Abstract
Objectives:
A high proportion of children with Down syndrome (DS) have the obstructive sleep apnea syndrome (OSAS). Although adults with DS have many predisposing factors for OSAS, this population has not been well studied. We hypothesized that OSAS is common in adults with DS, and that the severity of OSAS is worse in DS adults who are more obese.
Design:
Cohort study
Setting:
Sleep laboratory
Participants:
16 adults with DS underwent evaluation for sleep disordered breathing.
Interventions:
Polysomnographic were compared to a retrospective sample of adult patients referred for clinically suspected OSAS.
Measurements and Results:
Polysomnograms were abnormal in 94% of DS subjects. The median apnea hypopnea index (AHI) was 37/h (range 0–118). The median arterial oxygen saturation nadir was 75% (23% to 95%), and the median peak end-tidal CO2 was 58 (47–66) mm Hg. There was a significant correlation between body mass index and AHI (r = 0.53, p < 0.05). Sixty-three percent had an Epworth score > 10. The AHI and saturation nadir were significantly worse in DS than non-DS patients.
Conclusions:
Adults with DS frequently have OSAS, with obstructive apnea, hypoxemia, hypoventilation, and sleep fragmentation. The severity of OSAS correlated with obesity. We speculate that the complications of untreated OSAS (cardiovascular disease, increased mortality, and neurobehavioral morbidities including daytime sleepiness and impaired cognitive function) commonly overlap with the manifestations of DS and therefore may not elicit a prompt investigation in these patients. We speculate that OSAS is an important, but potentially treatable, cause of morbidity in adults with DS.
Citation:
Trois MS; Capone GT; Lutz JA; Melendres MC; Schwartz AR; Collop NA; Marcus CL. Obstructive Sleep Apnea in Adults with Down Syndrome. J Clin Sleep Med 2009;5(4):317-323.
Keywords: Sleep disordered breathing, Trisomy 21, hypothyroidism
The obstructive sleep apnea syndrome (OSAS) is a common condition, affecting approximately 2% to 4% of adults.1 It has significant neurological and cardiovascular complications.
Down syndrome (DS; trisomy 21) is the most common known chromosomal cause of mental retardation. It is a frequent genetic disorder, with an incidence of 1 per 660 live births.2 It is therefore important to fully understand the syndrome and to provide information to adequately manage and treat patients, so that individuals can achieve their full potential in terms of mental and physical health and their place in society. Thus, treatable conditions such as OSAS should be addressed.
OSAS has a higher prevalence in children with DS (30% to 55%)3–5 than in otherwise healthy children (2%).6 Patients with DS have many predisposing factors for OSAS, including midfacial hypoplasia and mandibular hypoplasia,7,8 glossoptosis, an abnormally small upper airway with superficially positioned tonsils and relative tonsillar and adenoidal encroachment, increased secretions, an increased incidence of lower respiratory tract anomalies,9 and a reduced tracheal diameter.10 In addition, in DS there is generalized hypotonia. As children with DS grow up, they maintain their anatomic abnormalities and generalized hypotonia. In addition, they have an increased risk of developing hypothyroidism and obesity, which are also risk factors for OSAS. Despite this high preponderance of risk factors for OSAS, there are few studies evaluating OSAS in adults with Down syndrome.
We hypothesized that OSAS was common in adults with DS. We also hypothesized that the severity of OSAS would be worse in those adults with DS who were most obese. We therefore performed sleep studies in adults with DS to better characterize the nature and severity of sleep disordered breathing in this population.
METHODS
Adults with DS were recruited and underwent polysomnography, measurement of thyroid function, and the Epworth Sleepiness Scale. Polysomnographic data were compared to data from clinical patients without DS, referred for evaluation of OSAS. Treatment was not part of this research study. However, the caregivers of subjects with abnormal thyroid function or sleep study results were informed of the results, and clinical evaluation was recommended.
Study Group
Adults with DS, aged ≥ 18 years, were eligible if they had no acute intercurrent infection at the time of the study and had not undergone prior treatment for OSAS during adulthood (such as continuous positive airway pressure therapy or uvulopalatopharyngoplasty). Subjects who were treated during childhood (e.g., with tonsillectomy and adenoidectomy) were eligible for participation because certain risk factors for OSAS, such as obesity and hypothyroidism, can become manifest during adulthood in the DS population. Furthermore, OSAS has also been shown to recur during adolescence in the general population.11
Subjects were recruited from the local Association of Retarded Citizens (ARC), Parents of Down Syndrome (PODS) group meetings and the Kennedy Krieger Down Syndrome Clinic. The Kennedy Krieger Institute serves the needs of individuals with developmental disabilities. In addition, one subject was referred for clinical evaluation. Talks were given and fliers were distributed at PODS and ARC group meetings in the greater Baltimore and Washington D.C. area, and advertisements were submitted to their publications. Subjects and their families were told that this was a study on sleep, but were not told that the study was to assess sleep disorders or apnea. Subjects received $50 as reimbursement for their time and expenses, such as transportation and meals. Twenty-seven local ARC and PODS groups were contacted, and 97 letters were sent to patients with DS followed at the Kennedy Krieger Down Syndrome Clinic, of which 20 were returned as undeliverable.
The study was explained personally by one of the investigators. Informed consent was obtained from the parents or legal guardian, and assent from the patient. When the subject was their own legal guardian, then informed consent was obtained from the subject him/herself. The study was approved by the Western Institutional Review Board.
Sixteen adults with DS were recruited. Eight subjects at the clinic were approached, all of whom agreed to participate. The other 8 subjects responded to the fliers/letters. The demographic characteristics of the study population are shown in Table 1. The subjects were 19 to 56 (median 33) years of age. Eight of the subjects were female (of whom 4 were postmenopausal), 12 were obese, and an additional 2 were overweight. One patient was Asian and the others were Caucasians. One subject had a previous diagnosis of diabetes mellitus, one had albinism, three had a history of depression, three had obsessive compulsive disorder and one had Alzheimer's disease. One subject had undergone scoliosis surgery. Six subjects were receiving serotonin reuptake inhibitor drugs and one subject was on an unknown type of antidepressant, one was on an anti-psychotic drug and another used insulin. None of the patients had a history of chronic lung diseases, smoking or alcohol intake.
Table 1.
Study Group
| DS | Controls | |
|---|---|---|
| N | 16 | 48 |
| Age (years) | 33 (19-56) | 33 (17-56) |
| Male, (N, %) | 8 (50) | 24 (50) |
| Body mass index (kg/m2) | 31 (22-51) | 29 (20-52) |
| Hypothyroid at the time of study (N, %) | 8 (57)* | NA |
| Epworth Sleepiness Scale | 12 (3-20) | NA |
All data are displayed as median and range where appropriate There were no significant differences between groups.
NA, not available.
N = 14
Control Group
Controls were obtained retrospectively from a clinical database of 3,934 patients who underwent standard diagnostic nocturnal polysomnography12 at the Johns Hopkins University adult Sleep Center for evaluation of suspected OSAS. Three controls were selected for each subject with DS, based on the first 3 sequential controls in the database that most closely matched the DS subjects for age, sex, and body mass index (BMI).
Forty-eight matched controls were obtained from the database. These subjects were well-matched to the DS cohort, with 50% being male, a median (range) age of 33 (17–56) years (nonsignificant), and mean BMI of 29 (20–52) kg/m2 (nonsignificant).
History and Physical Examination
A sleep specialist performed a standardized history and physical examination, including measurement of blood pressure, on all subjects with DS in the evening prior to the sleep study. As per standard clinical practice, the presence of Down syndrome was verified by the clinical characteristics of the syndrome. Height and weight were obtained. The subject was defined as overweight if the BMI was ≥ 25 kg/m2, and obese if the BMI was ≥ 30 kg/m2.
The Epworth Sleepiness Scale,13 a subjective measure of the propensity to fall asleep in 8 situations, was administered via proxy to the accompanying caregiver. The Epworth scale was modified to adjust to the reality of these subjects, who do not drive. Thus, question 8 was taken to refer to the subject as a passenger in the car, while stopped for a few minutes in traffic.
Thyroid Function Testing
Thyroid-stimulating hormone (TSH) and free levothyroxine (T4) levels were tested the morning following the sleep study, unless there was a prior accessible result from the past 6 months.
Polysomnography
Subjects with DS underwent an overnight polysomnogram in the sleep laboratory located within the General Clinical Research Center at Johns Hopkins University. No sedation or sleep deprivation was used prior to the study. An adult parent or guardian was encouraged to accompany the subject. Subjects arrived at the laboratory at 21:00, and studies were terminated at 07:00. The following parameters were recorded: electroencephalogram (C3/A2,01/A2), right and left electroculograms, submental electromyogram, tibial electromyogram, electrocardiogram, chest and abdominal wall motion (piezoelectric transducers or inductance plethysmography), nasal pressure and oral airflow (nasal pressure cannula with oral thermistor bead, Pro-Tech, Woodinville, WA), end-tidal CO2 measured at the nose by infrared capnometry (Novametrix CO2SMO, Wallingford, CT), arterial oxygen saturation by pulse oximetry (Masimo Quartz Q-400 pulse oximeter, Louisville, CO), and oximeter pulse waveform. Subjects were also monitored and recorded on videotape, using an infrared video camera. They were continuously observed by a trained polysomnography research technician.
All polysomnograms on subjects with DS were scored by a registered experienced sleep technologist and subsequently reviewed by a single investigator, a physician experienced in sleep medicine, to ensure consistency.
The following parameters were measured:
Sleep architecture: Assessed by standard techniques.14 Arousals were defined as recommended by the American Sleep Disorders Association.15
Apnea: Obstructive apneas and hypopneas (partial obstructions) were scored according to standard adult criteria.12,16 Obstructive apneas were defined as the presence of chest/abdominal wall motion in the absence of airflow, for ≥ 10 sec. Hypopneas were defined as events ≥ 10 sec in which there was a decrease in oronasal airflow of at least 50%, or a smaller decrease associated with desaturation ≥ 4% or arousal. Mixed apneas were defined as apneas having both central and obstructive components. The apnea hypopnea index (AHI) was defined as the number of obstructive apneas, hypopneas and mixed apneas per hour of sleep. Central apneas were defined as cessation of airflow and respiratory effort ≥ 10 sec. The central apnea index was defined as the number of central apneas per hour of sleep.
Arterial oxygen saturation (SpO2): The SpO2 nadir, mean SpO2, and percentage of total sleep time during which SpO2 was < 90% were quantitated using the Alice software. SpO2 measurements associated with a poor pulse waveform were omitted.
End-tidal carbon dioxide (ETCO2): The mean and peak ETCO2 were measured using the Alice software. The percentage of total sleep time during which ETCO2 was ≥ 50mm Hg was quantified.
Control polysomnography results were obtained retrospectively. The control polysomnograms were performed at the Johns Hopkins Bayview Medical Center using the same techniques for performing and scoring polysomnography,12,14–16 except that ETCO2 was not measured and the arousal index was not scored. Blood pressure, Epworth Sleepiness Scale scores, and thyroid function tests were not available for controls.
Statistical Methods
Nonparametric methods were used, as most data were not normally distributed. All results were expressed as median (range) unless otherwise specified. Differences were compared between subjects and controls using the Wilcoxon rank sum test.
RESULTS
Polysomnography
Polysomnographic results are shown in Table 2. Most subjects with DS tolerated the procedure well and did not try to remove the monitoring equipment. However, several had difficulty sleeping in the laboratory situation. Sleep efficiency was < 85% in 69% of the subjects. In the morning, caregivers filled out a questionnaire commenting on whether the night's sleep was representative of a typical night's sleep or not. Seven caregivers stated that it was typical, 4 that it was atypical (3 subjects slept worse than usual and one slept better than usual), and 5 did not respond.
Table 2.
Polysomnography Results
| DS | Controls | P value | |
|---|---|---|---|
| Sleep efficiency (%) | 67 (16-95) | 88 (15-99) | 0.000 |
| Total sleep time (min) | 307 (71-455) | 380 (84-698) | 0.003 |
| Arousal index (N/hr) | 19 (4-98) | NA | NA |
| Stage 1 (% TST) | 9 (3-31) | 13 (3-62) | 0.028 |
| Stage 2 (%TST) | 58 (29-85) | 63 (25-79) | 0.317 |
| Slow wave sleep (%TST) | 15 (0-30) | 1 (0-38) | 0.019 |
| REM sleep (% TST) | 19 (0-48) | 17 (0-33) | 0.364 |
| Obstructive apnea hypopnea index (N/hr) | 37 (0-118) | 16 (0-148) | 0.036 |
| Central apnea index (N/hr) | 0 (0-10) | 0 (0-10) | 0.680 |
| SpO2 nadir (%) | 75 (23-95) | 93 (77-100) | 0.000 |
| Peak ETCO2 (mm Hg) | 58 (47-66) | NA | NA |
All data are displayed as median and range where appropriate.
NA, not available; REM, rapid eye movement; TST, total sleep time.
Sleep Architecture
The median amount of slow wave sleep in subjects with DS was 15% of total sleep time (Table 2); however, slow wave sleep was absent in 4 of the 16 subjects. One of these patients was 56 years old, but the others were in their 20s; two of these patients had very low sleep efficiency. Although the median amount of REM sleep was normal, the percentage of REM time as a proportion of total sleep time was markedly increased in 3 of the subjects (38% to 48%). Two patients did not have any REM sleep, but both slept very poorly overall, with a total sleep time of 106 minutes and 76 minutes, respectively. The arousal index varied from 4–98/h, with a median of 19/h, and correlated with the apnea hypopnea index (r = 0.64, p < 0.02).
Apnea
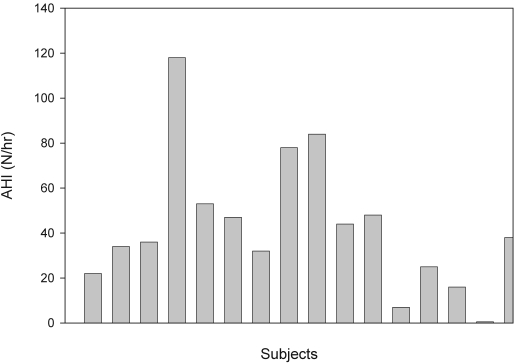
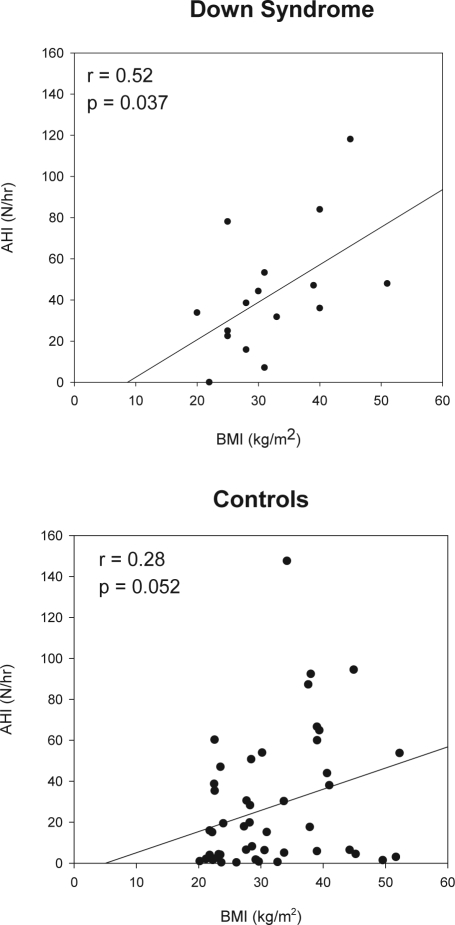
Most subjects with DS had severe obstructive apnea (Figure 1; Table 2). One subject, who slept for only 106 minutes and had no REM sleep, had an AHI of 0/h. Another subject had an AHI of 7/h. All the remaining subjects had an AHI > 15/h, and 69% had severe OSAS, with an AHI > 30/h. The AHI correlated significantly with the BMI (r = 0.52, p = 0.037; Figure 2). There was no correlation between age and AHI, and no clear relationship between hypothyroidism and the severity of obstructive apnea. There was no significant difference in any polysomnographic parameter between males and females. The AHI varied from 0–56/h on those receiving serotonin reuptake inhibitor medications compared to 1–120/h in the remaining subjects. The one subject with Alzheimer's disease had an AHI of 45/h.
Figure 1.
The apnea hypopnea index (AHI) for each individual subject is shown.
Figure 2.
The correlation between the body mass index (BMI) and apnea hypopnea index (AHI) is shown.
Central apneas were relatively rare. Only one patient had a central apnea index > 5/h. He had a central apnea index of 10/h, but the main abnormality on his polysomnogram was obstructive apnea, with an obstructive AHI of 47/h.
Oxygen Saturation
Most subjects had normal baseline arterial oxygen saturation during wakefulness (median 96%, range 89% to 98%). During sleep, the median baseline SpO2 was 93% (range 58% to 96%), but episodic desaturation in association with obstructive events was frequent. The median saturation nadir was 75%, and was as low as 23% in one subject. Three-quarters of subjects had a saturation nadir < 85%. Nine of the 16 patients spent ≥ 30 minutes with desaturation below 90%.
Hypercapnia
Hypercapnia was common. The median awake ETCO2 was 45 (range 41–54) mm Hg. The median ETCO2 during sleep was 48 (range 39–56) mm Hg. The peak ETCO2 ranged from 47 to 66 mm Hg, with a median of 58 mm Hg. Sixty-three percent of the subjects had ETCO2 > 50 mm Hg for more than 10% of total sleep time.
Control Polysomnographic Data
In general, controls had better sleep efficiency but more stage 1 and less slow wave sleep than subjects with DS (Table 2). Controls had less severe apnea than the DS subjects. In the control group, the median AHI was 16/h (range 0-48) (p = 0.036 vs DS). Fifty-four percent had an AHI > 15/h, compared to 88% of the DS subjects; and 38% had an AHI > 30/h, versus 69% of DS. The correlation between AHI and BMI (r = 0.28, p = 0.052) was not as strong as in the subjects with DS (Figure 2). The SpO2 nadir was 93% (77% to 100%) for controls versus 75% (23% to 95%) for DS (p < 0.001); 10% of controls had a saturation nadir < 85%.
Thyroid Status
Two DS subjects refused thyroid testing. Six subjects were known to be hypothyroid and were receiving treatment. Of these, 3 were found to be euthyroid (on medication), and 3 were hypothyroid on medication (TSH > 4.5 IU/mL). An additional 5 subjects without a history of hypothyroidism were found to be hypothyroid upon testing.
Blood Pressure
None of the DS subjects was hypertensive. In fact, all had slightly low blood pressure, with a median systolic pressure of 100 (90–110) and median diastolic pressure of 60 (50–70) mm Hg.
Epworth Sleepiness Scale
The results of the Epworth Sleepiness Scale are shown in Table 1. Scores covered a wide range, with one subject having a score as high as 20.
Treatment
Although not part of this research protocol, the 14 subjects with abnormal sleep studies were referred for treatment. Of these, 9 followed up in the sleep clinic at our institution. An additional subject's family stated that they would consult with a sleep physician who had treated the subject's father. The remaining 4 subjects were not known to have received treatment, although it is possible that they received treatment elsewhere.
Continuous positive airway pressure (CPAP) treatment was recommended for all of the subjects seen in our clinic. Patients underwent CPAP titration studies. Based on this, CPAP levels of 7–10 cm H2O were recommended for most patients, and bilevel pressure of 18/12 cm H2O was recommended for one patient with severe OSAS. Of these 9 patients, 5 had excellent use (6–8 h/night). This was documented objectively by download of the equipment compliance meter in 4 cases; the 5th patient did not have downloadable equipment. One of these patients required behavioral therapy.17 Subjectively, family members reported that these patients had an improvement in daytime functioning and a decrease in excessive daytime sleepiness on treatment.
Of the remaining 4 patients, one used CPAP for 2 h/night (as documented with objective monitoring), one was too anxious to accept CPAP treatment, one had problems with nasal congestion and CPAP tolerance and was undergoing formal behavioral modification, and one went to clinic but did not return for a CPAP titration study.
DISCUSSION
OSAS is common in children with DS, with a prevalence of 30% to 55%.3–5 Adults with DS have even more predisposing factors for OSAS than children with DS, as they still have the craniofacial anomalies, but are more likely to be obese or hypothyroid. Furthermore, the prevalence of OSAS tends to increase with age. Nevertheless, the type and severity of OSAS in the adult Down syndrome population has not been well characterized. A few case reports have described OSAS in adults with DS.18 Resta et al19 recently described OSAS in 6 adults with DS. A 1987 study using rarely used technology (static charge sensitive beds) suggested an increased prevalence of apnea in adults with Down syndrome.20 However, factors such as oxygenation, and whether apneas were central or obstructive in nature, could not be determined. Nevertheless, this study showed that patients with DS who were older than 40 years of age had periodic breathing for 24% of the night.
The purpose of the current study was to evaluate the presence of OSAS in the adult DS population. The findings from this sample, of whom only one had been referred for clinical evaluation, showed that OSAS was common and occurred in both males and females. Ninety-four percent of subjects had obstructive sleep apnea. The degree of OSAS and desaturation was much more severe than that found in the clinical control group, and very abnormal when compared to normative data in the literature. For example, 88% of subjects with DS in the current study had an AHI > 15/h, as compared to only 9% of normal adults in a population-based study,1 and 75% of the subjects with Down syndrome had a saturation nadir < 85%, as compared to 8% of a normal population.21
The major limitation of our study is that it was not population based. Unfortunately, there is no national registry or database for Down syndrome. In order to minimize selection bias, patients were not recruited preferentially based on a history of sleep or respiratory complaints. The talks and literature supplied to the DS associations or parent groups did not emphasize problems with sleep or breathing. These groups are not affiliated with a hospital, and their members were unaware of the exact objectives of the study. All the subjects at the Kennedy Krieger Down Syndrome Clinic were approached sequentially for consent, regardless of their presenting complaint. Nevertheless, it is possible that subjects with sleep or respiratory issues were more likely to agree to the study. Even so, the frequency and severity of sleep disordered breathing was much higher than would be expected from a community sample,1 and was even higher than expected from a symptomatic population. In order to assess this, we compared the sample to sequential matched controls obtained from our clinical database. All of the controls presented to a tertiary care sleep clinic for evaluation of symptoms of obstructive sleep apnea. Despite the fact that the controls were presenting because they were symptomatic, these clinical patients had a much lower AHI, and better oxygenation, than the DS population.
The Epworth scores were elevated, suggesting the presence of excessive daytime sleepiness in the study population. However, it should be noted that the Epworth score has not been validated in the DS population, and its sensitivity in detecting sleepiness in this population is unknown. The score was modified by one question, as has previously been done when using the scale in children.22 Scores were obtained from the guardian rather than the subject. Although proxy measurements of the score have been used successfully in other patient populations,22,23 it may not have accurately reflected daytime sleepiness in the subjects with DS, especially in subjects residing in institutions with multiple caregivers. Further studies evaluating the presence of sleepiness and cognitive dysfunction in adults with DS and OSAS need to be conducted.
In normal adults, the major risk factor for sleep disordered breathing is obesity. In the current study, 12 of the 16 subjects with DS were obese and an additional 2 were overweight; only 2 subjects were of normal weight. The apnea hypopnea index was highly correlated with the degree of obesity. Thus, obesity, a common and potentially treatable problem in DS, appears to play an important role in the pathophysiology of OSAS in this population.
A significant proportion of the subjects with DS were found to be hypothyroid, and some patients on treatment for hypothyroidism were found to have subtherapeutic levels. Although hypothyroidism is a risk factor for OSAS, a clear relationship between thyroid status and OSAS could not be discerned in this study. Nevertheless, this is one treatable risk factor that should be carefully monitored in patients with DS.
Several subjects with Down syndrome were receiving medications that could affect the central nervous system, in particular serotonin reuptake inhibitor drugs. This could have affected the study results. Unfortunately, information on medication use was unavailable for the controls. In general, serotonin reuptake inhibitor drugs affect sleep architecture by increasing stage 1 sleep and decreasing total sleep time, although this varies with specific drugs.24 However, there is evidence that SSRI medications actually increase upper airway motor tone,25 and the therapeutic effects of these drugs in sleep apnea are being explored. Thus, it would not be expected that these drugs would worsen OSAS in the study subjects. It is possible that some of the differences seen in sleep architecture between DS and controls were due to medication effects.
OSAS is often associated with hypertension. Surprisingly, all of the subjects in this study had low blood pressure, despite the presence of severe OSAS. This is consistent with previous reports of low blood pressure in patients with DS.26
It is surprising that the subjects in this study had such severe OSAS, yet medical evaluation had been sought in only one case. Many of the sequelae of OSAS, such as cognitive dysfunction and pulmonary hypertension, are frequently associated with DS. Thus, the presence of these complications might not prompt caregivers to seek further diagnostic testing. Further, many adults with DS live in facilities such as group homes, where their sleep may not be closely observed at night.
In otherwise healthy adults, OSAS can result in neurologic impairment. It has been associated with excessive daytime sleepiness, impaired reaction time, impaired executive function27 and ischemic stroke.28 Adults with DS have an increased prevalence of dementia and Alzheimer's disease,29 and it is theoretically conceivable that this could be related, at least in part, to the hypoxemia and sleep fragmentation associated with OSAS.30
Patients with OSAS have increased mortality.31 The high mortality rate of untreated patients is believed to be due to the consequences of systemic arterial hypertension, pulmonary hypertension, right heart insufficiency, bradycardic and tachycardic arrhythmias and stroke.28,32 These cardiovascular complications are likely to be even more hazardous in patients with DS, who may have residual sequelae from congenital heart disease.26 Both Loughlin33 and Levine34 demonstrated that pulmonary hypertension in children with DS could be reversed by relieving upper airway obstruction. Despite improvements in the care of patients with DS, they still die early. A recent study showed that the median age of death is 49 years.35 It is possible that untreated OSAS is contributing to this early mortality.
Treatment was not a part of this study. Nevertheless, a significant proportion of subjects were able to tolerate CPAP, with a subjective improvement in daytime function and alertness. Further studies evaluating functional, neurocognitive and cardiovascular sequelae of OSAS in this population, and their response to treatment, are needed. In addition, studies are required to assess the best form of treatment for adults with DS, including the potential utility of surgery, and ways to enhance compliance with CPAP.
CONCLUSION
This study has shown that adults with Down syndrome frequently have OSAS, with obstructive apnea, hypoxemia, hypoventilation, and sleep fragmentation. This could put them at risk for cardiovascular and neurologic complications. Whereas previously individuals with Down syndrome tended to be institutionalized or received supportive care only, they can now be functional members of society. Efforts have been made to improve not only their longevity but also their quality of life. More and more studies are showing the consequences of untreated OSAS. Therefore, diagnosing and treating a common disorder such as OSAS prior to the development of serious complications is both reasonable and desirable. Based on the data from this pilot study, we recommend that a population based study be performed to determine the true prevalence of OSAS in this population, and that consideration be made of screening all subjects with DS for OSAS.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Marcus has received research support and the use of equipment from Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We are extremely grateful to the individuals, parents and case managers who participated in this study. We would especially like to thank Dr. Haydee de Paula from the Association of Retarded Citizens for her interest and support. We would also like to thank Benilton Carvalho, MSc. for performing the statistical analysis; Andrew Warren, M.D., for help recruiting subjects; Laurie Karamessinis, RPFT, for assistance with data management, and the sleep technologists who performed and scored the studies.
Financial Support: Dr. Marcus was supported by grants NHLBI grant HL58585, and NIH/National Center for research resources grant M01-RR00052 to the Johns Hopkins University School of Medicine.
ABBREVIATIONS
- AHI
Apnea hypopnea index
- ARC
Association of Retarded Citizens
- BMI
Body mass index
- CPAP
Continuous positive airway pressure
- DS
Down syndrome
- OSAS
Obstructive sleep apnea syndrome
- ETCO2
End-tidal CO2
- PODS
Parents of Down Syndrome
- SpO2
Arterial oxygen saturation measured by pulse oximetry
- T4
Levothyroxine
- TSH
Thyroid-stimulating hormone
- TST
Total sleep time
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Smith DW, editor. Recognizable patterns of human malformation. Philadelphia: W B Saunders; 1982. pp. 10–13. [Google Scholar]
- 3.Marcus CL, Keens TG, Bautista DB, Von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics. 1991;88:132–9. [PubMed] [Google Scholar]
- 4.Stebbens VA, Dennis J, Samuels MP, Croft CB, Southall DP. Sleep related upper airway obstruction in a cohort with Down's syndrome. Arch Dis Child. 1991;66:1333–8. doi: 10.1136/adc.66.11.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Miguel-Diaz J, Villa-Asensi JR, Alvarez-Sala JL. Prevalence of sleep-disordered breathing in children with Down syndrome: polygraphic findings in 108 children. Sleep. 2003;26:1006–9. doi: 10.1093/sleep/26.8.1006. [DOI] [PubMed] [Google Scholar]
- 6.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 7.Fink GB, Madaus WK, Walker GF. A quantitative study of the face in Down's syndrome. Am J Orthod. 1975;67:540–53. doi: 10.1016/0002-9416(75)90299-7. [DOI] [PubMed] [Google Scholar]
- 8.Uong EC, McDonough JM, Tayag-Kier CE, et al. Magnetic resonance imaging of the upper airway in children with Down syndrome. Am J Respir Crit Care Med. 2001;163:731–6. doi: 10.1164/ajrccm.163.3.2004231. [DOI] [PubMed] [Google Scholar]
- 9.Strome M. Obstructive sleep apnea in Down syndrome children: a surgical approach. Laryngoscope. 1986;96:1340–2. doi: 10.1288/00005537-198612000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Aboussouan LS, O'Donovan PB, Moodie DS, Gragg LA, Stoller JK. Hypoplastic trachea in Down's syndrome. Am Rev Respir Dis. 1993;147:72–5. doi: 10.1164/ajrccm/147.1.72. [DOI] [PubMed] [Google Scholar]
- 11.Guilleminault C, Partinen M, Praud JP, Quera-Salva MA, Powell N, Riley R. Morphometric facial changes and obstructive sleep apnea in adolescents. J Pediatr. 1989;114:997–9. doi: 10.1016/s0022-3476(89)80447-0. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Indications and standards for cardiopulmonary sleep studies. Am Rev Respir Dis. 1989;139:559–68. doi: 10.1164/ajrccm/139.2.559. [DOI] [PubMed] [Google Scholar]
- 13.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales A. A manual of standardized terminology: techniques and scoring systems for sleep stages of human subjects. Bethesda, MD: NINDB Neurological Information network (US); 1968. [Google Scholar]
- 15.Sleep disorders atlas task force. Guilleminault C. C. EEG arousals: scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 16.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 17.Koontz KL, Slifer KJ, Cataldo MD, Marcus CL. Improving pediatric compliance with positive airway pressure therapy: the impact of behavioral intervention. Sleep. 2003;26:1010–5. doi: 10.1093/sleep/26.8.1010. [DOI] [PubMed] [Google Scholar]
- 18.Clark RW, Schmidt HS, Schuller DE. Sleep-induced ventilatory dysfunction in Down's syndrome. Arch Intern Med. 1980;140:45–50. [PubMed] [Google Scholar]
- 19.Resta O, Barbaro MP, Giliberti T, et al. Sleep related breathing disorders in adults with Down syndrome. Downs Syndr Res Pract. 2003;8:115–9. doi: 10.3104/reports.138. [DOI] [PubMed] [Google Scholar]
- 20.Telakivi T, Partinen M, Salmi T, Leinonen L, Harkonen T. Nocturnal periodic breathing in adults with Down's syndrome. J Ment Defic Res. 1987;31:31–9. doi: 10.1111/j.1365-2788.1987.tb01340.x. Pt 1. [DOI] [PubMed] [Google Scholar]
- 21.Catterall JR, Calverley PM, Shapiro CM, Flenley DC, Douglas NJ. Breathing and oxygenation during sleep are similar in normal men and normal women. Am Rev Respir Dis. 1985;132:86–8. doi: 10.1164/arrd.1985.132.1.86. [DOI] [PubMed] [Google Scholar]
- 22.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 23.Kingshott RN, Sime PJ, Engleman HM, Douglas NJ. Self assessment of daytime sleepiness: patient versus partner. Thorax. 1995;50:994–5. doi: 10.1136/thx.50.9.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweitzer PK. Drugs that disturb sleep and wakefulness. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: WB Saunders; 2000. pp. 441–461. [Google Scholar]
- 25.Sunderram J, Parisi RA, Strobel RJ. Serotonergic stimulation of the genioglossus and the response to nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:925–9. doi: 10.1164/ajrccm.162.3.9907077. [DOI] [PubMed] [Google Scholar]
- 26.Marino B. Cardiac aspects. In: Pueschel SM, Pueschel JK, editors. Biomedical concerns in persons with Down syndrome. Baltimore: Paul H. Brookes Publishing; 1992. pp. 91–103. [Google Scholar]
- 27.Berry DT, Webb WB, Block AJ, Bauer RM, Switzer DA. Nocturnal hypoxia and neuropsychological variables. J Clin Exp Neuropsychol. 1986;8:229–38. doi: 10.1080/01688638608401315. [DOI] [PubMed] [Google Scholar]
- 28.Mohsenin V. Sleep-related breathing disorders and risk of stroke. Stroke. 2001;32:1271–8. doi: 10.1161/01.str.32.6.1271. [DOI] [PubMed] [Google Scholar]
- 29.Hollandt JH, Hon J, Huppert FA, Stevens F, Watson P. Population-based study of the prevalence and presentation of dementia in adults with Down's syndrome. Br J Psychiatry. 1998;172:493–8. doi: 10.1192/bjp.172.6.493. [DOI] [PubMed] [Google Scholar]
- 30.Gibson GE, Pulsinelli W, Blass JP, Duffy TE. Brain dysfunction in mild to moderate hypoxia. Am J Med. 1981;70:1247–54. doi: 10.1016/0002-9343(81)90834-2. [DOI] [PubMed] [Google Scholar]
- 31.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 32.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 33.Loughlin GM, Wynne JW, Victorica BE. Sleep apnea as a possible cause of pulmonary hypertension in Down syndrome. J Pediatr. 1981;98:435–7. doi: 10.1016/s0022-3476(81)80716-0. [DOI] [PubMed] [Google Scholar]
- 34.Levine OR, Simpser M. Alveolar hypoventilation and cor pulmonale associated with chronic airway obstruction in infants with Down syndrome. Clin Pediatr (Phila) 1982;21:25–9. doi: 10.1177/000992288202100104. [DOI] [PubMed] [Google Scholar]
- 35.Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population-based study. Lancet. 2002;359:1019–25. doi: 10.1016/s0140-6736(02)08092-3. [DOI] [PubMed] [Google Scholar]