Abstract
Each of the five cellular layers of the cerebral neocortex is composed of a specific number of a single predominant ‘class’ of projection neuron. The projection neuron class is defined by its unique morphology and axonal projections to other areas of the brain. Precursor cell populations lining the embryonic lateral ventricles produce the projection neurons. The mechanisms regulating precursor cell proliferation also regulate total numbers of neurons produced at specific developmental periods and destined to a specific neocortical layer. Because the newborn neurons migrate relatively long distances to reach their final layer destinations, it is often assumed that the mechanisms governing acquisition of neuronal-class-specific characteristics, many of which become evident after neuron production, are independent of the mechanisms governing neuron production. We review evidence that suggests that the two mechanisms might be linked via operations of Notch1 and p27Kip1, molecules known to regulate precursor cell proliferation and neuron production.
The neocortical proliferative process: an overview
The neocortex is the largest subdivision of the human brain and the seat of higher cognitive functions. The majority of its neurons are projection neurons that send axonal projections relatively distant cortical and subcortical targets. The other type of neocortical neuron is the interneuron that makes up ~35% of neocortical neurons. The axons of the interneurons terminate locally, generally contacting nearby projection neurons. The neocortex is a six-layered structure. However, layer I, adjacent to the pial membrane, is cell poor and neocortical neurons are distributed across cellular layers II, III, IV, V and VI.
Neocortical precursor cell populations and neocortical protomap
The projection neurons fall into the following five classes based on their morphology and patterns of axonal projections: the large, medium and small pyramids of layers II, III, V; the granule cells of layer IV; and the polymorphic cells of layer VI. The projection neurons are produced initially from precursor cells organized as a pseudostratified ventricular epithelium (PVE) [1] that comprises the bulk of the ventricular zone (VZ) [2–4]. At later times in development, as PVE precursors pass through each cell cycle, a proportion of the precursors exits the PVE to undergo additional divisions away from the lateral ventricular border whereas the complementary proportion migrates directly to the cortex [5,6]. The precursor cells that escape from the PVE but are to undergo further divisions are called intermediate progenitor cells (IPCs) or basal progenitors (BPs). The IPCs ascend to the basal fringe of the VZ and at later developmental stages (e.g. at embryonic days 13 to 14 [E13–E14] in mice) are found in the subventricular zone (SVZ) and intermediate zone (IZ) [5–13]. The IPCs amplify the output of PVE precursor cells and therefore are the most proximate source of neocortical projections.
The foundation of our understanding of neocortical development is that the regional variations in numbers, cytological features and functional properties of projection neuron classes that characterize sensory, motor and association areas of the mature cortex are represented as a nascent ‘protomap’ within the PVE [14,15]. The mechanisms giving rise to the protomap and defining the transcriptional profiles of neurons arising according to the protomap are little understood other than that these are somehow instantiated as gradients of large set of transcription factors and signal transduction systems antedating neocortical neuron production [16–20]. What is less widely recognized is that properties of the protomap are instantiated during proliferation of the PVE precursors and propagated secondarily to the IPCs. This is supported by retroviral labeling analyses, which show that each PVE lineage is competent to give rise to all five projection neuron classes [21,22]. This type of analysis would include the IPCs also that would have arisen from the PVE [5,6,23]. That is, the retroviral-based studies indicate that projection neuron classes are specified in the course of PVE precursor cell proliferation. Further proliferation of IPCs principally serves to expand precursor cell numbers (while keeping the specification properties acquired in the PVE intact) and ultimately to expand the size of the neocortex [6,9,23]. In other words, the protomap is a property of the PVE that is faithfully translated to the IPCs.
Precursor cell heterogeneity
The PVE in general cell stains has a deceptive homogeneity and simplicity that reveals little of its complex role in neocortical histogenesis. Even at the earliest stages of development the local proliferative output of the PVE is heterogeneous at a molecular level. Thus, multiple classes of projection neurons arise simultaneously from the PVE within any given radial sector of the neocortex as shown by S-phase cell labeling methods using a pulse of tritiated thymidine [24]. It does not imply that the PVE is composed of class-specific proliferative lineages. On the contrary, retroviral labeling show that each proliferative lineage of the PVE is competent to give rise to the full succession of neuron classes [21,22]. This suggests that whether the neuron undergoes its terminal division within the PVE or undergoes one or more amplifying divisions as an IPC/BP, class specific properties of its daughter cells, once instantiated in the PVE, are propagated faithfully [16–20].
There is considerable heterogeneity of PVE progenitor lineages including variation in cytology, molecular constitution and cell-cycle kinetics [9]. Specifically, the PVE includes the classical radial glial cell (RGC) with ascending process extending to the pial surface and a non-polarized cell whose processes do not extend to the pial surface but are contained within the VZ [25,26]. The RGC is the stem cell population that maintains the proliferative pool. The nonpolarized cell is the ‘short neuron precursor’ arising from an RGC division as daughter that exits the PVE [25,27,28]. These cell forms differ from the RGCs with respect molecular profiles and cell-cycle kinetics [27–29]. Molecular heterogeneity pertaining structural components, signal transduction systems or transcription factor profiles distinguishes not only glial and neuronal precursors but also diverse lineages within two cell classes [5,6,8,25,29–35].
Gradients of cell proliferation and cell output in the PVE
Neocortical projection neurons of corresponding class do to not arise synchronously across entire cerebral hemisphere. Indeed, as in many parts of the central nervous system there is clear morphogenetic gradient in neocortical development. In the neocortex, the neurogenetic gradient follows a spatial-temporal sequence that is initiated rostrolaterally under the influence morphogens diffusing from the anterior neural ridge [17,36,37]. The initiation of neurogenesis propagates posteriorly along the transverse neurogenetic gradient (TNG) requiring almost 40 hours to traverse the hemisphere in mouse [38,39]. Progression of the TNG relies upon the integrity of gap- and hemi-junctional communications linking adjacent PVE cells [40–42].
Along the TNG two proliferative parameters, the duration of the G1 phase the cell cycle (TG1) and the fraction of post-proliferative cells that exit the PVE (Q) vary systematically with each successive cell cycle. IN mouse, the only species in which progression of proliferative parameters in PVE has been worked out in detail, average TG1 increases 3–4-fold during the embryonic day 11 (E11) to E17 interval of neurogenesis. During the same interval, average Q ascends from 0.0 to 1.0 [1,43,44]. Once a given cell cycle is initiated at the anterior margin of the PVE, it propagates down the long axis of epithelium at a rate that is a function of overall duration of the cell cycle (TC), a rate that progressively slows as neurogenesis proceeds and TC lengthens [38]. The values for these parameters have been worked out PVE population averages from which individual progenitor lineages vary [21,27,28]. Moreover, we remind the reader that these parameters apply only to the PVE and not to the IPC/BP populations.
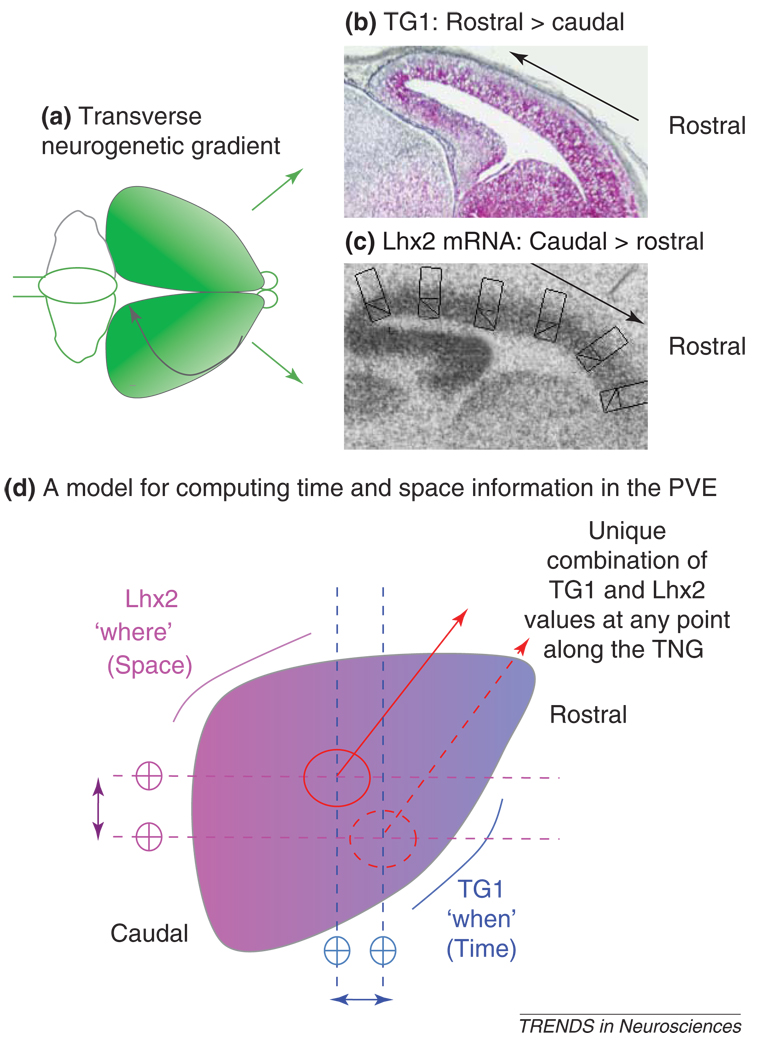
The neurogenetic gradient is not the only gradient in the PVE. The expression patterns of mRNAs for transcription factors implicated in neuron specification are also distributed as gradients across the PVE. Interestingly, the axes of all the gradients align with one another [17,19,45–47]. Recently, we discovered that intersection of two of these gradients serve to link mechanisms regulating PVE precursor cell proliferation with those regulating projection neuron class specification. Specifically we found that information about the position of a precursor cell with respect to its neighbors in the PVE coupled with information about the sequence of origin of the projection neuron classes is sufficient to orchestrate the TNG. Thus, the graded increase in TG1 together with graded expression of mRNA for the LIM homeodomain transcription factor Lhx2 provide both sets of information to the PVE precursor cells (Figure 1). Specifically, mechanisms regulatory to TG1 are sufficient to inform a PVE lineage ‘when’ it is in its layer VI to II projection neuron class production sequence. The expression level of Lhx2 mRNA is sufficient to encode the ‘where’ information [48] needed to locate a PVE cells with respect to the other PVE cells. This type of combinatorial signaling scheme provides the first insight into how the information needed for the protomap to become translated into the mature neocortex might be coded in the precursor cells and executed during neurogenesis.
Figure 1.
Neocortical neurogenesis proceeds according to a transverse neurogenetic gradient (TNG) that is initiated rostrolaterally and propagates caudomedially in the direction of the curved black arrow in (a). The TNG can be represented as a gradient in the length of the G1 phase of the cell cycle (TG1) as well as a gradient in the expression of mRNA for the transcription factor Lhx2 in the ventricular zone. Thus, in midsagittal sections from an E14 mouse forebrain, with the section plane approximately aligned with the direction of the TNG, the TG1 values are expressed as a high rostral to low caudal gradient (b), while the Lhx2 mRNA expression runs in the opposite direction: high caudal to low rostral (c). The points of intersections of these two gradients in the neocortical pseudostratified ventricular epithelium (PVE) represent unique values along the TNG (d), which might be sufficient to inform a neocortical cell lineage at all times both the time ('when' in the layer VI to II neuron origin sequence) and space ('where' in the PVE with respect to a lineage’s neighbor along the TNG). In (d) the PVE is represented as a flat sheet and the TG1 and Lhx2 mRNA expression gradients are colored blue and magenta, respectively. Two points of intersections of the TG1 and Lhx2 mRNA gradient are illustrated to show that each intersection can represent a unique value.
Linkage of prolilferative gradients and projection neuron class specification
Armed with the idea that a combination of cell-cycle parameter and transcription-factor gradients encodes the workings of the protomap, we return to our central theme, a regulatory linkage of mechanisms of proliferation with those of projection neuron class specification. Here, we begin by drawing a distinction between mechanisms of specification and differentiation. In support of this distinction, recent studies have shown that the transcriptional mechanisms of specification go forward principally in the S phase through the M phase interval of the terminal cell cycle [49,50], whereas those of differentiation principally follow the terminal division in the PVE or SVZ [51–53]. This is important because it indicates that the molecular bases of specification and differentiation are set up separately at two distinct times during the cell cycle. This separation also implies that there are two distinct sets of processes that regulate specification versus differentiation.
A given progenitor lineage shifts its output from one projection neuron class to the next: ‘graded or intermediate forms’ are not evident. The lack of intermediate forms suggests that each projection neuron class represents a stable transcriptional steady state that by some mechanism ‘comes as a transcriptional class defining set’ [54]. The initial steps in neuron differentiation must also be dependent upon a class defining transcriptional set. These steps will be further dependent upon translational mechanisms and protein synthesis [52] following the requirements appropriate to the histogenetic fate of cells [49–53].
proliferation, specification, Notch1 signaling
Neuronal specification is regulated by the actions of a set of transcription factors [15,19,55,56,17]. These transcription factors activate proneural genes including Neurog2 (Ngn2) and Ascl1 (Mash1), which, in turn, activate the transcriptional cascade specific to each neuron class [17,37,46,57]. A crucial regulator of this transcription cascade is the Notch signal-transduction system represented mammals by four receptors and five ligands [8]. The Notch1 receptor and its delta-like 1 (Dll1) ligand have been most emphasized in studies of neocortical neurogenesis. Since we suggest that mechanisms of proliferation and specification are closely related, the role of Notch1 signaling in neuronal specification must be related to its role in the regulation of cell proliferation, especially in the regulation of cell output or Q over the course of neocortical neurogenesis.
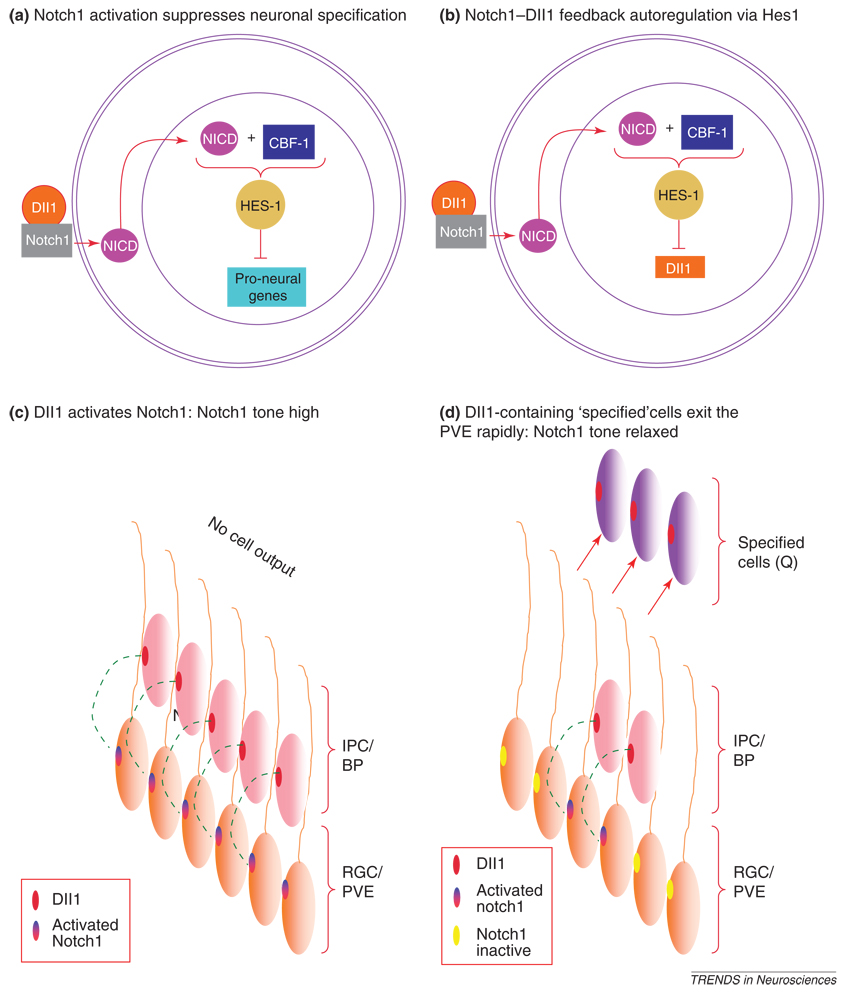
The Notch1 signaling cascade in RGCs of PVE might be taken to begin with activation of the Notch1 transmembrane receptor by the Dll1 ligand [58–60]. This activation triggers cleavage of the Notch1 intracellular domain (NICD) from the transmembrane receptor followed by transport of NICD to the nucleus. There, among its diverse functions, NICD might bind to and convert the CBF1 (CRT/DRE binding factor 1) repressor complex into a transcriptional activator [58,59] (Figure 2a). CBF1 then activates transcription of a set of transcriptional repressors, of which the bHLH (basic helix-loop-helix) transcription factor Hes1 has had principal emphasis with respect to neocortical neurogenesis. Members of the Hes1 family suppress transcription of at least genes including the proneural genes Ngn2 and Mash1 as well as the delta and jagged Notch ligands [46,60–62,52,53] (Figure 2a). The NICD–CBF1 mediated transcriptional suppression seems to be principally regulatory to PVE progenitor proliferation and specification [34]. NICD operations in the nucleus, independent of CBF1, by contrast, have a crucial role in direction of lineages toward the glial fate within the IPC/BP cells [34]. The importance of these observations is that Notch1–CBF mediated signaling mechanisms might serve to link together mechanisms of cell proliferation and specification in the neocortex.
Figure 2.
Upon activation by its ligand Delta1 (Dll1) secreted by intermediate precursor cell (IPC) or basal precursor (BP) population, the Notch1 membrane receptor on the radial glial cell (RGC) in the pseudostratified ventricular epithelium (PVE) is cleaved into a Notch intracellular domain (NICD), which binds to CBF-1 to upregulate Hes1. Hes1 represses transcription of proneural genes (a) and also Dll1 (b). Prior to the onset of cell-cycle exit and cell specification, Dll1 activation of Notch1 maintains a high Notch1 tone in the PVE (c). Once cell-cycle exit begins, Dll1-containing cells rapidly exit the PVE and reduce the availability of Dll1 (c). Therefore, Notch1 tone is relaxed facilitating increased cell production and specification in a self-perpetuating cycle.
Q and suppression of Notch1 tone
The NICD–CBF1 mediated transcriptional suppression favors cell proliferation rather than cell-cycle exit. Yet, during neocortical neurogenesis, PVE cells exit the cell cycle and the rate of cell output per cycle, or Q, rises. Therefore, Notch1–CBF mediated suppression Hes1 must be gradually attenuated in order for cell output to increase. Hes1, as noted earlier, suppresses transcription of Dll1 [53] (Figure 2b) and, in principle, relaxes Notch1 tone allowing cell output and specification to go forward. The specified cells will carry Dll1 as they exit the PVE, thus attenuating Notch1 tone in the PVE [32,33] (Figure 2c). These cells correspond to the short neuronal precursor cell, the population that does not express Hes1 [25,27,28]. A component of this population exits the PVE as the IPC/BP. The IBC/BP contact the ascending processes of the RGCs enabling Dll1 signaling to augment Notch1 tone and sustain RGC proliferation [33,34,63].
Paradoxically, however, the advance of specification indicates that Dll1–Notch1–Q system is dynamic. The plausible mechanism mediating the dynamism is progressive weakening of the Dll1 drive of Notch1 signaling. Various mechanisms of relaxation of Notch1 tone have been recognized [64–67]. In the PVE [68], one mechanism might be, in part, inherent in the kinetics of Dll1 ligand transcription, translation and membrane insertion into the IPC/BP so that there is variation in effective Notch1 signaling. Possibly it is because only a proportion of the cells completing terminal divisions in PVE become IPC/BP [5,69]. The complementary proportion that exits the cell cycle as young projection neurons and enters the developing cortical plate might not contribute to Notch1 signaling in the RGC [63]. Thus, some cells leave the PVE rapidly after terminal divisions, in only an hour or two, whereas their sister cells remain in the PVE [9,70,1,39,32,33]. The daughter cells that exit rapidly might be the cells that migrate directly to the cortex rather than becoming IPC/BP. The interval before migration of the rapidly exiting cells is very likely to be much too brief to accommodate the time delay needed for the feedback replenishment of Dll1–Notch1 signaling in adjacent cells Dll1 [9,32,33,70] (Figure 2c). Whatever the actual mechanism or set of mechanisms, these must act with closely regulated precision to assure the cycle-by-cycle relaxation of Notch1 tone and commensurate advance of Q.
P27Kip1: a link between Notch1 signaling and cell-cycle exit
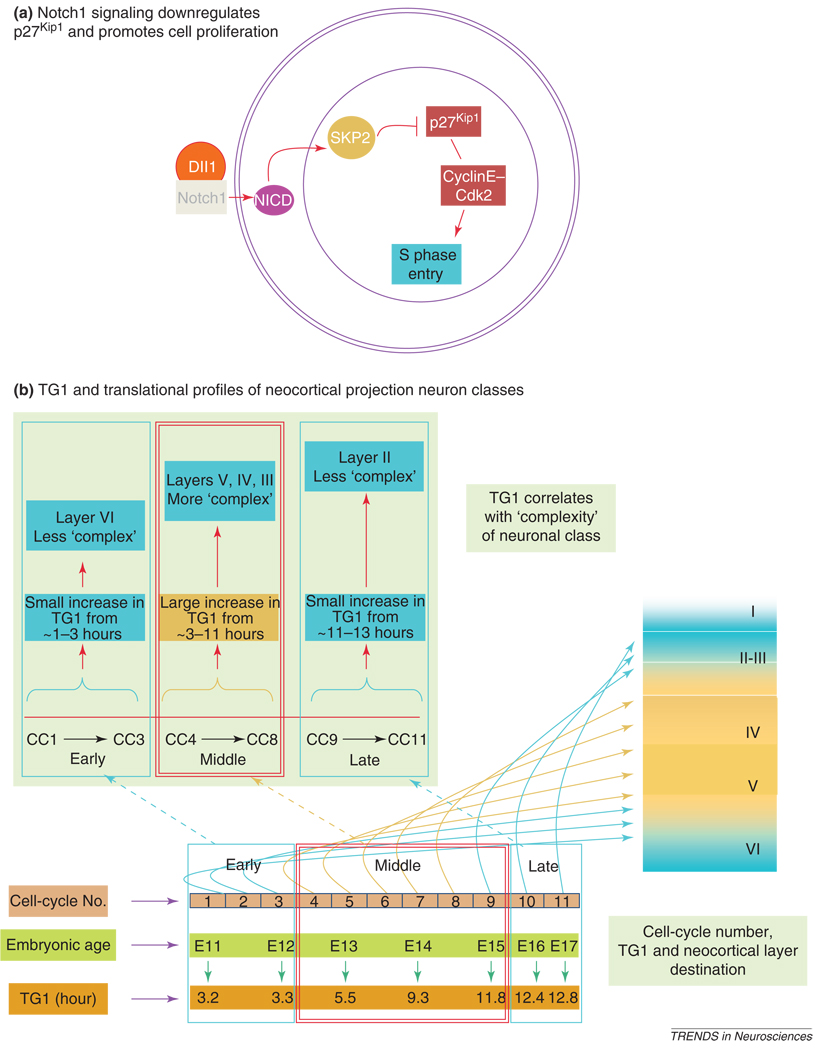
Cells, once specified, must also accomplish initial steps in differentiation to migrate from the VZ. They must also be released from the cycle itself by mechanisms that act to block cycle progression at and beyond the G1 restriction point. The cell-cycle inhibitor p27Kip1 is a plausible intermediary agent linking Notch1 signaling and cell-cycle exit. The reason for suggesting this is that expression of p27Kip1 and possibly other cell-cycle inhibitory agents rises in the PVE [29], with each cell cycle associated with the cycle-by-cycle advance in cell output (Q) and relaxation of Notch1 tone. p27Kip1 facilitates cell-cycle exit by holding cyclin E/Cdk2 levels below thresholds that drive cells through the G1–S transition [71–73]. There appears to be a regulatory relationship between Notch1 and p27Kip1 in that Notch1 suppresses p27Kip1 [74–77] by, among other mechanisms, ubiquitination and proteolysis via a CBF1-mediated upregulation of Skp2-dependent mechanism (Figure 3a) [74]. Thus, Notch1-p27Kip1 interactions seem to be intimately related to the regulation of cell proliferation and cell-cycle exit in the PVE.
Figure 3.
Notch1 activation by Delta1 (DII1) releases NICD, which upregulates Skp2-dependent mechanisms to downregulate p27kip1. Downregulation of p27Kip1 promotes entry of cells into S phase of the cell cycle by promoting cyclinE–cdk2 activity (a). During the embryonic day 11 (E11) to E17 interval of neurogenesis in mice, there is on average sufficient time for 11 cell cycles (CC) to be executed. The increase in the length of the G1 phase of the cell cycle (TG1) from CC 1 to 11 occurs in three phases: early (CC 1– 3), mid (CC4–8) and late (CC9–11). The early and late phases involve slow increase in TG1 while the middle phase involves a rapid increase (from 3.3 to 11.8 hours). The rapid increase correlates with the specification of the most 'complex' projection neuron classes – those of layers V and VI. Thus, a given value of TG1 corresponds to the protein synthesis 'burden' associated with specification of the projection neuron class being produced. Therefore, higher the complexity of neuron class being specified, the greater would be the value of TG1. We emphasize that the values of TG1 are population averages estimated for precursor cells in the mouse PVE.
Differentiation, p27Kip1 and linkage to TG1
Relaxation of Notch1 tone would commensurately be associated with relaxation of the inhibition of p27Kip1 activity. Thus, the fine-tuned control of Notch1 activity reflecting a shifting balance of the Dll drive plausibly lies at the core of the overall PVE regulatory process. However, this formulation makes no provision for mechanisms necessary to initiate the differentiation steps essential to cycle exit. Moreover it ignores the linkage of the gradient TG1 to the ‘when’ information, reviewed in an earlier section. The cell cycle lengthens dramatically during neocortical development owing to lengthening of the G1 phase. In fact, only G1 is variable in duration, especially early G1 [39,54,78,79]. Variations in TG1 change the protein synthetic time available in the pre-rather than the post-restriction check point interval of G1 [78,80,81]. The importance of this becomes clear with realization that: (i) the expression patterns of Hes1 and other downstream components of the Notch1 signaling pathway are oscillatory, paced by a Jak3–Stat3, Stat3–Soc3 oscillatory loop [53] with a period of some 2–3 hours, and (ii) it is in the early G1 that the oscillatory pattern of expression of Hes1 is turned off in cells that enter G1 phase committed to neuronal class and destined for cycle exit. In other words, the cycle-by-cycle lengthening of G1 implies a cycle-by-cycle lengthening of the time available for protein synthesis by the cell. In support of this formulation, the duration of the G1 phase for any given cell cycle has been found to be longer in cells completing their terminal divisions than in those that continue to proliferate [27,28].
By analogy with the expression of Hes1, it seems reasonable that other proteins might similarly require some time to reach a level of expression to exert their functions. Thus, consider that a given neuronal class expresses many proteins [82–84] and, although the five neocortical projection neuron classes might be expected to share major components of their translational profiles, each neuronal class must have its own class-specific protein constitution. We suggest that synthesis of class-specific protein profiles requires the small and regular cycle-by-cycle increments in TG1 observed experimentally in the course of neurogenesis [39,43]. The hypothesis that G1 duration operates in some way in neuronal class specification is anticipated by the observations that proliferative cells have progressively shorter TG1 than do cells in their terminal cycle poised to exit the cycle [27,28]. It might also be related to the more widely experienced phenomenon that PVE cells in culture rapidly exit the cell cycle [42]. Overall, the link between TG1 and cell class output is rather tight. The rise in TG1 with cycle is 'S-shaped' rather than exponential [39,43], and the most rapid ascent of the curve corresponds to the time of origin of the most complex forms – those of layers V–III (Figure 3) [85]. Early and late flat segments of the curve, by contrast, correspond to origin of layers VI and III/II neurons, respectively (Figure 3b) [24], both of which are relatively homogeneous. The rapid ascent of TG1 is associated with maximum complexity of differential features of neurons, whereas minimal change in TG1 occurs over intervals of minimal change in neuron class features. This implies correspondence between the complexity of neuronal class, the complexity of the requirement for protein synthesis and associated cycle-by-cycle increment in TGI (Figure 3b).
p27Kip1 is not only a major driver of cell-cycle exit but also has been found to contribute to the control of protein synthesis in early G1 phase. As evidence for this, when p27Kip1 is overexpressed prematurely it advances the neuronal class specification sequence such that the PVE produces cell classes destined for more superficial layers that it would normally, as if reprogrammed to operate at a TG1 in advance of the actual cycle of reference [48]. For an understanding of this, we consider the recent finding that p27Kip1 has two additional roles that can be dissociated from its role as a cell-cycle inhibitor [52]. First, in early G1, p27Kip1 stabilizes the proneural protein Ngn2 and thereby facilitates transcription and translation of an array of differentiation proteins, represented by HuC/D and TuJ1. Second, p27Kip1 promotes migration by directly inhibiting the signaling of RhoA, an inhibitor of migration, but only in cells that undergo terminal divisions in the PVE. This is an integrative component of our overall formulation in that the same molecule that has key role in cell-cycle exit also has a key role in the transcription of differentiation proteins and in implementation of the cycle exit of postmitotic cells primed by specification and differentiation to leave the PVE. It is further integrative that the abundance of p27Kip1, like that of Dll1, is critically regulated by the Notch1 signal transduction system.
Conclusion
The present model of neocortical neurogenesis is brought together from discrete blocks of observations. Those blocks of observations that have been worked through in detail and repeatedly confirmed include the neurogenetic sequence itself as expressed in the TNG, the general mechanisms of eukaryotic cell-cycle operation and the mechanisms of the Notch signal-transduction system. The components that link these large blocks are only the wave front of current investigations and include the functions of p27kip1 as a regulator of protein synthesis and the role of Notch1 as a regulator of p27Kip1 activity. Detailed studies will be required to test our hypothesis that relaxation of Notch1 suppression of projection neuron class specification is controlled by Dll1 and Q. Finally, we note that the present formulation stops short of full exploration of current work on the IPC/BP, yet another block emerging that is of central importance to cerebral histogenesis. To the extent that work ahead bears upon the validity of this or modified models, the models will contribute to our understanding of both the normal and pathological development of the organ system that most distinctly defines the human species.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takahashi T, et al. Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J. Neurosci. 1993;13:820–833. doi: 10.1523/JNEUROSCI.13-02-00820.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cajal SRy. Histologie du Systeme Nerveux de l'Homme et des Vertebres. Consejo Superior de Investigaciones Cientificas; 1952. [Google Scholar]
- 3.His W. Die Entwicklung des Menschlichen Gehirns wahrend der ersten Monate. von S. Hirzel; 1904. [Google Scholar]
- 4.Sauer FC. The interkinetic migration of embryonic epithelial nuclei. J. Morphol. 1936;60:1–11. [Google Scholar]
- 5.Englund C, et al. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pontious A, et al. Role of intermediate progenitor cells in cerebral cortex development. Dev. Neurosci. 2008;30:24–32. doi: 10.1159/000109848. [DOI] [PubMed] [Google Scholar]
- 7.Attardo A, et al. Live imaging at the onset of cortical neurogenesis reveals differential appearance of the neuronal phenotype in apical versus basal progenitor progeny. PLoS One. 2008;3:e2388. doi: 10.1371/journal.pone.0002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbin JG, et al. Regulation of neural progenitor cell development in the nervous system. J. Neurochem. 2008;106:2272–2287. doi: 10.1111/j.1471-4159.2008.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotz M, Huttner WB. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 10.Iacopetti P, et al. Expression of the antiproliferative gene TIS21 at the onset of neurogenesis identifies single neuroepithelial cells that switch from proliferative to neuron-generating division. Proc. Natl. Acad. Sci. U. S. A. 1999;96:4639–4644. doi: 10.1073/pnas.96.8.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kriegstein AR, Gotz M. Radial glia diversity: a matter of cell fate. Glia. 2003;43:37–43. doi: 10.1002/glia.10250. [DOI] [PubMed] [Google Scholar]
- 12.Noctor SC, et al. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 13.Smart IH, et al. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 15.O'Leary DD, et al. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Echevarria D, et al. Neuroepithelial secondary organizers and cell fate specification in the developing brain. Brain Res. Brain Res. Rev. 2003;43:179–191. doi: 10.1016/j.brainresrev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu. Rev. Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- 18.Machon O, et al. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev. Biol. 2007;311:223–237. doi: 10.1016/j.ydbio.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 19.Puelles L, Rubenstein JL. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 20.Storm EE, et al. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–1844. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- 21.Cai L, et al. Size distribution of retrovirally marked lineages matches prediction from population measurements of cell cycle behavior. J. Neurosci. Res. 2002;69:731–744. doi: 10.1002/jnr.10398. [DOI] [PubMed] [Google Scholar]
- 22.Walsh C, Cepko CL. Cell lineage and cell migration in the developing cerebral cortex. Experientia. 1990;46:940–947. doi: 10.1007/BF01939387. [DOI] [PubMed] [Google Scholar]
- 23.Cubelos B, et al. Cux-2 controls the proliferation of neuronal intermediate precursors of the cortical subventricular zone. Cereb. Cortex. 2008;18:1758–1770. doi: 10.1093/cercor/bhm199. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, et al. Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J. Neurosci. 1999;19:10357–10371. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gal JS, et al. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J. Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misson J-P, et al. Mitotic cycling of radial glial cells of the fetal murine cerebral wall: a combined autographic and immunohistochemical study. Brain Res. 1988;466:183–190. doi: 10.1016/0165-3806(88)90043-0. [DOI] [PubMed] [Google Scholar]
- 27.Calegari F, et al. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J. Neurosci. 2005;25:6533–6538. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J. Cell Sci. 2003;116:4947–4955. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- 29.Farkas LM, et al. Insulinoma-associated 1 has a panneurogenic role and promotes the generation and expansion of basal progenitors in the developing mouse neocortex. Neuron. 2008;60:40–55. doi: 10.1016/j.neuron.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Heins N, et al. Emx2 promotes symmetric cell divisions and a multipotential fate in precursors from the cerebral cortex. Mol. Cell. Neurosci. 2001;18:485–502. doi: 10.1006/mcne.2001.1046. [DOI] [PubMed] [Google Scholar]
- 31.Heins N, et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi A, et al. Single-cell gene profiling defines differential progenitor subclasses in mammalian neurogenesis. Development. 2008;135:3113–3124. doi: 10.1242/dev.022616. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi D, et al. Selection of differentiating cells by different levels of delta-like 1 among neural precursor cells in the developing mouse telencephalon. Development. 2008;135:3849–3858. doi: 10.1242/dev.024570. [DOI] [PubMed] [Google Scholar]
- 34.Mizutani K, et al. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- 35.Tarabykin V, et al. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;128:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- 36.Cholfin JA, Rubenstein JL. Frontal cortex subdivision patterning is coordinately regulated by Fgf8, Fgf17, and Emx2. J. Comp. Neurol. 2008;509:144–155. doi: 10.1002/cne.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Leary DD, Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr. Opin. Neurobiol. 2002;12:14–25. doi: 10.1016/s0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- 38.Caviness V, et al. Neuronogenesis and the early events of neocortical histogenesis. In: Goffinet A, Rakic P, editors. Development of the Neocortex. Springer Verlag; 2000. pp. 107–143. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi T, et al. The cell cycle of the pseudostratified ventricular epithelium of the murine cerebral wall. J. Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissman TA, et al. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Bittman K, et al. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J. Neurosci. 1997;17:7037–7044. doi: 10.1523/JNEUROSCI.17-18-07037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goto T, et al. Developmental regulation of the effects of fibroblast growth factor-2 and 1-octanol on neuronogenesis: implications for a hypothesis relating to mitogen-antimitogen opposition. J. Neurosci. Res. 2002;69:714–722. doi: 10.1002/jnr.10361. [DOI] [PubMed] [Google Scholar]
- 43.Miyama S, et al. A gradient in the duration of the G1 phase in the murine neocortical proliferative epithelium. Cereb. Cortex. 1997;7:678–689. doi: 10.1093/cercor/7.7.678. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi T, et al. The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J. Neurosci. 1996;16:6183–6196. doi: 10.1523/JNEUROSCI.16-19-06183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai L, et al. Misexpression of basic helix-loop-helix genes in the murine cerebral cortex affects cell fate choices and neuronal survival. Development. 2000;127:3021–3030. doi: 10.1242/dev.127.14.3021. [DOI] [PubMed] [Google Scholar]
- 46.Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr. Opin. Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 47.Monuki ES, et al. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 48.Suter B, et al. Navigating neocortical neurogenesis and neuronal specification: a positional information system encoded by neurogenetic gradients. J. Neurosci. 2007;27:10777–10784. doi: 10.1523/JNEUROSCI.3091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dehay C, et al. Modulation of the cell cycle contributes to the parcellation of the primate visual cortex. Nature. 1993;366:464–466. doi: 10.1038/366464a0. [DOI] [PubMed] [Google Scholar]
- 50.McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- 51.Tokunaga A, et al. Mapping spatio-temporal activation of Notch signaling during neurogenesis and gliogenesis in the developing mouse brain. J. Neurochem. 2004;90:142–154. doi: 10.1111/j.1471-4159.2004.02470.x. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen L, et al. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimojo H, et al. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 54.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat. Rev. Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 55.Rubenstein JL, Beachy PA. Patterning of the embryonic forebrain. Curr. Opin. Neurobiol. 1998;8:18–26. doi: 10.1016/s0959-4388(98)80004-4. [DOI] [PubMed] [Google Scholar]
- 56.Schuurmans C, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guillemot F. Cell fate specification in the mammalian telencephalon. Prog. Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 58.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 59.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 60.Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu. Rev. Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- 61.Scardigli R, et al. Crossregulation between Neurogenin2 and pathways specifying neuronal identity in the spinal cord. Neuron. 2001;31:203–217. doi: 10.1016/s0896-6273(01)00358-0. [DOI] [PubMed] [Google Scholar]
- 62.Ahmad I, et al. Neural stem cells in the mammalian eye: types and regulation. Semin. Cell Dev. Biol. 2004;15:53–62. doi: 10.1016/j.semcdb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 63.Yoon KJ, et al. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron. 2008;58:519–531. doi: 10.1016/j.neuron.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 64.Giudicelli F, Lewis J. The vertebrate segmentation clock. Curr. Opin. Genet. Dev. 2004;14:407–414. doi: 10.1016/j.gde.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 65.Giudicelli F, et al. Setting the tempo in development: an investigation of the zebrafish somite clock mechanism. PLoS Biol. 2007;5:e150. doi: 10.1371/journal.pbio.0050150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis J, Ozbudak EM. Deciphering the somite segmentation clock: beyond mutants and morphants. Dev. Dyn. 2007;236:1410–1415. doi: 10.1002/dvdy.21154. [DOI] [PubMed] [Google Scholar]
- 67.Lewis J. Autoinhibition with transcriptional delay: a simple mechanism for the zebrafish somitogenesis oscillator. Curr. Biol. 2003;13:1398–1408. doi: 10.1016/s0960-9822(03)00534-7. [DOI] [PubMed] [Google Scholar]
- 68.Ross SE, et al. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- 69.Kowalczyk T, et al. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb. Cortex. 2009 doi: 10.1093/cercor/bhn260. http://cercor.oxfordjournals.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haubensak W, et al. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 72.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 73.Cheng M, et al. The p21(Cip1) and p27(Kip1) CDK 'inhibitors' are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarmento LM, et al. Notch1 modulates timing of G1-S progression by inducing SKP2 transcription and p27 Kip1 degradation. J. Exp. Med. 2005;202:157–168. doi: 10.1084/jem.20050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murata K, et al. Hes1 directly controls cell proliferation through the transcriptional repression of p27Kip1. Mol. Cell. Biol. 2005;25:4262–4271. doi: 10.1128/MCB.25.10.4262-4271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borriello A, et al. Retinoic acid induces p27Kip1 nuclear accumulation by modulating its phosphorylation. Cancer Res. 2006;66:4240–4248. doi: 10.1158/0008-5472.CAN-05-2759. [DOI] [PubMed] [Google Scholar]
- 77.Borriello A, et al. p27Kip1 accumulation is associated with retinoic-induced neuroblastoma differentiation: evidence of a decreased proteasome-dependent degradation. Oncogene. 2000;19:51–60. doi: 10.1038/sj.onc.1203231. [DOI] [PubMed] [Google Scholar]
- 78.Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 79.Pardee AB. Cell fates. In: Stein G, Pardee AB, editors. Cell Cycle and Growth Control: Biomolecular Regulation and Cancer. 2nd edn. John Wiley and Sons; 2004. pp. 3–15. [Google Scholar]
- 80.Zetterberg A, et al. What is the restriction point? Curr. Opin. Cell Biol. 1995;7:835–842. doi: 10.1016/0955-0674(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 81.Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation of quiescence of Swiss 3T3 cells. Proc. Natl. Acad. Sci. U. S. A. 1985;82:5365–5369. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arlotta P, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 83.Molyneaux BJ, et al. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 84.Hevner RF, et al. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev. Neurosci. 2003;25:139–151. doi: 10.1159/000072263. [DOI] [PubMed] [Google Scholar]
- 85.Zilles K. Cortex. In: Paxinos G, editor. The Human Nervous System. Academic Press; 1990. pp. 757–802. [Google Scholar]