Abstract
Study Objectives:
Osteoarthritis pain affects more than half of all older adults, many of whom experience co-morbid sleep disturbance. Pain initiates and exacerbates sleep disturbance, whereas disturbed sleep maintains and exacerbates pain, which implies that improving the sleep of patients with osteoarthritis may also reduce their pain. We examined this possibility in a secondary analysis of a previously published randomized controlled trial of cognitive behavioral therapy for insomnia (CBT-I) in patients with osteoarthritis and co-morbid insomnia.
Methods:
Twenty-three patients (mean age 69.2 years) were randomly assigned to CBT-I and 28 patients (mean age 66.5 years) to an attention control. Neither directly addressed pain management. Twelve subjects crossed over to CBT-I after control treatment. Sleep and pain were assessed by self-report at baseline, after treatment, and (for CBT-I only) at 1-year follow-up.
Results:
CBT-I subjects reported significantly improved sleep and significantly reduced pain after treatment. Control subjects reported no significant improvements. One-year follow-up found maintenance of improved sleep and reduced pain for both the CBT-I group alone and among subjects who crossed over from control to CBT-I.
Conclusions:
CBT-I but not an attention control, without directly addressing pain control, improved both immediate and long-term self-reported sleep and pain in older patients with osteoarthritis and co-morbid insomnia. These results are unique in suggesting the long-term durability of CBT-I effects for co-morbid insomnia. They also indicate that improving sleep, per se, in patients with osteoarthritis may result in decreased pain. Techniques to improve sleep may be useful additions to pain management programs in osteoarthritis, and possibly other chronic pain conditions as well.
Citation:
Vitiello MV; Rybarczyk B; Von Korff M; Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med 2009;5(4):355–362.
Keywords: CBT-I, sleep, pain, osteoarthritis, insomnia, co-morbid
Osteoarthritis is a common cause of pain and disability among older adults, affecting 20 million Americans. The prevalence of osteoarthritis is rapidly increasing with the accelerating growth of the older portion of the US population.1 Osteoarthritis is characterized by joint degeneration, pain, and dysfunction, with 80% of patients with osteoarthritis experiencing limitations of movement.1 Osteoarthritis demonstrates a broad spectrum of symptom severity, ranging from intermittent aching and joint stiffness to loss of motion and severe chronic pain.2 Severity and disability tend to increase with age, although severity can fluctuate markedly over short periods of time.
Sleep quality is a major concern among persons with osteoarthritis, with 60% of people with osteoarthritis reporting pain during the night.3 In fact, pain secondary to arthritis is the most common factor predicting sleep disturbance in the population at large.4 It is well established that pain interferes with sleep5 and, more recently, that disturbed sleep lowers the pain threshold.6–8 Whether sleep disturbance precedes or follows pain onset is unclear, but reciprocal effects are likely.5 Patients with osteoarthritis who report having pain and stiffness in the morning have more sleep-related muscle spasms and objectively assessed sleep disturbance.9 Even after treatment with anti-inflammatory medications, patients with osteoarthritis show significantly greater objective sleep disturbance, as compared with age-matched control subjects.10 Chronic sleep disturbance, so common among older patients with osteoarthritis, is itself associated with impaired daytime function, daytime sleepiness and fatigue, reduced quality of life, and increased health care utilization.11–12
Given the likely reciprocal effects between pain and sleep disturbance, teasing apart unique causal pathways is difficult. Chronic pain initiates and exacerbates sleep disturbance; disturbed sleep in turn maintains and exacerbates chronic pain and related dysfunction.5,13–14 Sleep disruption, fragmentation, or restriction produces hyperalgesia6–8 and can interfere with analgesic treatments involving opioidergic and serotonergic mechanisms of action.13 The basis for this reciprocal relationship may be the modulation of pain during sleep and waking by reciprocally active neurons in the raphe magnus of the brainstem, providing a potential neural substrate for the reciprocal relationship of chronic pain and sleep disruption.14 Given this reciprocal relationship between sleep and pain, a question with major clinical implications is whether an intervention that improves sleep, per se, in individuals with disturbed sleep and a co-morbid pain state, such as osteoarthritis, might reduce pain as well.
A recent randomized controlled trial of cognitive behavioral therapy for insomnia (CBT-I) versus an attention control in a group of older adults with co-morbid illnesses—osteoarthritis, coronary artery disease, or chronic obstructive pulmonary disease—reported clinically significant improvements in sleep quality.15 Although CBT-I has been shown to achieve high levels of efficacy when treating insomnia in otherwise healthy populations,16 prior to the study of Rybarczyk et al.,15 CBT-I was not tested in a well-controlled study of individuals with insomnia and co-morbid chronic medical illnesses. Until recently, the assumption has been that such insomnias usually had medical causes and that the best approach to correcting the insomnia was to treat the medical condition.17
Rybarczyk and colleagues' CBT-I treatment protocol did not specifically address pain management. However, the study investigated the hypothesis that improvements in sleep would result in improvements in daytime functioning, so a broad array of measures were included in their analyses. The CBT-I–treated group showed no reductions in pain report on the McGill Pain Questionnaire (MPQ) across the 3 co-morbid medical illnesses, or for the osteoarthritis group alone, relative to an attention-control group.15 However, Rybarczyk and colleagues analyzed neither a second available pain measure (ie, SF-36 pain subscale) nor the within-group effects. Given the possibility that the attention-control group might have received some pain benefits, examining within-group effects is an important analytic consideration. To better explore the potential impact of improved sleep on osteoarthritis pain, we reanalyzed the Rybarczyk et al. data, using within-group analyses to examine both available measures of pain, as well as previously unavailable 1-year follow-up sleep and pain data, for osteoarthritis participants only. We also examined effects among participants who crossed over from the control group to the CBT-I treatment.
METHODS
A full description of the parent study—detailing patient recruitment and screening, etc.—may be found in the report by Rybarczyk et al.15 Here we report only the methodological information relevant to our above-stated purpose. The study protocol was approved by the Rush University Medical Center Institutional Review Board, and all participants gave written informed consent at the time of enrollment.
PARTICIPANTS
Twenty-three patients with osteoarthritis (mean age 69.2, 18 women and 5 men) were randomly assigned to CBT-I and 28 patients with osteoarthritis (mean age 66.5, 27 women and 1 man) to an attention-control stress management and wellness (SMW) intervention. Demographic and other characteristics of study subjects by treatment group are reported in Table 1. Subjective ratings of sleep quality and pain were assessed by patient self-report before treatment, after treatment, and at 1-year follow-up.
Table 1.
Demographic and Other Baseline Characteristics by Treatment Group
| CBT-I (n = 23) | SMW (n = 28) | |
|---|---|---|
| Sex, no. | ||
| Men | 5 | 1 |
| Women | 18 | 27 |
| Age, ya | 69.2 (8.9) | 66.5 (7.7) |
| Education, ya | 14.7 (3.8) | 13.8 (2.6) |
| Race, no. | ||
| Caucasian | 16 | 17 |
| African American | 5 | 11 |
| Hispanic | 2 | 0 |
| Chronic illnesses, noa | .57 (.66) | .59 (.46) |
| Insomnia duration, yb | 3.5 (0.6–7.5) | 4.8 (0.6–49.0) |
| Medicationsc | ||
| Sleep | 30% (7) | 29% (8) |
| Pain | 35% (8) | 50% (14) |
CBT-I refers to cognitive behavioral therapy for insomnia; SMW, stress management and wellness.
Data are presented as mean (SD).
Data are presented as median (range).
Percent of subjects (n) taking over-the-counter or prescription medications for sleep at least once in a 2-week period, or daily medications for pain.
Procedures
Participants were recruited by placement of brochures, memos, and flyers in places where medical patients who qualified for the study might see them, ie, community presentations (e.g., senior centers) and letters to individuals from mailing lists obtained from non-patient sources (e.g., American Arthritis Foundation membership mailing list) and physicians supporting the study. Participants were paid up to $200, based on how much of the protocol they completed. Enrollment occurred between January 2001 and October 2003.
Potential volunteers who contacted the investigators about the study were initially screened by telephone. The inclusion criteria used at this stage were (1) age 55 or older; (2) at least 3 episodes of insomnia per week for at least 6 months (problems with sleep onset, sleep maintenance, or a combination of both were allowed, defined as taking at least 30 minutes to fall asleep, being awake for at least 60 minutes after falling asleep, or accumulating less than 6.5 hours of sleep per night); and (3) daytime consequences of insomnia, such as fatigue, irritability, or difficulty concentrating. After passing the telephone screening, participants were required to undergo a night of home polysomnographic assessment to exclude individuals with sleep apnea or sleep disorders other than insomnia. Twenty-two individuals with suspected sleep apnea were eliminated from the study, and 2 additional individuals were eliminated based on t-scores of 70 or higher on subscales other than anxiety and somatization on the Brief Symptom Inventory.18
To meet criteria for osteoarthritis, an individual needed to report physician-diagnosed osteoarthritis (confirmed by a radiograph or magnetic resonance imaging study) in 1 or more joints (including the knees, hips, lower back, neck, fingers, thumb, or big toe), ongoing treatment from a physician, and at least “moderate” pain ratings on the SF-36 pain item addressing the degree of “bodily pain” or the pain-rating item from the Arthritis Impact Measurement Scales 2.19 One exception was made for an osteoarthritis participant who reported mild pain but had the highest possible Arthritis Impact Measurement Scales 2 scores for the number of joints that are painful and the degree of joint stiffness.
Per the design of the study, participants in the SMW treatment who continued to demonstrate sleep difficulties at the post-treatment assessment were offered the opportunity to cross over to CBT-I treatment after the post-treatment assessment. This design was based on previous experience by the authors suggesting that study retention over a 1-year follow-up period in this population was likely to be substantially compromised if or when sleep was not improved after treatment. Twenty-three of the 28 SMW subjects continued to have sleep problems that met study criteria. Of these, 13 began CBT-I treatment, and 12 completed both the treatment and the 1-year follow-up assessment. The 2 primary reasons for subjects choosing not to cross over were schedule conflicts and not wanting to participate in another class. Data from these additional 12 subjects were added to the CBT-I group for analyses presented in Table 3.
Table 3.
Comparison of Sleep Parameters and Pain Scores at 1-Year Follow-Up in Older Patients with Osteoarthritis and Co-Morbid Insomnia
| Parameter | CBT-I (n = 19)c |
CBT-I with Cross-overs (n = 31)d |
||||||
|---|---|---|---|---|---|---|---|---|
| Pre-treatment | 1-year | P Valueb | ESb | Pre-treatment | 1-year | P Valuea | ESb | |
| TST, min | 361 (51) | 393 (48) | 0.034 | 0.457 | 363 (47) | 390 (54) | 0.010 | 0.377 |
| SE, % | 74.3 (9.3) | 82.8 (6.9) | 0.001 | 0.734 | 74.7 (9.7) | 82.7 (9.2) | 0.000 | 0.598 |
| SLAT, min | 35.4 (17.9) | 24.0 (17.7) | 0.006 | 0.453 | 34.7 (22.0) | 23.7 (18.1) | 0.000 | 0.386 |
| WASO, min | 48.1 (29.0) | 28.7 (20.7) | 0.015 | 0.545 | 49.1 (29.3) | 29.2 (24.4) | 0.000 | 0.522 |
| Naps, min/wk | 11.6 (13.9) | 8.5 (11.2) | 0.470 | 0.174 | 11.9 (14.6) | 8.7 (12.6) | 0.221 | 0.166 |
| MPQ scoree | 9.1 (8.5) | 7.4 (7.7) | 0.129 | 0.148 | 9.6 (7.6) | 7.4 (6.7) | 0.029 | 0.217 |
| SF-PAIN scoree | 58.5 (19.8) | 64.2 (23.4) | 0.081 | 0.186 | 59.1 (20.4) | 63.8 (23.5) | 0.114 | 0.151 |
| GDS score | 5.9 (3.7) | 5.6 (4.6) | 0.668 | 0.051 | 5.6 (3.8) | 5.5 (4.4) | 0.794 | 0.017 |
Data are presented as mean (SD), significance level, and effect sizes (ES) for pre-treatment and post-treatment sleep measures. TST refers to total sleep time; SE, sleep efficiency; SLAT, sleep latency; WASO, wake after sleep onset; Geriatric Depression Scale (GDS) scores for. Data extracted from Rybarczyk et al., 2005.15 The cognitive behavioral therapy for insomnia (CBT-I) group contained the 19 subjects originally randomly assigned to CBT-I who participated in 1-year follow-up. The CBT-I with cross-overs group included those 19 subjects plus 12 subjects originally randomly assigned to the stress management and wellness (SMW) condition, who subsequently crossed-over and received CBT-I treatment.
Significance levels for pre-treatment to 1-year paired t-tests (2-tailed).
Effect size (ES) refers to within-subjects Cohen d.28
One subject had missing data on both follow-up pain measures.
Two subjects had missing data for the McGill Pain Questionnaire (MPQ), and 4 had missing data for the Medical Outcomes Study Short Form-36 Pain (SF-PAIN).
Higher MPQ scores indicate more pain. Higher SF-PAIN scores indicate less pain.
Interventions
The CBT-I and SMW (attention-control condition) interventions consisted of 8 weekly 2-hour classes matched in as many characteristics as possible. Class sizes ranged from 4 to 8 participants, averaging 5 members per class. All classes were conducted at an academic medical center in downtown Chicago and were spread out over the calendar year. Participants who missed a class were given make-up classes for up to 2 missed sessions.
CBT-I Protocol
The CBT-I intervention protocol closely followed Morin's insomnia treatment protocol,20 with the exception of an added relaxation-training component. Sessions were led by 2 experienced clinical psychologists. Each session included a didactic presentation, a question-and-answer period, a review of each individual's sleep log, and group discussion to solve problems encountered during implementation of the techniques. The 2 main behavioral components, stimulus control21 and sleep restriction22 were introduced and emphasized during the first 3 sessions. A strict schedule of bedtimes and arising times was prescribed to consolidate sleep and decrease time spent awake during the night. Patients initially reduced their time in bed to the amount of time they were actually sleeping, according to their pretreatment sleep logs, but not less than 4.5 hours. Sleep logs were completed continuously during treatment, and the bedtime was moved earlier by a maximum of one-half hour each week if there was sufficient improvement in sleep efficiency, usually defined as achieving 85% sleep efficiency. Participants were also instructed to lie awake in bed no longer than 15 minutes, at which time they were to go to another room, engage in a non-stimulating task in a dimly lit room, and return to the bed only when they felt sleepy again. No activities were permitted in bed other than sleep and sex. The third component to be introduced was cognitive restructuring, which emphasized changing unrealistic beliefs and irrational fears regarding sleep or loss of sleep. The fourth component was relaxation training, designed to decrease anxiety and reduce cognitive and physiologic arousal at bedtime. Each participant was given a relaxation audiotape that included the following 4 commonly used modalities: deep breathing, progressive muscle relaxation, autogenic training, and imagery. A final component of the intervention was sleep-hygiene education, including the use of increased daytime bright-light exposure to address any circadian causes of insomnia. Topics included increasing natural-light exposure, daytime activity. and exercise; reducing caffeine and alcohol intake; keeping an appropriate bedroom temperature; reducing ambient noise in the bedroom; using warm baths in the evening; and using appropriate food choices and eating patterns. The CBT-I treatment condition made no mention of pain management.
SMW Treatment
The SMW treatment, adapted from Rybarczyk et al.,23 consisted of didactic presentations and corresponding skill training covered the following 6 topics: (1) the mind-body relationship, (2) modifying self-talk for the reduction of stress and anxiety, (3) effective communication and assertiveness, (4) problem solving and goal setting, (5) nutrition, and, (6) exercise for individuals with chronic conditions. Topics 1 and 2, covered over 4 separate class sessions, were presented by a physician with extensive training and speaking experience regarding mind-body health. Topics 3 and 4 by were presented by psychologists, and Topics 5 and 6 were presented by an expert nutritionist and exercise physiologist. At the beginning of the SMW class, participants were given a presentation of a treatment rationale based on the common public perception of an interrelationship between sleep problems and stress, nutrition, and exercise. This model suggests that improvements in daytime coping and wellness lead to improvements in sleep. As a substitute for an active treatment of relaxation training, participants were given brief instruction in breath awareness. Providing such a quasi-relaxation training procedure was deemed essential to increasing the credibility of this program as a treatment for insomnia.
The SMW intervention was designed as an attention control for CBT-I and did not include an active treatment for relaxation or specifically mention pain control, but it did have several components that have been included in effective multi-component interventions for management of chronic pain. Although the SMW intervention targeted coping with chronic medical conditions in general (not chronic pain, per se), it included problem-solving, goal-setting, cognitive approaches to reducing stress and anxiety, interpersonal skills training, and education about exercise enhancement. These components have been used in various forms in previous studies of CBT interventions for coping with chronic pain.24 We assumed that analgesic effects of these nonspecific interventions were likely to be modest, but SMW conceptually might have had some therapeutic benefit for pain outcomes.
Study Measures
Although the parent study employed an extensive battery of self-report measures, here we report only those measures directly relevant to our specific stated purpose.
Sleep log
Sleep logs were paper-and-pencil records that participants completed each morning for 2 weeks before treatment (during the month prior to treatment), after treatment (during the month after treatment ended), and at the 1-year follow-up. They were also completed on a weekly basis during the CBT-I class. The logs included sleep latency (SLAT), nighttime awakenings (quantity and duration), time in bed, naps, and any medication used for sleep. For the present study, average SLAT, total sleep time (TST), wake after sleep onset (WASO), and sleep efficiency (SE) were calculated for each of the 3 two-week assessment periods for each study subject.
Short-Form MPQ
The Short-Form MPQ was designed to be a brief version of the long-form MPQ, which has been widely used in the measurement and study of pain.25 The short-form MPQ has been shown to correlate highly with the standard MPQ and to be sensitive to pain-management interventions. For the present study, the total score was used.
The SF-36
The SF-36 is a 36-item scale designed to assess the following 8 health concepts: physical functioning, role limitations due to physical health problems, social functioning, general mental health, role limitations due to emotional problems, general health perceptions, vitality, and bodily pain.26 The SF-36 has demonstrated adequate reliability and validity. For the present study, we report only the Bodily Pain Subscale (SF-PAIN), which is comprised of a question addressing “How much bodily pain have you had during the past 4 weeks?” (ranging from 1 “none” to 6 “very severe”) and a question asking “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?” (ranging from 1 “not at all” to 5 “extremely”).
Geriatric Depression Scale
The Geriatric Depression Scale (GDS) was developed specifically for use with older adults and uses a simple “yes/no” answer format.27 The GDS scale comprises 30 items, none of which reflect the somatic and vegetative aspects of depression, thus reducing possible confounding of depressive and age-related medical-illness symptoms. We report GDS data to address the possibility that any observed reduction in pain might be the result of improvements in depression status.
Statistical Analysis
In light of the small sample of patients with osteoarthritis who were available, we assessed within-subject pre-treatment to post-treatment change in each of the 2 groups separately using paired t-tests to maximize the ability of the analysis to detect clinically important change. The α level was set at ≤ 0.05. The parent study only tested for treatment effects on pain by using a repeated measures analysis of variance examining group X time effects. Within-group effect sizes (Cohen d) for CBT-I and SMW treatments were also calculated.28
RESULTS
As shown in Table 2, CBT-I subjects reported significantly decreased SLAT and WASO and increased SE after treatment, compared with before treatment. They also reported significantly reduced pain on the SF-Pain and a non-significant trend for reduced pain on the MPQ. CBT-I subjects reported no significant change in TST, whereas SMW subjects reported no significant changes in any measure of sleep quality or pain from before to after treatment. Average within-group pretreatment to post-treatment effect sizes of the 5 sleep measures and of the 2 pain measures were 0.501 and 0.243 for CBT-I versus 0.189 and 0.060 for SMW (Table 2).
Table 2.
Comparison of Sleep Parameters and Pain Scores in Older Patients with Osteoarthritis and Co-Morbid Insomnia Treated with Stress Management and Wellness or Cognitive Behavioral Therapy for Insomnia
| Parameter | SMWc (n = 28) |
CBT-Id (n = 23) |
||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | p Valuea | ESb | Before | After | p Valuea | ESb | |
| TST, min | 342 (84) | 370 (71) | 0.059 | 0.255 | 351 (60) | 372 (59) | 0.069 | 0.250 |
| SE, % | 70.2 (14.1) | 75.2 (14.0) | 0.069 | 0.252 | 71.0 (12.3) | 84.0 (8.1) | 0.000 | 0.883 |
| SLAT, min | 36.9 (27.1) | 33.4 (31.0) | 0.360 | 0.085 | 40.4 (21.4) | 23.5 (22.0) | 0.014 | 0.551 |
| WASO, min | 67 (45) | 55 (41) | 0.134 | 0.197 | 62 (47) | 25 (21) | 0.000 | 0.719 |
| Naps, min/wk | 15.5 (20.7) | 11.4 (15.7) | 0.084 | 0.158 | 9.9 (31.1) | 5.4 (6.8) | 0.067 | 0.141 |
| MPQ scoree | 11.1 (9.6) | 11.6 (10.8) | 0.704 | 0.035 | 10.1 (9.6) | 8.0 (7.1) | 0.221 | 0.176 |
| SF-PAIN scoree | 50.3 (21.4) | 53.1 (25.0) | 0.371 | 0.085 | 56.4 (19.7) | 66.1 (24.3) | 0.010 | 0.310 |
| GDS score | 5.3 (4.5) | 4.6 (4.5) | 0.327 | 0.110 | 5.6 (3.8) | 5.1 (4.7) | 0.608 | 0.083 |
Data are presented as mean (SD), significance level (p), and effect sizes (ES) for pre-treatment and post-treatment sleep measures. SMW refers to stress management and wellness; CBT-I, cognitive behavioral therapy for insomnia; TST, total sleep time; SE, sleep efficiency; SLAT, sleep latency; WASO, wake after sleep onset; GDS, Geriatric Depression Scale. Data extracted from Rybarczyk et al., 2005.15
Significance levels for pre-treatment to post-treatment paired t-tests (2-tailed).
Effect size (ES) refers to within-subjects Cohen d.28
One subject had missing data for the McGill Pain Questionnaire (MPQ), and another had missing data for the Medical Outcomes Study Short Form-36 Pain (SF-PAIN).
One subject had missing data for both post-treatment pain measures.
Higher MPQ scores indicate more pain. Higher SF-PAIN scores indicate less pain.
The majority of CBT-I subjects (19 of 23) were further assessed for sleep quality and perceived pain at a 1-year follow-up visit. As shown in the left panel of Table 3, at 1-year follow-up, they reported significantly decreased SLAT and WASO and increased SE and TST relative to pre-treatment. They also reported non-significant trends for reduced perceived pain on both the SF-Pain (p = 0.08) and the MPQ (p = 0.13). Average within-group pre-treatment to 1-year follow-up effect sizes of the 5 sleep measures and of the 2 pain measures were 0.473 and 0.167 (Table 3). SMW subjects were not assessed at 1-year follow-up.
Data from the pretreatment versus 1-year follow-up analyses that included both the 19 original CBT-I subjects, as well as the 12 SMW subjects who chose to cross over to CBT-I and finished treatment and 1-year follow-up, are reported in the right panel of Table 3. This group of 29 CBT-I treated subjects reported significantly decreased SLAT and WASO and significantly increased SE and TST relative to pretreatment baselines. They also reported significantly less pain on the MPQ and a non-significant trend for reduced pain on the SF-PAIN (p = 0.11). Average within-group pre-treatment to 1-year follow-up effect sizes of the 5 sleep measures and of the 2 pain measures were 0.410 and 0.184 (Table 3).
GDS scores for both CBT-I and CBT-I plus crossover subjects were low at pre-treatment and essentially unchanged at 1-year follow-up.
DISCUSSION
CBT-I improved both immediate and long-term self-reported sleep quality in this sample of older patients with osteoarthritis and co-morbid insomnia. These results are unique in demonstrating the long-term durability of CBT-I effects for co-morbid insomnia. The other major finding of this study—that CBT-I, without specifically addressing pain management, appeared to reduce both immediate and long-term reported pain in these patients—is also unique. This last finding is in contrast with the failure of the SMW treatment to significantly reduce reported pain at post-treatment assessment, despite the fact that the SMW protocol contained several treatment components that have been included in effective multi-component interventions for management of chronic pain. There were non-significant trends for improved sleep in the SMW group, as might be expected for an intervention targeting stress, but SMW effect sizes were substantially smaller than those of the CBT-I group.
These findings extend those of the parent study15 by suggesting the durability of CBT-I treatment effects on both sleep and pain across a 1-year follow-up. Several previous studies of CBT-I for insomnia co-morbid with chronic medical illness have demonstrated durability of treatment effects,29 but the longest follow-up to date has been 4 months in a sample of older adults with mixed chronic medical conditions.30 This is a potentially important finding given the common view that insomnia in medical populations is considered to be largely secondary to medical factors, such as pain or discomfort, and any worsening of those symptoms over time is believed by some to result in relapse of the insomnia.17
The recent finding of Manber and colleagues, who reported that CBT-I enhanced depression outcome in patients with co-morbid insomnia and major depression, provides additional support that improving sleep can result in an improvement in a co-morbid disorder, be it medical (osteoarthritis) or psychiatric (major depression).31 Another recent study by Stepanski and colleagues found that increased difficulty sleeping predicted increased pain ratings in people with cancer.32 If pain management for people with cancer can be enhanced through successful treatment of sleep disturbance, this would have important clinical implications for improving quality of life in this patient population. These results support the need for large-scale randomized controlled trials in diverse patient populations in which chronic pain and insomnia commonly co-occur (e.g., osteoarthritis, chronic back pain, cancer).
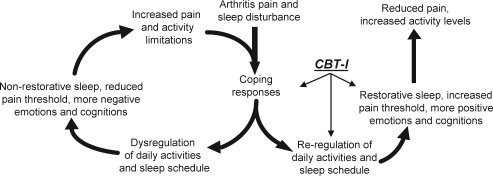
How might improving sleep decrease perceived pain among older patients with osteoarthritis? Figure 1 schematically depicts possible mechanisms through which osteoarthritis pain and associated sleep disturbance are believed to dysregulate daily activities and sleep schedule. As illustrated on the left side of Figure 1, when people with osteoarthritis must cope with arthritis pain and sleep disturbance, their daily activities and sleep schedules are altered. Sleep disturbance lowers the pain threshold and amplifies the transmission of pain signals, resulting in increased attention to pain, compromised function, and more negative pain-focused emotions and cognitions. Thus, via sleep disturbance-induced hyperalgesia, patients with osteoarthritis experience increased pain, reduced activity levels, and further disrupted sleep in a positive feedback-loop pattern.
Figure 1.
Conceptual model: Impact of cognitive behavioral therapy for insomnia (CBT-I).
Correspondingly, as shown on the right side of Figure 1, CBT-I enhances sleep, which raises the pain threshold, and amplification of pain-signal transmission is reduced. This, in turn, results in less perceived pain, less compromised function, and less pain-focused and more positive emotions and cognitions. This decreased pain and increased activity is then likely to further improve sleep, and the reciprocal interaction between sleep and pain helps to maintain both improved sleep quality and decreased perceived pain, again forming a positive feedback-loop.
Although the findings of this study support the need for further research, the current study has limitations. First, as noted previously, the parent study's SMW control group, which contained several components typically employed in CBT intervention protocols for pain control, was not an optimal control condition. Because the SMW control group received interventions that may be beneficial for pain, it was employed as a comparison group with separate within-subject analyses rather than as a control group employing a more rigorous between-groups analysis to control for type I error. Also, the SMW comparison group was not followed for 1 year. Even though pain is thought to progressively worsen in osteoarthritis, this study design was unable to control for spontaneous improvements in pain levels unrelated to improved sleep that may have occurred during the follow-up period. Second, the criteria for osteoarthritis in the present study allowed participants with only moderate pain levels in a single joint to participate in the study. The primary purpose of the parent study was to address co-morbid insomnia, so emphasis was placed on verifying that subjects had the requisite level of insomnia and a verifiable diagnosis of osteoarthritis (rather than a significant level of osteoarthritis pain). Third, although insomnia is more common among older women, as compared with men, this study sample had a preponderance of women, which might diminish the generalizability of the study findings to men with osteoarthritis and co-morbid insomnia. Fourth, although relaxation training was only a secondary component of the CBT-I and was only applied at bedtime, patients might have used such interventions to directly improve pain management, and the interventions may have had a possible direct effect on pain management. However, the potential impact of such relaxation is questionable, as a relatively large number of CBT-I studies have not included a relaxation component and have found no decrements in outcomes.16
Finally, greater confidence in the reliability of the results would require a larger sample size, which would also provide an opportunity to test related hypotheses, such as what factors predict successful sleep and pain outcomes. Given these limitations, we regard the results of this reanalysis of Rybarczk et al.'s data to yield only preliminary evidence in favor of the hypothesis that a successful sleep intervention has analgesic benefits among patients with osteoarthritis and co-morbid insomnia. Further randomized trials, with larger subject samples and intent-to-treat analyses, are needed to definitively evaluate this important clinical effect.
Such research is urgently needed because drugs used to control pain and inflammation remain the mainstay of osteoarthritis management. However, risks associated with long-term pharmacologic management constrain available treatment options and expose patients to health risks. Recently, COX-2 inhibitors were removed from the market due to increased risks of myocardial infarction.33 The use of other widely used drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs), is associated with health risks, including gastrointestinal and renal complications.34 The 2009 American Geriatrics Society clinical practice guideline, Pharmacological Management of Persistent Pain in Older Persons, “in a significant departure from the 2002 guideline—recommends that nonselective NSAIDs and COX-2 selective inhibitors be considered rarely, with caution, in highly selected individuals,” recommending opioid therapy instead.35 However, opioids carry their own significant risks, and the American Geriatrics Society guideline also states, “Clinicians should anticipate, assess for, and identify potential opioid-associated adverse effects.”35 These limitations have led to the evaluation of CBT-I for treatment of osteoarthritis pain and related dysfunction.
The evidence regarding the efficacy of cognitive-behavioral interventions specifically for arthritis pain and functioning is not strong. Astin et al.36 reported a meta-analysis of 25 trials of CBT-based pain interventions for rheumatoid arthritis and estimated an effect size of .22 for post-treatment benefits in pain, with somewhat larger effect sizes for improvements in functional disability, coping, and self-efficacy. The evidence regarding efficacy of cognitive behavioral interventions for osteoarthritis is also weak. Dixon and colleagues,24 in their meta-analysis of CBT-based pain interventions for osteoarthritis and rheumatoid arthritis, estimated a small pooled effect size of 0.18 for pain intensity and effect sizes of similar magnitude for functional outcomes. However, the effectiveness of a CBT-based sleep intervention for osteoarthritis pain has not been previously evaluated until the current study. Interestingly, the average effect size for the current study's CBT-I–related post-treatment reduction of pain was 0.24
Although some CBT-based pain interventions for osteoarthritis discuss sleep disturbances, sleep is typically not addressed in a systematic fashion, and interventions rarely go beyond basic sleep-hygiene recommendations that, by themselves, have little impact on sleep outcomes.37–38 Incorporating CBT-I, which specifically targets sleep, into behavioral interventions for osteoarthritis is a potential approach to increase the overall effectiveness for pain and functional outcomes that merits further research.
The ability of CBT-I to improve both short- and long-term sleep quality has been well demonstrated. The current study indicates that such long-term improvements may also be obtained in patients with insomnia and co-morbid osteoarthritis. The current study further suggests that, in addition to improving sleep, and even without directly addressing pain management, CBT-I appears to decrease pain in older patients with osteoarthritis and co-morbid insomnia both after treatment and at 1-year follow-up. These results, while preliminary, support the hypothesis that improving sleep, per se, in patients with osteoarthritis may be analgesic, such that perceived pain is reduced without being specifically targeted. These results further suggest that techniques to improve sleep, such as CBT-I, should be considered as additions to the various existing behavioral treatment programs for pain management in osteoarthritis, and possibly in other chronic pain conditions as well.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was supported by PHS grants AG017491 (BDR) and AG025515, AG031126, MH072736, NR001094, and CA116400 (MVV). The authors thank Dr. Laura D. Baker for her assistance with effect-size calculations.
REFERENCES
- 1.Buckwalter JA, Heckman JD, Petrie DP. Aging of the North American population: new challenges for orthopaedics. J Bone Joint Surg. 2003;85A:748–58. [PubMed] [Google Scholar]
- 2.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–73. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 3.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbance and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Moffit PF, Kalucy RS, Baum FE, Cooke RD. Sleep difficulties, pain and other correlates. J Intern Med. 1991;230:245–49. doi: 10.1111/j.1365-2796.1991.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 6.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–52. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 8.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–51. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 9.Moldofsky H, Lue FA, Saskin P. Sleep and morning pain in primary osteoarthritis. J Rheumatol. 1987;14:124–8. [PubMed] [Google Scholar]
- 10.Leigh TJ, Hindmarch I, Bird HA, Wright V. Comparison of sleep in osteoarthritic patients and age and sex matched health controls. Ann Rheum Dis. 1988;47:40–2. doi: 10.1136/ard.47.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+ Cochrane. Database Syst Rev. 2003:CD003161. doi: 10.1002/14651858.CD003161. [DOI] [PubMed] [Google Scholar]
- 12.Simon G, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417–23. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 13.Lautenbacher S, Kundermann B, Krieg J-C. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Foo H, Mason P. Brainstem modulation of pain during sleep and waking. Sleep Med Rev. 2002;7:145–54. doi: 10.1053/smrv.2002.0224. [DOI] [PubMed] [Google Scholar]
- 15.Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, Davis A. A placebo-controlled test of CBT for co-morbid insomnia in older adults. J Consult Clin Psychol. 2005;73:1164–74. doi: 10.1037/0022-006X.73.6.1164. [DOI] [PubMed] [Google Scholar]
- 16.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 17.Stepanski E, Rybarczyk B. Emerging research on the treatment and etiology of secondary or co-morbid insomnia. Sleep Med Rev. 2006;10:7–18. doi: 10.1016/j.smrv.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Derogatis LR, Nelisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;3:595–605. [PubMed] [Google Scholar]
- 19.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2: the content and properties of a revised and expanded Arthritis Impact Measurement Scales health status questionnaire. Arthritis Rheumat. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 20.Morin CM. New York, NY: Guilford Press; 1993. Insomnia: Psychological Assessment and Management. [Google Scholar]
- 21.Bootzin R. Effects of self-control procedures for insomnia. In: Stuart RB, editor. Behavioral Self-Management: Strategies, Techniques and Outcomes) New York, NY: Brunner/Mazel; 1977. pp. 176–95. [Google Scholar]
- 22.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- 23.Rybarczyk B, DeMarco G, DeLaCruz M, Lapidos S, Fortner B. A classroom mind-body wellness intervention for older adults with chronic illness: comparing immediate and one year benefits. Behav Med. 2001;27:15–27. doi: 10.1080/08964280109595768. [DOI] [PubMed] [Google Scholar]
- 24.Dixon KE, Keefe FJ, Scipio CD, Perri LCM. Psychological intervention for arthritis pain management in adults: a meta-analysis. Health Psychol. 2007;26:241–50. doi: 10.1037/0278-6133.26.3.241. [DOI] [PubMed] [Google Scholar]
- 25.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 26.Ware JE, Kosinski M, Keller SD. Boston, MA: New England Medical Center, The Health Institute; 1994. SF-36 Physical and Mental Health Summary Scales: A User's Manual. [Google Scholar]
- 27.Brink TL, Yesavage JA, Lum O, Heersema P, Adey M, Rose TL. Screening tests for geriatric depression. Clin Gerontol. 1982;1:37–43. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 28.Dunlop WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods. 1996;1:170–7. [Google Scholar]
- 29.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–92. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Rybarczyk B, Lopez M, Benson R, Alsten C, Stepanski E. Efficacy of two behavioral treatment Programs for co-morbid geriatric insomnia. Psychol Aging. 2002;17:288–298. [PubMed] [Google Scholar]
- 31.Manber R, Edinger JD, Gress JL, San Pedro-Dalcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with co-morbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stepanski E, Walker MS, Schwartzberg LS, et al. The relation of trouble sleeping, depressed mood, pain and fatigue in patients with cancer. J Clin Sleep Med. 2009;5:132–6. [PMC free article] [PubMed] [Google Scholar]
- 33.Juni P, Nartley L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364:20–1. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- 34.Pham K, Hirschberg R. Global safety of coxibs and NSAIDs. Curr Top Med Chem. 2005;5:465–73. doi: 10.2174/1568026054201640. [DOI] [PubMed] [Google Scholar]
- 35.Pharmacological Management of Persistent Pain in Older Persons American Geriatrics Society Panel on Persistent Pain in Older Persons. J Am Geriatr Soc. 2009 doi: 10.1111/j.1532-5415.2009.02376.x. Jul1 epub ahead of press. doi:10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 36.Astin JA, Beckner W, Soeken K, Hochberg MC, Berman B. Psychological interventions for rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum. 2002;47:291–302. doi: 10.1002/art.10416. [DOI] [PubMed] [Google Scholar]
- 37.McCurry SM, Logsdon RG, Teri L, Vitiello MV. Evidence-based psychological treatments for older adult sleep disorders. Psychol Aging. 2007;22:18–27. doi: 10.1037/0882-7974.22.1.18. [DOI] [PubMed] [Google Scholar]
- 38.Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Med Rev. 2003;7:215–25. doi: 10.1053/smrv.2001.0246. [DOI] [PubMed] [Google Scholar]