Abstract
Specific language impairment (SLI) is a common developmental disorder characterized by difficulties in language acquisition despite otherwise normal development and in the absence of any obvious explanatory factors. We performed a high-density screen of SLI1, a region of chromosome 16q that shows highly significant and consistent linkage to nonword repetition, a measure of phonological short-term memory that is commonly impaired in SLI. Using two independent language-impaired samples, one family-based (211 families) and another selected from a population cohort on the basis of extreme language measures (490 cases), we detected association to two genes in the SLI1 region: that encoding c-maf-inducing protein (CMIP, minP = 5.5 × 10−7 at rs6564903) and that encoding calcium-transporting ATPase, type2C, member2 (ATP2C2, minP = 2.0 × 10−5 at rs11860694). Regression modeling indicated that each of these loci exerts an independent effect upon nonword repetition ability. Despite the consistent findings in language-impaired samples, investigation in a large unselected cohort (n = 3612) did not detect association. We therefore propose that variants in CMIP and ATP2C2 act to modulate phonological short-term memory primarily in the context of language impairment. As such, this investigation supports the hypothesis that some causes of language impairment are distinct from factors that influence normal language variation. This work therefore implicates CMIP and ATP2C2 in the etiology of SLI and provides molecular evidence for the importance of phonological short-term memory in language acquisition.
Main Text
Developmental speech and language disorders are a heterogeneous group of childhood conditions with variable presentation and etiology. Together, they account for 40% of pediatric referrals1 and statements of educational need.2 The term specific language impairment (SLI) defines a category of speech and language disorders in which a profound language impairment represents the primary deficit.2 This disorder affects 5%–8% of preschool children2 and is highly heritable.3 Nonetheless, in contrast to other related developmental disabilities (e.g., dyslexia [MIM #127700] and attention deficit hyperactivity disorder [ADHD, MIM #143465]), relatively few genetic studies have been performed for SLI. SLI is a prototypical multifactorial disorder that is predicted to involve numerous genetic loci and environmental factors.3 Three primary sites of linkage have been described4, 5, the most robust of which is on chromosome 16q (SLI1, MIM #606711). This region is of interest because the linkage is highly specific to a single psychometric measure (nonword repetition).4, 6, 7 The test for nonword repetition involves the repetition of nonsensical words of increasing length and complexity and is regarded as a measure of phonological (speech sound) processing and short-term memory.8 Individuals with SLI typically perform particularly poorly on nonword repetition, even when their language difficulties have apparently resolved, leading to the postulation that a short-term memory deficit causes susceptibility to SLI9 by impairing the retention of novel verbal information.10 This paper incorporates two contingent investigations: an association screen of the SLI1 region in a cohort of language-impaired families and a subsequent replication study of detected association effects in an independent sample selected from the Avon Longitudinal Study of Parents and Children (ALSPAC) general-population cohort.11, 12
The association screen utilized 806 individuals from 211 families ascertained by the SLI Consortium (SLIC). This nuclear-family cohort was collected from five sites across the UK (The Newcomen Centre at Guy's Hospital, London; the Cambridge Language and Speech Project (CLASP)13; the Child Life and Health Department at the University of Edinburgh14; the Department of Child Health at the University of Aberdeen; and the Manchester Language Study15, 16) and included the families in whom the SLI1 linkage was originally identified. Ethical permission for each collection was granted by local ethics committees. SLIC families were all selected on the basis of a single proband with receptive and/or expressive language skills more than 1.5 SD below the normative mean for his or her age. A more detailed description of these samples and the exclusionary criteria applied to the SLIC collection can be found in previous publications.4, 6, 7
Genotyping for the association screen was performed in two phases with a combination of Sequenom and Illumina technologies. We performed an initial high-density screen involving 1906 SNPs to tag all 58 genes (including introns, exons, and 5 Kb 5′ and 2 Kb 3′ of coding sequences) mapped to the 10.29 Mb SLI1 region of linkage (D16S3138–D16S413. Chromosome 16 position 76.16 Mb–86.45 Mb [B35]). Haplotype blocks were built within Haploview17 via the Gabriel method.18 Any between-block gap that was more than 15 Kb in size was tagged with the Tagger algorithm. Two genes that mapped to the region (CDH13 [MIM #601364] and WWOX [MIM #605131]) were found to be larger than 1 Mb in size. For these two genes, blocks were built to cover the exonic regions only. Any region containing a SNP that met our predefined significance threshold (p < 0.001 in any one analysis or p < 0.01 across both analyses) was then supplemented with additional markers in a follow-up panel that included 138 SNPs, eight of which had previously been genotyped. Both phases of genotyping were completed prior to the replication study and were subjected to consistent quality-control procedures. The total genotype mismatch rate was 0.73% for duplicated SNPs and 0.76% for duplicated samples. Across both phases, 261 (12.7%) of SNPs were excluded at the quality-control stage. These included SNPs with a genotype rate of <80%, a minor-allele frequency of <2.5%, SNPs with unusual Beadstudio cluster patterns (Illumina) or atypical peaks in MassArray TyperAnalyser (Sequenom), SNPs with a GenTrain score of <0.5 (Illumina), and markers that showed consistent bad inheritances (>10 errors after data clean up). Across the entire region, the merged data set consisted of, on average, one SNP every 6.4 Kb. Across the known genes, there was on average one SNP every 4.5 Kb, and the largest remaining gap between blocks was 19,579 bp. Details of SNP coverage can be found in Table S1. Q-Q plots can be found in Figure S1. Given the consistent linkage between SLI1 and nonword repetition, all association analyses were based upon this measure. Our principal analysis involved the variance-components modeling of 28-item nonword repetition scores8 within 211 SLIC families (ao option) as a quantitative trait and was performed within QTDT.19 In addition, we performed a categorical case-control allelic test of association within PLINK.20 In this case-control analysis, SLIC individuals with low nonword-repetition scores (>2 SD below population mean, n = 79) were chosen as cases, and family members with above-average performance (>0.5 SD above population mean, n = 71) were used as controls. To avoid interdependence, we selected only one case or control from each family unit.
The initial screen involved 1678 SNPs, of which thirteen (0.77%) exceeded our significance threshold, highlighting two primary regions of association (Table 1 and Figure 1). The follow-up panel chiefly included SNPs in these two regions and supported the association seen in the screen while reducing the evidence for association at other loci (Table 2 and Figure 1). Of the 105 SNPs tested in the follow-up panel, five (4.8%) were found to be significantly associated (Table 2 and Figure 1). The first identified cluster of association lay across 26 Kb (exons 2–4) of the CMIP gene (MIM #610112; seven significant SNPs, minP = 5 × 10−7). This gene encodes an adaptor protein and has two isoforms, the shorter of which is involved in cell signaling pathways and is upregulated in minimal change nephrotic syndrome (MCNS), a childhood kidney disease.21 Little is known about the function of the longer transcript. Both isoforms are expressed in the brain.21 The second region of association was observed between exons 7 and 12 (10.8 Kb) of the ATP2C2 gene (six significant SNPs, minP = 2 × 10−5). This gene is one of two secretory-pathway Ca2+-ATPases (SPCAs) that move cytosolic calcium and manganese ions into the golgi.22 Its expression is limited to the brain, testis, gastrointestinal tract, and respiratory tissues and mammary, salivary, and thyroid glands.22 In the mammary gland, ATP2C2 expression facilitates the secretion of Ca2+ into casein micelles during lactation.23
Table 1.
Significant Association in the SLIC Association Screen
| SNP | Chromosome Position (bp – B36) | Gene | Alleles (A1/A2) | A1 CEPH Frequency | Typed Strand | p Quant | Effect Size | Heritability | p Emp QTDT | p Case-Cont | Frequency of A1 Cases | Frequency of A1 Controls | Odds ratio (95% CI) | p Emp PLINK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs8051754 | 78,554,834 | intergenic | T/C∗ | 0.46 | − | 0.0931 | −0.28 ± 0.11 | 0.019 | 0.0892 | 0.0007∗ | 0.64 | 0.85 | 3.1 (1.6–6.0) | 0.0018∗ |
| rs4417561 | 78,568,860 | intergenic | G∗/C | 0.26 | − | 0.0244 | −0.30 ± 0.11 | 0.022 | 0.0252 | 0.0004∗ | 0.37 | 0.15 | 3.2 (1.7–6.3) | 0.0011∗ |
| rs2316184 | 79,204,885 | CDYL2 | G/A∗ | 0.14 | + | 0.0032∗ | -0.48 ± 0.12∗ | 0.045 | 0.0034∗ | 0.0096∗ | 0.15 | 0.30 | 2.5 (1.2–4.9) | 0.0126 |
| rs12927866 | 80,209,823 | CMIP | A/G∗ | 0.47 | − | 0.4104 | −0.27 ± 0.10 | 0.019 | 0.3581 | 0.0003∗ | 0.29 | 0.49 | 2.4 (1.5–3.9) | 0.0004∗ |
| rs4265801 | 80,222,553 | CMIP | T∗/G | 0.43 | + | 0.3446 | −0.09 ± 0.09 | 0.030 | 0.5065 | 4 × 10−5∗ | 0.61 | 0.29 | 3.9 (2.0–7.6) | 0.0393∗ |
| rs7201632 | 80,234,949 | CMIP | C/T∗ | 0.49 | + | 0.8966 | −0.25 ± 0.09 | 0.017 | 0.7975 | 0.0004∗ | 0.36 | 0.56 | 2.3 (1.4–3.7) | 0.0004∗ |
| rs3785054 | 82,918,978 | WFDC1 | C∗/T | 0.36 | − | 0.0044∗ | −0.29 ± 0.10∗ | 0.019 | 0.0033∗ | 0.0089∗ | 0.34 | 0.20 | 2.0 (1.2–3.4) | 0.0102 |
| rs8053211 | 83,011,254 | ATP2C2 | A∗/G | 0.46 | + | 5 × 10−5∗ | −0.38 ± 0.09∗ | 0.040 | 3 × 10−5∗ | 0.0014∗ | 0.61 | 0.43 | 2.1 (1.3–3.3) | 0.0029∗ |
| rs11860694 | 83,014,948 | ATP2C2 | C∗/G | 0.54 | − | 2 × 10−5∗ | −0.37 ± 0.09∗ | 0.039 | 9 × 10−6∗ | 0.0018∗ | 0.61 | 0.43 | 2.1 (1.3–3.3) | 0.0027∗ |
| rs16973771 | 83,018,079 | ATP2C2 | G/A∗ | 0.48 | − | 0.0003∗ | −0.35 ± 0.09∗ | 0.034 | 0.0006∗ | 0.0025∗ | 0.34 | 0.51 | 2.0 (1.3–3.2) | 0.0036∗ |
| rs2875891 | 83,021,410 | ATP2C2 | T/C∗ | 0.44 | + | 0.0057∗ | −0.34∗ ± 0.10∗ | 0.031 | 0.0063∗ | 0.0022∗ | 0.30 | 0.47 | 2.1 (1.3–3.4) | 0.0026∗ |
| rs8045507 | 83,022,078 | ATP2C2 | T/C∗ | 0.48 | − | 0.0017∗ | −0.33 ± 0.09∗ | 0.029 | 0.0020∗ | 0.0022∗ | 0.34 | 0.51 | 2.1 (1.3–3.3) | 0.0028∗ |
Three significant SNPs fell within the CMIP gene, and five fell within ATP2C2. The remaining four significant SNPs were either intergenic or isolated signals of association. SNP alleles are given with the minor allele in the SLIC sample first. Putative risk alleles are marked with an asterisk. P Quant gives the p value for the quantitative, family-based analysis. p case-cont gives the p value for the case-control analysis. p values <0.01 are marked with an asterisk. The odds ratios indicate the ratio of case/control odds for each additional copy of the putative risk allele. Odds ratios were calculated within PLINK. The effect size is the estimated effect of each risk allele on the nonword repetition score (in SD ± SE). Effect sizes were calculated with MERLIN. Heritability gives the proportion of total variance explained by the SNP. Heritability estimates were calculated with MERLIN. The p Emp column gives empirical p values for the given SNP; these values were derived from permutations within QTDT or PLINK.
Figure 1.
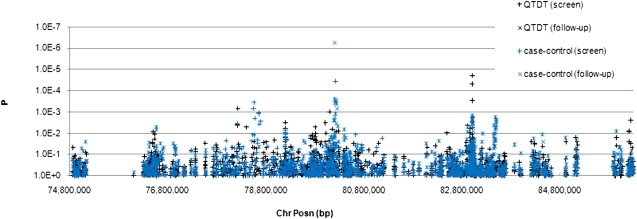
Association in SLIC Cohort
Association results for family-based quantitaive analysis and case-control analysis of nonword repetition across the SLI1 region. In the case-control analysis, cases and controls were selected on the basis of their nonword-repetition performance (see text). Gaps in data represent regions where there are no mapped genes. SNPS included in the screen genotype panel are shown as +, and SNPs included in the follow-up genotype panel are shown as x.
Table 2.
Significant Association in the SLIC Cohort with the Follow-up Panel
| SNP | Chromosome Position (bp – B36) | Gene | Alleles (A1/A2) | A1 CEPH Frequency | Typed Strand | P Quant | Effect Size | Heritability | p Emp QTDT | p Case-Cont | Frequency of A1 Cases | Frequency of A1 Controls | Odds Ratio (95% CI) | p Emp PLINK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs6564903 | 80,211,158 | CMIP | C∗/T | 0.48 | + | 0.1279 | −0.37 ± 0.10 | 0.038 | 0.1225 | 5 × 10−7∗ | 0.79 | 0.38 | 3.5 (2.1–5.9) | 1 × 10−6∗ |
| rs3935802 | 80,219,068 | CMIP | G∗/C | 0.46 | − | 0.2667 | −0.31 ± 0.10 | 0.025 | 0.2486 | 0.0003∗ | 0.71 | 0.49 | 2.5 (1.5–4.2) | 0.0006∗ |
| rs16955705 | 80,230,851 | CMIP | C/A∗ | 0.50 | + | 0.3916 | −0.25 ± 0.10 | 0.017 | 0.3627 | 0.0003∗ | 0.31 | 0.54 | 2.6 (1.5–4.4) | 0.0003∗ |
| rs4243209 | 80,247,592 | CMIP | C/T∗ | 0.22 | + | 0.0065∗ | −0.42 ± 0.12 | 0.027 | 0.0043∗ | 0.0007∗ | 0.11 | 0.26 | 3.0 (1.6–5.8) | 0.0012∗ |
| rs12149426 | 83,022,607 | ATP2C2 | A/C∗ | 0.26 | + | 0.0064∗ | −0.31 ± 0.12 | 0.017 | 0.0082∗ | 0.0082∗ | 0.14 | 0.27 | 2.3 (1.2–4.2) | 0.0039∗ |
Of the 105 SNPs analyzed in the follow-up panel, 16 lay in CMIP, 76 lay in the ATP2C2 gene, and the remaining 13 lay in other regions that had shown association in the screen (see Table 1). Eight SNPs were genotyped in both the screen and follow-up panels. All of these markers showed some evidence of association in the screen phase (p < 0.01) but had genotype success rates of <95%, and none lay within CMIP or ATP2C2. Each of the duplicated SNPs showed increased success rates and decreased association levels in the follow-up panel. SNP alleles are given with the minor allele in the SLIC sample first. Putative risk alleles are marked with an asterisk. p Quant gives the p value for the quantitative, family-based analysis. p case-cont gives the p value for the case-control analysis. p values <0.01 are shown in bold. The odds ratios indicate the ratio of case/control odds for each additional copy of the putative risk allele. Odds ratios were calculated within PLINK. The effect size is the estimated effect of each risk allele on the nonword-repetition score (in SD ± SE). Effect sizes were calculated with MERLIN. Heritability gives the proportion of total variance explained by the SNP. Heritability estimates were calculated with MERLIN. The p Emp column gives empirical p values for the given SNP; these values were derived from permutations within QTDT or PLINK.
Three lines of evidence indicate that the associations at CMIP and ATP2C2 represent separate effects. First, we did not see any indication of long-range linkage disequilibrium between the two loci (which lie almost 3 Mb apart) in the SLIC cohort or public data (Figure S2). Second, the inclusion of a CMIP covariate in the linkage or association model did not affect the level of linkage or association seen at ATP2C2 (or vice versa for ATP2C2 covariates) (Figure S3). Finally, in a stepwise regression model, the group mean for SLIC individuals carrying a double-risk genotype was found to be significantly lower than those who were homozygous for risk at a single locus (p = 3.7 × 10−6, Table 3). In this model, the group mean for double-risk individuals was 15.8 points (1.05 SD) below that of individuals carrying nonrisk variants at both loci (Table 3). We therefore propose that CMIP and ATP2C2 independently regulate nonword repetition performance and together underlie the linkage seen between SLI and chromosome 16.
Table 3.
Nonword-Repetition Group Means for CMIP and ATP2C2 Risk Variants
| Genotype (Number of Risk Alleles) | Single SNP | rs6564903 (CMIP) |
|||
|---|---|---|---|---|---|
| TT (0) | CT (1) | CC (2) | |||
| Single SNP | 96.62 | 92.57 | 86.30 | ||
| rs11860694 (ATP2C2) | GG (0) | 96.54 | 99.14 | 99.85 | 89.65 |
| CG (1) | 91.77 | 99.40 | 93.10 | 85.84 | |
| CC (2) | 87.03 | 88.44 | 88.33 | 83.32 | |
The effects of CMIP (rs6564903) and ATP2C2 (rs11860694) on nonword-repetition performance were modeled as additive effects within a regression framework in the R package. This regression model included all available SLIC children with genotype and nonword-repetition data (n = 503). Group means were calculated for each SNP in isolation (“Single SNP” entries) and in combinations of genotypes (3 × 3 grid) across risk SNPs. Note that individuals carrying combinations of risk alleles performed significantly worse than those carrying risk variants at a single locus. Nonword-repetition scores are age adjusted and standardized against normal population controls with a mean of 100 and a SD of 15.
Our replication sample consisted of 490 cases selected from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort.11, 12 This is a general-population sample that follows the development of 14,062 live-born individuals born in the southwest of England. The ALSPAC group periodically performs an assessment of the development of consenting individuals, and these measurements include tests of language ability. Informed written consent was obtained from the parents at the time of enrolment. Ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees. Because the current study focuses upon language impairment, we selected individuals from the lower extreme of language-related phenotype distributions (Children's Communication Checklist (CCC)24 and Wechsler Objective Language Dimensions (WOLD)25) for our replication sample. This included 665 individuals (10.3%) with a CCC pragmatic composite 1–3 SD below the ALSPAC population mean (123 ≤ × ≤ 145) or a WOLD listening comprehension score ≥2 SD below the ALSPAC population mean (≤3). Of these individuals, 490 had completed a 12-item nonword repetition test. Because the genotyping in the replication sample was restricted to a single individual from each family, we performed a quantitative association analysis within PLINK20 by using nonword repetition in a linear-regression framework. In addition, we used PLINK20 to carry out a case-control analysis analogous to that described for SLIC. We selected cases and controls from the extremes of the nonword repetition performance distribution of the 490 selected individuals. As expected, given the extreme nature of the language impairment in the SLIC samples, the distribution of nonword repetition differed between the SLIC and ALSPAC cohorts. Therefore, in the replication cohort, the cut-offs used for cases and controls were less extreme than those applied for the association screen. Cases were selected from the identified replication sample to have nonword repetition scores ≥1 SD below the general-population mean (n = 112), and controls had nonword repetition scores ≥1 SD above the general-population mean (n = 72). Data were analyzed for three CMIP and three ATP2C2 SNPs (rs12927866, rs4265801, and rs16955705; and rs16973771, rs2875891, and rs8045507, respectively), and significant associations (p < 0.05) were seen for two CMIP and two ATP2C2 SNPs (Table 4 and Figure 2). Regression trends for ATP2C2 followed those seen in SLIC, replicating the previously described association. Association to CMIP was in an opposite direction from that described above (Table 4 and Figure 2). Although this result might represent a type I error, the consistency of significant association in light of the low number of SNPs tested supports a role for CMIP. Associations can occur in opposite directions if the relationship between the observed and causal variants differs between populations.26 This is particularly true if multiple risk loci interact in an additive or multiplicative fashion26, as is predicted for CMIP. Identification of the causal variant will enable the further characterization of the relationship between risk variants in different populations.
Table 4.
Association in the Replication Cohort
| SNP | Chromosome Position (bp – B36) | Gene | Alleles (A1/A2) | SLIC Risk Allele | A1 CEPH Frequency | Typed Strand | p Quant | Effect Size | p Case-Cont | Frequency of A1 Cases | Frequency of A1 controls | Odds Ratio (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs12927866 | 80,209,823 | CMIP | T/C | C | 0.47 | + | 0.1623 | −0.08 | 0.0955 | 0.39 | 0.30 | 1.5 (0.9-2.3) |
| rs4265801 | 80,222,553 | CMIP | T/G∗ | T | 0.43 | + | 0.0182∗ | −0.15 | 0.0214∗ | 0.43 | 0.56 | 1.6 (1.1-2.5) |
| rs16955705 | 80,230,851 | CMIP | C∗/A | A | 0.50 | + | 0.0238∗ | −0.14 | 0.0257∗ | 0.48 | 0.36 | 1.6 (1.1-2.5) |
| rs16973771 | 83,018,079 | ATP2C2 | C/T∗ | T | 0.48 | + | 0.0079∗ | −0.14 | 0.0135∗ | 0.32 | 0.45 | 1.7 (1.1-2.7) |
| rs2875891 | 83,021,410 | ATP2C2 | T/C | C | 0.44 | + | 0.0668 | −0.06 | 0.0802 | 0.29 | 0.37 | 1.5 (1.0-2.3) |
| rs8045507 | 83,022,078 | ATP2C2 | A/G∗ | G | 0.48 | + | 0.0058∗ | −0.15 | 0.0110∗ | 0.31 | 0.44 | 1.8 (1.1-2.7) |
SNP alleles are given with the minor allele first. Putative risk alleles in the replication cohort are marked with an asterisk. p Quant shows the p value for the quantitative analysis. p < 0.05 are highlighted in bold. The odds ratio indicates the ratio of case/control odds for each additional copy of the putative risk allele. The 95% confidence intervals for the odds ratios of all significantly associated SNPs exceeded 1.0. The effect size is the estimated effect of each risk allele on the nonword-repetition score (in SD).
Figure 2.
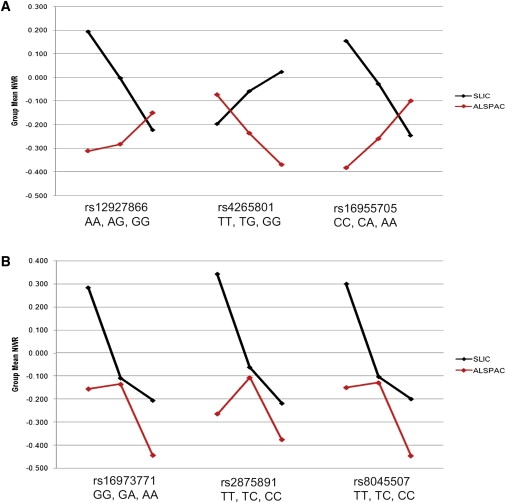
Nonword-Repetition Means for CMIP and ATP2C2 in SLIC and Replication Cohorts
(A) CMIP.
(B) ATP2C2.
All means are for age- and sex-adjusted nonword-repetition scores standardized with a mean of 0 and a SD of 1. The three CMIP SNPs (rs12927866, rs4265801, and rs16955705) show genotype trends in the opposite direction from SLIC (A), whereas the three ATP2C2 SNPs (rs16973771, rs2875891, and rs8045507) show genotype trends in the same direction as SLIC (B).
Given the partial replication of association, we investigated whether the primary associated SNPs in ATP2C2 and CMIP had an effect upon additional language- and memory-related measures (Table S2). In SLIC, we found borderline association for ATP2C2 with measures of receptive language (oral directions27 [p = 0.006], word classes27 [p = 0.04], and comprehension28 [p = 0.03]), expressive language (formulating sentences27 [p = 0.04]), and vocabulary28 (p = 0.04). In the replication cohort, aside from nonword repetition, we only observed borderline association between ATP2C2 and counting span, a measure of working memory (p = 0.01). In the replication sample, nonword repetition performance had been scored according to the number of syllables the nonword contained. For both CMIP and ATP2C2, the majority of association came from the five-syllable nonwords (p = 0.016 and p = 6 × 10−4, respectively) (Table S2). In neither sample did we observe association to reading-related tasks, which have been reported to show linkage to SLI1.6 Nor did we find any association to digit span28 or recalling sentences,27 two measures that have a high memory load. This is consistent with the finding that nonword repetition correlates with SLI to a higher degree than other short-term memory tests (e.g., digit span). The sensitivity of nonword repetition to SLI could be because it places heavier demands on processing of speech sounds than other memory tests as a result of the child's having to perceive and produce an unfamiliar sequence.29 It is important to note that, although nonword repetition is a good marker for SLI, poor performance on nonword repetition is not a perfect correlate of this disorder.30 In our study, 50% of SLIC probands performed poorly (>1 SD below the expected population mean) on nonword repetition, but a significant number (27%) scored above the expected population mean. These findings support recent opinion that deficits across multiple domains are required to cause persistent language impairments.31
A recent genome-wide association study of ADHD listed a SNP (rs10514604; p = 8 × 10−7) in ATP2C2 within the top 30 significant associations.32 Despite distinct defining characteristics, ADHD and SLI show a high level of comorbidity both with each other32 and with disorders such as developmental coordination disorder, speech-sound disorder (SSD; MIM #608445), and dyslexia.33, 34, 35 For example, individuals with SLI, SSD, ADHD, or dyslexia often present with linguistic deficits and impairments in short-term memory.33 It has therefore been suggested that certain aspects of these disorders might share a common etiology. Given the high levels of co-occurrence, we did not exclude children affected by ADHD and dyslexia from our study samples. However, in some of our SLIC samples, data were available for the presence of hyperactivity, coordination, and reading problems. From this, we estimate that approximately one-third of our SLIC samples showed some evidence of ADHD or developmental coordination disorder and that approximately one-half of our probands had reading problems. In the entire ASLPAC sample, 1.3% of individuals met criteria for ADHD. In the selected ALSPAC replication sample, the rate of ADHD increased to 3.7%. Thus, as expected, it is clear that the rate of developmental disorders across our cohorts is elevated over that expected in a population sample. Nonetheless, the association detected in our samples shows a strong correlation to nonword-repetition ability which has repeatedly been shown to be a strong indicator of language impairment.9, 10 Furthermore, in ADHD samples, performance on the nonword-repetition task is correlated with linguistic ability rather than the presence of hyperactivity.33, 36 Thus, we conclude that variants in ATP2C2 might account for shared aspects of the linguistic deficit in SLI and ADHD. Given this possibility, we also postulate that ATP2C2 might contribute to phonological short-term memory in other developmental disorders.
Finally, we investigated the effects of ATP2C2 and CMIP on nonword-repetition performance at the population level. Across the entire unselected ALSPAC population (n = 3612), there was no evidence for quantitative association between nonword-repetition ability and either locus (minP = 0.48). Moreover, there were no differences in allele frequency for ATP2C2 or CMIP SNPs between either SLIC or replication-sample individuals and unselected European population controls (data not shown). Taken together, these data indicate that ATP2C2 and CMIP do not modulate nonword-repetition performance across the entire population, nor, in isolation, do they cause a predisposition to SLI. Instead, we propose that when combined with additional, as-yet-unidentified, susceptibility factors (either genetic or environmental), variants in ATP2C2 and CMIP have a detrimental effect upon nonword repetition performance and thus heighten the risk of developmental language impairments. This situation demonstrates a fundamental principle often overlooked in the mapping of complex disorders: that genetic variants might have selective effects in specific populations depending upon the genetic and environmental background. The question as to whether SLI constitutes a qualitatively distinct disorder caused by abnormal development of language abilities or merely represents the tail end of normal linguistic development is a matter of recent debate.37 Although the absence of association in our population sample could reflect insufficient sample sizes or the insensitivity of psychometric tests to quantify variation beyond the lower extremes of the spectrum, it is obvious that the effects of ATP2C2 and CMIP upon nonword-repetition performance are particularly pertinent to individuals with language difficulties. As such, this investigation provides molecular evidence that, at least in terms of the effects described here, SLI represents a distinct disorder caused by genetic variants discrete from those that influence language ability in the general population.
In summary, we have used a positional fine-mapping approach to demonstrate association between ATP2C2 and CMIP and nonword repetition performance across two independent language-impaired populations. We propose that variants in both loci combine to modulate nonword-repetition performance in language-impaired populations. Both genes are expressed in the brain and represent good candidates for language- and memory-related processes. ATP2C2 is involved in the translocation of cytosolic calcium and manganese ions to the golgi.22 Calcium homeostasis is important for the regulation of many neuronal processes, including working memory, synaptic plasticity, and neuronal motility38, and manganese dysregulation has been linked to Parkinsonism (MIM #168600), Alzheimer disease (MIM #104300), and disordered memory.39 The functional role of CMIP is less defined, but it is known to interact with filamin A (MIM #300017)40 and the NF-kappaB subunit RelA (MIM #164014).41 The filaminA protein is involved in the reorganization of the actin cytoskeleton, which is of importance in the formation of the dendritic spine.40 The NF-κB family of transcription factors plays a central role in many neuronal processes, including synaptic activity and memory formation, and members of this family have been implicated in neurodegenerative disorders.42 Further characterization of the observed associations has enabled us to infer that SLI represents a qualitatively distinct disorder caused by a combination of genetic variants that disrupt multiple pathways important to the development of language. It is anticipated that the functional characterization of ATP2C2 and CMIP will promote a better understanding of the molecular basis of language acquisition and aid in the diagnosis and treatment of individuals affected by language disorders.
Acknowledgments
We thank all the families and professionals who participated in the study, Caroline Durrant and Jean-Baptiste Cazier for statistical advice, members of the Monaco lab for support, and Leila Jannoun, Jane Addison, Clare Craven, Deborah Jones, Tilly Storr, Til Utting-Brown, Margaret Main, Jane Steele, and Alan MacLean for assistance with data collection and management. We are extremely grateful to all the families who took part in the ALSPAC study, to the midwives for their help in recruiting them, and to the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The UK Medical Research Council, the Wellcome Trust, and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors, and D.F. Newbury and A.P. Monaco will serve as guarantors for this paper's contents. The Wellcome Trust specifically funded this research. All laboratory work and the collection of data from families ascertained by Guy's Hospital and the University of Manchester were funded by The Wellcome Trust. CLASP was funded by The Wellcome Trust, British Telecom, Isaac Newton Trust, National Health Service (NHS) Anglia & Oxford Regional R&D Strategic Investment Award, and an NHS Eastern Region R&D Training Fellowship Award. The Edinburgh group was supported by the Chief Scientist's Office, Scotland. The Aberdeen group was supported by Grampian Healthcare Trust and Grampian Primary Care NHS Trust. D.V.M. Bishop is a Wellcome Trust Principal Research Fellow, and S.E. Fisher is a Royal Society Research Fellow.
Published online: July 30, 2009
Footnotes
Supplemental Data include three figures and two tables and can be found with this article online at http://www.ajhg.org/.
Web Resources
The URLs for data presented herein are as follows:
Illumina, www.illumina.com/
Sequenom, http://www.sequenom.com/
GE Healthcare, http://www6.gelifesciences.com/
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim
Haploview, http://www.broad.mit.edu/mpg/haploview/
HAPMAP, http://www.hapmap.org/
The Monaco Group at the Wellcome Trust Centre for Human Genetics (Neurogenetics), http://www.well.ox.ac.uk/monaco/
ALSPAC, http://www.bristol.ac.uk/alspac/
Manchester Language Study, http://www.manchesterlanguagestudy.co.uk/
Supplemental Data
References
- 1.Harel S., Greenstein Y., Kramer U., Yifat R., Samuel E., Nevo Y., Leitner Y., Kutai M., Fattal A., Shinnar S. Clinical characteristics of children referred to a child development center for evaluation of speech, language, and communication disorders. Pediatr. Neurol. 1996;15:305–311. doi: 10.1016/s0887-8994(96)00222-6. [DOI] [PubMed] [Google Scholar]
- 2.Law J., Boyle J., Harris F., Harkness A., Nye C. Prevalence and natural history of primary speech and language delay: Findings from a systematic review of the literature. Int. J. Lang. Commun. Disord. 2000;35:165–188. doi: 10.1080/136828200247133. [DOI] [PubMed] [Google Scholar]
- 3.Bishop D.V.M., North T., Donlan C. Genetic basis of specific language impairment: Evidence from a twin study. Dev. Med. Child Neurol. 1995;37:56–71. doi: 10.1111/j.1469-8749.1995.tb11932.x. [DOI] [PubMed] [Google Scholar]
- 4.SLI Consortium A genomewide scan identifies two novel loci involved in specific language impairment. Am. J. Hum. Genet. 2002;70:384–398. doi: 10.1086/338649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett C.W., Flax J.F., Logue M.W., Vieland V.J., Bassett A.S., Tallal P., Brzustowicz L.M. A major susceptibility locus for specific language impairment is located on 13q21. Am. J. Hum. Genet. 2002;71:45–55. doi: 10.1086/341095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SLI Consortium Highly significant linkage to the SLI1 locus in an expanded sample of individuals affected by specific language impairment. Am. J. Hum. Genet. 2004;74:1225–1238. doi: 10.1086/421529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falcaro M., Pickles A., Newbury D.F., Addis L., Banfield E., Fisher S.E., Monaco A.P., Simkin Z., Conti-Ramsden G., The SLI Consortium Genetic and phenotypic effects of phonological short-term memory and grammatical morphology in specific language impairment. Genes Brain Behav. 2008;7:393–402. doi: 10.1111/j.1601-183X.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- 8.Gathercole S.E., Willis C.S., Baddeley A.D., Emslie H. The children's test of nonword repetition: A test of phonological working memory. Memory. 1994;2:103–107. doi: 10.1080/09658219408258940. [DOI] [PubMed] [Google Scholar]
- 9.Gathercole S.E., Baddeley A.D. Phonological memory deficits in language disordered children: Is there a causal connection? J. Mem. Lang. 1990;29:336–360. [Google Scholar]
- 10.Gathercole S.E., Briscoe J., Thorn A., Tiffany C., ALSPAC Study Team Deficits in verbal long-term memory and learning in children with poor phonological short-term memory skills. Q. J. Exp. Psychol. (Colchester) 2008;61:474–490. doi: 10.1080/17470210701273443. [DOI] [PubMed] [Google Scholar]
- 11.Jones R.W., Ring S., Tyfield L., Hamvas R., Simmons H., Pembrey M., Golding J., ALSPAC study team A new human genetic resource: A DNA bank established as part of the Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) Eur. J. Hum. Genet. 2000;8:653–660. doi: 10.1038/sj.ejhg.5200502. [DOI] [PubMed] [Google Scholar]
- 12.Golding J., Pembrey M., Jones R. ALSPAC – The Avon Longitudinal Study of Parents and Children I. Study methodology. Paediatr. Perinat. Epidemiol. 2001;15:74–78. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 13.Burden V., Stott C.M., Forge J., Goodyer I. The Cambridge Language and Speech Project (CLASP). I. Detection of language difficulties at 36 to 39 months. Dev. Med. Child Neurol. 1996;38:613–631. doi: 10.1111/j.1469-8749.1996.tb12126.x. [DOI] [PubMed] [Google Scholar]
- 14.Clark A., O'Hare A., Watson J., Cohen W., Cowie H., Elton R., Nasir J., Seckl J. Receptive language disorder in childhood: Familial aspects and long term outcomes: Results from a Scottish study. Arch. Dis. Child. 2007;92:614–619. doi: 10.1136/adc.2006.101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti-Ramsden G., Crutchley A., Botting N. The extent to which psychometric tests differentiate subgroups of children with specific language impairment. J. Speech Lang. Hear. Res. 1997;40:765–777. doi: 10.1044/jslhr.4004.765. [DOI] [PubMed] [Google Scholar]
- 16.Conti-Ramsden G., Botting N. Characteristics of children attending language units in England: A national study of 7-year old. Int. J. Lang. Commun. Disord. 1999;34:359–366. doi: 10.1080/136828299247333. [DOI] [PubMed] [Google Scholar]
- 17.Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;15:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel S.B., Schaffner S.F., Nguyen H., Moore J.M., Roy J., Blumenstiel B., Higgins J., DeFelice M., Lochner A., Faggart M. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 19.Abecasis G.R., Cardon L.R., Cookson W.O. A general test of association for quantitative traits in nuclear families. Am. J. Hum. Genet. 2000;66:279–292. [Google Scholar]
- 20.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.L., Bender D., Maller J., Sklar P., deBakker P.I., Daly M.J. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimbert P., Valanciute A., Audard V., Pawlak A., Le Gouvelo S., Lang P., Niaudet P., Bensman A., Guellaën G., Sahali D. Truncation of C-mip (Tc-mip), a new proximal signaling protein, induces c-maf Th2 transcription factor and cytoskeleton reorganization. J. Exp. Med. 2003;198:797–807. doi: 10.1084/jem.20030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Missiaen L., Dode L., Vanoevelen J., Raeymaekers L., Wuytack F. Calcium in the Golgi apparatus. Cell Calcium. 2007;41:405–416. doi: 10.1016/j.ceca.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Faddy H.M., Smart C.E., Xu R., Lee G.Y., Kenny P.A., Feng M., Rao R., Brown M.A., Bissell M.J., Roberts-Thomson S.J. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem. Biophys. Res. Commun. 2008;369:977–981. doi: 10.1016/j.bbrc.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bishop D.V.M. Development of the Children's Communication Checklist (CCC): A method for assessing qualitative aspects of communicative impairment in children. J. Child Psychol. Psychiatry. 1998;39:879–891. [PubMed] [Google Scholar]
- 25.Rust J. The Psychological Corporation; London, UK: 1996. WOLD Wechsler Objective Language Dimensions Manual. [Google Scholar]
- 26.Lin P.I., Vance J.M., Pericak-Vance M.A., Martin E.R. No gene is an island: The flip-flop phenomenon. Am. J. Hum. Genet. 2007;80:531–538. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semel E.M., Wiig E.H., Secord W. Psychological Corporation; San Antonio: 1992. Clinical Evaluation of Language Fundamentals–Revised. [Google Scholar]
- 28.Wechsler D., Golombok S., Rust J. Third Edition. The Psychological Corporation; Sidcup, UK: 1992. Wechsler Intelligence Scale for Children: UK Manual. [Google Scholar]
- 29.Archibald L.M., Gathercole S.E. Nonword repetition in specific language impairment: More than a phonological short-term memory deficit. Psychon. Bull. Rev. 2006;14:919–924. doi: 10.3758/bf03194122. [DOI] [PubMed] [Google Scholar]
- 30.Conti-Ramsden G., Durkin K. Phonological short-term memory, language and literacy: Developmental relationships in early adolescence in young people with SLI. J. Child Psychol. Psychiatry. 2007;48:147–156. doi: 10.1111/j.1469-7610.2006.01703.x. [DOI] [PubMed] [Google Scholar]
- 31.Bishop D.V.M. What causes Specific Language Impairment in children? Curr. Dir. Psychol. Sci. 2006;15:217–221. doi: 10.1111/j.1467-8721.2006.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesch K.P., Timmesfeld N., Renner T.J., Halperin R., Röser C., Nguyen T.T., Craig D.W., Romanos J., Heine M., Meyer J. Molecular genetics of adult ADHD: Converging evidence from genome-wide association and extended pedigree studies. J. Neural Transm. 2008;115:1573–1585. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- 33.Cohen N.J., Vallance D.D., Barwick M., Im N., Menna R., Horodezky N.B., Isaacson L. The interface between ADHD and language impairment: An examination of language, achievement, and cognitive processing. J. Child Psychol. Psychiatry. 2000;41:353–362. [PubMed] [Google Scholar]
- 34.Pennington B.F., Bishop D.V.M. Relations among speech, language and reading disorders. Annu. Rev. Psychol. 2009;60:283–306. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- 35.Kibby M.Y., Cohen M.J. Memory functioning in children with reading disabilities and/or attention deficit/hyperactivity disorder: A clinical investigation of their working memory and long-term memory functioning. Child Neuropsychol. 2008;14:525–534. doi: 10.1080/09297040701821752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gathercole S.E., Alloway T.P. Practitioner review: Short-term and working memory impairments in neurodevelopmental disorders: Diagnosis and remedial support. J. Child Psychol. Psychiatry. 2006;47:4–15. doi: 10.1111/j.1469-7610.2005.01446.x. [DOI] [PubMed] [Google Scholar]
- 37.Hayiou-Thomas M.E. Genetic and environmental influences on early speech, language and literacy development. J. Commun. Disord. 2008;41:397–408. doi: 10.1016/j.jcomdis.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng J.Q., Poo M.M. Calcium signalling in neuronal motility. Annu. Rev. Cell Dev. Biol. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- 39.Normandin L., Hazell A.S. Manganese neurotoxicity: An update of pathophysiologic mechanisms. Metab. Brain Dis. 2002;17:375–387. doi: 10.1023/a:1021970120965. [DOI] [PubMed] [Google Scholar]
- 40.Grimbert P., Valanciute A., Audard V., Lang P., Guellaën G., Sahali D. The Filamin-A is a partner of Tc-mip, a new adapter protein involved in c-maf-dependent Th2 signaling pathway. Mol. Immunol. 2004;40:1257–1261. doi: 10.1016/j.molimm.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 41.Kamal M. C-mip interacts physically with RelA and inhibits nuclear factor kappa B activity. Mol. Immunol. 2009;46:991–998. doi: 10.1016/j.molimm.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 42.Mémet S. NF-kappaB functions in the nervous system: From development to disease. Biochem. Pharmacol. 2006;72:1180–1195. doi: 10.1016/j.bcp.2006.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


