Abstract
Introduction
Adherence to low-carbohydrate, ketogenic diets has been associated with greater weight loss in the short-term than low-fat, calorie-restricted diets. However, consumption of ketogenic diets may result in decreased voluntary exercise and thus render long-term weight loss and maintenance of weight loss difficult.
Methods
Rats were maintained on either a non-ketogenic chow (CH) diet or a low-carbohydrate, ketogenic diet (KD) for 6 weeks. Half of each dietary group was sedentary, while the other half was given access to a running wheel. Running wheel activity (total distance and meters/minute), plasma leptin and insulin, adiposity, and hypothalamic mRNA for neuropeptide Y (NPY) and proopiomelanocortin (POMC) were measured to assess activity-related effects in animals maintained on KD.
Results
With access to a running wheel, rats on KD engaged in similar levels of voluntary activity as CH rats and both dietary groups decreased caloric intake. Caloric intake increased over time such that it was significantly greater than sedentary controls after one month of access to the wheels, however body weight remained decreased. Sedentary rats maintained on KD had increased adiposity and plasma leptin levels and decreased hypothalamic POMC mRNA, as compared to sedentary CH rats. KD rats with access to a running wheel had similar levels of adiposity and plasma leptin levels as CH rats with access to running wheels, but significantly increased POMC mRNA in the arcuate.
Conclusion
We demonstrate that maintenance on KD does not inhibit voluntary activity in a running wheel. Furthermore, prevention of KD-related increased adiposity and plasma leptin, as measured in sedentary KD rats, significantly increases levels of the anorexigenic neuropeptide POMC mRNA.
Keywords: proopiomelanocortin, leptin, low-carbohydrate diet, ketosis, running wheel
Introduction
The prevalence of overweight and obesity among adults in the United States has increased significantly over the past four decades (1). Numerous diets have been proposed to induce weight loss, and it is estimated that between 30 and 45% of adults in the United States use specific dietary approaches in an attempt to lose weight (2). Although low fat, reduced-calorie diets coupled with increased energy expenditure are generally recommended for weight loss, the use of low-carbohydrate diets has increased in recent years (3). Proponents of low-carbohydrate, ketogenic diets promote the concept that caloric intake and dietary fat are not the culprits in obesity, and that ultimately minimization of carbohydrate intake leads to weight loss, decreased hunger, and maintenance of reduced body weight (4, 5). Low carbohydrate diets have, in fact, been associated with greater weight loss in the short-term as compared to weight loss associated with low fat diet users (6–11). However, long-term evaluations found no significant differences in weight loss between low-carbohydrate dieters and controls at one year (11, 12).
While many individuals engage in dieting strategies in an attempt to lose weight, increased physical activity is an important component of the weight-loss equation. Studies comparing weight loss with diet alone as compared to those that included increased physical activity demonstrate increased weight-loss maintenance in individuals who regularly exercised (13, 14). Individuals consuming carbohydrate-restricted diets report fatigue and reduced vigor in response to exercise significantly more than individuals consuming high-carbohydrate diets (11, 15). Furthermore, maintenance on a ketogenic diet results in reduced free- living physical activity, a response that can be counter-productive in individuals trying to lose weight (16). Fatigue and perceived effort during exercise have been compared in trained, overweight adults adhering to a low-carbohydrate diet that was ketogenic or to a control diet that was also low in carbohydrate but non-ketogenic (5% vs. 40% carbohydrate). While weight loss was similar among diet groups, there was a positive correlation between feelings of fatigue while exercising and blood ketone concentrations. These data suggest that ketogenic diets enhance fatigability and can reduce the desire to exercise (16).
Given reported differences in levels of physical activity by individuals consuming low carbohydrate diets (16), the increased fat content of low carbohydrate diets (8, 17) and the known effects of consuming a high fat diet on adiposity (18–23), we hypothesized that rats maintained on a ketogenic diet would engage in less voluntary activity when given access to a running wheel than chow-fed rats. We evaluated running wheel activity, caloric intake and body weight in rats given access to a running wheel for four consecutive weeks, as well as adiposity and plasma insulin and leptin. We have previously reported that maintenance on a ketogenic diet affects neuropeptide Y (NPY) and proopiomelanocortin (POMC) mRNA in the arcuate nucleus of the hypothalamus (24), therefore we included measures of these parameters after exposure to running wheels.
Methods
Thirty-two male Long Evans rats (Harlan, Indianapolis, IN) weighing 225–250 g were individually housed in clear plexiglass tub cages and maintained at a constant temperature (25°C) on a 12:12 h light/dark cycle (lights on at 4:00 AM). The tub cages were attached to running wheels, (Lafayette Instruments, Lafayette, IN) however access to wheels was blocked and the wheels were in a locked position unless otherwise noted. All rats had 24 h access to food and tap water during all portions of the experiment, except for 1 h each day during which time food was weighed and replaced. Procedures were approved by the Purdue University Animal Care and Use Committee.
Food intake, body weight, and running wheel access
After one week of acclimation to the laboratory, during which time rats were given ad libitum access to rodent chow (Harlan Teklad 2018 18% protein diet, 3.4 kcal/g), rats were weight-matched and divided into two groups. The first group maintained ad libitum access to chow (CH) and the second group was placed on a ketogenic diet (KD) from which 5% of the calories were derived from carbohydrate, 80% from fat, and 15% from protein (Table 1, Research Diets D06040601, 6.1 kcal/g, Research Diets Inc., New Brunswick, NJ). Caloric intake and body weights were measured and recorded daily.
Table 1.
Components of the ketogenic diet (Research Diets, D06040601).
| Gram % | Kcal % | |
|---|---|---|
| Protein | 23 | 15 |
| Carbohydrate | 8 | 5 |
| Fat | 55 | 80 |
| Total | 100 | |
| Kcal/g | 6.1 | |
| Ingredient | gram | kcal |
| Casein, 80 Mesh | 151 | 604 |
| L-Cysteine | 1.5 | 6 |
| Corn Starch | 40 | 160 |
| Cellulose | 50 | 0 |
| Soybean Oil | 25 | 225 |
| Lard | 336 | 3024 |
| Mineral Mix, S10026 | 10 | 0 |
| Dicalcium Phosphate | 13 | 0 |
| Calcium Carbonate | 5.5 | 0 |
| Potassium Citrate, 1 H20 | 16.5 | 0 |
| Vitamin Mix, V10001 | 10 | 40 |
| Choline Bitartrate | 2 | 0 |
| FD&C Yellow Dye #5 | 0.025 | 0 |
| FD&C Blue Dye #1 | 0.025 | 0 |
| TOTAL | 660.55 | 4059 |
Following one week of access to CH or KD, rats were weight-matched within their diet groups. Running wheels were unlocked for half of each group at this point (CH-RUN (n=8) and KD-RUN (n=8)), and remained locked for the other half of the rats who served as sedentary controls for each diet group (CH-SED (n=8) and KD-SED (n=8)). For rats with running wheel access, running wheel activity was computer monitored consecutively and recorded every 30 minutes for 30 days.
Blood and Tissue Collection
At the end of the experiment, rats were killed between 12:00 and 2:00 PM by decapitation under ether inhalation anesthesia. Brains were rapidly removed, submerged into iced isopentane for 25 seconds and immediately stored in dry ice. Fifty μl of trunk blood was collected for β-hydroxybutyrate analysis (Stat-site ketosis meter, GDS Diagnostics, Elkhart, IN), and the remaining trunk blood was collected into chilled K+EDTA vacutainer tubes, briefly placed in ice, and then centrifuged at 4°C for 15 m at 2000 rpm. Plasma was aspirated into eppendorf tubes. Blood and brains were stored at −80°C until processing. Epidydimal, subcutaneous, and retroperitoneal fat pads were also removed and weighed.
Radioimmunoassay
Radioimmunoassays (Millipore, St. Charles, MO) were used to determine baseline levels of plasma insulin and leptin. The rat insulin RIA kit had a sensitivity of 0.1 ng/ml. The intra- and interassay coefficients of variation (CV) were below 2.2% and 9.4%, respectively, and the correlation coefficient for the standard curve was 0.85. The rat leptin RIA sensitivity was 0.5 ng/ml. The intra- and interassay CV were below 2.4% and 5.7%, respectively, and the correlation coefficient for the standard curve was 0.94. Volumes of 100 μl of plasma were used in duplicate samples for each assay, as directed by the manufacturer.
In situ hybridization
Brains were coronally sectioned at 14 μm, mounted onto electrostatically charged Superfrost Plus slides (Fisher Scientific), and stored at −80°C. Brain slices were fixed with 4% paraformaldehyde and dehydrated with an ascending series of alcohols. Sections from each rat containing the arcuate nucleus of the hypothalamus were selected and stored at −80°C for future processing. Plasmids of NPY and POMC were linearized with the appropriate restriction enzymes. Antisense riboprobes were labeled with 35S-labeled UTP (Perkin Elmer), using in vitro transcription systems with T3 polymerase, according to protocols provided by the manufacturer (Promega). Probes were then purified using Quick Spin RNA columns (Roche Diagnostics).
For processing, slides were warmed and rinsed in triethylamine (TEA) buffer (pH 8.0) and TEA with acetic anhydride. Sections were incubated in hybridization buffer comprised of 50% formamide, 0.3 M NaCl, 10 mM Tris HCl (pH 8.0), 1 nM EDTA (pH 8.0), 1 × Denhardt’s solution (Eppendorf), 10% dextran sulfate, 10 mM DTT, 500 μg/mL yeast tRNA, and 108 cpm/μL 35S-UTP, and incubated overnight in a 56°C humid chamber. After hybridization, sections were washed three times in 2XSSC followed by one wash in 2XSSC + DTT at 56°C. Slides were then treated with 20 μg/mL RNase A (Sigma) in buffer containing 5 M NaCl, 0.5 M EDTA, 1 M Tris, pH 7.5 and ddH20. Sections were washed twice in 2XSSC + DTT, and then twice in 0.1XSSC + DTT, and dehydrated in an ascending series of alcohols. Slides were exposed to Kodak Biomax film for 2 days. Autoradiographic images were then scanned, and quantified with Scion Image software (National Institute of Health), utilizing autoradiographic 14C-microscales (Amersham Pharmacia Biotech) as a standard. An experimentally blinded observer performed scanning and quantification. Data for each animal were means of the product of hybridization area × density, with the background density subtracted from the three sections, reflecting the region-specific levels of gene expression. Data for each animal were normalized to CH-SED controls as 100%, and are expressed as mean ± standard error (SEM).
Data analyses
Data are represented as mean ± SEM. Caloric intake and body weight were first analyzed by 2-way ANOVA for repeated measures (diet × exercise over time), followed by planned t tests. Activity data were analyzed by repeated measures ANOVA, with time as the repeated measure, and further analyzed with post hoc Bonferroni comparisons when significant differences were found. Because terminal body weights were different between the sedentary and running groups, fat pads were analyzed as percentage of overall body weight for each depot measured. These values, and the RIA data were assessed by ANOVA and further analyzed by Tukey’s Multiple Comparison Test where appropriate. A value of P < 0.05 was considered significant.
Results
Caloric intake, body weight and activity levels
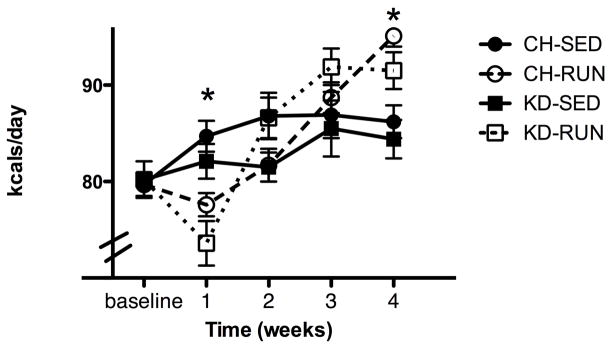
Prior to gaining access to the running wheels, there were no differences in daily mean caloric intake between rats maintained on CH and those maintained on KD (79.7 ± 1.2 and 80.2 ± 1.9 kcals/day, respectively). As depicted in Figure 1, access to a running wheel resulted in decreased caloric intake in CH-RUN as compared to CH-SED in the first week (CH-SED: 84.7 ±1.6 and CH-RUN: 77.6 ± 1.2 kcals/day (P <0.01)). Mean daily caloric intake during the second and third weeks of running wheel access did not differ between CH-RUN and CH-SED rats, however at 4 weeks CH-RUN rats consumed significantly more calories per day than did CH-SED rats (CH-SED: 86.2 ±1.7 and CH-RUN: 95.1 ±1.1 kcals/day (P <0.05)). Access to running wheels had a similar effect in KD-RUN, as compared to KD-SED. After one week of access to the wheels, KD-RUN rats consumed significantly fewer calories per day than KD-SED rats (KD-SED: 82.1 ±1.8 and KD-RUN: 73.6 ±2.3 kcals/day (P < 0.01)). This difference was abolished in the second week of running and KD-RUN rats consumed significantly more calories per day than KD-SED rats in the 4th week of access to the running wheels (KD-SED: 84.4 ± 2.0 and KD-RUN (P < 0.01)). There were no differences in mean daily caloric intake between CH-SED and KD-SED or CH-RUN and KD-RUN at any of the weekly intervals. Cumulative caloric intake is represented in Table 2. While differences in caloric intake were measured on a week-by-week basis with access to a running wheel, cumulatively there were no differences between groups.
Figure 1. Caloric intake in sedentary and exercising rats.
Rats were maintained on CH or KD. Half of each group was allowed access to a running wheel (CH-RUN and KD-RUN), whereas the other half remained sedentary (CH-SED and KD-SED). Average caloric intake (kcal/day) during each week of the experiment is given for sedentary (SED) and exercising (RUN) rats that were maintained on CH or KD. At baseline, all rats were sedentary. Weeks 1–4 represent mean caloric intake during each of the 4 weeks the exercising rats had access to running wheel. Data are means ± SEM (kcal/day). *P < 0.05 when sedentary rats were compared to exercising rats.
Table 2.
Effects of exercise and diet-type on cumulative caloric intake, body weight gain and adiposity in rats maintained on chow or a ketogenic diet. Values with differing symbols are significantly different from one another (P < 0.05).
| CH-SED | CH-RUN | KD-SED | KD-RUN | |
|---|---|---|---|---|
| Cumulative intake during experiment (pre- and post-wheel kcals) | 3069.1 ± 95.0 | 2940.6 ± 82.0 | 2876.4 ± 100.7 | 2967.1 ± 36.3 |
| Cumulative intake with wheel access (kcals) | 2496.8 ± 84.4 | 2401.1 ± 64.3 | 2308.5 ± 86.5 | 2432.9 ± 27.3 |
| Terminal body weight (g) | 455.03 ± 16.01 | 412.78 ± 10.98* | 442.73 ± 32.6 | 392.48 ± 15.43* |
| Body weight gain from start of experiment (g) | 86.6 ± 4.00 | 67.68 ± 6.70* | 88.27 ± 12.09 | 51.77 ±8.17* |
| Total fat pad weight (g) | 9.51 ± 1.11 | 6.93 ±0.96 | 16.66 ± 2.86* | 8.82 ± 0.51 |
| Total fat pad (% body weight) | 2.18 ± 0.26 | 1.66 ± 0.18 | 3.78 ± 0.46* | 2.26 ± 0.14 |
| Epidydimal fat (% body weight) | 0.76 ± 0.09 | 0.59 ± 0.07 | 1.23 ± 0.16* | 0.75 ± 0.06 |
| Retroperitoneal fat (% body weight) | 0.73 ± 0.09 | 0.57 ± 0.09 | 1.31 ± 0.17* | 0.84 ± 0.19 |
| Subcutaneous fat (% body weight) | 0.70 ± 0.08 | 0.49 ± 0.04* | 1.24 ± 0.16# | 0.67 ± 0.05 |
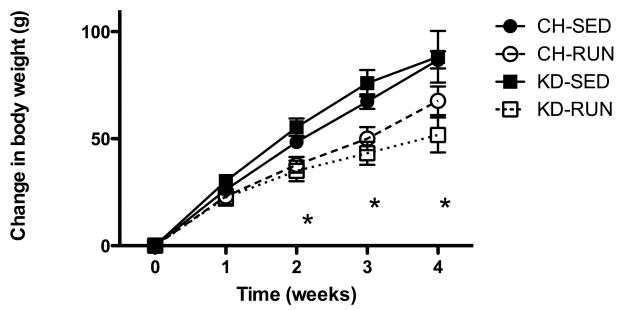
Access to running wheels resulted in decreased body weight gain in CH-RUN and KD-RUN rats, as compared to rats without access to wheels (Figure 2). After two weeks of access to the running wheels, CH-RUN rats had gained significantly less body weight than CH-SED rats (P < 0.05). This difference persisted for the duration of the experiment such that CH-RUN body weights were 24.2% less than those of CH-SED. Similar effects were measured in KD rats. Body weight gain in KD-RUN was significantly less than in KD-SED after two weeks of access to the wheels, (P <0.01), and this difference persisted throughout the experiment such that after one month, KD-RUN body weights were 30.8% less than KD-SED. The type of diet on which the rats were maintained had no effect on body weight change over time.
Figure 2. Effects of access to a running wheel on body weight in chow- or ketogenic diet-fed rats.
Body weights were also recorded daily for the duration of the experiment. After 2 weeks with access to a running wheel, rats on CH and rats on KD gained significantly less body weight than did sedentary controls. This difference persisted for the duration of the experiment. Data are means ± SEM body weight change (g) from the time access to the running wheel was allowed in the RUN groups. *P <0.05 when RUN rats were compared to SED rats on the same diet.
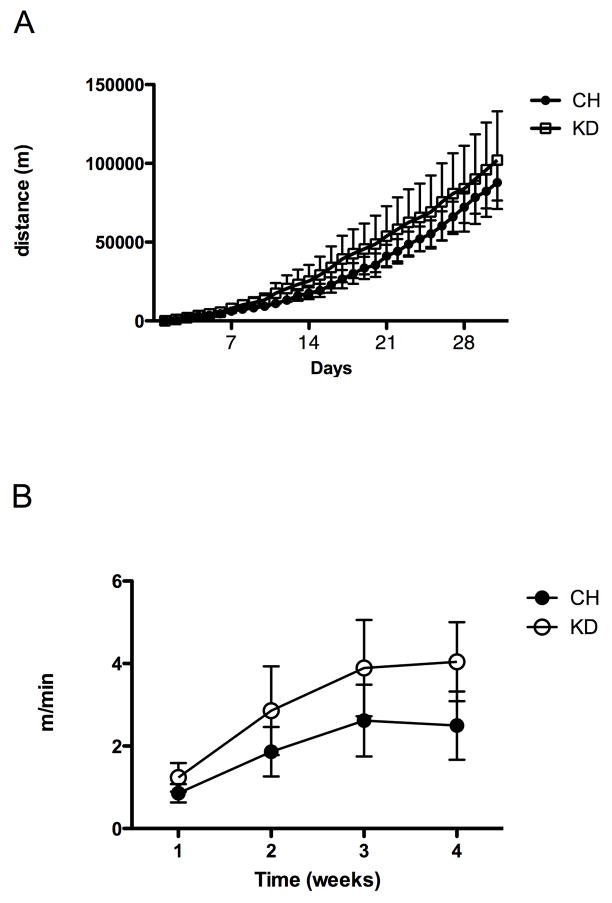
Activity levels were recorded every 30 minutes for the month during which rats had access to the running wheels. As depicted in Figures 3A and 3B, CH-RUN and KD-RUN rats had similar levels of activity (total distance (m)) and ran at similar rates (m/min) for the duration of the experiment.
Figure 3. Cumulative running wheel activity.
Rats on CH or KD were allowed continuous access to running wheels attached to their home cages. (A) Total distance (m) over time and (B) rate of running (m/min) are given as mean ± SEM for CH or KD. There were no statistically significant differences between dietary groups for either measure.
Plasma profiles and body composition
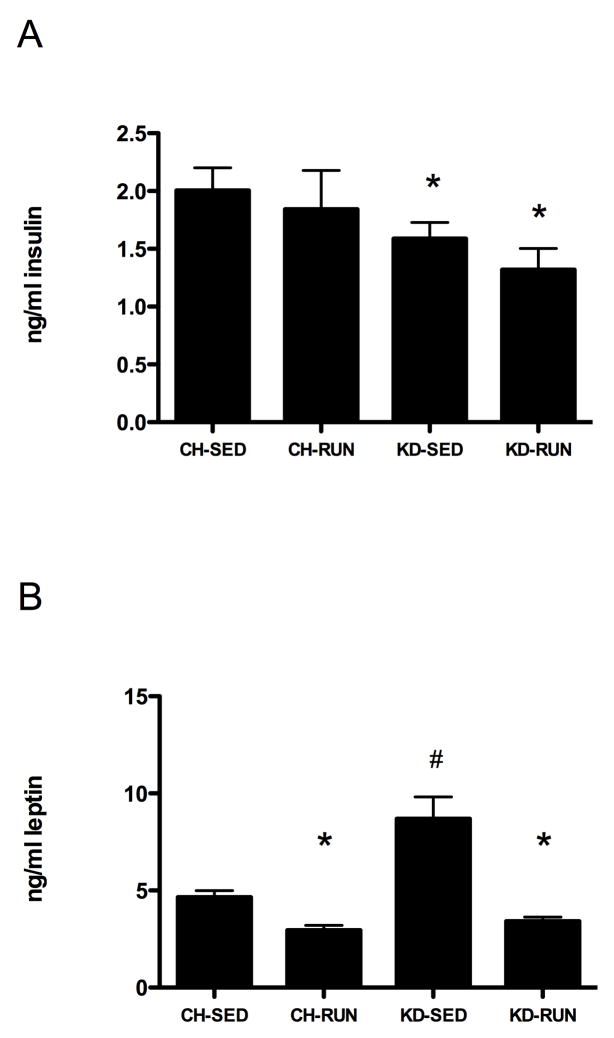
Maintenance on KD resulted in significantly increased levels of s-hydroxybutyrate, as compared to rats maintained on CH. β-hydroxybutyrate levels were elevated in both KD-SED (0.36 ± 0.07 mM) and KD-RUN (0.31 ± 0.04 mM), as compared to chow-fed rats (CH-SED: 0.18 ± 0.02 and CH-RUN: 0.16 ± 0.02 mM (P <0.01 for both groups, data not shown)). Rats maintained on KD (SED and RUN) had lower levels of plasma insulin than chow-fed rats (P < 0.05 in both cases, Figure 4A). There were no running wheel-related effects on insulin levels for either dietary group. Figure 4B demonstrates differences in plasma leptin levels. Access to the running wheels resulted in decreased plasma leptin in both CH-RUN and KD-RUN rats, as compared to their sedentary counterparts (P <0.05 in both cases). After 1 month of access to the running wheels, CH-RUN leptin levels were 36.6% less than CH-SED. The difference was much greater in KD rats. Plasma leptin was 60.8% lower in KD-RUN than in KD-SED. While the KD-SED rats had significantly higher levels of leptin than CH-SED, there were no diet-related differences after access to the wheels such that CH-RUN and KD-RUN had similar plasma leptin levels. In sedentary rats, maintenance on KD resulted in increased epidydimal fat, retroperitoneal and subcutaneous fat, as compared to maintenance on chow (Table 2, P < 0.05 in all cases). After 1 month of access to running wheels, CH-RUN and KD-RUN groups had levels of epidydimal and retroperitoneal fat that were statistically indistinguishable from CH-SED controls, whereas subcutaneous fat was significantly less in CH-RUN rats (P < 0.05). Overall, KD-RUN rats had 47.7% less body fat than KD-SED (P < 0.05) after 1 month of access to the running wheels, and CH-RUN had 27.2% less total body fat than CH-SED.
Figure 4. Plasma analyses of sedentary and exercising rats.
After 1 month during which rats on CH or KD were sedentary or allowed access to running wheels, plasma was analyzed by RIA for levels of (A) insulin and (B) leptin. Data are given as mean ± SEM and bars with different symbols are significantly different from one another (P <0.05).
Hypothalamic gene expression
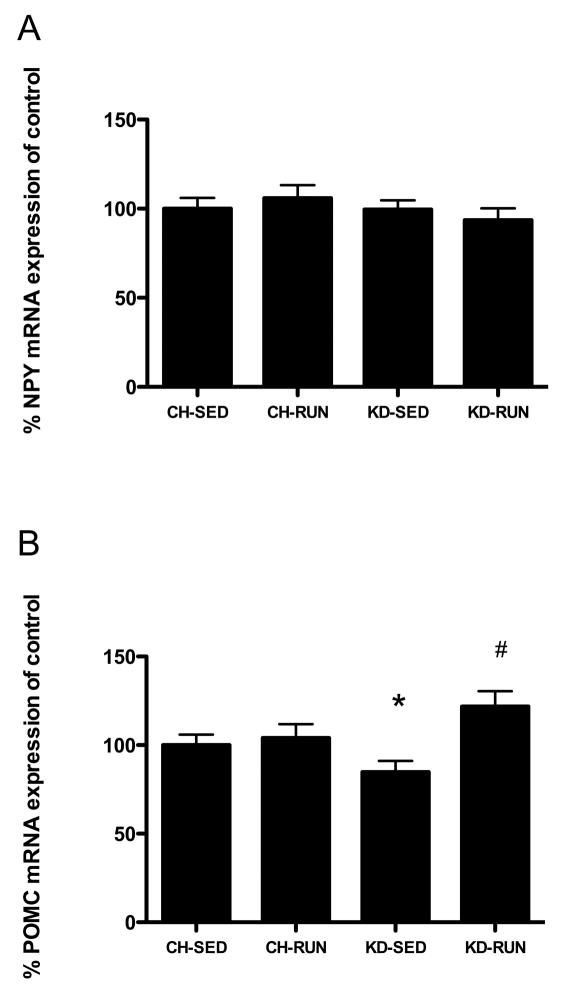
In the arcuate nucleus of the hypothalamus, there were no differences in expression levels of mRNA for NPY between any of the dietary or exercise groups (Figure 5A). As for POMC, there was no effect of exercise in the CH-fed animals such that CH-SED and CH-RUN were not different from one another. Maintenance on KD, however, did affect levels of POMC mRNA expression. POMC mRNA expression was significantly lower in KD-SED, as compared to CH-SED (P <0.05), and was significantly greater in KD-RUN (P <0.05, Figure 5B).
Figure 5. Hypothalamic neuropeptide mRNA expression in exercising and sedentary rats maintained on chow or a ketogenic diet.
Neuropeptide mRNA expression levels were assessed by in situ hybridization for arcuate nucleus (A) NPY and (B) POMC. Data are means ± SEM and *P < 0.05 as compared to CH-SED.
Discussion
Studies in human subjects maintained on low carbohydrate diets suggest an association between these types of diets and a reduction in voluntary physical activity. In the current studies, we demonstrate that maintenance on a ketogenic diet does not result in decreased voluntary activity when rats were given access to a running wheel, as compared to activity levels of rats maintained on a non-ketogenic, chow diet. Running wheel access resulted in similar effects on body weight, caloric intake and activity levels in rats maintained on CH or KD. Interestingly, exercise in rats maintained on KD had a significant effect on hypothalamic POMC mRNA expression levels that was not observed in exercising rats maintained on chow.
In the sedentary groups, rats on KD consumed the same number of calories per day as CH controls. This finding is consistent with our previous work, in which rats maintained on a ketogenic diet had similar rates of weight gain and caloric intake as did chow-fed rats (24). Previous research has demonstrated that rats maintained on CH and given voluntary access to running wheels decrease food intake and body weight gain as compared to sedentary controls over a 7- to 10-day period (25–27) and that over a 4 week period, rats with access to a running wheel gain significantly less than sedentary rats (28). Whereas low-carbohydrate and ketogenic diets have been associated with increased fatigue in response to exercise and reductions in free- living physical activity in human subjects (16, 29, 30), our current results demonstrate that access to a running wheel induced changes in caloric intake and body weight, regardless of diet-type. After one week of access to a running wheel, both CH and KD rats had a significant decrease in daily caloric intake. This deficit was attenuated during the second week, and rats in both groups consumed more calories per day than did sedentary controls by the 4th week. Differences in body weight were evident after two weeks of wheel running. Despite increased caloric intake in the 4th week, body weight remained significantly lower in CH-and KD-fed rats with access to wheels, as compared to their sedentary counterparts. This is likely due to the similarities in cumulative caloric intake over the course of the experiment, which demonstrates that overall, the reduced body weight in CH-RUN and KD-RUN is associated with increased physical activity.
Several lines of evidence indicate that chronic consumption of diets high in fat contributes to obesity. Body fat storage occurs at a greater rate when excess energy comes from fat than when it comes from carbohydrate or protein (19, 20). Animals maintained on high fat diets increase energy intake, increase efficiency of body fat gain, and become obese (23). Cross-sectional studies in humans have demonstrated that the concentration of dietary fat is correlated with increased body weight and body mass index (18, 21, 22). We have previously reported that rats on KD have increased adiposity despite no difference in body weight as compared to CH-fed rats (24). With the exception of subcutaneous fat, chow-and KD rats with access to running wheels had similar levels of body fat, as compared to sedentary, chow-fed rats. These data demonstrate that body fat deposition is reduced to the same degree in chow and KD, regardless of the increased dietary fat and propensity for fat storage in KD rats.
We have previously reported ketogenic diet-related effects on the central melanocortin system, including decreased POMC mRNA expression, increased sensitivity to the anorexigenic effects of agonism of the MC3/4R by central administration of MTII, and increased expression levels of the MC4R (24). In the current experiments, we replicate and extend findings on the effects of KD on the hypothalamic melanocortin system. In sedentary rats fed KD, there was less POMC mRNA expression, however after 4 weeks of voluntary running POMC was increased, as compared to sedentary chow and sedentary KD rats. Overall, there are numerous factors that influence central neuropeptide systems involved in the regulation of energy that have not been investigated in animals maintained on a ketogenic diet. Our current experiments allow for speculation about how exercise-induced changes in leptin and caloric intake may affect hypothalamic POMC mRNA expression levels in rats maintained on KD.
It is known that leptin receptors are expressed on POMC neurons, and leptin administration stimulates POMC mRNA expression in CH-fed animals (31, 32). Additionally, increased POMC expression has been demonstrated in cases of overfeeding and decreased voluntary caloric intake (33). This relationship is inconsistent with both sedentary KD, in which leptin levels are high and POMC expression is reduced, and active KD rats in which leptin levels are reduced and POMC expression is elevated. These data suggest the possibility of a diet-related disconnect in the relationship between peripheral leptin and the central melanocortin system, as it has been characterized in animals maintained on non-ketogenic diets. Whether or not this is the case is currently unknown and admittedly speculative, and POMC responsivity to circulating leptin in animals maintained on a ketogenic diet requires further investigation.
A second possible explanation for increased POMC expression in KD-RUN is that these rats consumed more kcals per day than did sedentary controls on the same diet. Increased POMC mRNA expression may be a reflection of this increased caloric intake, as rats that are overfed display increased POMC mRNA expression (33). However, the pattern of intake, in terms of total kcals per day, was the same in CH-RUN and KD-RUN, yet there was no increase in POMC mRNA in CH-RUN. It is possible that this is due to differential sensitivity to increased caloric intake in rats consuming KD, as compared to those consuming CH, at the level of arcuate POMC neurons.
In conclusion, maintenance on a ketogenic diet does not inhibit voluntary activity on a running wheel, such that there are similar levels of activity in rats maintained on a ketogenic diet or chow. Whereas maintenance on a ketogenic diet leads to significantly increased adiposity in the rat, it is prevented with levels of exercise similar to those of chow-fed rats. The present results also indicate that voluntary exercise results in a significant increase in POMC expression in rats consuming a ketogenic diet, and highlight diet-related effects on the central melanocortin system that warrant attention for future study.
Acknowledgments
This research was supported by NIH grant DK078654 (KPK). The authors wish to thank Sara Hargrave, Mary Ann Honors, and Melissa McCurley for technical assistance.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. Jama. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 2.Serdula MK, Mokdad AH, Williamson DF, Galuska DA, Mendlein JM, Heath GW. Prevalence of attempting weight loss and strategies for controlling weight. Jama. 1999;282:1353–8. doi: 10.1001/jama.282.14.1353. [DOI] [PubMed] [Google Scholar]
- 3.Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–93. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 4.Atkins RC, Ornish D, Wadden T. Low-carb, low-fat diet gurus face off. Interview by Joan Stephenson. Jama. 2003;289:1767–8. 73. doi: 10.1001/jama.289.14.1767. [DOI] [PubMed] [Google Scholar]
- 5.Atkins RC. Avon Books. New York: 1992. Atkins’ New Diet Revolution. [Google Scholar]
- 6.Larosa JC, Fry AG, Muesing R, Rosing DR. Effects of high-protein, low-carbohydrate dieting on plasma lipoproteins and body weight. J Am Diet Assoc. 1980;77:264–70. [PubMed] [Google Scholar]
- 7.Westman EC, Yancy WS, Edman JS, Tomlin KF, Perkins CE. Effect of 6-month adherence to a very low carbohydrate diet program. Am J Med. 2002;113:30–6. doi: 10.1016/s0002-9343(02)01129-4. [DOI] [PubMed] [Google Scholar]
- 8.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–23. doi: 10.1210/jc.2002-021480. [DOI] [PubMed] [Google Scholar]
- 9.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–77. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- 10.McAuley KA, Hopkins CM, Smith KJ, et al. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia. 2005;48:8–16. doi: 10.1007/s00125-004-1603-4. [DOI] [PubMed] [Google Scholar]
- 11.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 12.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. Jama. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66:239–46. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 14.Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S547–52. doi: 10.1097/00005768-199911001-00010. [DOI] [PubMed] [Google Scholar]
- 15.Keith RE, O’Keeffe KA, Blessing DL, Wilson GD. Alterations in dietary carbohydrate, protein, and fat intake and mood state in trained female cyclists. Med Sci Sports Exerc. 1991;23:212–6. [PubMed] [Google Scholar]
- 16.White AM, Johnston CS, Swan PD, Tjonn SL, Sears B. Blood ketones are directly related to fatigue and perceived effort during exercise in overweight adults adhering to low-carbohydrate diets for weight loss: a pilot study. J Am Diet Assoc. 2007;107:1792–6. doi: 10.1016/j.jada.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Atkins R. Avon Books. New York: 1992. Atkins’ New Diet Revolution. [Google Scholar]
- 18.George V, Tremblay A, Despres JP, Leblanc C, Bouchard C. Effect of dietary fat content on total and regional adiposity in men and women. Int J Obes. 1990;14:1085–94. [PubMed] [Google Scholar]
- 19.Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. 1995;62:19–29. doi: 10.1093/ajcn/62.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Lean ME, James WP. Metabolic effects of isoenergetic nutrient exchange over 24 hours in relation to obesity in women. Int J Obes. 1988;12:15–27. [PubMed] [Google Scholar]
- 21.Lissner L, Heitmann BL. Dietary fat and obesity: evidence from epidemiology. Eur J Clin Nutr. 1995;49:79–90. [PubMed] [Google Scholar]
- 22.Macdiarmid JI, Cade JE, Blundell JE. High and low fat consumers, their macronutrient intake and body mass index: further analysis of the National Diet and Nutrition Survey of British Adults. Eur J Clin Nutr. 1996;50:505–12. [PubMed] [Google Scholar]
- 23.Woods SC, D’Alessio DA, Tso P, et al. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83:573–8. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 24.Kinzig KP, Scott KA, Hyun J, Bi S, Moran TH. Altered hypothalamic signaling and responses to food deprivation in rats fed a low-carbohydrate diet. Obes Res. 2005;13:1672–82. doi: 10.1038/oby.2005.205. [DOI] [PubMed] [Google Scholar]
- 25.Levitsky DA. Feeding patterns of rats in response to fasts and changes in environmental conditions. Physiol Behav. 1970;5:291–300. doi: 10.1016/0031-9384(70)90101-0. [DOI] [PubMed] [Google Scholar]
- 26.Looy H, Eikelboom R. Wheel running, food intake, and body weight in male rats. Physiol Behav. 1989;45:403–5. doi: 10.1016/0031-9384(89)90147-9. [DOI] [PubMed] [Google Scholar]
- 27.Mueller DT, Loft A, Eikelboom R. Alternate-day wheel access: effects on feeding, body weight, and running. Physiol Behav. 1997;62:905–8. doi: 10.1016/s0031-9384(97)00266-7. [DOI] [PubMed] [Google Scholar]
- 28.Levin BE, Dunn-Meynell AA. Differential effects of exercise on body weight gain and adiposity in obesity-prone and -resistant rats. Int J Obes (Lond) 2006;30:722–7. doi: 10.1038/sj.ijo.0803192. [DOI] [PubMed] [Google Scholar]
- 29.Keith RE, O’Keeffe KA, Alt LA, Young KL. Dietary status of trained female cyclists. J Am Diet Assoc. 1989;89:1620–3. [PubMed] [Google Scholar]
- 30.Butki BD, Baumstark J, Driver S. Effects of a carbohydrate-restricted diet on affective responses to acute exercise among physically active participants. Percept Mot Skills. 2003;96:607–15. doi: 10.2466/pms.2003.96.2.607. [DOI] [PubMed] [Google Scholar]
- 31.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–92. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MW, Seeley RJ, Woods SC, et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–23. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 33.Hagan MM, Rushing PA, Schwartz MW, et al. Role of the CNS melanocortin system in the response to overfeeding. J Neurosci. 1999;19:2362–7. doi: 10.1523/JNEUROSCI.19-06-02362.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]