Abstract
Objective
To assess the course of modified Rodnan skin score (MRSS) in patients with diffuse cutaneous systemic sclerosis (dcSSc) with different baseline disease durations (defined from the date of onset of first non-Raynaud’s phenomenon symptom) in 3 large randomized controlled trials (RCT).
Methods
Data from RCTs were pooled and analyzed: high dose versus low dose D-Penicillamine (D-Pen), recombinant human relaxin vs. placebo (relaxin), and oral bovine type I collagen vs. placebo (collagen) studies. Patients were divided into 5 groups according to their disease durations at baseline. The linear mixed model for correlated data was used to model the two predictors of MRSS: Time (in months) and disease duration.
Results
At entry, mean MRSS score was 21.0 units in the D-Pen, 27.3 units in the relaxin, and 26.1 units in the collagen studies. Time in study was a significant predictor of improvement of MRSS regardless of disease duration at baseline (P-value: < 0.0001). Patients with disease duration ≥ 2 years showed a greater rate of decline compared to patients with < 2 years (P-values: < 0.05). Similar results were obtained when disease duration was reclassified by including Raynaud’s phenomenon in the definition.
Conclusion
Our study confirms recent findings that patients entered in the clinical trials do not follow the same trend in natural history of skin thickening as seen in the dcSSc populations previously reported in early open longitudinal studies. These findings have important implications in study design where “prevention of worsening” is the main objective.
Keywords: Natural history, scleroderma, systemic sclerosis, clinical trials, modified Rodnan skin score
Introduction
Systemic sclerosis (SSc) is a connective tissue disorder characterized by fibrosis of the skin and it may involve the heart, lungs, kidneys, and gastrointestinal tract(1). SSc is divided into two major categories (diffuse cutaneous SSc vs. limited cutaneous SSc) based on the extent of skin involvement(2). Diffuse cutaneous systemic sclerosis (dcSSc), which can be associated with significant morbidity and mortality, is a subset of SSc in which more rapid change in skin involvement occurs, making it more feasible to study in relatively short clinical trials. It is for this reason that dcSSc has been the focus of investigation in many clinical trials(2;3). Measurement of skin thickness is used as surrogate measure of disease severity and mortality in patients with dcSSc— an increase in skin thickening is associated with involvement of internal organs and increased mortality(2). In addition, an improvement in skin score is associated with a more favorable outcome(4). Currently the best way to identify high-risk patients who are more likely to be recruited into clinical trials is by the severity and extent of skin involvement(5). The modified Rodnan skin score (MRSS), a measure of skin thickness has been used as the primary outcome measure in most of these trials, as it is feasible, reliable, valid, and responsive to change in multicenter clinical trials(6).
The natural history of dcSSc is well known. It is generally accepted that MRSS tends to worsen in early disease and improve in late disease, although time of peak involvement remains poorly defined. “Early” dcSSc is often defined as the rapid and severe increasing induration (“thickening”) of the skin, which has, until recently(7) been thought to peak at 1–3 years after disease onset(8). Late stage is defined based on lessening of cutaneous induration (perceived as “softening”), which occurs after skin induration has reached its peak in extent and severity(1). The natural history of skin involvement can be used by physicians to determine the probabilities of different events occurring in particular patients(9;10).
Based on the natural history (10;11) and previous experiences from randomized controlled trials (RCTs)(12), the American College of Rheumatology Committee on Design and Outcomes in Clinical Trials in Systemic Sclerosis(5) recommended that dcSSc patients with early disease be recruited into trials, as these patients are at the greatest risk for internal organ damage and mortality; patients with disease duration < 2 years from first scleroderma-related symptom (judged by the physician) were considered suitable for drug efficacy trials. In a recent small RCT of patients with early dcSSc (mean disease duration of 7.6 months; n= 45), average MRSS scores improved after entry into the clinical trial and did not follow the expected natural history course(13). Analysis of patients with early dcSSc cohort from the Royal Free Hospital showed decline in median MRSS over the first 5 years after disease onset(14). However, in-depth analyses have not been performed to assess the course of MRSS in large dcSSc RCTs.
In the present study we examined the course of MRSS in 3 double-blind, RCTs of patients with dcSSc. Study 1 examined the effects of high dose versus low dose D-Penicillamine(15) (D-Pen study), study 2 investigated the effects of recombinant human relaxin (relaxin study)(16) and study 3 assessed the efficacy of oral bovine type I collagen (collagen study)(17). The aim of this study was: 1) assess the course of MRSS scores in dcSSc patients with different baseline disease durations defined from the date of onset of first symptom characteristic of dcSSc (other than Raynaud’s phenomenon) and 2) assess the course of MRSS scores when the disease duration was reclassified by including the first symptom related to scleroderma (including Raynaud’s phenomenon in the disease duration).
Patients and Methods
Patients and data
We used data from 3 different double-blind RCTs in patients with dcSSc.
D-Penicillamine
This trial was a prospective, controlled study examining the efficacy of high-dose versus low dose D-Pen(15). Previous open label studies had shown an improvement in MRSS when treated with D-Pen(18;19). One hundred and thirty four patients with dcSSc who had a disease duration ≤ 18 months from the onset of the first SSc manifestation (other than Raynaud’s phenomenon) participated in the study and 68 completed the study; 25% of the observations had missing MRSS data during the course of the study Patients were given either the conventional (high-dose: 750–100 mg daily) or a low dosage of D-Pen (125 mg every other day) for 24 months. As part of the study, skin scores were obtained by evaluating the patients’ degree and extent of skin thickening at baseline and every 6 months thereafter for the duration of the study. In this study, there were no statistically significant differences in the three primary outcomes (change in the skin score, incidence of renal crisis, and survival) between treatment groups at baseline and 2 years(15).
Recombinant human relaxin
Subjects with dcSSc were enrolled in this clinical trial evaluating the safety, efficacy and dose-response effect of continuous subcutaneously infused recombinant human relaxin(16). All participants had SSc disease duration of ≤ 5 years since the onset of the first sign or symptom of SSc other than Raynaud’s phenomenon. Other inclusion criteria included moderately severe skin disease with a skin score of ≥ 20 or ≥ 16 if truncal skin was involved. Patients were randomized to receive either relaxin (25 µg/kg/day, 10 µg/kg/day) or placebo in a 2:1:2 ratio for 24 weeks. Two-hundred and thirty one patients were randomized and 195 (84%) patients completed the 24-week study. MRSS data was available for 5 additional patients who returned for their last study visit; 12% of observations had missing MRSS during course of the study. MRSS was obtained at baseline, 4, 12 and 24 weeks. Analysis of the trial results revealed that there was no statistically significant difference in the change in the skin score and functional disability between groups who received recombinant human relaxin and those who received placebo.
Oral bovine type I collagen
This trial randomized 168 dcSSc patients with baseline disease duration of up to 10 years to receive 12 months of oral bovine type I collagen (500 ug/day) or placebo(17). One hundred and twenty four patients (74%) had MRSS data available at 12 month; 15% of observations had missing MRSS during course of the study.. MRSS was obtained at 4, 8 and 12 month follow-up visits. The results showed no statistically significant difference in the change in the skin score at month 12 in the two groups.
Measure
In all 3 clinical trials, skin thickness was evaluated using the MRSS(20). Skin thickening was assessed by palpation of the skin in 17 areas of the body (fingers, hands, forearms, arms, feet, legs and thighs, face, chest and abdomen) using a 0–3 scale, where 0=normal, 1=mild thickness, 2= moderate thickness, and 3= severe thickness. Total skin score can range from 0 (no thickening) to 51 (severe thickening in all 17 areas).
Statistical Analysis
For the purpose of the present study, data from the 3 RCTs were pooled and analyzed irrespective of treatment assignments because none of the active therapies differed from placebo group in the primary and secondary outcomes. In the pooled dataset, 17% of observations had missing MRSS during course of the study. After the data were pooled, patients were divided into 5 groups according to their disease duration at baseline: group 1 (< 6 months), group 2 (6-<12 months), group 3 (12-<24 months), group 4 (24-<48 months) and group 5 (≥ 48 months). Disease duration was calculated from the date of onset of first symptom characteristic of dcSSc (other than Raynaud’s phenomenon). Box-and-whisker plots were used to illustrate the trend in MRSS decline in different disease duration categories by plotting the median MRSS at different scheduled visits. MRSS was obtained during 4 follow-up visits in the relaxin and collagen studies, while the D-Pen study included a fifth visit. The visits were conducted at the following intervals during the course of each study: D-Pen: baseline, 6, 12, 18 and 24 months; in the relaxin study, the visits were conducted at baseline, 1, 3 and 6 months; and in the collagen study the visits were conducted at baseline, 4, 8 and 12 months.
MRSS was modeled as a continuous variable with baseline disease duration and time in study (expressed in months after baseline) as covariates. Interaction terms between disease duration category and time in study were introduced in order to allow the rate of MRSS change to vary between disease duration groups. To account for the within-subject correlation of skin score over multiple visits, a mixed effects model with random intercept for subject was used. Mixed effects models allow all data points to be included in the analysis and are appropriate to use when data are missing at random and even remain relatively robust to data that are not missing at random.
We also analyzed the absolute change in MRSS in all 3 RCTs using one-way ANOVAs. For the D-Pen study, we assessed the MRSS change from baseline to 12 months and from 12 to 24 months; the change in MRSS was assessed from baseline to 6 months and from baseline to 12 months in the relaxin and collagen studies, respectively. Proportions of patients with an overall MRSS improvement and worsening were calculated in each study and in the pooled data. Based on a previous analysis of the D-Pen study(21), improvement and worsening in the MRSS was defined as a change of ≥ 5.3 points (minimum clinically important differences or MCID) in MRSS and analyzed in patients who completed the 3 RCTs .
We conducted 3 additional analyses: 1) disease duration was reclassified by including Raynaud’s phenomenon as part of disease duration in the D-Pen and relaxin studies (the collagen study did not capture the onset of Raynaud’s phenomenon), 2) course of MRSS was assessed in the placebo groups in the relaxin and collagen studies; D-Pen study compared the effects of low dose vs. high dose D-Pen and therefore was not included in the placebo group analysis, and 3) Since MRSS data was missing in 17% of observations during course of the trials in all 3 studies, we performed multiple imputation on the data. Multiple imputation uses a regression-type approach to estimate each missing datum. Imputed values are generated taking into account responses from the same participant on other correlated variables and responses to the same domain from participants who responded similarly. The three multiply-imputed datasets were then pooled and the pooled multiply-imputed dataset was analyzed using the mixed-effects regression model presented earlier.
All computations were achieved using the statistical SAS System Release 8.2 (SAS Institute Inc., Cary, NC, USA) and STATA 9.2 (College Station, TX, USA) software package.
Results
Baseline Characteristics
Detailed baseline characteristics have previously been published for all three trials(15;17;22–24). In all 3 studies, the majority of participants were female and Caucasian (Table 1). Mean (SD) age in the D-Pen, relaxin and collagen studies was 43.7 (12.4), 47.3 (10.3) and 50.8 (12.2) years, respectively. The mean (SD) MRSS scores in the D-Pen, relaxin and collagen studies were 21.0 (8.0) units, 27.3 (6.9) units and 26.1 (7.8) units, respectively. All 3 studies recruited patients with moderate functional disability as assessed by Health Assessment Questionnaire-Disability Index (HAQ-DI)score of ≥ 1.0(25).
Table 1.
Baseline Characteristics in the D-Penicillamine (D-Pen), recombinant human relaxin (relaxin) and oral bovine type I collagen (collagen) studies.
| Variable | D-Pen (n= 134) |
Relaxin (n=231) |
Collagen (n=168) |
|---|---|---|---|
| Age (years), mean± SD† | 43.7 ± 12.4 | 47.3 ± 10.3 | 50.8 ± 12.2 |
| Female (%) | 77.6 | 85.2 | 79.2 |
| Ethnicity: | |||
| Caucasian (%) | 67.9 | 74.0 | 76.2 |
| African American (%) | 19.4 | 13.3 | 16.1 |
| Other (%) | 12.7 | 12.8 | 7.7 |
| Disease Duration (months), mean± SD † | 9.5 ± 4.1 | 26.4 ± 16.4 | 41.8 ± 31.9 |
| Modified Rodnan Skin Score (MRSS, 0–51), mean± SD *† | 21.0 ± 8.0 | 27.3 ± 6.9 | 26.1 ± 7.8 |
| Health Assessment Questionnaire-Disability Index (HAQ-DI, 0–3), mean± SD **† | 1.04 ± 0.67 | 1.18 ± 0.71 | 1.22 ± 0.72 |
Age, disease duration and MRSS were significantly different among the three studies (P-values: < .0001), whereas HAQ-DI scores were not (P-value: 0.07).
MRSS of 0 represents no skin thickening; MRSS of 51 represents severe skin thickening involving all 17 areas of the body.
HAQ-DI score of 0 represents no disability and HAQ-DI score of 3 represents severe disability.
Mean baseline age, disease duration and MRSS were significantly different among the 3 studies (P-values < .0001), whereas baseline HAQ-DI scores were not (P-value: 0.07).
MRSS
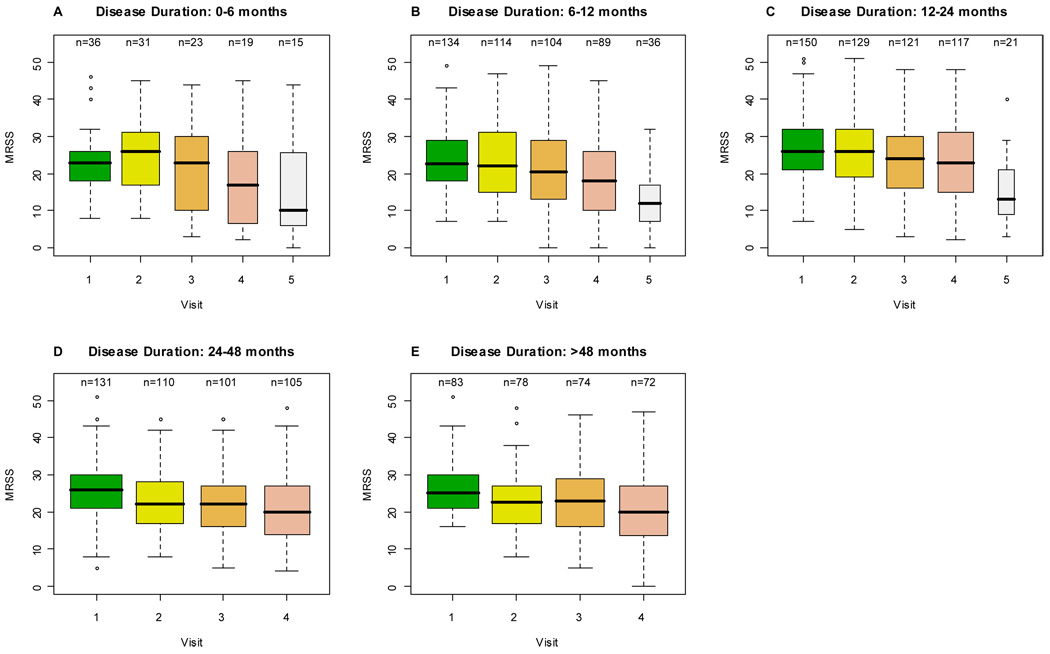
On average, MRSS improved in all 3 RCTs. Figure (A–E) illustrates the decline in MRSS over the course of the studies in all disease duration categories. Median MRSS declined in all 5 disease duration groups with time (Figure and Table 2). Patients with disease duration of < 6 months had a small but significant increase in the median MRSS scores from visit 1 to visit 2 (mean score 22.3 to 24.6; P-value: 0.03), followed by a decrease in MRSS thereafter. No other disease duration category showed an increase in their median MRSS. Analysis of individual RCTs showed a similar pattern of decline in MRSS scores (data not shown). Table 2 describes the results of the linear mixed effects model. Time in study (in months) was a significant predictor of decline in MRSS (P-value: <0.0001). In other words, irrespective of disease duration, MRSS declined significantly during the course of the trials. Overall, the rate of decline of MRSS in the 6-<12 month and 12-<24 month disease duration groups was similar to the reference group of < 6 months (P-values: 0.33 and 0.83 respectively), while the 24-<48 month and ≥ 48 month groups declined at a more rapid rate (P-values: 0.007 and 0.006 respectively) when compared to the reference group (< 6 month).
Figure 1.
Mean course of the modified Rodnan skin score across different disease duration categories. The rectangle shows the interquartile range (IQR); it goes from the first quartile (the 25th percentile) to the third quartile (the 75th percentile). The whiskers go from the minimum value to the maximum value unless the distance from the minimum value to the first quartile is more than 1.5 times the IQR. In that case the whisker extends out to the smallest value within 1.5 times the IQR from the first quartile. A similar rule is used for values larger than 1.5 times IQR from the third quartile. A special symbol shows the values, called outliers, which are smaller or larger than the whiskers.
Table 2.
Linear Mixed modeling of disease duration and time as “predictors” of MRSS in he D- Penicillamine (D-Pen), recombinant human relaxin (relaxin) and oral bovine type I collagen (collagen) studies. The table shows that time in study was a significant predictor of change in skin score and patients with longer disease duration (≥24 months) show greater decline in skin score compared to short duration patients.
| Unadjusted Regression Coefficient* | SE | P-Value | ||
|---|---|---|---|---|
| Overall (n=534) | Time (Months after baseline) | −0.249 | 0.055 | < 0.0001 |
| Baseline Disease Duration Category (months) † | 6-<12 | −0.061 | 0.063 | 0.332 |
| 12-<24 | 0.014 | 0.066 | 0.834 | |
| 24-<48 | −0.229 | 0.084 | 0.007 | |
| ≥ 48 | −.232 | 0.084 | 0.006 | |
Coefficients for disease duration are from interaction terms and represent the additional rate of change for each group beyond that given by the time covariate.
Reference group is disease duration of < 6 months. Disease duration was calculated from the onset of first non-Raynaud’s phenomenon.
We also assessed the absolute overall and between groups changes in MRSS in all 3 RCTs (Table 3). In the D-Pen study, the MRSS decline was similar among the three subgroups (based on baseline disease duration) within the study (P-value: > 0.05). In the relaxin and collagen trials, the skin score declined by 4.83 (6.99) over the 6-month trial and by 3.40 (7.12) over the 12 month trial, respectively. The decline was greater in the groups with disease duration ≥ 2 years compared to groups with disease duration < 2 years in the relaxin and collagen trials (P-value: <0.05).
Table 3.
Overall and between group assessments of MRSS change in the D-Penicillamine (D-Pen), recombinant human relaxin (relaxin) and oral bovine type I collagen (collagen) studies.
| Baseline Disease Duration Category | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | <6 months* | 6–12 Months* | 13–18 Months* | -- | Global F-test for equality of Means | Early vs. Late Disease†† | |||||||
| MRSS Change #† | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |||
| 0–12 month | 98 | −2.47 (8.60) | 17 | −2.18 (9.65) | 55 | −2.82 (8.82) | 26 | −1.92 (7.70) | -- | --- | 0.9 | NA‡ | |
| 12–24 month | 71 | −2.76 (4.88) | 14 | −2.07 (3.93) | 36 | −3.28 (4.19) | 21 | −2.33 (6.45) | -- | --- | 0.66 | NA‡ | |
| Baseline Disease Duration Category | |||||||||||||
| < 12 Months** | 12–24 Months** | 24–48 Months** | > 48 Months** | ||||||||||
| 0–6 month | 200 | −4.83 (6.99) | 39 | −2.69 (8.14) | 65 | −4.09 (6.94) | 69 | −6.13 (6.41) | 27 | −6.33 (5.96) | 0.04 | 0.007 | |
| 0–12 month | 124 | −3.40 (7.12) | 14 | −1.07 (10.93) | 29 | −1.45 (7.39) | 36 | −3.17 (5.80) | 45 | −5.58 (5.99) | 0.05 | 0.03 | |
MRSS changes are computed for the total population in each study and for different subgroups (based on baseline disease duration) within each study.
Early disease was defined as having baseline disease duration < 18 months in the D-pen and < 24 months in the relaxin and collagen studies. Late disease was defined as having baseline disease durations of 24–48 and > 48 months in the relaxin and collagen studies.
NA= Not applicable.
Baseline disease duration subgroups in the D-Pen study.
Baseline disease duration subgroups in the relaxin and collagen studies.
Although, on average, the MRSS score improved over time (Table 2), there were individual patients who had no improvement or even worsening of their MRSS scores (Figure and Table 4). At an individual level, 44% of patients showed improvements (a decrease of ≥ 5.3 points) in their MRSS, while 8% had worsening of the skin (an increase of > 5.3 points) in the pooled analysis (Table 4).
Table 4.
Proportion of patients with MRSS improvement and worsening (MCID ≥ 5.3 units) from baseline to last visit.
| Improved (≥ 5.3 units)* | Worsened (≥ 5.3 units)** | ||||
|---|---|---|---|---|---|
| N (Total) | N | Percentage | N | Percentage | |
| All subjects | 396 | 176 | 44 | 30 | 8 |
| D-Pen | 72 | 34 | 47 | 7 | 10 |
| Relaxin | 200 | 95 | 48 | 14 | 7 |
| Collagen | 124 | 47 | 38 | 9 | 7 |
Improvement is defined as a decline in MRSS of ≥ 5.3 units between baseline and last study visit.
Worsening is defined as increase in MRSS of ≥5.3 units between baseline and last study visit.
Results were similar when onset of first Raynaud’s phenomenon was used as part of the definition of disease duration in the D-Pen and relaxin trials (Appendix Table 1). In addition, similar results were obtained when analysis was repeated for patients in placebo groups in the relaxin and collagen studies (decline in MRSS for patients with ≥ 2 years of baseline disease duration was significant and greater than that of groups with < 2 years of baseline disease duration: P-values: < 0.05). In addition, analysis of the imputed data showed that time was still a significant predictor of MRSS, suggesting that all disease duration groups show a statistically significant rate of decline (Appendix Table 2).
Appendix Table 1.
Sensitivity analysis (disease duration defined using onset of first symptom including Raynaud’s symptom). Linear Mixed modeling of disease duration and time as “predictors” of MRSS in the D- Penicillamine (D-Pen) and recombinant human relaxin (relaxin) studies.
| Unadjusted Regression Coefficient* | SE | P-Value | ||
|---|---|---|---|---|
| Overall (N=290) | Time (Months after baseline) | −0.21 | 0.07 | 0.003 |
| Baseline Disease Duration Category (months) † | 6-<12 | −0.10 | 0. 08 | 0.2 |
| 12-<24 | −0.08 | 0.08 | 0.3 | |
| 24-<48 | −0.31 | 0.09 | 0.002 | |
| ≥ 48 | −0.26 | 0.10 | 0.01 | |
Coefficients for disease duration are from interaction terms and represent the additional rate of change for each group beyond that given by the time covariate.
Reference disease duration was < 6 months.
Appendix Table 2.
Sensitivity analysis based on imputed data. Linear Mixed modeling of disease duration and time as “predictors” of MRSS in the D- Penicillamine (D-Pen), recombinant human relaxin (relaxin), and oral bovine type I collagen (collagen) studies.
| Unadjusted Regression Coefficient* | SE | P-Value | ||
|---|---|---|---|---|
| Overall (N=534) | Time (Months after baseline) | −0.21 | 0.06 | 0.001 |
| Baseline Disease Duration Category (months) † | 6-<12 | −0.06 | 0. 07 | 0.388 |
| 12-<24 | −0.004 | 0.06 | 0.953 | |
| 24-<48 | −0.15 | 0.09 | 0.103 | |
| ≥ 48 | −0.16 | 0.09 | 0.058 | |
Coefficients for disease duration are from interaction terms and represent the additional rate of change for each group beyond that given by the time covariate.
Reference group is disease duration of < 6 months. Disease duration was calculated from the onset of first non-Raynaud’s phenomenon.
Discussion
We present pooled analysis from 3 large RCTs examining the course of skin thickening using data from D-Penicillamine, recombinant human relaxin and oral bovine type I collagen on MRSS trials. We found that MRSS improves after entering the clinical trials, irrespective of the SSc disease duration (defined either from the first non-Raynaud’s sign or symptom or from the first symptom including Raynaud’s phenomenon). Our results are of considerable interest for the design of clinical trials in dcSSc as they demonstrate that patients, irrespective of their disease duration showed an overall improvement in MRSS after entering in the trials. This challenges the American College of Rheumatology(5) guidelines which recommends including patients with early dcSSc (less than 2 years of disease duration) in RCTs, where “prevention of worsening” is the main objective.
Our study is consistent with reports from other investigators. In a recent pilot study evaluating the use of human recombinant transforming growth factor antibody in treating early-stage dcSSc patients (n=45; baseline disease duration of < 18 months; median disease duration of 6.4 months)(13), Denton et al. noted that patients with early dcSSc improved after entering the study; the improvement in the MRSS correlated with the disease duration (correlation coefficient= −0.54). In particular, patients with shorter disease duration at baseline showed greater change in their average MRSS score from baseline to 6 month. Recent analysis in patients with early diffuse SSc followed at the Royal Free Hospital showed a similar decline in median MRSS scores during the first 5 years(14). In another analysis examining the course of skin involvement in a RCT, Clements et al. (12) demonstrated that a skin tethering score (a measure of tethering and not skin thickness) in patients with dcSSc was stable during the first year and improved by the second and third years, regardless of disease duration (< 3 years, 3–8 years, and > 8 years) at baseline; it should be noted that, this study only had 11 patients with disease duration of < 3 years who completed the 3 year trial. Also, in an analysis comparing the characteristics of SSc patients in the D-Pen trial with those who entered other SSc clinical trials, Furst et al. (26) suggested that the use of ACR guidelines for SSc trials might change the nature of patient populations entering studies; patients who decided not to participate or were excluded from the study would have a different course and might even have followed the timeline generally accepted for the natural history of skin involvement. Our analyses of 3 large RCT’s confirm these preliminary observations(7;12;13)—there was a general tendency for skin to soften over time, and there was no difference in this tendency among patients with different disease durations at baseline. On average, having “early disease” was not associated with worsening of MRSS during the trials as seen in previously reported natural history cohorts. The only significant predictor of improvement in skin scores was time in the study confirming the observations by others(12–14).
In our study, patients with ≥ 2 years of disease duration generally had a greater decline in their MRSS compared to patients with < 2 years (Table 3). This trend in MRSS decline is accepted as part of the natural history of skin involvement in dcSSc patients(10;14). Previous studies have shown that earlier disease duration is associated with a greater change in MRSS compared to baseline. The earlier the disease duration the more likely it is for skin to worsen. In our study although the cohort as a whole had an improvement in MRSS, a proportion of patients did show worsening of the skin. This can be explained by the fact that individual patients can have changes that are different than that of the cohort when analyzed as a whole. Individual patients can have their MRSS peaks at different time points and this may explain the difference in MRSS decline that is observed between early versus late disease groups(13). However, from a clinical trial design and analysis perspective, average rather than individual MRSS scores are important and form the basis for future sample size calculation.
Several factors could have contributed to this earlier than expected skin improvement seen in dcSSc patients participating in the RCTs. First, the patients who participate in RCTs may experience a “placebo effect” in which both physician and patient perceive improvement secondary to being in a study. Second, patients who participate in clinical trials tend to enter studies when their disease is active and at its worst; it is when they sense this that they may be interested in trying new interventions through clinical trials. These patients, once in the trial, may improve as part of the natural disease process, a statistical phenomenon known as “regression to the mean”. A third possibility relates to the definition of disease duration. The natural history of skin involvement in diffuse SSc is based on the first sign or symptom attributable to SSc. In early diffuse SSc, Raynaud’s phenomenon may precede, coincide, or develop after other signs or symptoms associated with SSc(9) and, when using Raynaud’s phenomenon as the first sign or symptom (rather than the more common first non-Raynaud’s sign or symptom), disease duration may differ by the 2 definitions. However, our sensitivity analysis showed similar results when Raynaud’s phenomenon was included in the definition of disease duration compared to when disease duration was assessed from the first non-Raynaud’s symptom or sign attributed to SSc.
Our analyses have several strengths. First, we included 3 large RCTs, which were conducted in multiple major US Scleroderma Centers. Second, 2 of the 3 trials (relaxin and collagen) recruited patients with both early and late disease providing us an opportunity to compare the pattern of early versus late-stage MRSS over time.
Our study also has some limitations. Our analysis is post-hoc rather than based on a priori hypothesis. However, this and other analyses(13;14) using the ACR-recommended guidelines have given similar findings giving us confidence in our results. In addition, the trial design may have influenced our results. The D-Pen trial was a “prevention of progression” trial design; the relaxin and collagen studies sought patients with moderate-to-severe disease to assess “reversal of skin thickening.”
In conclusion, our study suggests that patients recruited in dcSSc clinical trials show an improvement in their average MRSS, independent of disease duration. Our findings confirm previously published results. These findings have important implications in the “prevention of worsening” study designs, when using skin softening as an endpoint.
Acknowledgment
Dr. P. Khanna was supported by 1 T32 AR 053463 and Dr. D. Khanna was supported by a National Institutes of Health Award (NIAMS K23 AR053858-01A1) and a New Investigator Grant from the Scleroderma Foundation. We thank Dr. James Seibold, Principal Investigator of the human recombinant relaxin trial for providing the data.
Reference List
- 1.Medsger TA, Jr, Steen V. Classification prognosis. In: Clements PJ, Furst DE, editors. Systemic Sclerosis. Baltimore: Williams and Wilkins; 1996. pp. 51–64. [Google Scholar]
- 2.Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000;43(11):2445–2454. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 3.Khanna D, Merkel PA. Outcome measures in systemic sclerosis: an update on instruments and current research. Curr Rheumatol Rep. 2007;9(2):151–157. doi: 10.1007/s11926-007-0010-5. [DOI] [PubMed] [Google Scholar]
- 4.Steen VD, Medsger TA., Jr Improvement in skin thickening in systemic sclerosis associated with improved survival. Arthritis Rheum. 2001;44(12):2828–2835. doi: 10.1002/1529-0131(200112)44:12<2828::aid-art470>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.White B, Bauer EA, Goldsmith LA, Hochberg MC, Katz LM, Korn JH, et al. Guidelines for clinical trials in systemic sclerosis (scleroderma). I. Disease-modifying interventions.The American College of Rheumatology Committee on Design and Outcomes in Clinical Trials in Systemic Sclerosis. Arthritis Rheum. 1995;38(3):351–360. doi: 10.1002/art.1780380309. [DOI] [PubMed] [Google Scholar]
- 6.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22(7):1281–1285. [PubMed] [Google Scholar]
- 7.Nihtyanova SI, Denton CP. Current approaches to the management of early active diffuse scleroderma skin disease. Rheum Dis Clin North Am. 2008;34(1):161–179. doi: 10.1016/j.rdc.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Clements P, Medsger TA, Feghali C. Cutaneous involvement in systemic sclerosis. In: Clements P, Furst DE, editors. Sytemic sclerosis. Second ed. Philadelphia: Lippincott Willaims and Wilkins; 2004. pp. 129–150. [Google Scholar]
- 9.Medsger TA., Jr. Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheum Dis Clin North Am. 2003;29(2):255–273. doi: 10.1016/s0889-857x(03)00023-1. vi. [DOI] [PubMed] [Google Scholar]
- 10.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43(11):2437–2444. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 11.Shand L, Lunt M, Nihtyanova S, Hoseini M, Silman A, Black CM, et al. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: application of a latent linear trajectory model. Arthritis Rheum. 2007;56(7):2422–2431. doi: 10.1002/art.22721. [DOI] [PubMed] [Google Scholar]
- 12.Clements P, Lachenbruch P, Furst D, Paulus H. The course of skin involvement in systemic sclerosis over three years in a trial of chlorambucil versus placebo. Arthritis Rheum. 1993;36(11):1575–1579. doi: 10.1002/art.1780361112. [DOI] [PubMed] [Google Scholar]
- 13.Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: A multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2006;56(1):323–333. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 14.Shand L, Lunt M, Nihtyanova S, Hoseini M, Silman A, Black CM, et al. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: application of a latent linear trajectory model. Arthritis Rheum. 2007;56(7):2422–2431. doi: 10.1002/art.22721. [DOI] [PubMed] [Google Scholar]
- 15.Clements PJ, Furst DE, Wong WK, Mayes M, White B, Wigley F, et al. High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum. 1999;42(6):1194–1203. doi: 10.1002/1529-0131(199906)42:6<1194::AID-ANR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Khanna D, Clements P, Furst D, Korn J, Ellman M, Rothfield N, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Recombinant Human Relaxin in the Treatment of Systemic Sclerosis with Diffuse Scleroderma. Arthritis Rheum. 2009 doi: 10.1002/art.24380. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postlethwaite AE, Wong WK, Clements P, Chatterjee S, Fessler BJ, Kang AH, et al. A multicenter, randomized, double-blind, placebo-controlled trial of oral type I collagen treatment in patients with diffuse cutaneous systemic sclerosis: I. Oral type I collagen does not improve skin in all patients, but may improve skin in late-phase disease. Arthritis Rheum. 2008;58(6):1810–1822. doi: 10.1002/art.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steen VD, Medsger TA, Jr, Rodnan GP. D-Penicillamine therapy in progressive systemic sclerosis (scleroderma): a retrospective analysis. Ann Intern Med. 1982;97(5):652–659. doi: 10.7326/0003-4819-97-5-652. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez SA, Sigal SH. A 15-year prospective study of treatment of rapidly progressive systemic sclerosis with D-penicillamine [see comment] J Rheumatol. 1991;18(10):1496–1503. [PubMed] [Google Scholar]
- 20.Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20(11):1892–1896. [PubMed] [Google Scholar]
- 21.Khanna D, Furst DE, Hays RD, Park GS, Wong WK, Seibold JR, et al. Minimally Important Difference in Diffuse Systemic Sclerosis-Results from the D-Penicillamine Study. Ann Rheum Dis. 2006;65(10):1325–1329. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seibold J, Clements P, Korn JH, Ellman M, Rothfield N, Wigley F, et al. U.S. phase III trial of relaxin in diffuse scleroderma. J Rheumatol. 2001;63(T-64) Ref Type: Abstract. [Google Scholar]
- 23.Khanna D, Furst DE, Clements PJ, Park GS, Hays RD, Yoon J, et al. Responsiveness of the SF-36 and the Health Assessment Questionnaire Disability Index in a systemic sclerosis clinical trial. J Rheumatol. 2005;32(5):832–840. [PubMed] [Google Scholar]
- 24.Khanna D, Furst DE, Wong WK, Tsevat J, Clements PJ, Park GS, et al. Reliability, validity, and minimally important differences of the SF-6D in systemic sclerosis. Qual Life Res. 2007 doi: 10.1007/s11136-007-9207-3. [DOI] [PubMed] [Google Scholar]
- 25.Khanna D, Clements PJ, Postlethwaite AE, Furst DE. Does Incorporation of Aids and Devices Make a Difference in the Score of the Health Assessment Questionnaire-Disability Index? Analysis from a Scleroderma Clinical Trial. J Rheumatol. 2008;35(3):466–468. [PubMed] [Google Scholar]
- 26.Furst DE, Clements PJ, Wong WK, Mayes MD, Wigley F, White B, et al. Effects of the American College of Rheumatology systemic sclerosis trial guidelines on the nature of systemic sclerosis patients entering a clinical trial. Rheumatology (Oxford) 2001;40(6):615–622. doi: 10.1093/rheumatology/40.6.615. [DOI] [PubMed] [Google Scholar]