Abstract
Study Objectives:
to analyze the agreement between effective CPAP–determined on the basis of a 7-night auto-adjusting positive airway pressure (APAP) trial at home with that obtained through 5 different predictive equations.
Methods:
Data were collected from consecutive CPAP-naïve patients with OSA who underwent a 7-night non-attended home-setting APAP trial. The 95th percentile APAP pressure was considered as the effective CPAP and also as the reference variable against which the equation-based predictions were compared. All patients fulfilled the following criteria: residual respiratory disturbances index (RDI) < 10 events/h, average air leak < 0.4 L/sec and > 4 h of use per night during the APAP trial.
Results:
A total of 100 consecutive patients (70 men) with the following characteristics were included: mean age 49 ± 11 years, body mass index 34 ± 4 kg/m2, diagnostic Epworth Sleepiness Scale score 14 ± 7, diagnostic RDI 56 ± 28 events/h, 95th percentile APAP 11 ± 2 cm H2O, hours of use per night 6.2 ± 1.3, and residual RDI 5 ± 2 events/h. A poor level of agreement between the 95th percentile pressure and the pressures obtained through 5 predictive equations was observed (the intra-class correlation coefficient ranged from 0.17 to 0.32).
Conclusions:
The disagreement observed between the effective CPAP determined through a 7-night APAP trial and the pressures obtained by the predictive equations suggest that long-term CPAP prescriptions based on predictive equations may be improper.
Citation:
Torre-Bouscoulet L; Castorena-Maldonado A; López-Escárcega E; Vázquez-García JC; Pérez-Padilla R. Agreement between 95th percentile pressure based on a 7-night auto-adjusting positive airway pressure trial vs. equation-based predictions in sleep apnea. J Clin Sleep Med 2009;5(4):311-316.
Keywords: sleep apnea, titration, CPAP, APAP, treatment, equations
Obstructive sleep apnea syndrome is characterized by repetitive episodes of complete (apnea) or partial (hypopnea) upper airway obstruction occurring during sleep. These events often result in reductions in blood oxygen saturation and are usually terminated by brief arousals from sleep.1 Continuous positive airway pressure (CPAP) is the treatment of choice,2 and randomized, placebo-controlled studies have shown that this form of therapy improves subjective and objective daytime sleepiness, quality of life, cognitive performance, and psychological well-being in patients with this syndrome.3–6 In addition, when CPAP is used appropriately, it has been proven to reduce the risk of fatal and non-fatal cardiovascular events7 and traffic accidents,8 in addition to improving metabolic control in insulin-resistant patients.9
Full-night, attended polysomnography performed in the laboratory is the preferred approach for titration to determine optimal positive airway pressure;10,11 however, simplified methods including auto-adjusting positive airway pressure (APAP) titration12 and CPAP-prescriptions based on predictive equations,13 have been introduced as practical strategies designed to save time and reduce costs.
Even though the American Academy of Sleep Medicine recently approved APAP devices as a reliable method of CPAP titration in patients with moderate-to-severe OSA without significant comorbidities,12 it is not known if the attended or unattended APAP titration conducted on only one night offers advantages over that determined over longer periods of time. Intuitively, one would consider as “effective” a CPAP pressure that, over time, eliminates respiratory pauses, results in an improved perception of symptoms, and reduces the risks associated with this disorder. In addition, an effective CPAP would be associated with adequate adherence and minimal air leakage.
CPAP prescription based on predictive equations is an even simpler strategy for determining CPAP pressure, and one that has proven to be as effective as the standard method and APAP titrations.13 Indeed, an interesting study by Masa et al.14 demonstrated that 3 different methods of determining effective CPAP (manual, APAP, and predictive equations) were equally effective in terms of decreasing the excessive daytime sleepiness and the apnea-hypopnea index, while at the same time attaining indistinguishable adherence rates. It would appear, then, that the method of determining effective CPAP does not play a decisive role in improving a patient's clinical condition; however, little is yet known as to whether these methods of CPAP titration would generate different results if other therapeutic targets, such as cardiovascular risk factors, were taken into account.15
Given that APAP devices have recently been approved as a reliable strategy for titration, and because of the availability of several predictive equations that have been proposed as a prescription-based approach, the purpose of this study was to analyze the agreement between effective CPAP determined on the basis of a 7-night APAP at-home trial, and the values of 5 different predictive equations, one of which was derived from the same ethnic group. The hypothesis of this study was that a reduced agreement between 7-night APAP pressure and equation-based predictions would be found.
METHODS
The study was conducted in a single tertiary care center in Mexico City (7,350 feet) and was approved by the institutional review board. Data were collected retrospectively, and appropriate care was taken to protect patients' privacy and identity. We analyzed data from consecutive CPAP-naïve patients with OSA who underwent a 7-night non-attended home-setting APAP trial.
All patients completed a standardized questionnaire on sleep symptoms that included the Spanish-validated Epworth Sleepiness Scale (ESS),16 and a physical examination with anthropometric measurements (weight, height, neck circumference) was performed. In addition, the clinical probability of OSA was determined on the basis of the adjusted neck circumference,17 wherein an index > 48 cm was considered indicative of a high pre-test probability. According to Flemons,17 the adjusted neck circumference is obtained as follows: neck circumference (measured in centimeters) is adjusted if the patient has hypertension (4 cm are added), is a habitual snorer (3 cm are added), or is reported to choke or gasp most nights (3 cm are added). A low clinical probability corresponds to an adjusted neck circumference of less than 43 cm; an intermediate probability (4 to 8 times as probable as a low probability) to a neck circumference of 43 to 48 cm; and a high probability (20 times as probable) to a neck circumference of more than 48 cm.
Following current recommendations,18 all patients with a high probability of OSA but no comorbid conditions—such as cardiac failure, chronic obstructive pulmonary disease or morbid obesity—were scheduled to complete a simplified respiratory polygraphy (SRP) using a portable device in an attended setting at the Sleep Unit. The SRP was done using the Remmers Sleep Recorder (SagaTech Electronics Inc., Calgary, Alberta, Canada), which measures oxygen saturation, respiratory movements, airflow, snoring, and leg movements.19 This device calculates an RDI on the basis of oxygen desaturation events. The algorithm sequentially scans each recorded oxygen saturation value (1 Hz). Whenever a drop in a sampled oxygen saturation value is detected, the program assigns an event marker to that reading. Because oxygen saturation values are sampled and recorded at 1 Hz, event markers are separated by no less than one second. When an increase in oxygen saturation is detected, the program determines if at least 3 consecutive event markers—that is, 3 consecutive falls in recorded oxygen saturation readings—were present prior to this rise. If this criterion is met and one of the event markers is associated with an oxygen saturation value > 4% lower than the baseline oxygen saturation, then a respiratory disturbance is designated. The quality of the SRP was ascertained through a visual analysis performed by an experienced sleep technician. An RDI ≥ 15 events/h has a sensitivity of 98% and a specificity of 88% for diagnosis of moderate-to-severe obstructive sleep apnea.19 We had previously verified the performance of this particular model of portable monitor in Mexico City at an altitude of 2,240 meters (7,350 feet) above sea level, where an intra-class correlation coefficient between the polysomnographic apnea hypopnea index (AHI) and the RDI of 0.89 (95% confidence interval, 0.83−0.96) was found.20 Data from patients examined using polysomnography were not included in this study. Patients that were considered apt candidates for the APAP trial (n = 152) were: (1) those with severe OSA (ESS ≥ 11 and RDI ≥ 30 events/h); (2) those with no sleepiness (ESS < 11) but an RDI ≥ 30 events/h; and, (3) patients with RDIs between 5 and 30 and ESS ≥ 11. The APAP trial lasted 7 consecutive nights in a non-attended home setting. All APAP trials were done using the same commercial brand of APAP device, the ResMed, Autoset Spirit (ResMed Inc, San Diego, CA, USA). The Autoset device measures mask airflow using a pneumotachograph, which processes the flow to determine respiratory airflow, leaks, and snoring. The pressure increases then in response to apneas, snoring, and episodes of flow limitation suggested by the shape of the inspiratory flow wave.21
All patients received detailed information concerning CPAP treatment and the nasal mask to be used was selected on an individual basis. One week after initiating APAP, the patients made a routine visit to the CPAP clinic, where the information stored in the APAP machines was downloaded to provide data on usage, mask leakage, pressure and AHI, all of which were entered in a database for later analysis.
We have reported previously on our clinical experience with APAP trials performed on patients with moderate-to-severe OSA who were evaluated using a simplified diagnostic approach.22 For the current study, we included data from a cohort of patients who, once they had completed the APAP trial, fulfilled the following criteria: residual RDI < 10 events/h, average air leak < 0.4 L/sec, and > 4 h of use per night during the APAP trial. These parameters were obtained from the APAP device. The 95th percentile APAP pressure during the 7-night APAP trial was considered as the effective CPAP pressure and also as the reference variable against which the equation-based predictions were compared. The predicted CPAP pressures were obtained using the following equations:
Hoffstein23 CPAP = (BMI × 0.16) + (NC × 0.13) + (AHI × 0.04) – 5.12
Stradling24 CPAP = (NC × 0.128) + (ODI × 0.048) + 2.1
Series25 CPAP = (BMI × 0.193) + (NC × 0.007) + (AHI × 0.02) – 0.611
Loredo26 CPAP = 30.8 + RDI × 0.03 - nadir saturation × 0.05 - mean saturation × 0.2)
-
Torre27 Male: CPAP = (BMI × 0.09) + (ODI × 0.01) – (mean SpO2 × 0.06) + 11.9
Female: CPAP = (BMI × 0.07) + (ESS × 0.1) + (ODI × 0.02) + 5.33
Where BMI is body mass index, NC is neck circumference, AHI is the apnea-hypopnea index, ODI is the oxygen desaturation index, RDI is the respiratory disturbance index, and ESS is the Epworth Sleepiness Score.
Statistical Analysis
Data are summarized and presented in the form of mean ± standard deviation. Agreement analysis was performed by calculating the intra-class correlation coefficient (ICC) and its 95% confidence interval (95% CI). The average difference between the 95th percentile APAP minus the estimated CPAP calculated on the basis of each equation and the 95% limits of agreement are presented. Agreement is shown graphically using modified Bland & Altman plots. The level of statistical significance was p < 0.05. Analysis was performed using a commercial statistics package (Stata, release 9.2, StataCorp, College Station, TX, USA).
RESULTS
A total of 152 patients were considered as apt candidates for a 7-night non-attended home-setting APAP trial; however, 52 of them failed to meet the inclusion criteria. Therefore, only data from 100 patients (30 women, 70 men) were included. The general characteristics of the entire group as well as the main results of the SRP and APAP trials are shown in Table 1.
Table 1.
General Characteristics of the Subjects, n = 100
| Parameter | Values |
|---|---|
| Women/Men n | 30 / 70 |
| Age (years) | 49 ± 11 |
| BMI (kg/m2) | 34 ± 4 |
| Neck size (cm) | 43 ± 4 |
| Diagnostic ESS score | 14 ± 7 |
| Diagnostic RDI (events/hr) | 56 ± 28 |
| Mean SpO2 during the night (%) | 85 ± 7 |
| 95th percentile APAP (cm H2O) | 11 ± 2 |
| Mean air leakage (L/sec) | 0.1 ± 0.08 |
| Hours used per night (h/night) | 6.2 ± 1.3 |
| Residual RDI (events/h) | 5 ± 2 |
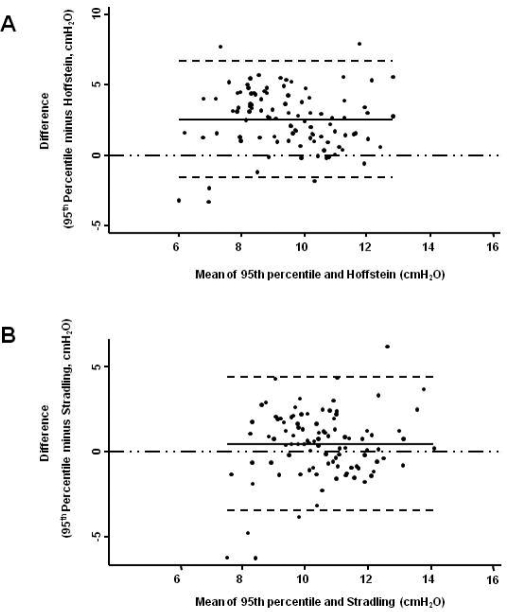
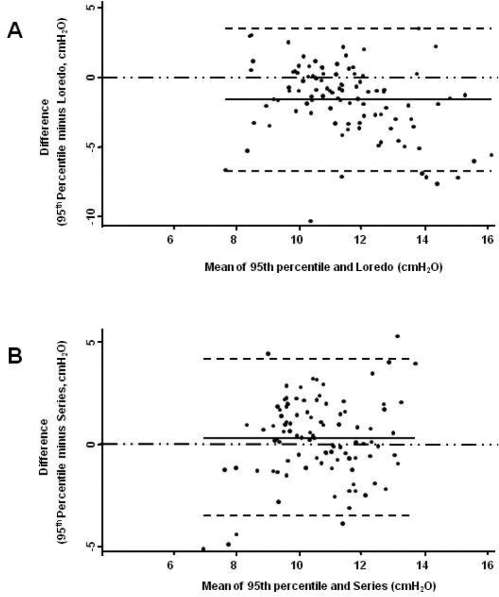
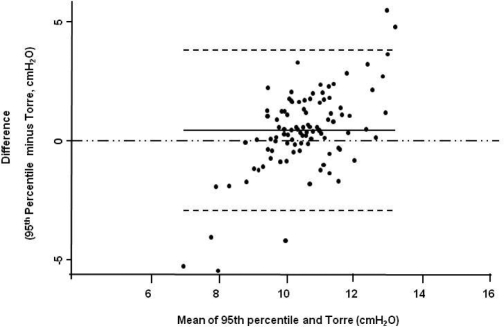
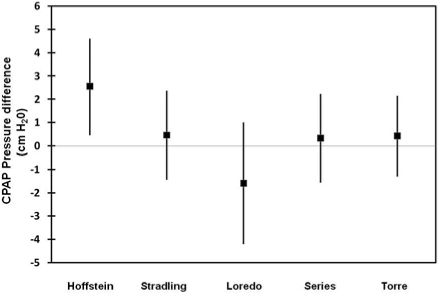
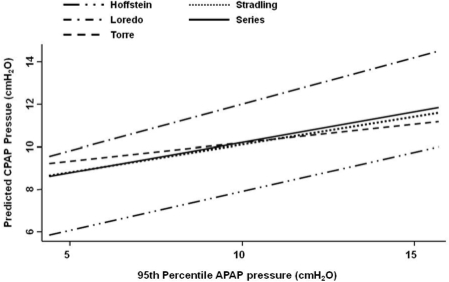
The agreement analysis between the 95th percentile APAP and the figures obtained from the predictive equations is shown in Table 2. The Stradling, Series, and Torre equations were found to have the narrowest 95% limits of agreement and the smallest average difference between the 95th percentile APAP and the estimated pressure. In comparison to the 95th percentile APAP, Hoffstein's equation predicted lower CPAP pressures, while Loredo's predicted higher ones. In addition, the 95% limits of agreement for the Hoffstein and Loredo equations ranged from −1.5 to 6.6 and −6.7 to 3.5 cm H2O, respectively. Figures 1-3 show the modified Bland & Altman plots for each equation. The average differences between the 95th percentile APAP and that obtained by each one of the equations used (95th percentile APAP minus estimated CPAP pressure) are shown in Figure 4. Finally, Figure 5 shows the linear prediction for each equation. Note that for a given CPAP pressure of 10 cm H2O (x axis), the Stradling, Series, and Torre equations predict almost identical pressures.
Table 2.
Agreement between 95th Percentile APAP Pressure and Equation-Based Predictions
| ICC | 95% CI | Difference‡ | 95% Limits of Agreement | |
|---|---|---|---|---|
| Hoffstein | 0.17* | 0.07–0.27 | 2.56 | -1.5 to 6.6 |
| Stradling | 0.29* | 0.11–0.46 | 0.46 | -3.4 to 4.3 |
| Loredo | 0.23* | 0.09–0.37 | -1.60 | -6.7 to 3.5 |
| Series | 0.32* | 0.15–0.49 | 0.33 | -3.4 to 4.1 |
| Torre | 0.26* | 0.12–0.40 | 0.43 | −2.9 to 3.8 |
The 95th percentile APAP pressure was considered as the reference value. ICC, Intraclass correlation coefficient; 95% CI, 95% confidence interval.
Difference = 95th percentile APAP pressure – CPAP predicted by the equation;
p < 0.01.
Figure 1.
Modified Bland & Altman Plots. The 95th percentile APAP was considered as the reference value. Negative figures imply that the values predicted by the equation were greater than the 95th percentile APAP. Panel A is for Hoffstein's predicted values and Panel B for Stradling's. The continuous line is the observed average agreement; dash lines are 95% limits of agreement, and y = 0 is the line of perfect average agreement.
Figure 2.
Modified Bland & Altman Plots. The 95th percentile APAP was considered as the reference value. Panel A is for Loredo's predicted values and Panel B for Series'. The continuous line is the observed average agreement; dash lines are 95% limits of agreement, and y = 0 is the line of perfect average agreement.
Figure 3.
Modified Bland & Altman Plot. The 95th percentile APAP was considered as the reference value. The predicted values are from Torre's equation. The continuous line is the observed average agreement; dash lines are 95% limits of agreement, and y = 0 is the line of perfect average agreement.
Figure 4.
Mean and standard deviation of the predicted values for each equation. The 95th percentile APAP was considered as the reference value. In general and compared to the reference (95th percentile APAP), Loredo's equation predicts higher pressure, whereas Hoffstein's predicts lower ones. The line of perfect average agreement is y = 0.
Figure 5.
Linear predictions of CPAP pressure for each equation. According to the fitted values, Hoffstein's equation consistently predicts the lowest CPAP pressures, whereas Loredo's predicts the highest ones.
In order to explore the potential practical implications of our data, we classified the patients according to the CPAP predicted by each equation as “low,” “high,” or “appropriate” predicted CPAP (Table 3). CPAP was considered low when the prediction was ≥ 1.5 cm H2O lower than the 95th percentile APAP pressure, and high when the prediction was ≥ 1.5 cm H2O higher than the 95th percentile APAP pressure. We believe, albeit intuitively, that a difference of 1.5 cm H2O or greater between the predicted value and the APAP pressure may have detrimental clinical implications in terms of residual sleep apnea, adherence, and sleep fragmentation.
Table 3.
Percentage of Patients Classified According to the Predicted CPAP by Each Equation as “Low,” “High,” or “Appropriate” Predicted CPAP
| Equation | Low CPAP | Appropriate CPAP | High CPAP |
|---|---|---|---|
| Hoffstein | 69% | 27% | 4% |
| Stradling | 28% | 62% | 10% |
| Loredo | 9% | 46% | 45% |
| Series | 28% | 58% | 14% |
| Torre | 24% | 67% | 9% |
Low CPAP was considered when the prediction was ≥ 1.5 cm H2O lower than the 95th percentile APAP pressure, and high when the prediction was ≥ 1.5 cm H2O higher than the 95th percentile APAP pressure.
DISCUSSION
The main findings in this study were: (1) a poor agreement between the 95th percentile pressure obtained by the 7-night APAP trial and the pressures calculated by the 5 predictive equations; therefore, predicted values do not agree well with this choice for prescription; and, (2) though overall agreement was low, the Stradling, Series, and Torre equations proved to have better predictive ability than those from Hoffstein and Loredo (the Hoffstein equation consistently predicted lower pressures compared to the 95th percentile pressure, whereas in most cases, predictions based on the Loredo equation were higher).
The best method of calculating long-term effective CPAP pressure has not yet been definitively determined. Though manual titration under attended polysomnography is still considered the gold standard,11 the 95th percentile pressure obtained over 7 nights may provide a better estimate than a titration conducted on only one night.28 In fact, one night of titration may yield a positive pressure higher than that needed, reflecting the well-known effect of CPAP on the way in which the upper airway soft tissues resolve the vibration-induced edema.29 In addition, we accepted 95th percentile pressure as the “effective” level as long as it was not associated with any residual sleep apnea or air leaks, and with at least 4 hours of use per night. We believe that this strict definition may better represent the long-term requirements of positive pressure.
The predictive equations assessed in this study are frequently used to determine effective CPAP and were derived from different population bases. While each equation has its own predictive ability, the parameters used in generating them were different; for instance, while AHI is included in the Series and Hoffstein equations, Stradling's and Torre's use ODI, and Loredo's combines RDI with nadir and mean saturation. Also, manual titration was performed in those studies for several different targeted outcomes, such as eliminating snoring, eliminating flow limitation, normalizing sleep architecture, and/or normalizing gas exchange. Taken together, these factors may explain, at least in part, the disagreement observed between 95th percentile pressure and the equation-based predictions.
While the equations that better predicted effective CPAP were those of Stradling, Series, and Torre, none of the equations tested were found to consistently predict effective CPAP at different pressure levels. For example, Hoffstein's equation properly estimated effective CPAP at low pressures but failed at high ones ( > 10 cm H2O). In contrast, the Loredo equation consistently predicted higher pressures than those considered “effective.” Furthermore, the equation derived from the same ethnic group (Torre) also failed at the extremes of the distribution of effective CPAP. Interestingly, at 10 cm H2O of effective CPAP, 3 of the 5 equations (Stradling, Series, Torre) predicted the effective pressure reasonably well (Figure 5). This last finding is of some practical importance, as the closer to 10 the predicted pressure comes, the better is the estimate of effective CPAP; however, the “best fit” around 10 cm H2O could be statistically expected because most patients (and therefore more observations) require pressure close to that value. Table 3 shows that the predicted values were considered appropriate in a range from 27% to 67%. Though these figures may appear to be “good,” this is not true if we consider that other methods exist, even in a simplified setting, to determine the “effective” pressure. Even in the best case scenario, inadequate pressure readings could be prescribed for perhaps one-third of all patients.
Based on our results, and taking into account previous studies,15 we believe that there is not yet sufficient evidence to support the use of predictive equations as a reasonable strategy for determining optimum long-term CPAP pressure. Certainly, one could suggest that CPAP estimates based on equations may constitute a temporary approach that can be used until other more efficient methods of titration become available; however, even as a short-term strategy, there are certain issues that should be taken into account, as the evidence currently available suggests that patients' initial experience with CPAP treatment during the titration process may be a crucial factor in determining their subsequent use of this treatment modality.30 It is possible, at least theoretically, that if the CPAP figure predicted by the equation were higher or lower than effective CPAP, patients might feel uncomfortable at that pressure level and thus decrease long-term CPAP adherence; however, this issue is merely speculative, as the results of this study do not allow us to affirm whether the pressures determined by APAP improve adherence. In addition, it is not yet known whether the disagreement observed between effective CPAP and equation-based predictions might have implications for the well-known effect of long-term CPAP use. Moreover, the differences in pressure observed among the methods used to determine effective CPAP, while perhaps of little importance for certain purposes14 (i.e., daytime sleepiness, quality of life) could, in fact, turn out to be highly significant when achieving other, even more specific, targets is considered (i.e., serological markers of cardiovascular risk, or metabolic parameters). Further studies are needed to elucidate whether these titration methods are equally efficient in decreasing the cardiovascular morbidity associated with OSA.
Finally, in comparison to manual or APAP titrations, a higher residual AHI has been reported when CPAP was determined using equation-based predictions.14 While the extent of health damage associated with residual OSA has yet to be determined, efforts should be made to assure optimum positive airway pressure. We believe, therefore, that there is little reason to use predictive equations and that caution is to be recommended when prescribing long-term CPAP based on such predictions.
The main limitation of our study is that we are not able to state categorically just what the potential relevance of the disagreement observed between effective CPAP and the equation-based estimates may be, as the potential implications of this disagreement are still only theoretical and have yet to be demonstrated. The lack of manual-determined pressure is also a limitation because it is considered to be the standard. In addition, we recognize that the definition of the 95th percentile APAP as the “gold standard” has inherent limitations because no other clinical outcomes (e.g., sleepiness, quality of life) other than CPAP adherence and residual RDI, were taken into account.
In conclusion, the disagreement observed between the effective CPAP determined on the basis of a 7-night APAP trial and the pressures obtained through the predictive equations suggests that long-term CPAP prescriptions based on predictive equations may be improper and, therefore, only acceptable when better methods of estimation are not available. Further studies are needed to elucidate whether that disagreement would negatively affect the well-known advantages of the long-term use of CPAP in patients with OSA, mainly on cardiovascular morbidity and mortality.7
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Lourdes Galicia-Polo, Rocío Baños-Flores, Elisa Sánchez-Gallén, Sandra Anaya-Ramírez, and Raúl Peñuelas-Baldenebro for their technical support, and Mr. Paul Kersey for correcting language use.
REFERENCES
- 1.American Academy of Sleep Medicine. Diagnostic and classification manual. 2nd edition. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders. [Google Scholar]
- 2.Loube DI, Gay PC, Strohl KP, et al. Indications for positive airway pressure treatment of adult obstructive sleep apnea patients: a consensus statement. Chest. 1999;115:863–6. doi: 10.1378/chest.115.3.863. [DOI] [PubMed] [Google Scholar]
- 3.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 4.Engleman HM, Martin SE, Kingshott RN, et al. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53:341–5. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engleman HM, Kingshott RN, Wraith PK, et al. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:461–7. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 6.Munoz A, Mayoralas LR, Barbe F, Pericas J, Agusti AG. Long-term effects of CPAP on daytime functioning in patients with sleep apnoea syndrome. Eur Respir J. 2000;15:676–81. doi: 10.1034/j.1399-3003.2000.15d09.x. [DOI] [PubMed] [Google Scholar]
- 7.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 8.Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. Am J Respir Crit Care Med. 2000;161:857–9. doi: 10.1164/ajrccm.161.3.9812154. [DOI] [PubMed] [Google Scholar]
- 9.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2, diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–52. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- 10.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 11.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 12.Morgenthaler T, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine Report. Sleep. 2008;31:141–7. doi: 10.1093/sleep/31.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West SD, Jones DR, Stradling JR. Comparison of three ways to determine and deliver pressure during nasal CPAP therapy for obstructive sleep apnoea. Thorax. 2006;61:226–31. doi: 10.1136/thx.2005.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masa JF, Jimenez A, Duran J, et al. Alternative methods of titrating continuous positive airway pressure: a large multicenter study. Am J Respir Crit Care Med. 2004;170:1218–24. doi: 10.1164/rccm.200312-1787OC. [DOI] [PubMed] [Google Scholar]
- 15.Marrone O, Salvaggio A, Romano S, Insalaco G. Automatic titration and calculation by predictive equations for the determination of therapeutic continuous positive airway pressure for obstructive sleep apnea. Chest. 2008;133:670–6. doi: 10.1378/chest.07-1372. [DOI] [PubMed] [Google Scholar]
- 16.Chiner E, Arriero JM, Signes-Costa J, Marco J, Fuentes I. [Validation of the Spanish version of the Epworth Sleepiness Scale in patients with a sleep apnea syndrome] Archivos de bronconeumologia. 1999;35:422–7. doi: 10.1016/s0300-2896(15)30037-5. [DOI] [PubMed] [Google Scholar]
- 17.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 18.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 19.Vazquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torre-Bouscoulet L C-MA, Baños-Flores R, Vázquez-García JC, Meza-Vargas MS, Pérez-Padilla R. Agreement between oxygen desaturation index and apnea–hypopnea index in adults with suspected obstructive sleep apnea at an altitude of 2240 m. Archivos de bronconeumologia. 2007;43:646–51. doi: 10.1016/s1579-2129(07)60150-5. [DOI] [PubMed] [Google Scholar]
- 21.Kessler R, Weitzenblum E, Chaouat A, Iamandi C, Alliotte T. Evaluation of unattended automated titration to determine therapeutic continuous positive airway pressure in patients with obstructive sleep apnea. Chest. 2003;123:704–10. doi: 10.1378/chest.123.3.704. [DOI] [PubMed] [Google Scholar]
- 22.Torre Bouscoulet L, Meza-Vargas MS, Castorena-Maldonado A, Reyez-Zúñiga M, Pérez-Padilla R. Autoadjusting positive pressure trial in adults with sleep apnea assessed by a simplified diagnostic approach. J Clin Sleep Med. 2008;4:341–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffstein V, Mateika S. Predicting nasal continuous positive airway pressure. Am J Respir Crit Care Med. 1994;150:486–8. doi: 10.1164/ajrccm.150.2.8049834. [DOI] [PubMed] [Google Scholar]
- 24.Stradling JR, Hardinge M, Paxton J, Smith DM. Relative accuracy of algorithm-based prescription of nasal CPAP in OSA. Respir Med. 2004;98:152–4. doi: 10.1016/j.rmed.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Series F. Accuracy of an unattended home CPAP titration in the treatment of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:94–7. doi: 10.1164/ajrccm.162.1.9908023. [DOI] [PubMed] [Google Scholar]
- 26.Loredo JS, Berry C, Nelesen RA, Dimsdale JE. Prediction of continuous positive airway pressure in obstructive sleep apnea. Sleep Breath. 2007;11:45–51. doi: 10.1007/s11325-006-0082-x. [DOI] [PubMed] [Google Scholar]
- 27.Torre Bouscoulet L, Lopez Escarcega E, Castorena Maldonado A, et al. [Continuous positive airway pressure used by adults with obstructive sleep apneas after prescription in a public referral hospital in Mexico City] Arch Bronconeumol. 2007;43:16–21. doi: 10.1016/s1579-2129(07)60015-9. [DOI] [PubMed] [Google Scholar]
- 28.Bachour A, Virkkala JT, Maasilta PK. AutoCPAP initiation at home: optimal trial duration and cost-effectiveness. Sleep Med. 2007;8:704–10. doi: 10.1016/j.sleep.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Ryan CF, Lowe AA, Li D, Fleetham JA. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after chronic nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1991;144:939–44. doi: 10.1164/ajrccm/144.4.939. [DOI] [PubMed] [Google Scholar]
- 30.Drake CL, Day R, Hudgel D, et al. Sleep during titration predicts continuous positive airway pressure compliance. Sleep. 2003;26:308–11. doi: 10.1093/sleep/26.3.308. [DOI] [PubMed] [Google Scholar]