,17)=57.5, p<0.001 (Figure 3).
Figure 3.
Affective ratings in response to outcome receipt. Outcomes occurring in the positive context were rated as more positive than those occurring in the negative context. Favorable outcomes were rated as more positive than unfavorable outcomes, regardless of the context in which they occurred.
A comparison of the total money amounts that were won in the favorable context and lost in the unfavorable context revealed no significant difference, t(17) = .61, p=.54. In the favorable context, participants won an average of $59.87 (SD = 8.33), while in the unfavorable context they lost an average of $60.77 (SD = 7.76).
Whole brain analysis
An exploratory whole-brain analysis was conducted to examine regions recruited by the interaction of Context and Outcome [favorable outcomes/positive context - unfavorable outcomes/positive context] vs. [favorable outcomes/negative context - unfavorable outcomes/negative context]). At a relatively stringent statistical threshold of p<.00001, significant BOLD responses were observed exclusively in NAc, amygdala, insula, OFC and medPFC, and nowhere else (Figure 4). Coordinates of the peak voxels for these regions can be found in Table 1. These results reaffirmed the critical role of this set of regions in the coding of outcomes modulated by context.
Figure 4.
Regions showing significant BOLD responses in a whole-brain analysis of the contrast comparing the most extreme responses in the positive context with the most extreme outcomes in the negative context ([favorable outcomes/positive context - unfavorable outcomes/positive context] vs. [favorable outcomes/negative context - unfavorable outcomes/negative context]). Activations were significant at p<.00001.
Table 1.
Regions showing a significant response in the whole brain analysis contrasting the most extreme outcome in the positive context with the most extreme outcome in the negative context ([favorable outcomes/positive context - unfavorable outcomes/positive context] vs. [favorable outcomes/negative context - unfavorable outcomes/ negative context]). Activations were significant at a threshold of p<.00001; x,y,z are MNI coordinates (mm).
| Region | Peak T-Score | k (cluster) | x | y | z |
|---|---|---|---|---|---|
| NAc (right) | 10.21 | 1179 | 14 | 12 | -12 |
| NAc (left) | 7.65 | -14 | 10 | -12 | |
| amygdala (right) | 4.90 | 20 | -10 | -10 | |
| insula (left) | 7.27 | 783 | -62 | -20 | 16 |
| OFC | 5.80 | 87 | -24 | 34 | -22 |
| insula (right) | 5.69 | 402 | 64 | -12 | -2 |
| medPFC | 5.19 | 67 | 4 | 52 | 0 |
Region of interest (ROI) analyses
The mean BOLD signal change values associated with each of the four outcome events, relative to baseline, were extracted from each a priori selected ROI. These events were (1) favorable-outcome/positive-context, (2) unfavorable-outcome/positive-context, (3) favorable-outcome/negative-context, and (4) unfavorable-outcome/negative-context. Preliminary analyses showed no effect of laterality in any of the ROIs, and thus laterality was not included as a factor in subsequent analyses. A five Region (NAc, OFC, medPFC, amygdala, insula) by two Context (positive, negative) by two Outcome (favorable, unfavorable) repeated measures ANOVA was conducted on ROI BOLD values.
The Region by Context by Outcome ANOVA yielded a significant 3-way interaction, F(4,68)=3.88, p<0.01, indicating that BOLD responses were differentially modulated by Context and Outcome across ROIs (Figure 5). We first describe the results for each context separately, and then for each type of outcome separately.
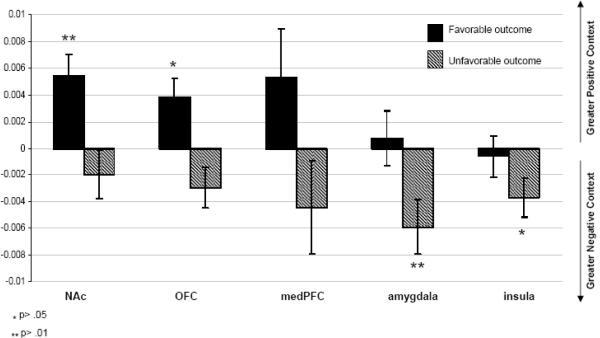
Figure 5.
Images showing activation location and extent in each ROI during the contrast between the most extreme responses in the positive context and the most extreme outcomes in the negative context ([favorable outcomes/positive context - unfavorable outcomes/positive context] - [favorable outcomes/negative context - unfavorable outcomes/negative context]). Graphs present the Mean BOLD response levels in each ROI for each context/outcome condition.
Outcome Coding Within Context
A comparison between favorable and unfavorable outcomes that occurred within the same context provided information concerning the specific influence of outcome valence on neural response.
In the positive context, ROI activation was significantly greater to favorable outcomes than to unfavorable outcomes in all five regions (NAc, p<.001; OFC, p<.001; medPFC, p<.001; amygdala, p<.001; insula, p<.05). Additionally, all ROIs showed strong responses to favorable outcomes relative to baseline, with these responses being highly significant in the NAc, OFC, medPFC and Insula (p's <0.001 - 0.008), and at a trend level in the amygdala (p=0.097).
In the negative context, three patterns of response emerged. The first pattern described the amygdala and insula responses. Both regions showed a significant response to both favorable (amygdala p<0.05; insula p< 0.01) and unfavorable (amygdala p<0.01; insula p< 0.001) outcomes relative to baseline. However, neither region showed a difference in the strength of response between the favorable and unfavorable outcomes. The second pattern described the medPFC response. The medPFC did not significantly respond to either outcome relative to baseline, and (similarly to the amygdala and insula) this region did not distinguish between favorable and unfavorable outcomes. The third pattern described the NAc and the OFC. Here, both regions showed a significant response to unfavorable outcomes relative to baseline (NAc, p<.05; OFC, p<.05), and also showed a significant discrimination between favorable and unfavorable outcomes (NAc, p<0.05; OFC, p<0.01).
Outcome Coding Between Contexts
A comparison of similar outcome types that occurred across different contexts provided information on the influence of context valence on outcome coding. To illustrate how outcome types were coded across contexts, Figure 6 presents the difference between positive and negative context responses for both favorable and unfavorable outcomes. Much like the above findings, three response patterns emerged. In the first pattern, both amygdala and insula coded unfavorable outcomes differently as a function of context. These regions showed a stronger response for unfavorable outcomes in the negative context than in a positive context (amygdala, p<0.01; insula, p<0.05). However, these regions coded favorable outcomes similarly in the positive and negative contexts. In the second pattern, the medPFC showed no differences in how favorable or unfavorable outcomes were coded across contexts. Finally, the third pattern was found in the NAc and OFC, and was a mirror of the pattern seen in the amygdala and insula. Both the NAc and OFC coded favorable outcomes differently as a function of context. These regions showed a stronger response for favorable outcomes in the positive context than in the negative context (NAc, p<0.01; OFC, p<0.01). However, there was no significant difference across contexts in how these regions coded an unfavorable outcome. These response patterns are consistent with a potentiating effect of a positive context on favorable outcomes coded in the NAc and the OFC, and a potentiating effect of a negative context on unfavorable outcomes coded in the amygdala and the insula. The medPFC appeared to be insensitive to the effect of context on outcome.
Figure 6.
The difference in mean activation level for favorable outcomes occurring in the positive context and in the negative context (favorable/positive minus favorable/negative); and the difference in mean activation level for unfavorable outcomes occurring in the positive context and in the negative context (unfavorable/positive minus unfavorable/negative). Values that are greater than zero indicate a greater peak response in the positive context, while values less than zero indicate a greater peak response in the negative context.
Exploratory correlations with performance scores
A number of exploratory correlations were conducted between task performance variables and the mean BOLD response levels that were extracted from each ROI.
In the positive context, more positive affective ratings for favorable outcomes were correlated with stronger medPFC activation (R=.53, p<.05), but with weaker insula activation (R=-.49, p<.05).
In the negative context, more negative affective ratings for unfavorable outcomes were correlated with higher activation in the amygdala (R=-.46, p=.05), insula (R=-.46, p=.05), and OFC (R=-.50, p<.05). In addition, shorter reaction times for rating unfavorable outcomes were correlated with higher activation in the amygdala (R=-.70, p<.001), OFC (R=-.59, p<.05), and NAc (R=-.46, p=.05).
Given the number of correlations performed, it is not clear whether these correlations are chance findings or reflect true associations. Nevertheless, they may suggest medPFC response maps the intensity of positive outcomes; amygdala, insula and OFC map intensity of negative outcomes; and amygdala, OFC and NAc map increased arousal (i.e., salience) to negative outcomes as indexed by reaction time.
Discussion
The purpose of this study was to examine the notion of a relative functional specialization of neural networks supporting a regionally-based preferential coding of positive and negative outcomes. Because most previous studies have not systematically assessed the context in which outcomes occur, some of the literature discrepancies could reflect this aspect of experimental designs. The present work was designed to include the influence of context on the coding of outcomes. Accordingly, this study assessed the neural responses to favorable and unfavorable monetary outcomes that resulted from probabilistic decisions made in a positive context (gain and no gain) and in a negative context (loss and no loss). With this design, the valence of a favorable outcome was potentiated by the positive context and the valence of an unfavorable outcome was potentiated by the negative context. This approach was expected to optimize the dissociation of neural regions implicated in preferentially coding positive valence and those implicated in preferentially coding negative valence.
Two main points emerged from the current findings. First, the regions a priori hypothesized to be recruited during response to outcomes were indeed the most activated regions in the brain, as revealed by the whole brain analysis. The main contrast of interest, which examined the influence of context on favorable vs. unfavorable outcomes, showed selective recruitment of the predicted forebrain structures at a stringent statistical threshold (see Figure 4). These regions included the amygdala, the ventral striatum, the ventral prefrontal cortex, (including OFC and medial PFC), and the insula. This result stands as one of the clearest demonstrations of the unique neural circuitry that supports the coding of outcomes.
The second point addressed the regional patterns of response to outcomes when modulated by context, and focused more specifically on the putative functional specialization of circuits for the coding of outcomes. First, all five regions were significantly more activated by a favorable outcome than by an unfavorable outcome when the outcome occurred in a positive context (see Figure 5). By itself, this finding suggested (at least in a positive context) that there was no regional differentiation in the coding of outcome valence, as all regions preferentially responded to a rewarding rather than a non-rewarding event. However, it was not clear whether this preferential response was to the valence (favorable vs. unfavorable), or to the salience of the outcome (higher affective intensity to a gain than to a null outcome). Findings from the negative context shed some light on this question.
In the negative context, responses to outcomes followed three different patterns. One pattern showed a stronger response to unfavorable outcomes than to favorable outcomes. This pattern was exhibited by the NAc and the OFC and, as they also showed greater activation to the favorable outcome in the positive context, suggested that these structures responded to the salience of outcomes. A second pattern was exhibited, by the amygdala and the insula, and showed no differentiation when coding the type of outcome in the negative context. However, these structures significantly responded to both the favorable and the unfavorable events occurring in the negative context. Taken together, these findings suggested that the amygdala and insula coded the negative context, rather than the events occurring during this context. A third pattern was seen in the medPFC which showed no significant response to either favorable or unfavorable events occurring in the negative context. However, this result should be qualified by the high inter-individual variability seen in the medPFC BOLD responses (see Figure 5). Such high inter-individual variability suggested that a number of individual factors could influence the responses of this region to events occurring in a negative context, and not necessarily that this region was insensitive to these events. This raises interesting questions about the nature of these individual factors.
A direct comparison of the outcome responses within the negative context to the outcome responses within the positive context (illustrated in Figure 6) provided further refinement of the characteristics of the three activation patterns described above. The first pattern confirmed that the NAc and OFC appeared most sensitive to the salience of outcomes across contexts. In addition, when comparing similar outcomes across contexts, the response to favorable outcomes by the NAc and OFC appeared to be potentiated by a positive context, as the response to favorable outcomes was significantly stronger in the positive than negative context. In contrast, the NAc and OFC did not show a similar potentiation of their response to unfavorable outcomes in the negative context.
Together, these findings regarding NAc and OFC responses suggest that these structures coded the salience of events, and additionally responded with a preferential sensitivity to the modulation of events by a positive context. This may explain findings in the literature that report engagement of these structures in reward paradigms more readily than the engagement of other regions such as amygdala or insula (Delgado et al., 2000; Knutson et al., 2001; Knutson et al., 2003; O'Doherty, Critchley, Deichmann, & Dolan, 2003; O'Doherty et al., 2001). These findings are also in line with other studies reporting that NAc activation in reward-related tasks is modulated by outcome salience (e.g., Zink, Pagnoni, Martin-Skurski, Chappelow, & Berns, 2004; Zink, Pagnoni, Martin, Dhamala, & Berns, 2003).
The second pattern that emerged when similar outcomes were compared across contexts was found in the amygdala and the insula. Here, the amygdala and the insula response to unfavorable outcomes appeared to be potentiated in the negative context. This pattern consisted of a greater response in these regions for unfavorable outcome in the negative than in the positive context (unfavorable/negative context > unfavorable/positive context), but no enhancement of their responses to favorable outcomes in the positive context (favorable/positive context = favorable/negative context). Taken together, these findings suggested that the amygdala and insula were activated by any events occurring in a negative context. Furthermore, their responses to unfavorable outcomes appeared to be weaker in the positive context than in a negative context, suggesting that these regions were biased towards coding negative events. This sensitivity to negative outcomes suggests that the response of the amygdala and insula to favorable outcomes occurring in the positive context may be related to the salience rather than the valence of these outcomes.
The third pattern was observed in the medPFC, and was characterized by an absence of significant modulation of outcomes by either the positive or negative context. Response in the medPFC to a favorable outcome did not differ whether the outcome occurred in a positive or a negative context. The same was true for an unfavorable outcome. However, as previously mentioned the inter-individual variability in the medPFC response was particularly high and may have prevented the detection of any effects. A substantial literature implicates the medPFC in self-referential processes (Gusnard, Akbudak, Shulman, & Raichle, 2001; Northoff et al., 2006), and it is conceivable that individual responses to outcomes vary as a function of the cognitive and self-referential interpretations of these outcomes, leading to great variability in activation. A detailed debriefing might have provided clues about the nature of the individual factors that modulated the medPFC activation.
The current study should be considered with some caveats. First, our analysis did not parse out outcomes by magnitude of losses or gains (i.e., when participants won or lost larger amounts of money, compared to when participants won or lost smaller amounts of money). Although this strategy could have provided more detailed information on the function of each region, such approach would have required many more task trials with significantly lengthened scan time. Future work would benefit from expanding on the current findings by conducting studies that employ parameterized outcome values in positive and negative contexts. A related, additional caveat concerns the use of only monetary outcomes, which prevents us from being able to generalize the current findings beyond economic situations or outcomes. Whether non-economic outcomes are coded differently from monetary outcomes needs to be empirically tested.
In conclusion, this work indicates that there is no simple dissociation by valence of the neural circuits underlying the coding of outcomes. There is no unique circuitry dedicated to the coding of favorable outcomes (rewards), or to the coding of unfavorable outcomes (punishments). However, there seems to be unique modulation of regional activation associated with the interaction of outcome and context. The NAc and OFC appeared to code the most extreme (salient) outcome in each context, with a potentiated response to favorable outcomes by a positive context. Conversely, the amygdala and insula activation appeared to be driven by a negative context, more than by the nature of the events occurring within this context. These structures also showed a potentiated response to unfavorable outcomes by a negative context, relative to a positive context, and responded preferentially to the most salient (favorable) outcome in the positive context. The medPFC, on the other hand, appeared to code favorable responses occurring in a positive context, and was not significantly activated by events in a negative context.
The findings from this study demonstrate how the surrounding contextual environment can influence the neural response to outcomes. Not only is this quantitative demonstration of contextual modulation important for better understanding the neural coding of outcomes, but the current findings also hold implications for both typical and atypical development. For example, adolescence is a period of heightened risk taking and sensitivity to reward (Arnett, 1992; Casey, Jones, & Hare, 2008; Ernst et al., 2005; Spear, 2000; Steinberg, 2004), as well as increased risk for the onset of psychopathology (see Ernst, Pine, & Hardin, 2006). Typical adolescent behavior has been associated with functional changes in NAc and amygdala (e.g., Ernst et al., 2005; Eshel et al., 2007; Galvan et al., 2005), and psychopathology (particularly affective disorders) which also involves striatal and amygdala -related neural circuitry (Casey et al., 2008; Pine, 2007). Future work will be necessary to understand whether and how context modulates outcome processing during this period of development, and how this contextual modulation could be involved in the adolescent onset of psychopathology
Supplementary Material
Table 2.
Location and t value of the peak voxel in each ROI when contrasting the most extreme outcome in the positive context with the most extreme outcome in the negative context ([favorable outcomes/positive context - unfavorable outcomes/positive context] vs. [favorable outcomes/negative context - unfavorable outcomes/ negative context]); x,y,z are MNI coordinates (mm).
| Region | Peak T-Score | k (cluster) | x | y | z |
|---|---|---|---|---|---|
| rNAc | 10.21 | 172 | 14 | 12 | -12 |
| lNAc | 7.00 | 105 | -12 | 10 | -10 |
| OFC | 5.26 | 82 | -24 | 36 | -18 |
| medPFC | 5.19 | 66 | 4 | 52 | 0 |
| rAmygdala | 5.52 | 96 | 22 | 4 | -16 |
| lAmygdala | 5.81 | 81 | -20 | 2 | -12 |
| rInsula | 5.02 | 587 | 50 | -12 | 14 |
| lInsula | 6.02 | 441 | -38 | -14 | 2 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akitsuki Y, Sugiura M, Watanabe J, Yamashita K, Sassa Y, Awata S, et al. Context-dependent cortical activation in response to financial reward and penalty: an event-related fMRI study. Neuroimage. 2003;19(4):1674–1685. doi: 10.1016/s1053-8119(03)00250-7. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless behavior in adolescence: A developmental perspective. Developmental Review. 1992;12:339–337. [Google Scholar]
- Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32(5):927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30(2):619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Event-related fMRI and the hemodynamic response. Hum Brain Mapp. 1998;6(56):373–377. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<373::AID-HBM8>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne RM. Mental models and counterfactual thoughts about what might have been. Trends in Cognitive Science. 2002;6:426–431. doi: 10.1016/s1364-6613(02)01974-5. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20(16):6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23(1):303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, et al. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42(12):1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58(8):597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Davidson M, Spicer J, Glover G, Casey BJ. The role of ventral frontostriatal circuitry in reward-based learning in humans. J Neurosci. 2005;25(38):8650–8656. doi: 10.1523/JNEUROSCI.2431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Harris LT, McClure SM, van den Bos W, Cohen JD, Fiske ST. Regions of the MPFC differentially tuned to social and nonsocial affective evaluation. Cogn Affect Behav Neurosci. 2007;7(4):309–316. doi: 10.3758/cabn.7.4.309. [DOI] [PubMed] [Google Scholar]
- Holmes A, Friston KJ. Generalisability, random effects, and population inference. Neuroimage. 1998;7 [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40(6):1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27(17):4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellers BA, Schwartz A, Ho K, Ritov I. Decision affect theory: Emotional reactions to the outcomes of risky options. Psychological Acience. 1997;8(6):423–429. [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11(11):489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Heslenfeld DJ, von Geusau NJ, Mars RB, Holroyd CB, Yeung N. Activity in human reward-sensitive brain areas is strongly context dependent. Neuroimage. 2005;25(4):1302–1309. doi: 10.1016/j.neuroimage.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55(6):594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. J Neurosci. 2007;27(18):4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, et al. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatry. 1999;56(10):913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Strous RD, Goldman RS, Ashtari M, Knuth KH, Lieberman JA, et al. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. Am J Psychiatry. 2002;159(2):217–226. doi: 10.1176/appi.ajp.159.2.217. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar sterotaxic atlas of the human brain. Thieme Medical Publishers Inc.; New York: 1988. [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5(3):277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Glascher J, Schroeder K, Sommer T, Braus DF, Buchel C. Dissociable systems for gain- and loss-related value predictions and errors of prediction in the human brain. J Neurosci. 2006;26(37):9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E. Testing for neural responses during temporal components of trials with BOLD fMRI. Neuroimage. 2000;11(6 Pt 1):783–796. doi: 10.1006/nimg.2000.0560. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42(3):509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23(22):8092–8097. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.