Abstract
Sporozoites are the infective stage of the malaria parasite. They are deposited in the skin by infected Anopheles mosquitoes and must penetrate cell barriers in the skin and liver sinusoid to reach their target cell, the hepatocyte, where they enter in a vacuole and begin development into the next life cycle stage, the exoerythrocytic form. Recent advances in our understanding of sporozoite biology in the dermal inoculation site, the role of cell traversal and the mechanism by which sporozoites productively invade hepatocytes will be highlighted in this review.
Plasmodium Sporozoites: The Same but Different
Apicomplexa is a phylum of obligate intracellular protists to which Plasmodium and other human pathogens such as Toxoplasma and Cryptosporidium belong. The invasive stages of these protists, called zoites, are structurally similar, possessing an apical ring of microtubules and specialized secretory organelles called micronemes and rhoptries. Work with Plasmodium merozoites shows that they invade cells in distinct stages, beginning with initial reversible attachment followed by irreversible attachment and the formation of a tight junction through which the parasite moves forward into the cell (reviewed in [1]). Upon entry into the host cell, the junction is sealed off and the zoite is in a parasitophorous vacuole within the cell. There is now evidence that similar to other zoites, sporozoites enter cells through the formation of a tight junction with the host cell, suggesting that the overall process is similar [2,3].
Host cell invasion by Apicomplexan zoites, including Plasmodium sporozoites, is an active process that requires motility [4,5]. Zoites move by gliding motility which is powered by a subpellicular actomyosin motor that is linked to the zoite surface through one or more members of the TRAP family of transmembrane proteins (reviewed in [6]). Thus, the force of the motor proteins results in the posterior movement of the TRAP-aldolase-actomyosin assembly and forward movement of the zoite.
Despite these similarities, sporozoites are different from other Apicomplexan zoites in that they are inoculated at some distance from their target cell and must make their way from the dermis to the liver in order to successfully infect the mammalian host. Thus, in contrast to many zoites, the specific biology of the sporozoite requires it to move through tissues without concomitant activation of its invasion machinery, a process that we are only just beginning to understand.
Sporozoite inoculation into and exit from the dermis
Recent studies from several groups have clearly established that there is a skin stage of malaria infection (reviewed in [7]). Sporozoites are inoculated into the dermis by infected mosquitoes and contrary to the widely accepted notion that they rapidly leave the injection site, recent studies have shown that the majority of sporozoites that successfully reach the liver, take between 1 to 3 hours to leave [8,9]. On average, 100 sporozoites are injected by a single infected mosquito [10,11] and once in the skin, they display robust motility following what appears to be a random path rather than being targeted to blood vessels [8,12]. A proportion of these sporozoites will encounter a blood vessel, penetrate it and be carried away in the bloodstream [8,12]. The efficiency with which inoculated sporozoites exit the dermis and reach the liver has been difficult to study and awaits further investigation. What happens to sporozoites that do not go to the liver? Some are undoubtedly destroyed in the skin, some may escape destruction and remain in the skin, possibly by becoming intracellular, and approximately 20% go to the draining lymph node [8,9] where the adaptive immune response is initiated [13].
Sporozoites can migrate through cells, a process that is distinct from productive invasion and results in wounding of the traversed cell [14]. Cell traversal is required for sporozoite exit from the dermis because it enables sporozoites to penetrate cell barriers and to escape destruction by phagocytic cells in the dermis[2]. Several proteins required for this process have been identified (Table 1): SPECT (Sporozoite microneme protein essential for cell traversal;[15]), SPECT2 (also called perforin-like protein 1 or PLP1; [16,17]), CelTOS (Cell traversal protein for ookinetes and sporozoites; [18]) and PL (phospholipase; [19]). Deletion mutants of all four genes have been generated and in vitro, when placed directly on hepatocytes, these mutants invade and develop normally. However they exhibit little to no cell traversal activity in migration assays and exhibit a significantly decreased ability to exit the dermis in vivo [2,19]. The wounding of host cells by migrating sporozoites suggests that the host cell membrane is compromised during this process. How this occurs has not been elucidated but the sequence of two of the aforementioned proteins, PL and SPECT2, may provide some clues. PL has a carboxy-terminal domain with significant similarity to mammalian LCATs (lecithin:cholesterol acyl transferases) and when expressed as a recombinant protein has lipase and membrane lytic activity [19]. In addition, SPECT2 (or PLP1) contains a membrane attack complex/perforin-like domain that is similar to mammalian proteins that lyse or make holes in membranes [17]. It is clear from these studies that sporozoites have a dedicated machinery for cell traversal, further highlighting its importance to the sporozoite. Nonetheless, our understanding of precisely how sporozoites migrate through cells awaits further investigation.
Table 1.
Sporozoite Proteins involved in Cell Traversal
| Proteins | Method of Identification | Exit from Dermis | Exit from Sinusoid | Domains/Homologies | Reference |
|---|---|---|---|---|---|
| SPECT | Screening of EST databases | + | + | - | 2 & 15 |
| SPECT2 | Screening of EST databases | + | + | MACPF domain | 2, 16 & 17 |
| CelTOS | Screening of EST databases | n.k. | + | - | 18 |
| Phospholipase | Subtractive hybridization screen | + | − | LCAT | 19 |
| TLP | Microarray | + | +/− | A-domains & TSR | 20 |
Abbreviations: MACPF, membrane-attack complex/perforin; LCAT, lecithin: cholesterol acyl transferase; TSR, thrombospondin type 1 repeat; n.k., not known.
In addition to cell traversal machinery, it has been recently shown that a member of the TRAP family of motor- binding proteins, called TLP (Trap-Like Protein), has a role in dermal exit [20]. Sporozoites in which this protein has been deleted display normal gliding motility on glass slides but are less successful in dermal exit. One possibility is that the force required to move through extracellular matrix is greater than that required to move on glass surfaces such that the former process may require additional motor-associated proteins to transduce sufficient force to propel the sporozoite. Alternately, it is possible that the adhesive domains of TLP interact with specific host molecules that may be required for cell traversal or crossing the endothelial cell barrier.
Crossing the Liver Sinusoid
After exiting the dermis and entering the blood circulation, sporozoites are arrested in the liver where they must traverse the sinusoidal barrier to access hepatocytes. The liver sinusoid is composed of fenestrated endothelial cells and Kupffer cells, which are resident macrophages. The endothelial cell fenestrae are too small to allow for free passage of sporozoites, therefore sporozoites must migrate through sinusoidal cells to access the liver parenchyma on the other side. The importance of cell traversal in crossing the liver sinusoid was demonstrated when cell traversal mutants were found to have decreased infectivity in vivo after intravenous (i.v.) inoculation, a result that contrasts with their infectivity in vitro when they are placed directly on hepatocytes [15,16,18]. Importantly, these mutants regain infectivity to wild type levels, in mice pretreated with liposome-encapsulated dichloromethylene diphosponate (CL) [15,16,18] which depletes the liver of Kupffer cells, leaving gaps in the sinusoidal barrier [21].
Switching from a Migratory to an Invasive Phenotype
How do sporozoites know they have contacted their target cell and switch from a migratory to an invasive phenotype? This is an area of some controversy. Initial studies on cell traversal raised the possibility that there is no liver-specific signal that initiates the invasion process but that after traversing a number of cells, repeated exposure to the intracellular environment activates sporozoites for invasion [22]. Thus cell traversal in and of itself is sufficient for activation for invasion. Further studies showed that the release of hepatocyte growth factor [23] or exposure to high concentrations of intracellular potassium [24] were specific events that occurred during cell traversal and led to sporozoite activation. However the generation of cell traversal mutants that cannot traverse cells yet retain full capacity to productively invade hepatocytes, raised doubts about this hypothesis, indicating that the story is likely more complex [15,16,18].
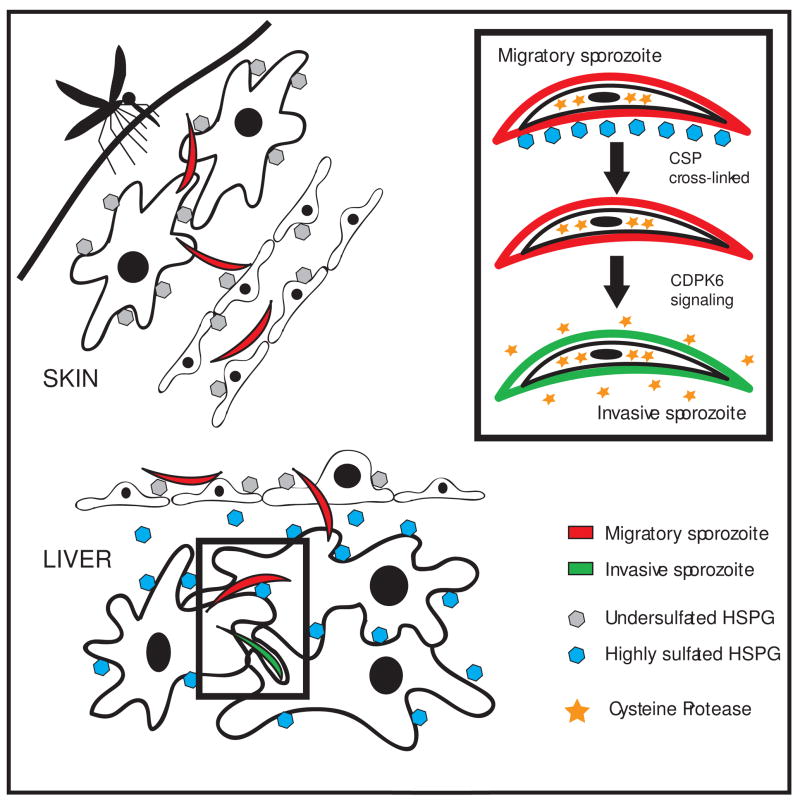
The recent finding that sporozoite residence in the dermis constitutes an important component of the malaria life cycle has led to studies of cell traversal mutants in the skin and as stated earlier, they exhibit decreased ability to exit the dermis [2]. We have found that when inoculated into the skin, sporozoites are in “migratory mode” and tend to migrate through, rather than invade, the cells they encounter. Specifically this is due to the low level of sulfation on the heparan sulfate proteoglycans (HSPGs) expressed by cells found in the dermis [25]. In contrast, when sporozoites contact hepatocytes expressing highly sulfated HSPGs they are activated via calcium dependent protein kinase 6 (CDPK6) to switch to an invasive mode [25], thus the sulfation level of HSPGs on different cell types serves as a type of “GPS” for sporozoites to know where they are and thereby control their infectivity for an organ that is far from their site of entry (Figure 1). These activated sporozoites continue to migrate through a few hepatocytes before productively invading one, thus further cell traversal may trigger other signals that render sporozoites fully competent for productive invasion. It is also possible that after initial activation by highly sulfated HPSGs, it may take some time for sporozoites to become fully competent for invasion and that further cell migration is not required. One group has proposed that the invasive capacity of the sporozoite is “masked” by the migratory phenotype so that specific signals lead not to activation for invasion but to suppression of the migratory phenotype [2]. Nonetheless, it is now clear that cell traversal is not sufficient for sporozoite activation and that there must be an initial signal that either activates or unmasks the invasive phenotype.
Model of the Sporozoite’s Journey in the Mammalian Host.
Migratory sporozoites (red) are injected into the dermis by an infected mosquito, where they encounter cells expressing undersulfated HSPGs (gray hexagons) and migrate through these cells to enter the blood circulation. In the liver they cross the sinusoidal barrier and encounter the highly sulfated HSPGs found in the loose basement membrane of the liver (space of Disse) and on hepatocytes (blue hexagons) and become activated for productive invasion (green sporozoites). The inset shows some of the specific steps involved in sporozoite activation, namely crosslinking of CSP by highly sulfated HSPGs, which results in CDPK6-dependent signaling that leads to the secretion of a cysteine protease (stars) that proteolytically processes surface CSP. Reproduced with modifications and permission from [25].
Invasion of Hepatocytes
Invasion by Apicomplexan zoites is an active process that requires the coordinated release of proteins from apical organelles (reviewed in [26]). Furthermore, exocytosis of apical organelles is stimulated by the mobilization of intracellular calcium and many secreted proteins contain cell-adhesive domains that function in zoite-host interactions [22,26]. Several adhesins of Plasmodium merozoites and Toxoplasma tachyzoites are proteolytically processed after their secretion onto the zoite surface (reviewed in [27]). Processing can occur in the N-terminus, C-terminus or both. N-terminal processing is thought to control exposure of adhesive domains whereas C-terminal processing is required for the release the adhesin/host cell receptor complexes from the zoite surface.
The limited amounts of sporozoite material that can be isolated has impeded the identification and functional characterization of sporozoite apical organellar proteins. To date, the best characterized proteins are CSP, the major surface protein of the sporozoite, and two microneme proteins, TRAP and AMA-1 (Table 2). CSP was originally thought to be a micronemal protein because early immuno-electron microscopy studies showed that it localized to both the plasma membrane and intracellular vesicles [28]. However, at that time there were no microneme markers to confirm the identity of these vesicles. Given that CSP is constitutively secreted onto the sporozoite surface where it forms a dense coat, it is likely that these CSP-containing vesicles are not subject to regulated exocytosis and so are not micronemes. Nonetheless, its precise intracellular localization awaits co-localization studies. The abundance of CSP on the sporozoite’s surface suggests that it participates in an early step in the invasion process and indeed the specific binding of recombinant CSP to HSPGs in the liver led to the model that CSP was responsible for initial, specific attachment of sporozoites to hepatocytes but had no role in the invasion process itself (reviewed in [1]). However, our finding that proteolytic processing of CSP is associated with invasion and that E-64, a cysteine protease inhibitor, abrogates CSP processing and invasion but not attachment to hepatocytes, challenges this hypothesis [29]. CSP processing is triggered after sporozoites contact highly sulfated HSPGs [25] and leads to removal of the N-terminal third of the protein [29]. Our recent studies have shown that this event unmasks the cell-adhesive type I thrombospondin repeat (TSR) in the C-terminus of the protein which then participates in the invasion process (A. Coppi and P. Sinnis, unpublished data).
Table 2.
Sporozoite Proteins Involved in Hepatocyte Invasion
| Proteins | Localization | Domains/Homologies | Reference |
|---|---|---|---|
| CSP | Surface | TSR | 25 & 29 |
| TRAP | Microneme | TSR & A-domain | 5 |
| AMA-1 | Microneme | PAN modules | 30 & 34 |
| P36/P36p | Microneme | 6 Cys domain | 41 & 42 |
| TRSP | unknown | TSR | 43 |
Abbreviations: TSR, thrombospondin type 1 repeat; PAN, Plasminogen Apple Nematode
Two microneme proteins whose secretion is upregulated after hepatocyte contact are TRAP and AMA-1 [30]. Both are type I transmembrane proteins with known adhesive motifs in their extracellular domains. TRAP is the primary motor-binding protein of sporozoites [5]. Its extracellular domain contains an A-domain and a TSR motif, which have been shown to bind to highly sulfated HSPGs [31] and its cytoplasmic domain binds to aldolase which links to actin [32]. Although mutations in the extracellular domains affect invasion but not gliding motility [33], it is not clear that TRAP’s role in invasion is distinct from its role in motility since altering the adhesive interactions between TRAP and its binding partners may impact on the ability of the sporozoite to move into the cell, a process which likely requires more motive force than gliding on glass.
The extracellular portion of AMA-1 has two domains with structural similarity to PAN modules which are found in a diverse array of adhesive proteins [34]. The role of AMA-1 in sporozoites has not been well studied, although studies in Toxoplasma tachyzoites and Plasmodium merozoites, suggest that it is a structural component of the moving junction [35,36]. Further studies should reveal if AMA-1 functions in sporozoites in the same manner.
After their secretion onto the sporozoite surface and possibly after binding to host cell receptors, TRAP and AMA-1 are proteolytically processed in their C-termini by one or more parasite serine proteases [30]. The protease responsible for TRAP cleavage has not yet been identified although in vitro data using a COS-cell assay suggests it is cleaved in the transmembrane domain by a rhomboid protease [37]. C-terminal cleavage of AMA-1 occurs in the juxtamembrane region and in the merozoite stage, the responsible protease has been identified as subtilisin-2 [30,38,39]. To date, the only protease that has been identified to have a role in sporozoite infection of hepatocytes is ROM1 (Rhomboid 1; [40]). The substrate(s) of ROM1 have not yet been identified and further studies are needed to determine the identities of the proteases that cleave TRAP and AMA-1 in sporozoites. CSP is N-terminally processed by a papain-family cysteine protease whose identity is also not yet known.
Transcription profiling of sporozoites followed by gene deletion studies have identified other proteins involved in sporozoite invasion (Table 2). P36p and P36, members of a small family of proteins with 6 conserved cysteine residues, have been found to have a role either in sporozoite commitment to invasion or in early events after invasion [41,42]. Additionally, thrombospondin-related sporozoite protein (TRSP), a transmembrane protein with a TSR motif in its N-terminus, has also been shown to have a role in sporozoite invasion of hepatocytes [43]. Studies using immune sera have identified several other sporozoite proteins which may function in invasion, as antibodies specific for these proteins can partially block invasion (reviewed in [44]).
What are the host proteins with which these parasite proteins interact? A recent study of tight junction formation during Toxoplasma tachyzoite entry into cells proposed that rhoptry proteins are secreted into the host cell, insert into the host cell membrane from the inside out, and serve as binding partners for adhesins on the zoite surface [45]. Thus, the zoite may provide both the ligands and receptors it needs for invasion. This is an appealing notion and explains why after so many years of investigation, relatively few host cell receptors have been identified. In the case of the sporozoite, it is likely that highly sulfated HSPGs of hepatocytes serve as initial attachment sites (reviewed in [44]). However, downstream binding partners for the TSR of CSP, or for TRAP and AMA-1 have not been identified. Two hepatocyte proteins which clearly have a role in sporozoite invasion are the tetraspanin CD81 and the scavenger receptor SRB1 [46–48]. Tetraspanins are proteins that associate with one another and with other proteins and lipids to form membrane microdomains. To date, it has not been possible to identify a sporozoite ligand which binds to CD81, suggesting that CD81 may not itself, function as a receptor but may facilitate invasion by organizing specific proteins and lipids in the host cell membrane [46]. Since SRB1 is a major provider of cholesterol to the hepatocyte, investigators examined the organization of tetraspanin-enriched microdomains on SRB1-/- hepatocytes and found that they were significantly decreased [48]. Thus, they hypothesized that SRB1 plays a role in the formation of CD81-enriched microdomains which in turn facilitate sporozoite entry. It is possible that rhoptry proteins required for tight junction formation can more easily insert into these regions of the plasma membrane. Further studies are required to confirm that this is indeed the mechanism by which CD81 and SRB1 facilitate sporozoite invasion.
Conclusion
Recent data suggests that the process of target cell invasion by Plasmodium sporozoites is broadly similar to the process in their more well-studied cousins, Toxoplasma tachyzoites, in that it likely involves regulated exocytosis and the formation of a tight junction between host and parasite [2,3,22]. Nonetheless, the sporozoite’s journey in the mammalian host dictates added layers of complexity and regulation. Its ability to migrate through nonpermissive cells [2,14] and to switch from a migratory to an invasive mode after contacting hepatic HSPGs [25] are critical to the sporozoite’s ability to retain infectivity for an organ that is far from its site of entry. The precise molecular events involved in the invasion process itself remain poorly characterized, however, recent transcription and proteomic analyses specifically focusing on preerythrocytic stages of Plasmodium [49–51], in conjunction with new techniques for conditional mutagenesis in Plasmodium [52], should lead to new insights in the near future.
Acknowledgments
The authors would like to thank Brandy Bennett and Dr. Marcelo Jacobs-Lorena for their helpful critiques of the manuscript and to acknowledge support from the National Institutes of Health (R01 AI056840).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest published during the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sinnis P, Sim BKL. Cell invasion by the vertebrate stages of Plasmodium. Trends in Microbiology. 1997;5:52–58. doi: 10.1016/s0966-842x(97)84657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 2.Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prévost MC, Ishino T, Yuda M, Ménard R. Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe. 2008;3:88–96. doi: 10.1016/j.chom.2007.12.007. This study demonstrates that cell traversal is critical for sporozoite exit from the dermis and also provides the first image of a tight junction during sporozoite invasion. [DOI] [PubMed] [Google Scholar]
- •• 3.Gonzalez V, Combe A, David V, Malmquist NA, Delorme V, Leroy C, Blazquez S, Ménard R, Tardieux I. Host cell entry by apicomplexa parasites requires actin polymerization in the host cell . Cell Host Microbe. 2009;5:259–272. doi: 10.1016/j.chom.2009.01.011. This study significantly expands upon the previously described model of invasion by Apicomplexan zoites and demonstrates for the first time, that host cell actin is critical for junction formation and zoite invasion. [DOI] [PubMed] [Google Scholar]
- 4.Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- 5.Sultan AA, Thathy V, Frevert U, Robson KJH, Crisanti A, Nussenzweig V, Nussenzweig RS, Menard R. Trap is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 6.Keeley A, Soldati D. The glideosome: A molecular machine powering motility and host-cell invasion by apicomplexa. Trends Cell Biol. 2004;14:528–532. doi: 10.1016/j.tcb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Sinnis P, Zavala F. The skin stage of malaria infection: Biology and relevance to the malaria vaccine effort. Future Microbiol. 2008;3:275–278. doi: 10.2217/17460913.3.3.275. [DOI] [PubMed] [Google Scholar]
- • 8.Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, Menard R. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. doi: 10.1038/nm1350. One of the first studies to demonstrate the power of intravital microscopy in elucidating the biology of Plasmodium in vivo. Working with P. berghei-GFP sporozoites, these investigators capture the behavior of sporozoites in the mammalian dermis and quantify the fate of these zoites. [DOI] [PubMed] [Google Scholar]
- • 9.Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9:1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. This study used the rodent malaria parasite P. yoelii and demonstrated that the majority of infectious sporozoites remain in the skin for 1 to 3 hours after their inoculation, a finding that led to the rejection of the commonly accepted notion that sporozoites left the inoculation site within 30 minutes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medica DL, Sinnis P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected Anopheline mosquitoes feeding on vertebrate hosts. Infec Immun. 2005;73:4363–4369. doi: 10.1128/IAI.73.7.4363-4369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Y, Kebaier C, Vanderberg J. Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infect Immun. 2007;75:5532–5539. doi: 10.1128/IAI.00600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderberg J, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- • 13.Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8(+) T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13:1035–1041. doi: 10.1038/nm1628. This study demonstrated that the “skin stage” of Plasmodium has a critical role to play in the development of the adaptive immune response to sporozoites. [DOI] [PubMed] [Google Scholar]
- 14.Mota M, Pradel G, Vanderberg JP, Hafalla JCR, Frevert U, Nussenzweig RS, Nussenzweig V, Rodriguez A. Migration of Plasmodium sporozoites through cells before infection. Science. 2001;291:141–144. doi: 10.1126/science.291.5501.141. [DOI] [PubMed] [Google Scholar]
- 15.Ishino T, Yano K, Chinzei Y, Yuda M. Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biology. 2004;2:77–84. doi: 10.1371/journal.pbio.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishino T, Chinzei Y, Yuda M. A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol. 2005;7:199–208. doi: 10.1111/j.1462-5822.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser K, Camargo N, Coppens I, Morrisey JM, Vaidya AB, Kappe SH. A member of a conserved Plasmodium protein family with membrane-attack complex/perforin (MACPF)-like domains localizes to the micronemes of sporozoites. Mol Biochem Parasitol. 2004;133:15–26. doi: 10.1016/j.molbiopara.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Kariu T, Ishino T, Yano K, Chinzei Y, Yuda M. Celtos, a novel malarial protein that mediates transmission to mosquito and vertebrate hosts. Mol Microbiol. 2006;59:1369–1379. doi: 10.1111/j.1365-2958.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- 19.Bhanot P, Schauer K, Coppens I, Nussenzweig V. A surface phospholipase is involved in the migration of Plasmodium sporozoites through cells. J Biol Chem. 2005;280:6752–6760. doi: 10.1074/jbc.M411465200. [DOI] [PubMed] [Google Scholar]
- 20.Moreira CK, Templeton TJ, Lavazec C, Hayward RE, Hobbs CV, Kroezec H, Janse CJ, Waters AP, Sinnis P, Coppi A. The Plasmodium TRAP/MIC2 family member, TRAP-Like Protein (TLP), is involved in tissue traversal by sporozoites. Cell Microbiol. 2008;10:1505–1516. doi: 10.1111/j.1462-5822.2008.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baer K, Roosevelt M, Clarkson AB, van Rooijen N, Schnieder T, Frevert U. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell Microbiol. 2007;9:397–412. doi: 10.1111/j.1462-5822.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- 22.Mota MM, Hafalla JCR, Rodriguez A. Migration through host cells activates Plasmodium sporozoites for infection. Nat Med. 2002;8:1318–1322. doi: 10.1038/nm785. [DOI] [PubMed] [Google Scholar]
- 23.Carrolo M, Giordano S, Cabrita-Santos L, Corso S, Vigario A, Silva S, Leiriao P, Carapau D, Armas-Portela R, Comoglio P, et al. Hepatocyte growth factor and its receptor are required for malaria infection. Nat Med. 2003;9:1363–1369. doi: 10.1038/nm947. [DOI] [PubMed] [Google Scholar]
- 24.Kumar KA, Garcia CR, Chandran VR, Van Rooijen N, Zhou Y, Winzeler E, Nussenzweig V. Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity. Mol Biochem Parasitol. 2007;156:32–40. doi: 10.1016/j.molbiopara.2007.07.004. [DOI] [PubMed] [Google Scholar]
- •• 25.Coppi A, Tewari R, Bishop J, Lawrence R, Esko J, Billker O, Sinnis P. Heparan sulfate proteoglycans provide a signal to sporozoites to stop migrating and to productively invade cells. Cell Host Microbe. 2007;2:316–327. doi: 10.1016/j.chom.2007.10.002. This study identifies the first molecule important in sporozoite activation for productive invasion of hepatocytes and provides a model for how sporozoites retain their infectivity for an organ that is far from their site of entry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carruthers V, Boothroyd JC. Pulling Together: an integrated model of Toxoplasma cell invasion. Curr Opin Microbiol. 2007;10:83–89. doi: 10.1016/j.mib.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 27.Carruthers VB, Blackman MJ. A new release on life: Emerging concepts in proteolysis and parasite invasion. Mol Microbiol. 2005;55:1617–1630. doi: 10.1111/j.1365-2958.2005.04483.x. [DOI] [PubMed] [Google Scholar]
- 28.Fine E, Aikawa M, Cochrane AH, Nussenzweig RS. Immuno-electron microscopic observations on Plasmodium knowlesi sporozoites: localization of protective antigen and its precursors. Am J Trop Med Hyg. 1984;33:220–226. doi: 10.4269/ajtmh.1984.33.220. [DOI] [PubMed] [Google Scholar]
- 29.Coppi A, Pinzon-Ortiz C, Hutter C, Sinnis P. The Plasmodium circumsporozoite protein is proteolytically processed during cell invasion. J Exp Med. 2005;201:27–33. doi: 10.1084/jem.20040989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silvie O, Franetich J, Charrin S, Mueller MS, Siau A, Bodescot M, Rubinstein E, Hannoun L, Charoenvit Y, Kocken C, et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. 2004;279:9490–6. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- 31.McCormick CJ, Tuckwell DS, Crisanti A, Humphries MJ, Hollingdale MR. Identification of heparin as a ligand for the A-domain of Plasmodium falciparum thrombospondin-related adhesion protein. Mol Biochem Parasitol. 1999;100:111–124. doi: 10.1016/s0166-6851(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 32.Bosch J, Buscaglia CA, Krumm B, Ingason BP, Lucas R, Roach C, Cardozo T, Nussenzweig V, Hol WG. Aldolase provides an unusual binding site for thrombospondin-related anonymous protein in the invasion machinery of the malaria parasite. Proc Natl Acad Sci U S A. 2007;104:7015–7020. doi: 10.1073/pnas.0605301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matuschewski K, Nunes AC, Nussenzweig V, Menard R. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO J. 2002;21:1597–1606. doi: 10.1093/emboj/21.7.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pizarro JC, Vulliez-Le Normand B, Chesne-Seck ML, Collins CR, Withers-Martinez C, Hackett F, Blackman MJ, Faber BW, Remarque EJ, Kocken CH, et al. Crystal structure of the malaria vaccine candidate apical membrane antigen 1. Science. 2005;308:408–411. doi: 10.1126/science.1107449. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GH, Thomas AW, Margos G, Dluzewski AR, Bannister LH. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect Immun. 2004;72:154–158. doi: 10.1128/IAI.72.1.154-158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander DL, Mital J, Ward GE, Bradley P, Boothroyd JC. Identification of the moving junction complex of Toxoplasma gondii: A collaboration between distinct secretory organelles. PLoS Path. 2005;1:e17. doi: 10.1371/journal.ppat.0010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker RP, Wijetilaka R, Urban S. Two Plasmodium rhomboid proteases preferentially cleave different adhesins implicated in all invasive stages of malaria. PLoS Path. 2006;2:e113. doi: 10.1371/journal.ppat.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howell SA, Withers-Martinez C, Kocken CHM, Thomas AW, Blackman MJ. Proteolytic processing and primary structure of Plasmodium falciparum apical membrane antigen-1. J Biol Chem. 2001;276:31311–31320. doi: 10.1074/jbc.M103076200. [DOI] [PubMed] [Google Scholar]
- 39.Harris PK, Yeoh S, Dluzewski AR, O’Donnell RA, Withers-Martinez C, Hackett F, Bannister LH, Mitchell GH, Blackman MJ. Molecular identification of a malaria merozoite surface sheddase. PLoS Path. 2005;1:241–251. doi: 10.1371/journal.ppat.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srinivasan P, Coppens I, Jacobs-Lorena M. Distinct roles of Plasmodium Rhomboid 1 in parasite development and malaria pathogenesis. PLoS Path. 2009;5:e1000262. doi: 10.1371/journal.ppat.1000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishino T, Chinzei Y, Yuda M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol. 2005;58 :1264–1275. doi: 10.1111/j.1365-2958.2005.04801.x. [DOI] [PubMed] [Google Scholar]
- 42.van Dijk MR, Douradinha B, Franke-Fayard B, Heussler V, van Dooren MW, van Schaijk B, van Gemert GJ, Sauerwein RW, Mota MM, Waters AP, et al. Genetically attenuated, p36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci U S A. 2005;102:12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labaied M, Camargo N, Kappe SH. Depletion of the Plasmodium berghei Thrombospondin-Related Sporozoite Protein reveals a role in host cell entry by sporozoites. Mol Biochem Parasitol. 2007;153:158–166. doi: 10.1016/j.molbiopara.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Sinnis P, Coppi A. A long and winding road: The Plasmodium sporozoite’s journey in the mammalian host. Parasitol Int. 2007;56:171–178. doi: 10.1016/j.parint.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Besteiro S, Michelin A, Poncet J, Dubremetz JF, Lebrun M. Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Path. 2009;5:e1000309. doi: 10.1371/journal.ppat.1000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silvie O, Rubinstein E, Franetich J, Prenant M, Belnoue E, Renia L, Hannoun L, Eling W, Levy S, Boucheix C, et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- • 47.Rodrigues CD, Hannus M, Prudencio M, Martin C, Goncalves LA, Portugal S, Epiphanio S, Akinc A, Hadwiger P, Jahn-Hofmann K, et al. Host scavenger receptor SR-B1 plays a dual role in the establishment of malaria parasite liver infection. Cell Host Microbe. 2008;4:271–282. doi: 10.1016/j.chom.2008.07.012. These investigators have elegantly demonstrated an important role for scavenger receptor B1 in sporozoite infectivity. [DOI] [PubMed] [Google Scholar]
- • 48.Yalaoui S, Huby T, Franetich JF, Gego A, Rametti A, Moreau M, Collet X, Siau A, Van Gemert G, Sauerwein R, et al. Scavenger Receptor B1 boosts hepatocyte permissiveness to Plasmodium infection. Cell Host Microbe. 2008;4:283–292. doi: 10.1016/j.chom.2008.07.013. This study provides compelling data suggesting that the role of SRB1 in sporozoite infection is through its role in cholesterol metabolism which modifies the microdomain architecture of the plasma membrane, thus facilitating sporozoite entry. [DOI] [PubMed] [Google Scholar]
- •• 49.Lasonder E, Janse CJ, van Gemert GJ, Mair GR, Vermunt AM, Douradinha BG, van Noort V, Huynen MA, Luty AJ, Kroeze H, et al. Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathogens. 2008;4:e1000195. doi: 10.1371/journal.ppat.1000195. The first comprehensive proteomic analysis that focuses on oocyst and salivary gland sporozoites. The wealth of information in this paper will be useful to everyone in the field for years to come. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, Daly TM, Bergman LW, Kappe SH. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A. 2008;105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y, Ramachandran V, Kumar KA, Westenberger S, Refour P, Zhou B, Li F, Young JA, Chen K, Plouffe D, et al. Evidence-based annotation of the malaria parasite’s genome using comparative expression profiling. PLoS ONE. 2008;3:e1570. doi: 10.1371/journal.pone.0001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combe A, Giovannini D, Carvalho TG, Spath S, Boisson B, Loussert C, Thiberge S, Lacroix C, Gueirard P, Ménard R. Clonal conditional mutagenesis in malaria parasites. Cell Host Microbe. 2009;5:386–96. doi: 10.1016/j.chom.2009.03.008. [DOI] [PubMed] [Google Scholar]