Abstract
N-truncated and N-modified forms of amyloid beta (Aß) peptide are found in diffused and dense core plaques in Alzheimer’s disease (AD) and Down’s syndrome patients as well as transgenic mouse models of AD. Although the pathological significance of these shortened forms Aβ is not completely understood, previous studies have demonstrated that these peptides are significantly more resistant to degradation, aggregate more rapidly in vitro and exhibit similar or, in some cases, increased toxicity in hippocampal neuronal cultures compared to the full-length peptides. In the present study we further investigated the mechanisms of toxicity of one of the most abundant Ntruncated/modified Aβ peptide bearing amino-terminal pyroglutamate at position 3 (AβN3(pE)). We demonstrated that AβN3(pE) oligomers induce phosphatidyl serine externalization and membrane damage in SH-SY5Y cells. Also, we produced AβN3(pE)-specific polyclonal antibodies in rabbit and identified an immunodominant epitope recognized by anti-AβN3(pE) antibodies. Our results are important for developing new immunotherapeutic compounds specifically targeting AβN3(pE) aggregates since the most commonly used immunogens in the majority of vaccines for AD have been shown to induce antibodies that recognize the N-terminal immunodominant epitope (EFRH) of the full length Aβ, which is absent in N-amino truncated peptides.
Keywords: N-truncated amyloid beta (Aß) peptide, Alzheimer’s disease immunotherapy, immunodominant epitope
INTRODUCTION
The accumulation of fibrillar and oligomeric forms of amyloid-beta (Aβ) peptide in the brain has been hypothesized to play a central role in the neuropathology of Alzheimer’s Disease (AD) (Masters et al., 1985; Hardy and Selkoe, 2002; Walsh and Selkoe, 2004; Haass and Selkoe, 2007). The main Aβ variants detected in the human brain are Aβ1-40 and Aβ1-42, however a significant proportion of AD brain Aβ consists also of N-terminal truncated/modified species (Mori et al., 1992, Seubert et al., 1992; Saido 1995; Saido 1996; Kuo et al., 1997; Tekirian et al 1998; Russo et al 2001, Miravalle et al., 2005). Although the pathological significance of N-terminally truncated/modified forms of Aβ is not completely understood, previous studies have demonstrated that these shortened Aβ forms are significantly more resistant to degradation, aggregate more rapidly in vitro and exhibit similar or, in some cases, increased toxicity in hippocampal neuronal cultures compared to the full-length peptides (Pike et al., 1995; Kuo et al, 1997; Tekirian 1999; Russo et al., 2002). Furthermore, N-truncated Aβ peptides progressively accumulate in the brain of sporadic AD patients, in early onset Familial Alzheimer’s disease (FAD) patients and in Down syndrome brain (Naslund et al., 1994; Saido et al., 1995; Tekirian et al., 1998; Huse et al., 2002; Kalback et al., 2002; Piccini et al., 2005; Vanderstichele et al., 2005; Guntert et al., 2006; Liu et al., 2006;). In a recent study, Sergeant and co-workers showed that Aβ amino truncated peptides aggregate at the earliest stages of AD even before the appearance of clinical symptoms (Sergeant et al., 2003). Moreover, diffuse non-fibrillar preamyloid aggregates contain N-truncated Aβ, which might play an essential role in neuronal loss and cognitive decline in AD patients (Kumar-Singh et al., 2000). Finally, the presence of intraneuronal pool of N-truncated Aβ peptides has been shown to correlate with the progression of pathology and neuronal loss in a transgenic mouse model APP/PS1KI, described earlier (Casas et al., 2004, Wirths et al., 2007; Bayer et al., 2008). Thus, the N-terminally truncated/modified Aβ peptides represent highly desirable and abundant therapeutic targets.
Anti-Aβ immunotherapy has been shown to disrupt Aβ aggregates, block aggregation, attenuate toxicity, as well as promote the clearance of the peptide in the central nervous system (CNS). Immunotherapy approaches, both active immunization with Aβ peptide, or passive transfer of anti-Aβ antibodies, have shown therapeutic efficacy in several amyloid precursor protein transgenic (APP/Tg) mouse models, which develop AD-like amyloid plaque pathology (Schenk et al., 1999; Bard et al., 2000; Town et al., 2001; Brody et al., 2008), as well as canine and primates models of amyloidosis (Lemere et al., 2004, Head et al., 2008). Interestingly, the majority of the previous studies used mainly Aβ1-40 or Aβ1-42 as an immunogen for active immunization, which induced antibodies specific for amino-terminal part (EFRH epitope) of Aβ. However, most of the N-truncated forms of the Aβ lack this critical B cell epitope. In the present study we have focused on one of the most abundant Ntruncated/modified Aβ peptide bearing amino-terminal pyroglutamate at position 3 (AβN3(pE)). Previously, this peptide has been found to accumulate in diffuse and mature senile plaques as well as in blood vessels in AD and Down’s syndrome brains. (Mori et al, 1992; Saido 1995, Kuo 1997; Harigaya et al., 2000; Russo et al., 2002; Guntert et al., 2006). Also, it has been shown that this peptide is more hydrophobic and has the increased aggregation potential (Pike,1995; He et al, 1999; Schilling et al., 2006). In addition, it has been demonstrated that soluble oligomers of AβN3(pE) impair learning and memory in mice after intracerebroventricular injection and induce a caspase-dependent neuronal apoptosis in vitro involving the activation of a cPLA2-arachidonic acid pathway (Youssef et al., 2007).
In the present study we further investigated the mechanisms of toxicity of AβN3(pE) oligomers and demonstrated that they induced phosphatidyl serine externalization and membrane damage in SH-SY5Y and IMR-32 cells. Finally, we produced AβN3(pE)-specific polyclonal antibodies in rabbits, and identified an immunodominant epitope recognized by anti-AβN3(pE) antibodies. We believe our results are potentially important for developing new immunotherapeutic compounds specifically targeting AβN3(pE) aggregates since the commonly used immunogens in the majority of vaccine strategies for AD have been shown to induce antibodies that recognized the amino-terminal part (EFRH epitope) of the full length Aβ, which is absent in N-amino truncated peptides.
2. MATERIALS AND METHODS
2.1. Materials
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). Synthetic human Aβ 1-42 and Aβ 35-25, as well as N-pyroglutamate modified peptides AβN3(pE) and AβN11(pE) were purchased from AnaSpec (San Jose, CA, USA). A non-related peptide (NRP; amino acid sequence: AALSPGSSAYPSATVLA) was synthesized in our laboratory and used as a negative control. A monoclonal anti-Aβ antibody (4G8) was from Sigma. HRPconjugated anti-mouse IgG2b and HRP-conjugated goat anti-rabbit IgG were from Zymed (San Francisco, CA, USA). Super Signal West Dura Extended Duration Substrate kit was from Pierce, Rockfird, IL, USA. Cell culture media (DMEM/F12, 1:1) were from GIBCO (Grand Island, NY, USA).
2.2. Peptide preparation and WB
Aβ1-42, AβN3(pE) and AβN11(pE) were dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) to allow a conversion to the monomer and, after evaporation of solvent, were stored in aliquots at -20°C. Oligomeric Aβ1-42, AβN3(pE) and AβN11(pE) were prepared essentially as described previously (Klein, 2002; Solorzano-Vargas et al., 2008) by incubation of monomers in DMEM/F12 at 4°C or at 37°C for 72 hrs. Aβ 35-25 and NRP were dissolved in a water at a concentration of 1 mg/ml. Formation of oligomeric Aβ1-42, AβN3(pE) and AβN11(pE) species was confirmed by the Western Blot. Briefly, after incubation, peptides were separated by electrophoresis on 4-12% polyacrilamide precast NuPAGE Bis-Tris gels (Invitrogen, Carlsbad, CA, USA) at 100 V for 1 h 45 mins and transferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA) using a semi dry blot system (Bio-Rad) at 25 V for 50 min. Membranes were blocked in PBS/2% non-fat dry milk/0.2% Triton X-100 overnight at 4°C and incubated overnight at 4°C with primary antibodies: 4G8 (1:2000) or rabbit anti-AβN3(pE) polyclonal IgG. After washing with PBS/0.2% Tween, the membranes were incubated with HRP-conjugated anti-mouse IgG2b, 1:2500 or anti-rabbit IgG, respectively, for 2 h at RT. Immunoreactive bands were detected by chemiluminescence using SuperSignal West Dura Extended Duration Substrate kit.
2.3. Immunization protocol
All experiments with animals were conducted using protocols approved by our Institutional Animal Care Committee. 3-month-old White New Zeland rabbits were immunized subcutaneously (s.c.) at 14 days intervals with 200 μg of human AβN3(pE) oligomers prepared at 37°C as described above. Freund’s complete adjuvant was used for primary injection followed by incomplete Freund’s adjuvant for three boost injections. Control rabbits were immunized with adjuvant alone or with a non-related peptide, respectively. Animals were bled on day 0 and 10 days after the third boost injection. The sera were stored at -20°C until use.
2.4. Purification of rabbit IgG
IgG were precipitated from rabbit sera with 1.7 M ammonium sulphate and dialyzed against PBS. Then this solution was applied to columns with Protein G-Sepharose (Zymed) and incubated for 1 h at room temperature. Non-bound proteins were removed by washing with PBS, and IgG was eluted with elution buffer (0.2 M glycine, adjusted to pH 2.8 with HCl). After dialysis against water, antibodies were lyophilized and stored at -20°C.
2.5. ELISA for evaluation of anti- AβN3(pE) antibodies
ELISA analysis was carried out using MaxiSorp microtiter plates (Nunc, Roskilde, Denmark) coated overnight with a synthetic peptides at a concentration of 20 μg/ml in carbonate buffer (pH 9.6). After washing with phosphate buffer containing 0.2% Tween-20 (PBS-Tween), plates were blocked with PBS/2% non-fat dry milk for 1 h at 37°C. Plates were washed, then rabbit sera diluted in PBS/2% non-fat dry milk/0.2% Triton X-100 were added and after incubation for 1 h at 37°C, plates were washed with PBS/0.2% Tween. Rabbit anti-human Aβ 1-42 polyclonal antibodies (Zymed) as well as rabbit polyclonal anti-AβN11(pE) antibodies obtained in our laboratory (unpublished data) were used as a positive control to confirm the peptide binding to well. HRPconjugated goat anti-rabbit IgG (Zymed) diluted in PBS/2% non-fat dry milk/0.2% Triton X-100 was added, and plates were incubated for 1 h at 37°C. Plates were washed and 2,2′-azino-bis- (3-ethyl-benzthiazoline-6-sulphonic acid (ABTS) single solution (Zymed) was added. The OD reading at 405 nm was registered using Opsys MR Microplate Reader (DYNEX Technologies, Chantilly, VA, USA). For competition ELISA, sera were preincubated overnight at 4°C with the preparations of serially two-fold diluted synthetic peptides, prior to adding to AβN3(pE)-coated wells. Then the assay was performed as described above.
2.6. Affinity selection of phages binding to anti- AβN3(pE) antibodies
Selection of phages by biopanning was performed as described in our previous studies (Gevorkian et al., 2000; Gevorkian et al., 2004). A Phage Display Peptide Library from New England Biolabs (Beverly, MA, USA) was used. The displayed 7-mer peptides are expressed at the N-terminus of the minor coat protein (cpIII) of M13 phage. MaxiSorp microtiter plates were coated with goat anti-rabbit IgG (Zymed) at a concentration of 5 μg/ml 1 h at 37°C, washed and blocked with PBS-2%BSA. After washing, polyclonal rabbit anti- AβN3(pE) antibodies diluted 1:200 in PBS-Tween-1% BSA were added and plates were incubated for 1 h at 37°C. Plates were washed, then 1011 plaqueforming units (PFU) from phage library diluted in PBS/BSA 1% were added and plates were incubated overnight at 4°C. Non-specific phages were washed off, and bound phage clones were eluted by triethilamine (0.1 M) and neutralized by Tris-HCl (1M, pH 7.5). Three rounds of biopanning were performed, and 21 individual clones were selected after the third round, amplified and used in ELISA to evaluate their binding to anti-AβN3(pE) antibodies. MaxiSorp microtiter plates were coated with goat anti-rabbit IgG and blocked as described above. Rabbit anti-AβN3(pE) antibodies diluted 1:100 in PBS-Tween-1% BSA were added and plates were incubated for 1 h at 37°C. After washing, 1010 PFU/ml of each phage clone diluted in PBS-1%BSA were added to plates and incubated overnight at 4°C. After washing, HRP-conjugated anti-M13 monoclonal antibody (Invitrogen) was added and incubated for 1 h at 37°C. After washing, ABTS single solution was added. The OD reading at 405 nm was registered using Opsys MR Microplate Reader (DYNEX Technologies).
Also, single-stranded DNA was prepared from all positive clones and one negative clone as described previously and used for DNA sequencing (Gevorkian et al., 2000). T7 Sequenase version 2.0 Quick Denature Plasmid Sequencing kit (Amersham Pharmacia Biotech, USA) and [α-35S] dATP (Amersham) were used according to the manufacturer’s instructions.
2.7. Cell culture
Human neuroblastoma SH-SY5Y and IMR-32 cells obtained from the American Type Culture Collection (ATCC, VA, USA) were maintained in DMEM/F12 (1:1) supplemented with 10% heat-inactivated fetal bovine serum (GIBCO) and penicillin-streptomycin (GIBCO) and differentiated for 8-10 days in the presence of 10 μM all-trans retinoic acid (SH-SY5Y) or 1mM dibutyryl cAMP (IMR-32 cells).
2.7. Annexin V — 7-Amino Actinomycin D (7-AAD) assay
SH-SY5Y cells were seeded in 6-well plates at a density of 106 cells/well and exposed to 10μM AβN3(pE), Aβ1-42 and Aβ1-16, diluted in DMEM/F12 without fetal bovine serum for 24 h. After treatment, supernatants were removed and adherent cells were harvested using 0.25% trypsin (Sigma). After dilution in PBS containing 1% fetal bovine serum, the adherent cells were added to detached population floating in supernatants and centrifuged at 600 g for 5 min at room temperature. Cells were resuspended in 500 μl of binding buffer (10 mM HEPES, 140 mM HCl and 2.5 mM CaCl2) and centrifuged again. Then 5 μl of annexin V-FITC (Invitrogen) diluted in 100 μl of binding buffer were added and cells were incubated for 40 min at room temperature in dark. After washing cells twice with binding buffer, 2.5 μl of 7-AAD (Sigma) were added and cells were incubated for 20 min at room temperature in dark. After washing three times, cells were resuspended in 400 μl of binding buffer and analyzed by flow cytometry. Flow cytometric analysis was performed using a FACScan Flow Cytometer (Becton-Dickinson, NJ, USA). Fluorescence was detected at an excitation wavelength of 488 nm and emission wave-lengths of 525 and 620 nm for the green (FITC) and red (7-AAD) fluorescence, respectively. A total of 10,000 events were acquired for each sample. Unstained cells, annexin V only stained cells and 7AAD only stained cells were run as controls to set gates.
For immunocytochemistry, cells were plated onto round glass coverslips (12 mm diameter) and placed in 24-well-plates (density was 105 cells/well). After treatment with peptides as described above, cells were washed with binding buffer and 2 μl of annexin V-FITC and 2 μl of 7-AAD diluted in binding buffer were added to each well. After incubation for 20 min, cells were washed again, fixed for 30 min at room temperature with 4% paraformaldehyde in PBS. After washing with PBS, cells were mounted onto glass slides in ProLong Gold antifade reagent with DAPI (Molecular Probes). Confocal images were collected sequentially at RT on a FV1000 confocal laser scanning microscope (Olympus, Tokyo, Japan) with a 60x oil immersion objective lents UPLSAPO (NA=1.35). FITC fluorescence was obtained after exciting the samples with a wavelength of 488 nm and reading the fluorescence at 519 nm. For the detection of 7-AAD and DAPI the excitation wavelengths were 543 and 405 and emissions were colleted at 619 y 461 nm, respectively. The fluorescence intensity was set optimally for control cells and this exposure was retained for cells treated with peptides in order to determine light intensity. Appropriate threshold was employed to eliminate background signal in the images employing Imaris 5.0.3 software (Bitplane AG, Zurich, Switzerland). Images from controls and treated cells were processed under similar conditions
2.8. BLAST homology search
A homology between peptide inserts of selected positive clones and known protein sequences was analyzed using BLASTP program (http://blast.ncbi.nlm.nih.gov).
2.9. Statistical analysis
Data were analyzed by ANOVA using SPSS statistical software program (Release 9.0).
3. RESULTS
3.1. Characterization of oligomeric AβN3(pE) and AβN11(pE) preparations
Oligomeric AβN3(pE) and AβN11(pE) were prepared from monomers essentially as described previously (Klein, 2002). All preparations were characterized by electrophoresis followed by silver staining (not shown) and immunoblotting. All oligomeric preparations of both AβN3(pE) and AβN11(pE) contained two prominent bands corresponding to trimers and tetramers as well as a monomers (Fig.1). Higher molecular weight bands corresponding to 12- and 24-mers were also observed mainly in oligomers preparations incubated at 37°C. All bands were identified by 4G8 antibody known to bind to an internal epitope of Aβ (aa17-24). It has been reported previously that oligomeric fulllength Aβ1-42 peptide also formed mainly trimers and tetramers under conditions used (Chang et al., 2003).
Fig.1.
Characterization of aggregated AβN3(pE) and AβN11(pE) peptides by PAGE and Western blot analysis. Oligomers were prepared and analyzed as described in Materials and methods section. (A) A monoclonal anti-Aβ antibody (4G8) binding to a central part of Aβ 1-42 was used to detect oligomers of AβN3(pE) and AβN11(pE) incubated at 4°C and at 37°C. (B) Rabbit antihuman AβN3(pE) polyclonal IgG were used to detect oligomers of AβN3(pE) incubated at 4°C and at 37°C. Rabbit preimmune serum was used as a negative control. Migration of the molecular mass standards is indicated by arrowheads.
3.2. Production of anti-AβN3(pE) antibodies and their binding to synthetic Aβ peptides
To produce anti-AβN3(pE) antibodies, rabbits were immunized with peptide preparation mixed with adjuvant. Animals were bled 10 days after the third boost injection and the production of specific antibodies was tested by ELISA. Anti-AβN3(pE) antibodies were found to bind specifically to AβN3(pE) peptide while no binding to Aβ 1-42 or AβN11(pE) was observed (Fig.2). No binding of preimmune serum or anti-NRP serum to AβN3(pE) was detected. Also, after pre-incubation of anti-AβN3(pE) antibodies with Aβ 1-42 and AβN11(pE) there were no inhibition of binding of anti-AβN3(pE) antibodies to AβN3(pE) (Fig.3).
Fig.2.
Rabbit anti-AβN3(pE) IgG are specifically binding in ELISA to AβN3(pE) peptide, while recognition of AβN11(pE) and Aβ 1-42 is negligible. AβN3(pE), AβN11(pE) and Aβ 1-42 were prepared as described in Materials and methods and used for covering microtiter plates. Optical densities (OD) were registered at 405. Data are means of three independent experiments ±SD.
Fig.3.
Rabbit anti-AβN3(pE) antibodies are highly specific against AβN3(pE) peptide as confirmed by competition ELISA. Sera were preincubated overnight at 4°C with the preparations of serially two-fold diluted synthetic AβN3(pE), AβN11(pE) and Aβ 1-42, prior to adding to AβN3(pE)-coated wells.
Anti-AβN3(pE) antibodies were tested in Western Blot and found to be specific for oligomeric AβN3(pE), while no binding to AβN11(pE) was observed (Fig.1).
3.3. Affinity selection of phages binding to anti- AβN3(pE) antibodies
To identify immunodominant region of AβN3(pE) peptide, the library of random heptapeptides displayed as a fusion to the minor coat protein of M13 phage was screened with rabbit anti-AβN3(pE) antibodies. Three rounds of biopanning were performed and 21 clones were randomly selected from the eluate after the third round. Binding of these clones to anti-AβN3(pE) antibodies was tested in ELISA. DNA sequences of heptapeptides coding inserts of 6 positive and 1 negative phage clones were determined and the deduced amino acid sequences are shown in Table 1. Peptide inserts of the three positive clones had the same sequence QFRHDWY, and two other positive clones (C11 and C12) had a homologous sequence QFRT(V)D(P)D(Y)P. Thus, after third round of biopanning five positive clones bearing inserts with peptide sequences homologous to AβN3(pE) were selected. One positive clone (C8) had a peptide sequence WPVGGEH with no homology with AβN3(pE). BLAST search revealed a homology of this sequence with a hexapeptide present in the growth factor receptor-bound protein 7 (GRB7), an adaptor molecule that mediates signal transduction from multiple cell surface receptors to various downstream signaling pathways.
Table 1.
Peptide sequences and reactivity of selected phage clones with rabbit anti-AβN3(pE) serum
| Phage clone |
Sequence |
OD 405nm |
|---|---|---|
| C5 |
T P S Y V S K | 0.03±0.001 |
| C8 |
W P V G G E H | 0.8±0.008 |
| C3, C10, C18 |
Q F R H D W Y | 1.42±0.04 |
| C11 |
Q F R H D D P | 1.39±0.046 |
| C12 |
Q F R V P Y P | 0.78±0.06 |
| C17 |
Q F R S D S T | 0.82±0.142 |
| C3.15 | S Y E F R H H | 0.03±0.005 |
Interestingly, neither anti-full length Aβ 1-42 antibodies nor anti- AβN11(pE) antibodies bound phage clones with a consensus sequence QFRH(T,V)D(P)W(D,Y)Y(P).
3.4. AβN3(pE) induces phosphatidyl serine (PS) externalization and increases the membrane permeability in SH-SY5Y and IMR-32 cells
To assess the cell death mechanisms induced by AβN3(pE), we used annexin V and 7-AAD dual staining approach to differentiate between early apoptotic, necrotic/late apoptotic and live cells. It is known that in cells undergoing apoptosis, the membrane PS translocates from the inner face of the plasma membrane to the cell surface and can bind to annexin V (Vermes et al., 1995). 7AAD is a fluorescent, cell-impermeable DNA-binding agent and is used to assess the cell membrane integrity. Both cell lines (SH-SY5Y and IMR-32) used in this study were characterized extensively in previous reports and shown to have biochemical properties of human neurons in vivo (Sherer et al., 2001) and, after differentiation, to have also all the characteristics of adult neurons in the brain and to be sensitive to degeneration evoked by Aβ (Lambert et al., 1994). That is why we differentiated cells for 7 to 10 days before conducting all experiments.
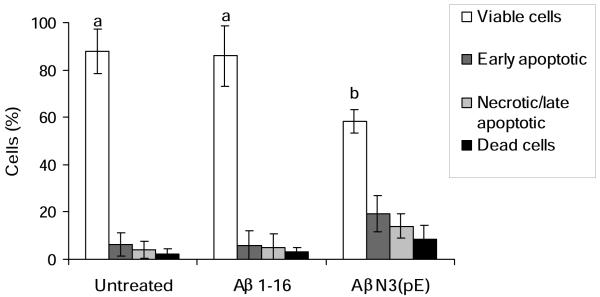
After treatment with AβN3(pE) for 24 h, differentiated SH-SY5Y cells were stained as described in Materials and Methods section and analyzed by flow cytometry. Four populations of cells were detected (Fig. 4). Live cells, which were not affected by the oligomeric AβN3(pE), are located in the lower left quadrant. The lower right quadrant represents early apoptotic (annexin V-positive and 7AAD-negative) cells while the upper right quadrant represents late apoptotic/necrotic cells (annexin V-positive and 7-AAD positive). A small number of dead cells were observed in the upper left quadrant (annexin V-negative and 7-AAD-positive). In summary, exposure to 10 μM AβN3(pE) induced a significant (p< 0.05) decrease in the number of viable cells (29.3% and 27.5%) compared with control untreated cells and control cells treated with Aβ 1-16 peptide, respectively (Fig. 5). Also, exposure to AβN3(pE) induced a 3-fold increase in the number of early apoptotic cells (annexin V positive and 7-AAD negative cells) compared with control cells. A three-fold increase in the number of annexin V-positive and 7-AAD-positive (necrotic/late apoptotic) cells compared with control untreated cells or Aβ 1-16 peptide treated cells was observed too.
Fig.4.
AβN3(pE) peptide toxicity in differentiated dopaminergic SH-SY5Y cell cultures. Representative dot-plots of annexin V-FITC and 7-AAD staining of untreated cells (A), cells treated with Aβ 1-16 (B), cells treated with Aβ1-42 (C), AβN3(pE) (D) and cells treated with ionomycin (E).
Fig.5.
Quantification by flow cytometry of AβN3(pE)-induced cell death. Data shown are means ± SD of 3 independent experiments. Means denoted with different letters are statistically different (P<0.05).
In the same assay, exposure to Aβ1-42 induced an increase (although less than in the case of AβN3(pE) ) in the number of early apoptotic cells compared with control untreated cells and cells treated with Aβ1-16 (Fig.4).
The same mechanisms of toxicity were observed by immunocytochemical analysis of cholinergic IMR-32 cells treated with oligomeric AβN3(pE). After 24 by incubation with 10 μM AβN3(pE) cells were stained with annexin V-FITC and 7-AAD. Cells treated with oligomeric AβN3(pE) showed both translocation of PS to the outer side of the plasma membrane (green color) and DNA staining with 7-AAD (red color) (Fig.6). At the same time, cells treated with a control peptide (Aβ 1-16) were annexin V/7AAD negative.
Fig.6.
AβN3(pE) induces phosphatidyl serine (PS) externalization and increases the membrane permeability as observed by immunocytochemical analysis. Cells were treated with AβN3(pE) as described above and stained with annexin V-FITC and 7-AAD (upper panel). A substantial incorporation of annexin V and 7-AAD is observed. Incubation of cells under same conditions with a negative control peptide (Aβ 1-16) (lower panel) did not induce toxicity. We observed similar immunostaining patterns in four independent experiments.
DISCUSSION
Soluble Aβ aggregates present in the brain of sporadic AD patients and in Down syndrome were significantly different and more toxic compared with Aβ present in normal brain, and this was correlated with the predominance of the N-truncated species over full length Aβ 1-42 (Kalback et al., 2002; Piccini et al., 2005; Vanderstichele et al., 2005). Importantly, AβN3(pE) is the most abundant one in AD brain and is absent in normal aging, while other N-truncated species were detected in both AD and aged control brains (Schilling et al., 2008). Moreover, the resistance toward proteolytic degradation by aminopeptidases decreases the rate of AβN3(pE) clearance and enhances its accumulation. In addition, pyroglutamate-containing Aβ species have been shown to be potential seeding species of aggregate formation in vivo and may play an important role during the initiation of the disease (Schilling et al., 2006). Importantly, it has been demonstrated that intracerebroventricular injection of a low dose of soluble AβN3(pE) oligomers impaired memory in male C57BL/6 mice suggesting that this peptide might cause impairments of synaptic integrity and plasticity (Youssef et al., 2007). Previously, it has been reported that in APPSLPS1KI transgenic mice, emerging of AβN3(pE) peptide is detected at an age when the first signs of hippocampal neuronal loss (Casas et al., 2004). Finally, it has been demonstrated recently that inhibition of glutaminyl cyclase, an enzyme responsible for AβN3(pE) formation, reduced plaque load in two different transgenic mouse models of AD accompanied by alleviated plaqueassociated inflammation and a significant memory improvement (Schilling et al., 2008). Thus collectively these results provide support for a role for AβN3(pE) in Aβ aggregate formation. We believe our results are important in the development of immunogens capable to inducing antibodies specifically targeting AβN3(pE), which may inhibit the formation, as well as enhance clearance of the more pathological forms of Aβ peptide in the CNS.
In the current study we demonstrated that the immunodominant region of one of the most abundant N-truncated/modified Aβ peptide, bearing aminoterminal pyroglutamate, AβN3(pE), is located at its amino terminus. Interestingly, although the epitope recognized by anti-AβN3(pE) antibodies contains FRH motif, anti-AβN3(pE) serum did not bind to phage clone bearing EFRH-containing peptide selected in our previous studies (Gevorkian et al., 2004). Also, rabbit anti-AβN3(pE) antibodies are highly specific against this modified peptide and do not recognize AβN11(pE) nor the full length Aβ1-42 peptides. These results emphasize the need to search for immunogens capable to target N-truncated/modified species, since previously reported immunotherapy studies are based on EFRH epitope.
The molecular mechanisms of neuronal death induced by AβN3(pE) oligomers were addressed in a few studies (Piccini et al., 2005, Youssef et al., 2007). It has been shown that AβN3(pE) oligomers altered the membrane structure and permeability of liposomes (Piccini et al., 2005). Also, it has been reported that AβN3(pE) oligomers induced a redox-sensitive neuronal apoptosis involving caspase activation and an arachidonic acid-dependent proinflammatory pathway (Youssef et al., 2007). In the present study we demonstrated in two human neuroblastoma cell lines - dopaminergic SH-SY5Y cells and cholinergic IMR-32 cells - that oligomeric AβN3(pE) induced PS externalization and loss of membrane integrity measured by annexin V/7AAD staining. Our results indicate that both apoptosis and necrosis can occur in these two cell lines after exposure to 10 μM oligomeric AβN3(pE).
Interestingly, the peptide insert of one of the positive clones selected in this study had a homology with a hexapeptide WPVGGD present in the growth factor receptor-bound protein 7 (GRB7), an adaptor molecule that mediates signal transduction from multiple cell surface receptors to various downstream signaling pathways (Daly, 1998; Shen and Guan, 2004). It has been reported previously that Aβ may alter the molecular composition of focal adhesions that would have multiple downstream effects such as rapid tyrosine phosphorylation of neuronal proteins and induction of an unscheduled cell cycle leading to death, among others (Zhang et al., 1994; Berg et al., 2002; Lambert et al., 1998; Williamson et al., 2002). Importantly, both proteins, Aβ and GRB7, were demonstrated to participate in the integrin signaling pathway by activating the focal adhesion proteins (Grace and Busciglio, 2002; Shen and Guan, 2004; Frasca et al., 2008). The sequence homology described in our study may explain, in part, these observations. However, further studies will have to be conducted.
In conclusion, by designing new immunogens (anti-AβN3(pE) epitope) capable of inducing antibodies against N-amino-truncated/modified Aβ peptides, one may specifically target the most pathological species of the Aβ peptide. This should significantly enhance the efficacy of immunotherapy in the CNS of AD patients, because only approximately 0.1% of the antibody in the blood gains entry into the brain.
ACKNOWLEDGMENTS
This work was supported by a grant from DGAPA-UNAM (IN200907), PASPA-DGAPA-UNAM, CONACyT ( 58081), Mexico to GG, by UC CONACYT MEXUS grant to DHC and GG, as well as NIH RO-1 grants (NIA AG20241 and NINDS NS50895), to DHC. The brain tissue used in this project was provided by the Institute for Brain Aging and Dementia and the University of California Alzheimer’s Disease Research Center (UCI-ADRC). Funding for the UCI-ADRC was provided by NIH/NIA Grant P50 AG16573. We thank Jesus Ramses Chavez Rios and Gabriel Orozco Hoyuela for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch K, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid-β peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer’s disease. Nature Medicine. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Breyahn H, Duan K, Rettig J, Wirths O. Intraneuronal β-amyloid is a major risk factor - novel evidence from the APP/PS1KI mouse model. Neurodegener. Dis. 2008;5:140–142. doi: 10.1159/000113684. [DOI] [PubMed] [Google Scholar]
- Berg MM, Krafft GA, klein WL. Rapid impact of β-amyloid on paxillin in a neural cell line. J. Neurosci. Res. 1997;50:979–989. doi: 10.1002/(SICI)1097-4547(19971215)50:6<979::AID-JNR8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu. Rev. Neurosci. 2008;31:175–193. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas C, Sergeant N, Itier J-M, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 Neuronal Loss with Intraneuronal and N-Terminal Truncated Aβ42 Accumulation in a Novel Alzheimer Transgenic Model. Am. J. Pathol. 2004;165:1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Bakhis L, Wang Z, Venton DL, Klein WL. Femtomole immunodetection of synthetic and endogenous amyloid-b oligomers and its application to Alzheimer’s disease drug candidate screening. J. Mol. Neurosci. 2003;20:305–313. doi: 10.1385/JMN:20:3:305. [DOI] [PubMed] [Google Scholar]
- Daly RJ. The Grb7 family of signaling proteins. Cell Signal. 1998;10:613–618. doi: 10.1016/s0898-6568(98)00022-9. [DOI] [PubMed] [Google Scholar]
- Frasca G, Carbonaro V, Merlo S, Copani A, Sortino MA. Integrins mediate β-amyloid-induced cell-cycle activation and neuronal death. J. Neurosci. Res. 2008;86:350–355. doi: 10.1002/jnr.21487. [DOI] [PubMed] [Google Scholar]
- Gevorkian G, Manoutcharian K, Govezensky T, Cano A, Dominguez V, Santamaria H, Larralde C. Identification of mimotopes of platelet autoantigens associated with autoimmune thrombocytopenic purpura. J. Autoimmunity. 2000;15:33–40. doi: 10.1006/jaut.2000.0389. [DOI] [PubMed] [Google Scholar]
- Gevorkian G, Petrushina I, Manoutcharian K, Ghochikyan A, Acero G, Vasilevko V, Cribbs DH, Agadjanyan MG. Mimotopes of conformational epitopes in fibrillar β-amyloid. J. Neuroimmunol. 2004;156:10–20. doi: 10.1016/j.jneuroim.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Grace EA, Busciglio J. Aberrant activation of focal adhesión proteins mediates fibrillar amyloid β-induced neuronal dystrophy. J. Neurosci. 2002;23:493–502. doi: 10.1523/JNEUROSCI.23-02-00493.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntert A, Dobeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience. 2006;143:461–475. doi: 10.1016/j.neuroscience.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harigaya Y, Saido TC, Eckman CB, Prada C-M, Shoji M, Younkin SG. Amyloid β protein starting pyroglutamate at position 3 is a major component of the amyloid deposits in the Alzheimers disease brain. Biochem. Biophys. Res. Comm. 2000;276:422–427. doi: 10.1006/bbrc.2000.3490. [DOI] [PubMed] [Google Scholar]
- He W, Barrow CJ. The Aβ 3-pyroglutamyl and 11-pyroglutamyl peptides found in senile plaque have greater b-sheet forming and aggregation propensities in vitro than full-length Aβ. 1999. [DOI] [PubMed] [Google Scholar]
- Head E, Pop V, Vasilevko V, Hill M, Saing T, Sarsoza F, Nistor M, Christie LA, Milton S, Glabe C, Barrett E, Cribbs D. A two-year study with fibrillar beta-amyloid (Abeta) immunization in aged canines: effects on cognitive function and brain Abeta. J. Neurosci. 2008;28:3555–3566. doi: 10.1523/JNEUROSCI.0208-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse JT, Liu K, Pijak DS, Carlin D, Lee VM-Y, Doms RW. β-Secretase processing in the trans-Golgi network preferentially generates truncated amyloid species that accumulate in Alzheimer’s disease brain. J. Biol. Chem. 2002;277:16278–16284. doi: 10.1074/jbc.M111141200. [DOI] [PubMed] [Google Scholar]
- Kalback W, Watson MD, Kokjohn TA, Kuo YM, Weiss N, Luehrs DC, Lopez J, Brune D, Sisodia SS, Staufenbiel M, Emmerling M, Roher AE. APP transgenic mice Tg2576 accumulate Abeta peptides that are distinct from chemically modified and insoluble peptides deposited in Alzheimer’s disease senile plaques. Biochemistry. 2002;41:922–928. doi: 10.1021/bi015685+. [DOI] [PubMed] [Google Scholar]
- Klein WL. Aβ toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem. Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh S, De Jonghe C, Cruts M, Kleinert R, Wang R, Mercken M, De Strooper B, Vanderstichele H, Lofgren A, Vanderhoeven I, Backhovens H, Vanmechelen E, Kroisel PM, Van Broeckhoven C. Nonfibrillar diffuse amyloid deposition due to a gamma(42)-secretase site mutation points to an essential role for N-truncated A beta842) in Alzheimer’s disease. Hum. Mol. Genet. 2000;9:2589–2598. doi: 10.1093/hmg/9.18.2589. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Emmerling MR, Woods AS, Cotter RJ, Roher AE. Isolation, chemical characterization, and quantitation of A beta 3-pyroglutamyl peptide from neuritic plaques and vascular amyloid deposits. Biochem. Biophys. Res. Commun. 1997;237:188–191. doi: 10.1006/bbrc.1997.7083. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, liosatos M, Morgand TE, Rosovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc. Natl.Acad. Sci. USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Stevens G, Sabo S, Barber K, Wang G, Wade W, Krafft G, Snyder S, Holzman TF, Klein WL. Beta/A4-evoked degeneration of differentiated SH-SY5Y human neuroblastoma cells. J. Neurosci. Res. 1994;39:377–385. doi: 10.1002/jnr.490390404. [DOI] [PubMed] [Google Scholar]
- Larner AJ. Hypothesis: amyloid beta-peptides truncated at the Nterminus contribute to the pathogenesis of Alzheimer’s disease. Neurobiol. Aging. 1999;20:65–69. doi: 10.1016/s0197-4580(99)00014-7. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, Zheng JB, Seabrook TJ, Louard D, Li D, Selkoe DJ, Palmour RM, Ervin FR. Alzheimer’s disease Aβ vaccine reduces central nervous system Aβ levels in a non-human primate, the Caribbean vervet. Am. J. Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Solano I, Mann D, Lemere C, Mercken M, Trojanowski JQ, Lee VM-Y. Characterization of Ab11-40/42 peptide deposition in Alzheimer’s disease and young Down’s syndrome brains: implication of N-terminally truncated Aβ species in the pathogenesis of Alzheimer’s disease. Acta neuropathol. 2006;112:163–174. doi: 10.1007/s00401-006-0077-5. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreyther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miravalle L, Calero M, Takao M, Roher AE, Ghetti B, Vidal R. Amino-Terminally Truncated Abeta Peptide Species Are the Main Component of Cotton Wool Plaques. Biochemistry. 2005;44:10810–10821. doi: 10.1021/bi0508237. [DOI] [PubMed] [Google Scholar]
- Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid beta protein in Alzheimer’s disease. J. Biol. Chem. 1992;267:17082–17086. [PubMed] [Google Scholar]
- Naslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, Silberring J, Gandy SE, Winblad B, Greengard P, Nordstedt C, Terenius L. Relative abundance of Alzheimer Aβ amyloid peptide variants in Alzheimer disease and normal aging. Proc. Natl. Acad. Sci. USA. 1994;91:8378–8382. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A, Russo C, Gliozzi A, Relini A, Vitali A, Borghi R, Giliberto L, Armirotti A, D’Arrigo, Bachi A, Cattaneo A, Canale C, Torrassa S, Saido TC, Markesbery W, Gambetti P, Tabaton M. β-Amyloid Is Different in Normal Aging and in Alzheimer Disease. J. Biol. Chem. 2005;280:34186–34192. doi: 10.1074/jbc.M501694200. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Overman MJ, Cotman CW. Amino-terminal Deletions Enhance Aggregation of beta-Amyloid Peptides in Vitro. J. Biol. Chem. 1995;270:23895–23898. doi: 10.1074/jbc.270.41.23895. [DOI] [PubMed] [Google Scholar]
- Russo C, Salis S, Dolcini V, Venezia V, Song X-H, Teller JK, Schettini G. Identification of amino-terminally and phosphotyrosine-modified carboxy-terminal fragments of amyloid precursor protein in Alzheiner’s Disease and Down Syndrome brain. Neurobiol. Dis. 2001;8:173–180. doi: 10.1006/nbdi.2000.0357. [DOI] [PubMed] [Google Scholar]
- Russo C, Violani E, Salis S, Venezia V, Dolcini V, Damonte G, Benatti U, D’Arrigo C, Patrone E, carlo P, Schettini G. Pyroglutamate-modified amyloid -peptides - A N3(pE) - strongly affect cultured neuron and astrocyte survival. J. Neurochem. 2002;82:1480–1489. doi: 10.1046/j.1471-4159.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- Saido TC, Iwatsubo T, Mann DMA, Shimada H, Ihara Y, Kawashima S. Dominant and differential deposition of distinct β-amyloid peptide species, AβN3(pE), in senile plaques. Neuron. 1995;14:457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- Saido TC, Yamao-Harigaya W, Iwatsubo T, Kawashima S. Aminoand carboxyl-terminal heterogeneity of beta-amyloid peptides deposited in human brain. Neurosci. Lett. 1996;215:173–176. doi: 10.1016/0304-3940(96)12970-0. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Schilling S, Lauber T, Schaupp M, Manhart S, Scheel E, Bohm G, Demuth H-U. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro) Biochemistry. 2006;45:12393–12399. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]
- Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, Jagla W, Sch lenzig D, landler C, Rudolph T, Reuter G, Cynis H, Montag D, Demuth HU, Rossner S. Glutaminyl cyclase inhibition attenuates pyroglutamate Ab and Alzheimer’s disease-like pathology. Nat. Medicine. 2008;14:1106–1111. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- Sergeant N, Bombois S, Ghestem A, Drobecq H, Kostanjevecki V, Missiaen C, Wattez A, David J-P, Vanmechelen E, Sergheraert C, Delacourte A. Truncated beta-amyloid peptide species in pre-clinical Alzheimer’s disease as new targets for the vaccination approach. J. neurochem. 2003;85:1581–1591. doi: 10.1046/j.1471-4159.2003.01818.x. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schiossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D, Lieberburg I, Schenk D. Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shen TL, Guan JL. Grb7 intracellular signaling and its role in cell regulation. Front Biosci. 2004;9:192–200. doi: 10.2741/1229. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Trimmer PA, Borland K, Parks JK, Bennett JP, Jr, Tuttle JB. Chronic reduction in complex I function alters calcium signaling in SH-SY5Y neuroblastoma cells. Brain Res. 2001;891:94–105. doi: 10.1016/s0006-8993(00)03203-0. [DOI] [PubMed] [Google Scholar]
- Solórzano-Vargas RS, Vasilevko V, Acero G, Ugen KE, Martinez R, Govezensky T, Vazquez-Ramirez R, Kubli-Garfias C, Cribbs DH, Manoutcharian K, Gevorkian G. Epitope mapping and neuroprotective properties of a human single Chain Fv antibody that binds an internal epitope of amyloid-beta 1-42. Mol. Immunol. 2008;45:881–886. doi: 10.1016/j.molimm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Tekirian TL, Markesbery WR, Russel MJ, Wekstein DR, Patel E, Geddes JW. N-terminal heterogeneity of parenchymal and cerebrovascular Abeta deposits. J. Neuropathol. Exp. Neurol. 1998;57:76–94. doi: 10.1097/00005072-199801000-00009. T.C. [DOI] [PubMed] [Google Scholar]
- Town T, tan J, Sansone N, Obregon D, Klein T, Mullan M. Characterization of murine immunoglobulin G antibodies against human amyloid-beta 1-42. Neurosci.Lett. 2001;307:101–104. doi: 10.1016/s0304-3940(01)01951-6. [DOI] [PubMed] [Google Scholar]
- Vanderstichele H, De Meyer G, Andreasen N, Kostanjevecki V, Wallin A, Olsson A, Blennow K, Vanmechelen E. Amino-truncated β-amyloid 42 peptides in cerebrospinal fluid and prediction of progression of mild cognitive impairment. Clin. Chem. 2005;51:1650–1660. doi: 10.1373/clinchem.2005.051201. [DOI] [PubMed] [Google Scholar]
- Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Williamson R, Scales T, Clark BR, Gibb G, Reynolds CH, Kellie S, Bird IN, Varndell IM, Sheppard PW, Everall I, Anderton BH. Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-β peptide exposure: involvement of src family protein kinase. J. Neurosci. 2002;22:10–20. doi: 10.1523/JNEUROSCI.22-01-00010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Weis J, Kayed R, Saido TC, Bayer TA. Age-dependent axonal degeneration in an Alzheimer mouse model. Neurobiol. Aging. 2007;28:1689–1699. doi: 10.1016/j.neurobiolaging.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Youssef I, Florent-Bechard S, malaplate-Armand C, Koziel V, Bihain B, Olivier J-L, Leininger-Muller B, Kriem B, Oster T, Pillot T. Ntruncated amyloid-b oligomers induce learning impairment and neuronal apoptosis. Neurobiol. Aging. 2007;29:1319–1333. doi: 10.1016/j.neurobiolaging.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lambert MP, Bunch C, Barber K, Wade WS, Krafft GA, Klein WL. Focal adhesion kinase expressed by nerve cell line shows increased tyrosine phosphorylation in response to Alzheimer’s A beta peptide. J. Biol. Chem. 1994;269:25247–25250. [PubMed] [Google Scholar]