Abstract
Study Objectives:
This study was designed to assess the effect of acid suppression on upper airway structure and function in patients with obstructive sleep apnea syndrome (OSAS) and gastroesophageal reflux disease (GERD).
Methods:
This is a single-site within-subjects design. Twenty five patients with documented mild OSAS and objectively documented GERD via 24-hour pH measurement were included in the study. Patients were studied before and after 8 weeks of treatment with rabeprazole, 20 mg, twice a day. Subjects underwent laryngoscopy, polysomnography, and 24-hour pH monitoring. Subjective assessments of sleep obtained included the Pittsburg Sleep Quality Index and the Epworth Sleepiness Scale.
Results:
Posterior commissure edema was significantly reduced (p < 0.05), and the Reflux Finding Score was improved (p < 0.07). Objective and subjective sleep parameters were significantly improved, sleep-onset latency was significantly reduced (26.2 vs 11.2, p < 0.05), and sleep-related acid contact time was significantly reduced (8.0% vs 1.7% p < 0.001). There was no significant change in the apnea-hypopnea index.
Conclusions:
In patients with mild OSAS and documented GERD, acid suppression improves upper airway abnormalities, as well as objective and subjective measures of sleep quality. Aggressive treatment of GERD in patients with OSAS may be helpful in the overall treatment of this select patient population
Citation:
Orr WC; Robert JJT; Houck JR; Giddens CL; Tawk MM. The effect of acid suppression on upper airway anatomy and obstruction in patients with sleep apnea and gastroesophageal reflux disease. J Clin Sleep Med 2009;5(4):330-334.
Keywords: Gastroesophageal reflux disease, obstructive sleep apnea, acid contact time, upper airway anatomy
Gastroesophageal reflux disease (GERD) and obstructive sleep apnea syndrome (OSAS) are often comorbid disorders.1–3 OSAS is a condition marked by pharyngeal narrowing, resulting in upper airway obstruction during sleep, which, in turn, produces repeated episodes of decreased oxygen saturation and brief arousals from sleep. It is well recognized clinically that patients with OSAS often complain of heartburn, and they clearly share a major overlapping risk factor, which is obesity. In one investigation, of the patients referred for overnight sleep studies, 74% reported GERD-related symptoms, including heartburn, acid regurgitation, or both.2 Other studies have also shown a significant increase in reflux symptoms in patients with demonstrated OSAS.1,4 The incidence of significant reflux symptoms in these studies ranges from 62% to 74%. In another study, patients whose OSAS was successfully treated with continuous positive airway pressure (CPAP) had a marked improvement in nighttime reflux, compared with those patients who were noncompliant or did not use CPAP.1
In a study that utilized polysomnography concurrent with distal esophageal pH monitoring, Ing and colleagues5 found that patients with OSAS exhibited more frequent nocturnal reflux than did similar patients with very mild OSAS. In this same study,5 esophageal acid clearance was also prolonged in patients with OSAS with a significantly greater proportion of time at an esophageal pH < 4.0. Similar results were found in a study by Berg et al.,6 who concluded that reflux events and obstructive apnea are not causally linked but may be the consequence of a coexisting pathology. It is also of interest that CPAP treatment itself will significantly lower the percentage of nighttime acid contact.7 Another study has shown that powerful acid inhibition with a proton pump inhibitor (pantoprazole) will markedly improve symptoms of sleepiness and reflux symptoms in patients with documented OSAS.8 Senior et al.3 have shown that a similar treatment in patients with documented OSAS and GERD via 24- hour pH monitoring will significantly reduce the apnea-hypopnea index (AHI). However, it should be noted that this study consisted of only 10 patients, and only 3 actually responded to treatment. The authors did conclude that further investigation is necessary to establish whether reducing gastroesophageal reflux (GER) may alter the upper airway, resulting in a reduction of upper airway obstruction. Thus, it appears that GERD is a common comorbid condition with OSAS and that symptoms are substantially improved by treating obstructive events. It remains to be determined definitely whether suppressing GER improves upper airway anatomy, physiology, or both anatomy and physiology in a way that could result in a reduction in upper airway obstructive events.
In the present study, we have undertaken an evaluation of patients with OSAS selected via both polysomnography and 24-hour pH recording to have both mild OSAS and documented GERD. We hypothesized that the treatment of GERD in patients with mild OSAS and objective evidence of significant reflux would reduce the number of obstructive events and that this would be accompanied by improvements in upper airway anatomy and sleep quality. Patients were subjected to powerful acid suppression (ie, rabeprazole 20 mg, twice a day) for 2 months. Patients were assessed with laryngoscopy, as well as full polysomnography and 24-hour esophageal pH monitoring, before and after treatment with powerful acid suppression.
METHODS
Participants
All participants had symptoms consistent with a diagnosis of OSAS, ie, snoring and daytime sleepiness or fatigue, and subjective complaints of GER. Participants were excluded if they had a history of Barrett esophagus, history of abdominal surgery, significant respiratory distress, neurologic or psychiatric disorder, or significant chronic renal or liver disease.
Participants were recruited from an internal database as well as through local advertising. One hundred and thirty-nine participants consented to participate. Ninety subjects did not meet screening criteria (45 screen for pH and 45 for polysomnography), 24 dropped out of the study, and 25 completed the study (see Table 1 for demographics on patients who completed the study). Of the 25 patients who completed both sleep and esophageal evaluations before and after treatment, only 15 completed the laryngoscopic evaluation under both conditions. All patients signed a consent form, which had been approved by the University of Oklahoma Health Sciences Institutional Review Board.
Table 1.
Participant Characteristics
| Participants (n = 25) | |
|---|---|
| Age, y | 42.8 ± 11.5 |
| Men, % | 72 |
| Race, no. | |
| African American | 1 |
| Caucasian | 21 |
| Other | 3 |
| BMI, kg/m2 | 31.4 ± 6.9 |
Data are shown as Mean ± SD unless otherwise indicated. BMI refers to body mass index.
Sleep and pH Measures
All participants underwent a full polysomnographic evaluation, as well as a 24-hour esophageal pH evaluation. To qualify, participants had to have an AHI of less than15 and percentage of esophageal acid contact time (percentage of total recording time with esophageal pH < 4.0) of greater than 6% total or 3% recumbent. Qualifying participants underwent 8 weeks of treatment with 20 mg of rabeprazole twice a day. At the end of 8 weeks, all subjects repeated the sleep and 24-hour esophageal pH evaluations.
Subjective Assessments
Subjective assessments included the Epworth Sleepiness Scale (ESS) and the Pittsburg Sleep Quality Index (PSQI), which were completed both before and after treatment.9,10
Anatomic Variables
Upper airway was evaluated at the Voice Laboratory of the Department of Otorhinolaryngology, University of Oklahoma Health Sciences Center. During the initial visit, a complete medical history and physical examination of the head and neck were performed. Fiberoptic nasopharyngoscopy was also performed. The nose was decongested with oxymetazoline 0.05%; the nose and pharynx were topically anesthetized with pontocaine 2%. The nose, pharynx, and larynx were examined using a fiberoptic nasopharyngoscope attached to a single-chip NTSC video camera (Olympus America, Melville, NY). The entire fiberoptic examination was recorded using a standard-definition VCR (Sony USA, New York, NY) Subsequent to treatment, an interim history was obtained. Fiberoptic examination was performed and recorded as described above.
The video clips were analyzed for pathologic changes of the larynx and hypopharynx by 3 blinded observers; 2 otolaryngologists (JRH and a senior otolaryngology resident) and a speech pathologist (CG). Training was performed by viewing 6 clips together and agreeing on the nature and stage of pathologic changes. The clips were then viewed during 2 sessions in a random and blinded manner. Specific indicators used were subglottic edema, ventricular obliteration, erythema, edema of the vocal cords, diffuse laryngeal edema (edema of larynx except vocal cords), posterior commissure hypertrophy, granulation tissue, and endolaryngeal mucus. The results from the 3 observers were averaged to give a score for each indicator for each exam. The Reflux Finding Score (scale 1-4) was calculated as a composite score for laryngeal findings, as assessed by the examining physician.11
RESULTS
Twenty-five participants met study criteria and completed both sleep evaluations and the 24-hour esophageal pH evaluations (Table 1). We have compared the data from the dropouts to those who completed the study in terms of baseline PSQI, ESS, and polysomnography variables. There were no significant differences.
Polysomnography
Sleep-onset latency was significantly (p < 0.05) shorter after treatment compared with baseline; however, total sleep time and other stages of sleep and arousals were not significantly different after treatment. There was no difference in the AHI index at baseline, compared to after treatment (Table 2).
Table 2.
Polysomnographic Parameters
| Pretreatment (n = 25) | Posttreatment (n = 25) | p Value | |
|---|---|---|---|
| TST, min | 357.0 ± 75.0 | 374.9 ± 53.2 | NS |
| SOL, min | 26.2 ± 35.3a | 11.2 ± 9.0a | < 0.05 |
| Sleep efficiency (TST/TIB) | 82.4 ± 13.9 | 86.3 ± 11.2 | NS |
| Sleep stage, % of TST | |||
| 1 | 5.14 ± 3.1 | 6.5 ± 5.4 | NS |
| 2 | 64.1 ± 7.0 | 63.3 ± 7.9 | NS |
| 3/4 | 9.5 ± 6.5 | 9.5 ± 6.9 | NS |
| REM | 20.3 ± 6.9 | 20.1 ± 5.1 | NS |
| REM-onset latency, min | 143.0 ± 65.5 | 129.0 ± 82.3 | NS |
| AHI, no/h | 9.3 ± 4.7 | 9.1 ± 8.7 | NS |
| Arousals, no. | 46.2 ± 20.0 | 41.4 ± 19.1 | NS |
Data are shown as Mean ± SD. TST refers to total sleep time; TIB, time in bed; REM, rapid eye movement; AHI, apnea-hypopnea index.
p<0.05
Twenty-Four–Hour pH Monitoring
Participants had significantly fewer reflux events and significantly less acid contact time in both upright and recumbent positions and a significantly lower percent acid contact time after treatment (Table 3). Participants also had significantly fewer episodes of long-duration (> 5 min) acid contact time after treatment.
Table 3.
24-Hour pH Monitoring Parameters
| Pretreatment (n = 25) | Posttreatment (n = 25) | p Value | |
|---|---|---|---|
| Total acid contact time, % | 8.2 ± 3.1 | 1.9 ± 5.4 | <0.001 |
| Waking | 8.7 ± 3.2a | 1.8 ± 5.2a | <0.001 |
| Sleep | 8.0 ± 7.0 | 1.7 ± 5.8 | <0.001 |
| Total number of events | 172.3 ± 72.2 | 21.1 ± 33.8 | <0.001 |
| Waking | 136.0 ± 63.8 | 17.6 ± 27.8 | <0.001 |
| Sleep | 35.4 ± 27.8 | 3.4 ± 8.1 | <0.001 |
| Total long-duration (< 5 min) events | 5.5 ± 9.1 | 0.8 ± 1.8 | <0.01 |
| Waking | 1.9 ± 2.5 | 0.4 ± 1.0 | <0.01 |
| Sleep | 4.3 ± 9.4 | 0.6 ± 1.6 | <0.057 |
Data are shown as mean ± SD.
Subjective Sleep Measures
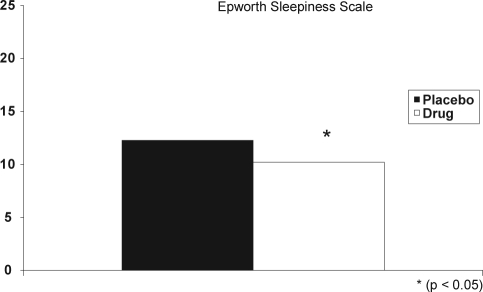
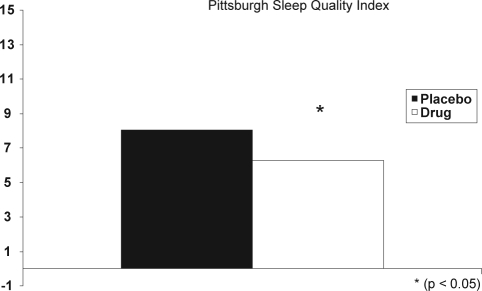
Participants reported significantly less daytime sleepiness after treatment, which was reflected in a significantly lower ESS score (Figure 1). The PSQI score was also significantly improved after treatment (Figure 2).
Figure 1.
Participants reported significantly less daytime sleepiness after treatment, reflected in a significantly lower Epworth Sleepiness Scale score. Placebo (dark bar); drug (open bar).
Figure 2.
The Pittsburgh Sleep Quality Index score was significantly improved after treatment. Placebo (dark bar); drug (open bar).
Anatomic and Physiologic Measures
Due to a variety of technical and medical issues (ie, upper respiratory infections, etc.) only 15 patients completed the laryngoscopic anatomic assessment before and after treatment (Table 4). The Reflux Finding Score was substantially improved after treatment, but this was not quite statistically significant (p < 0.07). Posterior commissure hypertrophy was significantly reduced after treatment.
Table 4.
Anatomic Changes
| Pretreatment (n = 15) | Posttreatment (n = 15) | p Value | |
|---|---|---|---|
| Posterior commissure hypertrophy | 1.3 ± .9 | 0.7 ± 0.5 | 0.04a |
| Ventricular obliteration false cord obliteration | 0.3 ± 0.7 | 0 ± 0 | NS |
| Vocal fold edema | 1.0 ± .9 | 0.5 ± 0.6 | NS |
| Erythema | 0.8 ± 1.0 | 0.7 ± 0.9 | NS |
| Laryngeal edema | 0.1 ± 0.5 | 0.0 ± 0.0 | NS |
| Subglottic edema | 0.1 ± 0.5 | 0 ± 0 | NS |
| Reflux Finding Score | 3.6 ± 3.2 | 1.9 ± 1.4 | 0.07a |
Data are shown as Mean ± SD.
p<0.05
DISCUSSION
This study is, to our knowledge, the first study to document anatomic changes in the upper airway associated with the treatment of GER in patients with OSA. We deliberately chose patients with mild OSA for 2 reasons: we felt that it would be more likely that upper airway changes due to GER could be improved, and we wanted patients not otherwise being treated (CPAP, oral appliance, etc.) The data revealed a strong trend in the direction of improvement in the Reflux Finding Score (p = 0.07), and there were significant reductions in posterior commissure hypertrophy. It is of interest that all other anatomic changes were in the direction of improvement, albeit small, subsequent to acid suppression treatment.
There was not, however, a significant reduction in the AHI. This is most likely due to the fact that, although the anatomic changes noted are certainly in the right direction, they are relatively small. This would be consistent with data reported by Steward.8 In this study, patients with OSAS were treated for 3 months with pantoprazole, 40 mg, once a day. In this study, he noted significant improvement in daytime sleepiness and total reflux symptoms, but there was no significant reduction in the AHI. It is possible that 2 months of treatment is not sufficient to resolve laryngeal and upper airway changes associated with GER.12,13 It has been reported that laryngeal abnormalities identified in patients with laryngopharyngeal reflux may take as long as 6 months of treatment with twice-a-day proton-pump-inhibitor therapy to completely resolve anatomic changes.13 It is also possible that laryngeal changes alone are not sufficient to produce changes in upper airway anatomy, which would result in a significant reduction in the AHI.
The pathophysiologic mechanisms that may link increased esophageal acid contact time and upper airway obstruction in patients with OSAS are not clearly delineated. It seems possible that the conditions may in fact interact, creating a self-perpetuating positive feedback loop. For example, it has been postulated that the very significant negative intrathoracic pressure created by airway obstruction in patients with OSAS predisposes the patient to develop GER. This notion is further supported by studies that have shown that CPAP reduces GER and esophageal acid contact time in patients with OSAS.14 Two very good studies have directly addressed this issue. Both of these studies did strongly suggest that, in patients with significant OSAS, there was an increase in esophageal acid contact time during the sleeping interval, although a direct relationship between reflux and obstructive events was not documented in either study.5,15
Further support for the link between GERD and OSAS is found from a study by Green and colleagues.1 This was an open-treatment, long-term study of patients with OSAS. The frequency of nocturnal GERD symptoms were reduced in patients treated with CPAP. They also noted a significant positive correlation between the degree of GERD-symptom improvement and the CPAP pressure used in treating the OSAS. Of interest is the fact that it has been shown that the treatment of GERD with an acid-suppressing agent may have a salubrious effect on OSAS.3 Although this was a study of only 10 patients, this group did confirm a relationship between OSAS and GERD via polysomnography and simultaneous esophageal pH monitoring. They showed that omeprazole treatment (20 mg, twice a day) for 1 month resulted in improvements in apnea and hypopnea scores, as well as symptoms of respiratory distress. However, only 3 of the 10 patients studied showed a distinct improvement in obstructive apnea, and an equal number actually got worse during treatment. The authors did not report any esophageal pH data or results on the resolution of either OSAS or GERD symptoms. Although this was a very small, open-treatment investigation, it provides the first evidence to suggest that there may be a positive effect of GERD treatment on the occurrence of upper airway obstruction.
In the current study, acid-suppression treatment did result in a significant reduction in the sleep-onset latency as well as improvement in subjective sleep measures (the ESS and PSQI). The reduction in GER and acid contact time during sleep resulted in a significant improvement in both objective and subjective sleep measures. The sleep-onset latency was significantly reduced, and it is notable that total reflux events during the sleeping interval were reduced form approximately 35 to 3. It is well known that, with powerful acid suppression, gastroesophageal reflux will continue, with many events becoming nonacidic. A study from our laboratory has shown that nonacidic reflux events continue during sleep after powerful acid suppression, and they can and do cause arousals.16 However, in the current study, the arousal responses were not significantly different with powerful acid suppression, so this effect does not appear to be important with regard to any effect on sleep patterns. Although our study did not include a placebo control, the congruence of both objective and subjective sleep measures strongly suggests that these findings are valid.
In patients with symptomatic GERD, it has been shown that acid suppression results in a significant improvement in the PSQI and other subjective sleep measures, to include reports of reduced nighttime heartburn.17 Furthermore, the findings of our study are in line with the previously cited study in which symptom resolution occurred within 2 months of treatment, but physical findings continued to resolve with up to 6 months of treatment.13 However, the AHI was not significantly reduced with powerful acid suppression, which would indicate that CPAP is both indicated and necessary to control obstructive events in this patient population.
Overall, this study is the first to demonstrate improved upper airway anatomic findings as well as improved subjective sleep quality and reduced sleep-onset latency associated with an objective reduction in acid contact time via powerful acid-suppression treatment in patients with OSAS.
DISCLOSURE STATEMENT
The research study was sponsored by Eisai Inc and Ortho-McNeil Scientific Affairs, LLC. Dr. Orr has received research support from Wyeth, Janssen Eisai; has participated in speaking engagements for Santarus and Astra Zeneca; and has consulted for Santarus and TAP. Dr. Robert is employed by a for-profit research organization. The other authors have indicated no other conflicts of interest.
ACKNOWLEDGMENTS
The research was conducted at Lynn Health Science Institute and at the University of Oklahoma Health Sciences Center. The research study was sponsored by Eisai Inc and Ortho-McNeil Scientific Affairs, LLC.
REFERENCES
- 1.Green BT, Broughton WA, O’Connor JB. Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea treated with continuous positive airway pressure. Arch Intern Med. 2003;163:41–5. doi: 10.1001/archinte.163.1.41. [DOI] [PubMed] [Google Scholar]
- 2.Valipour A, Makker HK, Hardy R, Emegbo S, Toma T, Spiro SG. Symptomatic gastroesophageal reflux in subjects with a breathing sleep disorder. Chest. 2002;121:1748–53. doi: 10.1378/chest.121.6.1748. [DOI] [PubMed] [Google Scholar]
- 3.Senior BA, Khan M, Schwimmer C, Rosenthal L, Benninger M. Gastroesophageal reflux and obstructive sleep apnea. Laryngoscope. 2001;111:2144–6. doi: 10.1097/00005537-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Guda N, Partington S, Vakil N. Sympomatic gastro-oesophageal reflux, arousals and sleep quality in patients undergoing polysomnography for possible obstructive sleep apnoea. Aliment Pharmacol Ther. 2004;20:1153–9. doi: 10.1111/j.1365-2036.2004.02263.x. [DOI] [PubMed] [Google Scholar]
- 5.Ing AJ, Ngu MC, Breslin AB. Obstructive sleep apnea and gastroesophageal reflux. Am J Med. 2000;108:S120–S5. doi: 10.1016/s0002-9343(99)00350-2. [DOI] [PubMed] [Google Scholar]
- 6.Berg S, Hoffstein V, Gislason Acidification of distal esophagus and sleep-related breathging disturbances. Chest. 2004;125:2101–6. doi: 10.1378/chest.125.6.2101. [DOI] [PubMed] [Google Scholar]
- 7.Tawk M, Goodrich S, Kinasewitz G, Orr W. The effect of 1 week of continuous positive airway pressure treatment in obstructive sleep apnea patients with concomitant gastroesophageal reflux. Chest. 2006;130:1003–8. doi: 10.1378/chest.130.4.1003. [DOI] [PubMed] [Google Scholar]
- 8.Steward DL. Pantoprazole for sleepiness associated with acid reflux and obstructive sleep disordered breathing. Laryngoscope. 2004;114:1525–28. doi: 10.1097/00005537-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF, Monk TH, et al. Psychiatry Res. 1989. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research; pp. 193–213. [DOI] [PubMed] [Google Scholar]
- 10.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 11.Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS) Laryngoscope. 2001:1313–7. doi: 10.1097/00005537-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: Position statement of the committee on speech, voice, and swallowing disorders of the American Academy of Otolaryngology-Head and Neck Surgery. Otolaryngol Head Neck Surg. 2002;127:32–5. doi: 10.1067/mhn.2002.125760. [DOI] [PubMed] [Google Scholar]
- 13.Belafsky PC, Postma GN, Koufman JA. Laryngeal reflux symptoms improve before changes in physical findings. Laryngoscope. 2001;111:979–81. doi: 10.1097/00005537-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Kerr P, Shoenut JP, Millar T, et al. Nasal CPAP reduces gastresophageal reflux in obstructive sleep apnea syndrome. Chest. 1992;101:1539–44. doi: 10.1378/chest.101.6.1539. [DOI] [PubMed] [Google Scholar]
- 15.Penzel T, Becker HR, Brandenburg U, et al. Arousal in patients with gastro-oesophageal reflux and sleep apnoea. Eur Respir J. 1999;14:1266–70. doi: 10.1183/09031936.99.14612669. [DOI] [PubMed] [Google Scholar]
- 16.Orr WC, Craddock A, Goodrich S. Acidic and non-acidic reflux during sleep under conditions of powerful acid suppression. Chest. 2007;131:460–5. doi: 10.1378/chest.06-1312. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DA, Orr WC, Crawley JA, et al. Effect of esomeprazole on nighttime heartburn and sleep quality in patients with GERD: a randomized, placebo-controlled trial. Am J Gastroenterol. 2005;100:1914–22. doi: 10.1111/j.1572-0241.2005.00285.x. [DOI] [PubMed] [Google Scholar]