Abstract
Study Objectives:
Recent meta-analyses raising concern about risks of hypnotics suggest a need for more clarification of these risks.
Methods:
Because of preliminary suggestions that eszopiclone causes infections, we studied US Food and Drug Administration files on the 4 most-recently approved hypnotics, combined with published studies, to compile the risk ratios of infections for groups randomly assigned to receive hypnotics versus those assigned to receive placebos in controlled trials. Parallel controlled clinical trials of eszopiclone, ramelteon, zaleplon, and zolpidem were included when data on subjects, duration of exposure, and adverse effects were available. Results of trials were combined by meta-analyses.
Results:
Of 8828 participants assigned to the 4 hypnotics and 4383 participants who randomly received placebos, 606 in the hypnotics groups and 200 in the placebo groups were reported to develop some kind of infection (risk ratio = 1.44, 95% confidence interval 1.25-1.64, p < 0.00001). Most infections were apparently mild and did not lead to dropouts. Subanalyses for individual drugs indicated that eszopiclone and zolpidem individually were associated with reported infections. There were insufficient data concerning individual studies of zaleplon and ramelteon for valid secondary meta-analyses of zaleplon or ramelteon by themselves.
Conclusions:
Research is needed to objectively determine whether the use of hypnotics increases the risk of infections. Immune compromise or esophageal reflux and aspiration should be studied as possible mechanisms.
Citation:
Joya FL; Kripke DF; Loving RT; Dawson A; Kline LE. Meta-analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. J Clin Sleep Med 2009;5(4):377-383.
Keywords: Eszopiclone, ramelteon, zaleplon, zolpidem, infection, inflammation, meta-analysis
On March 8, 2006, the New York Times estimated the US hypnotics market at about 43 million prescriptions per year.1 This would be sufficient for a nightly dose for more than 3,000,000 users throughout the year. The press has reported that usage has since grown and will continue to grow. Per capita consumption might be even higher in other wealthy countries.2 Up to 75% of prescriptions for hypnotics are written for users who chronically consume hypnotics for months or years at a time.2,3
Recently, 2 meta-analyses have emphasized the risks of contemporary hypnotics.4,5 Glass et al4 reported that risks were more common than benefits among patients over 60 years of age, who consume close to half of all hypnotics dispensed. Buscemi et al5 noted significantly more frequent harm (adverse medical symptoms) among those given hypnotics than among those randomly assigned to placebo.
In attempting to further understand the risks of hypnotics, our interest was attracted by the manufacturer's prescribing information for eszopiclone, which mentioned a dose-response association with reported adverse incidents of infection of several kinds.6 One of us remarked on the disturbing lack of published analysis of adverse effects of hypnotics, such as infection.7 Accordingly, we decided to systematically review data on reports of infection with the 4 most-recently introduced hypnotics, for which adequate data might be accessible, to see if increased reports of infection were associated with all 4 hypnotics.
METHODS
Several sources of data on reported infections occurring during randomized trials were surveyed. These included US Food and Drug Administration (FDA) New Drug Application (NDA) documents made available on the Drugs@FDA web site,8 published clinical trial reports listed in Pub Med, and official product prescribing information supplied by the manufacturers with FDA approval (Table 1). The FDA NDA sources provided data from the largest number of studies and often contained more detail about adverse effects than did the published literature. Whenever a study reported in FDA documents or prescribing information had been published, only the more detailed list of adverse effects (FDA, prescribing information or published) was used. The 4 most recently approved hypnotics were included in the study—zolpidem (Ambien), zaleplon (Sonata), eszopiclone (Lunesta), and ramelteon (Rozerem)—because much more extensive clinical trials data were available for these 4 than for older hypnotics and because they currently constitute most of the US hypnotics market. The zolpidem NDA, being the oldest of the 4, contained the least data, but, because zolpidem was used as a contrast drug in several zaleplon studies and because a large study of the zolpidem sustained-release preparation was recently published,9 sufficient zolpidem data were available.
Table 1.
Sources of Data
| Study | GROUPS RECEIVING HYPNOTICS |
GROUPS RECEIVING PLACEBO |
Source | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No.a | Retainedb | Durationc | Net yd | Age, ye | No.a | Retainedb | Durationc | Net yd | Age, ye | ||
| ESZOPICLONE | ESZOPICLONE PLACEBO | ||||||||||
| 26 | 338 | 1.000 | 0.0027 | 0.93 | 25-50 | 98 | 1.000 | 0.0027 | 0.27 | 25-50 | 11 |
| 46 | 204 | 1.000 | 0.1205 | 24.59 | 21-64 | 96 | 1.000 | 0.1205 | 11.57 | 21-64 | 11 |
| 49 | 593 | 0.800 | 0.5000 | 237.20 | 21-64 | 195 | 0.780 | 0.5000 | 76.05 | 21-64 | 11 |
| 47 | 136 | 0.990 | 0.0384 | 5.16 | 65-86 | 126 | 0.970 | 0.0384 | 4.69 | 65-86 | 11 |
| 48 | 143 | 0.950 | 0.0384 | 5.21 | 64-85 | 76 | 0.950 | 0.0384 | 2.77 | 64-85 | 11 |
| Eszopiclonef | 269 | 0.850 | 0.1534 | 6.73 | 21-64 | 274 | 0.840 | 0.1534 | 6.66 | 21-64 | 14 |
| Eszopiclone, 3 mg | 201 | 0.940 | 0.0833 | 4.98 | 40-60 | 209 | 0.933 | 0.0833 | 5.35 | 40-60 | 12 |
| Eszopiclone, 3 mg | 548 | 0.820 | 0.5000 | 10.41 | 21-64 | 280 | 0.740 | 0.5000 | 2.66 | 21-64 | 13 |
| Total Eszopiclone | 2432 | 295.22 | 1354 | 110.02 | |||||||
| RAMELTEON | RAMELTEON PLACEBO | ||||||||||
| PNFP002 | 252 | 1.000 | 0.0055 | 1.38 | 35-60 | 123 | 1.000 | 0.0055 | 0.67 | 35-60 | 15 |
| TL020 | 561 | 1.000 | 0.0959 | 53.79 | 18-64 | 287 | 1.000 | 0.0959 | 27.52 | 18-64 | 15 |
| TL021 | 274 | 1.000 | 0.0959 | 26.27 | 18-64 | 131 | 1.000 | 0.0959 | 12.56 | 18-64 | 15 |
| TL023 | 191 | 1.000 | 0.0027 | 0.52 | 18-64 | 97 | 1.000 | 0.0027 | 0.27 | 18-64 | 15 |
| TL025 | 555 | 1.000 | 0.0959 | 53.22 | 65+ | 274 | 1.000 | 0.0959 | 26.27 | 65+ | 15 |
| TL031 | 50 | 1.000 | 0.0767 | 3.84 | 18-45 | 49 | 1.000 | 0.0767 | 3.76 | 18-45 | 15 |
| TL032 | 57 | 0.720 | 0.5000 | 20.52 | 18-45 | 65 | 0.815 | 0.5000 | 26.49 | 18-45 | 15 |
| Ramelteon 8 mg, 16 mg | 274 | 0.958 | 0.0959 | 25.17 | 18-64 | 131 | 0.958 | 0.0959 | 12.03 | 16-64 | 16 |
| Total Ramelteon | 2214 | 184.72 | 1157 | 109.58 | |||||||
| ZALEPLON & ZOLPIDEM | ZALEPLON & ZOLPIDEM PLACEBO | ||||||||||
| 203 Zaleplon | 66 | 1.000 | 0.0384 | 2.53 | 18-60 | 33 | 1.00 | 0.0384 | 1.27 | 18-60 | 17 |
| 204 Zaleplon | 65 | 1.000 | 0.0767 | 4.99 | 18-60 | 32 | 1.000 | 0.0767 | 2.45 | 18-60 | 17 |
| 204 Zolpidem | 32 | 1.000 | 0.0767 | 2.45 | 18-60 | 17 | |||||
| 205 Zaleplon | 110 | 1.000 | 0.0137 | 1.51 | 18-60 | 27 | 1.000 | 0.0137 | 0.37 | 18-60 | 17 |
| 210 Zaleplon | 178 | 1.000 | 0.0027 | 0.49 | 26-60 | 89 | 1.000 | 0.0027 | 0.24 | 26-60 | 17 |
| 301 Zaleplon | 355 | 0.890 | 0.0767 | 24.24 | 18-65 | 119 | 0.930 | 0.0767 | 8.49 | 18-65 | 17 |
| 301 Zolpidem | 116 | 0.880 | 0.0767 | 7.83 | 18-65 | 17 | |||||
| 303 Zaleplon | 365 | 0.920 | 0.0767 | 25.76 | 18-65 | 126 | 0.930 | 0.0767 | 8.99 | 18-65 | 17 |
| 303 Zolpidem | 122 | 0.890 | 0.0767 | 8.33 | 18-65 | 17 | |||||
| 306 Zaleplon | 331 | 0.930 | 0.0384 | 11.81 | > 65 | 107 | 0.910 | 0.0384 | 3.73 | > 65 | 17 |
| 306 Zolpidem | 111 | 0.950 | 0.0384 | 4.04 | > 65 | 17 | |||||
| 307 Zaleplon | 484 | 0.880 | 0.0384 | 16.34 | 18-65 | 153 | 0.950 | 0.0384 | 5.58 | 18-65 | 17 |
| 308 Zaleplon | 270 | 0.970 | 0.0384 | 10.05 | ≥ 65 | 135 | 0.970 | 0.0384 | 5.02 | ≥ 65 | 17 |
| Zolpidem ER, 6.25 mg | 99 | 1.000 | 0.0575 | 5.70 | > 65 | 106 | 1.000 | 0.0575 | 6.10 | > 65 | 18 |
| Zolpidem ER, 12.5 mg | 102 | 1.000 | 0.0575 | 5.87 | 18-64 | 110 | 1.000 | 0.0575 | 6.33 | 18-64 | 18 |
| Zolpidem ER, 12.5 mg | 669 | 0.768 | 0.4603 | 236.46 | 18-64 | 349 | 0.830 | 0.4603 | 133.26 | 18-64 | 9 |
| Zolpidem, 10 mg | 68 | 0.926 | 0.0833 | 5.25 | 39-60 | 73 | 0.959 | 0.0833 | 5.83 | 39-60 | 19 |
| Zolpidem, < 10 mg | 152 | 1.000 | 0.0833 | 12.67 | 161 | 1.000 | 0.0833 | 13.42 | 20 | ||
| Zolpidem, 10 mg | 122 | 1.000 | 0.0767 | 9.36 | 16-85 | 126 | 1.000 | 0.0767 | 9.67 | 16-85 | 21 |
| Zaleplon 5-20 mg | 365 | 1.000 | 0.0767 | 28.00 | 16-85 | 126 | 1.000 | 0.0767 | 9.67 | 18-85 | 21 |
| Zaleplon Total | 2589 | 125.70 | 947 | 45.81 | |||||||
| Zolpidem Total | 1593 | 297.96 | 925 | 174.61 | |||||||
| ALL DRUGS | 8828 | 903.59 | 4383 | 440.01 | |||||||
Note: It was necessary to list zaleplon and zolpidem on consecutive lines to reflect trials in which there were 3-way randomizations of zaleplon, zolpidem, and placebo. The placebo results were tabulated opposite the corresponding zaleplon data. Studies in Table 1 were the same as those in Figure 1.
Refers to the number of patients receiving the drug or placebo.
To reflect dropout effects on duration of drug exposure, this retention factor was (total subjects – dropouts/2)/total subjects, assuming that the average dropout left the study half way through.
Duration in years of each subject's drug exposure, i.e., days of exposure / 365.
Total subject drug exposure in person years, computed as number of participants X retention factor X Duration in years
Refers to the age range of the participants in the trial.
Participants randomized to both eszopiclone and placebo received fluoxetine.
To be included, each study had to provide data from a randomized, parallel-group, controlled trial of the hypnotic contrasted with a placebo, with enumeration of the numbers of subjects, duration of dosage as fraction of a year, and numbers of incident adverse-event reports (mentions). There were no exclusions concerning age or health of participants, number of participants, or duration of drug administration. So far as could be determined, results from open-label nonrandomized portions of the trials were excluded from the compilations. Because some studies randomly allocated more participants to drug than to placebo groups, the size of drug and placebo groups often were not balanced. It can be assumed that all infection-susceptibility factors affecting the contrasts, such as age, sex, comorbidities, and duration of exposure, were balanced by randomization within each study. Although the vast majority of these reports may have used standardized terminology, when upper respiratory infection cases, sinusitis, viral infection, etc. were tabulated, the clinicians may have had little means of distinguishing infective from noninfective disorders, but the same diagnostic limitations would apply to drug and placebo groups. Insufficient data were available to assess the quality of infection ascertainments, if objective, or other aspects of the quality of these studies. Insufficient data were available to consider the severity of infections.
The contributions to total drug-exposure time from each study were estimated by multiplying the number of participants in drug and placebo groups (N) times the retention rate of the study (R) times the duration of the study (D). Duration in years was calculated from the number of days during the study when a participant was assigned to medication or placebo divided by 365. The retention rate (R), a decimal from 1.00 to 0.00, was estimated from the reported dropout rates, adjusted on the assumption that the average dropout occurred half way through the duration of drug administration. This was necessary because exact data on the drug-exposure intervals of each subject were not provided in the documentary sources. Accordingly, statistical techniques depending on exact dropout times, such as the Cox proportional hazards model, could not be used.
The compilation for each study was entered into the Review Manager 5.0 program for computation of meta-analyses.10 A random-effects Mantel-Haenszel analysis of infection risk ratios was computed, primarily for all 4 hypnotics combined, and then secondarily for each drug separately. The methodology weighted the studies, in part, by the numbers of infections observed, which may have reflected the durations of drug exposure, age and health of the subjects, and many unidentified factors.
RESULTS
Table 1 provides the sources of data for the 4 drugs and their placebos. In summary, there were 8828 participants in randomized trials assigned to hypnotics, for a total exposure duration of 904 patient years (10,843 months), and 4383 participants who randomly received placebos, for a total exposure of 440 patient years (5164 months). The mean drug exposure was 0.10 years per participant (about 1.2 months), and the mean placebo exposure was also 0.10 years.
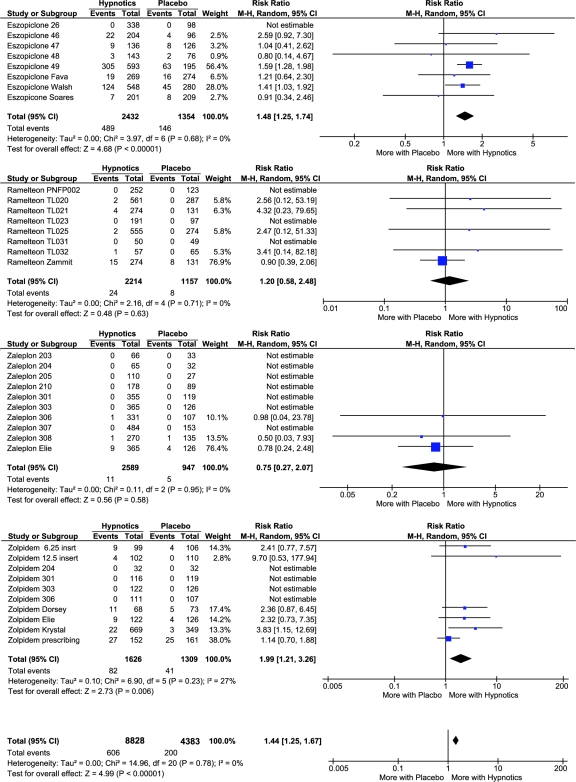
Table 2 lists the adverse events associated with the individual studies. The term infection was the most common term among various terms for infections, but a variety of other terms were used. In total, there were 606 infections reported among participants receiving hypnotics and 200 infections among those receiving placebo in the studies that listed infections (Table 3). Thus, over an average exposure of 36 days, roughly 1 participant in 15 receiving hypnotics reported an infection, versus roughly 1 participant in 22 receiving placebo. As might be expected, higher rates of infection were observed in the studies of longer duration, both in the drug and placebo groups (r = 0.59, p < 0.0002). The crude infection risk ratio for being randomly assigned to receive a hypnotic was 1.49. When the Review Manager meta-analysis was computed for the 4 drugs combined, the overall infection risk ratio was 1.44 (1.25-1.64, 95% confidence interval [CI], p < 0.00001) for being randomly assigned to receive a hypnotic, as contrasted with placebo. Figure 1 provides details of these analyses. Figure 1 indicates that, for studies in which no infection risks were reported in either drug or placebo groups, the software could not estimate a risk ratio and, therefore, gave studies listing no infection risks no weight in the meta-analyses. However, note that studies with a lower rate of infection in the drug group than in the placebo group were included.
Table 2.
Infections Associated with Individual Studies in Hypnotic and Placebo Groups
| Mention Term | Received Hypnotic N = 8828 | % Of 8828 | Received Placebo N = 4383 | % Of 4383 |
|---|---|---|---|---|
| Infection* | 211 | 2.39 | 63 | 1.44 |
| Laryngitis | 4 | 0.05 | 1 | 0.02 |
| Rhinitis | 44 | 0.50 | 15 | 0.34 |
| Pharyngitis | 149 | 1.69 | 53 | 1.21 |
| Sinusitis | 54 | 0.61 | 17 | 0.39 |
| Mouth ulcer | 1 | 0.01 | 2 | 0.05 |
| URI | 35 | 0.40 | 25 | 0.57 |
| Pneumonia | 2 | 0.02 | 0 | 0.00 |
| Bronchitis | 10 | 0.11 | 2 | 0.05 |
| Fever | 14 | 0.16 | 3 | 0.07 |
| Flu | 16 | 0.18 | 9 | 0.21 |
| Influenza | 8 | 0.09 | 0 | 0.00 |
| Viral infection | 18 | 0.20 | 4 | 0.09 |
| Conjunctivitis | 3 | 0.03 | 1 | 0.02 |
| Otitis media | 13 | 0.15 | 1 | 0.02 |
| Herpes simplex | 4 | 0.05 | 0 | 0.00 |
| Herpes zoster | 2 | 0.02 | 0 | 0.00 |
| UTI | 22 | 0.25 | 7 | 0.16 |
| Pyelonephritis | 2 | 0.02 | 0 | 0.00 |
| Cystitis | 4 | 0.05 | 2 | 0.05 |
| Diverticulitis | 1 | 0.01 | 0 | 0.00 |
URI refers to upper respiratory infection; UTI, urinary tract infection. *The general term infection was listed rather than a site-specific term.
Table 3.
Numbers of Participants and Infections Associated with Individual Studies for Each Hypnotic Drug and Parallel Placebo
| Medication | Received Drug |
Received Placebo |
||||
|---|---|---|---|---|---|---|
| No. | Infections, no. | Infections/participant, % | No. | Infections, no. | Infections/participant, % | |
| Eszopiclone | 2432 | 489 | 20.1 | 1354 | 146 | 10.8 |
| Ramelteon | 2214 | 24 | 1.1 | 1157 | 8 | 0.7 |
| Zaleplon | 2589 | 11 | 0.4 | 947 | 5 | 0.5 |
| Zolpidem | 1593 | 82 | 5.1 | 1309 | 41 | 3.1 |
| TOTALS | 8828 | 606 | 6.9 | 4383* | 200 | 4.6 |
The placebo total was corrected for placebo groups contrasted to both zaleplon and zolpidem in 3-way studies.
Figure 1.
Forest plots and details of meta-analyses for individual hypnotics and for the combination of the 4 drugs (bottom). Events were compiled infections, and Totals were for all 4 drugs. For studies with 3-way randomization of zaleplon, zolpidem, and placebo, the placebo groups were used twice (in the single-drug meta-analyses for zaleplon and zolpidem), but the grand total reflects the actual number of placebo participants.
Secondarily considering the data for each hypnotic individually, some heterogeneity appeared (Figure 1). For eszopiclone, the infection risk ratio was 1.48 (1.25-1.74, 95% CI, p < 0.00001). The risk ratio for the zolpidem studies was 1.99 (1.21-3.26, 95% CI, p = 0.006).
For ramelteon, the manufacturer provided the FDA with an aggregation of the adverse events for all trials combined.15 In the manufacturer's compilation, 313 infections were reported among 4100 ramelteon participants versus 85 among 1370 receiving placebo (p = 0.042, Fisher exact test, 1-tailed). Although the preponderance of infection in the ramelteon group appeared equivocally significant in this manufacturer's compilation (not a meta-analysis), it was uncertain if the durations of exposure were equivalent in the hypnotic and placebo groups, for some open-label ramelteon extension data may have been included in this compilation. Since we located only 24 mentions of infections linked to individual trials of ramelteon and only 8 for ramelteon placebo (Figure 1), it is evident that most of the infections aggregated in the manufacturer's overall report were not itemized in descriptions of the individual trials. Therefore, many of the notations of 0 infections for ramelteon studies as listed in Figure 1 may be erroneous because the infections were reported only in aggregate. Since meta-analysis requires itemization of the infection events by trial, the secondary meta-analysis for ramelteon alone may be unreliable because it could not include the aggregated data and its confidence limits were so broad as to be uninformative.
Similarly, the zaleplon manufacturer reported an aggregation of 3 randomized, parallel-group, 28-day trials that appeared to show about an 11% rate of infection mentions among participants receiving zaleplon versus a 6% rate among those receiving placebo, as summarized in Table 4 (p < 0.03). Because rounded percentages rather than exact numbers were supplied in this manufacturer's compilation, this statistical inference may be inexact. Although the manufacturer's compilation made clear that infections were frequent in 28-day studies of zaleplon, no information on infections associated with the individual 28-day studies could be located. Accordingly, it appears that much data on zaleplon infections were found only in aggregated form. Consequently, the secondary meta-analysis for zaleplon may also be unreliable. Likewise, its confidence limits were too broad to be informative.
Table 4.
Percentage of Subjects Reported to Have Infections with the Use of Zaleplon and Placeboa
| Zaleplon N = 786 | Placebo N = 277 | |
|---|---|---|
| Infection | 5 | 4 |
| Fever | 2 | 1 |
| Sinusitis | 2 | < 1 |
| Herpes simplex | 1 | < 1 |
| UTI | 1 | 0 |
| TOTAL (estimated) | 11* | 6* |
Data are presented as percentages. Estimated from Table 8.1.5.3.1 in the zaleplon New Drug Application, part 6, page 22 (1999b).22 These data apparently aggregated zaleplon 28-day parallel trials 204, 301, and 303 (see Table 1). UTI refers to urinary tract infection. *p < 0.04, χ2 = 5.1, estimating 11% of 786 and 6% of 277.
DISCUSSION
When meta-analyses are designed, there is always room for differences of opinion concerning what criteria for inclusion and exclusion of studies should be adopted. There is no right answer. Different criteria have different strengths and weaknesses. In these meta-analyses, we decided prospectively to focus on the 4 most-recently approved hypnotics for which relatively extensive FDA NDA reports are available on the Internet. This FDA source had more data and often offered more detail about adverse effects than did the published literature; however, postmarketing published studies on the same drugs were also included when available. We prospectively predicted that sufficient data could be located for the 4 hypnotics but not for other hypnotics.
In the result, for ramelteon and zaleplon, the information available was limited. A limitation to the meta-analyses was that, for 15 of the 36 studies overall, we could identify no by-study mentions of infections for either the drug or the placebo groups and, therefore, had to list these studies as reporting no infections. For ramelteon and zaleplon, the manufacturers had submitted compilations to the FDA aggregating infections for groups of studies. These compilations showed far more infections than the infection mentions located for the individual studies, so it is evident that, for at least some of the 15 studies (particularly the 3 studies contributing to Table 4), there were infection mentions during the studies that we could not ascertain. The individual studies for which we could identify no infection reports were mainly short studies in which presumably few infections occurred, since these studies comprised only 138 of the 1354 estimated person years of drug exposure in the total group of studies. To simulate the potential impact on the overall meta-analysis risk-ratio computation of studies listed with 0 infections, for the 3 studies aggregated in Table 4, we extrapolated infection numbers for each study from the aggregation. For the 12 other studies for which neither infection listings nor specific aggregated data were available, we simulated 1 infection per study in each of the drug and placebo groups. The overall risk ratio for all 36 studies in this simulation was 1.42 (1.24-1.63, p < 0.00001), hardly different from the 1.44 risk ratio computed for the 21 studies for which associated infections had been listed. Accordingly, it is unlikely that our inability to locate infection listings for some smaller and shorter-duration studies had any important influence on the outcome of the primary overall meta-analysis. The investigators considered trying to obtain missing study data from the manufacturers or the FDA but learned that this would probably be impossible. Some of the studies were performed more than 20 years ago, some in other countries, and, in 2 cases, the current manufacturer is not the company that had submitted the NDA to the FDA.
A method of meta-analysis was selected that tolerates heterogeneity between studies, provided that each is a randomized comparison of drug and placebo. This was essential, not only due to the inclusion of 4 quite different hypnotics, but also because the durations of the studies, drug doses, and types of subjects varied widely even for an individual drug, producing varying rates of infection for both drug and placebo groups. The prospective plan had been to compute the overall meta-analysis first, proceeding to consideration of the individual drugs only if the overall meta-analysis showed a significant risk for the group of hypnotics.
The overall analysis was robust, and the increased infection reports for the group of hypnotics was significant at the p < 0.00001 level. For the 4 modern hypnotics as a group, the overall meta-analysis showed that reports of infection were 44% more frequent in the hypnotic groups than in the placebo groups. In an average exposure of 36 days, a person randomly assigned to receive hypnotic had a 6.9% chance of reporting an infection, but, if assigned to placebo, the chance was 4.6%. Since these data were from randomized clinical trials designed by the manufacturers to determine if their hypnotic was efficacious, there is little reason to suspect any adverse bias. One interpretation would be that, as a group, the use of these hypnotics was associated with reported infections. However, we cannot exclude the possibility that trial participants and examining physicians would be more likely to report an infection if they guessed that the participant had received active hypnotic. Likewise, we cannot exclude the possibility that the infection rate would be more likely to be reported in a particular trial when the rate for the hypnotic exceeded that for placebo. Most reported infections were presumably mild, not leading to dropouts, but influenza and urinary tract infections, for example, occasionally prove lethal. The data did not include objective evidence of infections and could not exclude sterile inflammation or other sources of symptoms.
Our analysis found that sufficient data were available for eszopiclone and zolpidem, providing statistically robust evidence that random allocation to receive these hypnotics was associated with increased reports of infection. Three individual studies of eszopiclone and zolpidem also showed significant hypnotic association with infection reports within a single trial, demonstrating that the finding was no mere artifact of meta-analysis or selection of studies. In 2 studies of eszopiclone, a dose-response association has been clearly acknowledged in the prescribing information. It also seemed compelling for zolpidem. In a recent study,9 there were 22 mentions of sinusitis in the zolpidem extended-release group versus only 3 in the parallel placebo group, yielding a risk ratio greater than 3.
It was problematic that only a small number of infections were listed for individual trials of zaleplon and ramelteon. The argument could be made that zaleplon and ramelteon should have been dropped from the overall meta-analysis because of inadequate data, but, because removal of studies of these drugs might bias the overall meta-analysis toward a higher risk ratio, we thought it more conservative to include zaleplon and ramelteon, thus avoiding a potential retrospective bias.
It may be impossible to reach definite conclusions about individual effects of ramelteon and zaleplon. Because so many ramelteon and zaleplon studies listed few or no infections associated, though we know from the aggregations that there were more infections in both drug and placebo groups, we know our meta-analyses for these individual drugs were unreliable, even apart from the fact that, for these 2 drugs, the confidence limits were so broad as to make risk-ratio estimates uninformative. The manufacturer's aggregation of data for all ramelteon studies submitted to the FDA appeared comprehensive and displayed an equivocally significantly higher rate of infections for ramelteon subjects than for placebo recipients, but this was not a meta-analysis in which ramelteon and placebo groups necessarily had the same average durations of exposure, especially since open-label ramelteon data may have been included. The zaleplon aggregation in Table 4 appeared to show a significantly higher infection risk for zaleplon in 28-day parallel studies, but the bulk of zaleplon studies were not included in this aggregation, and the listing by percentages may make the statistical inference inaccurate. In summary, the infection risks for ramelteon and zaleplon individually were not sufficiently clarified.
To our knowledge, the literature regarding hypnotics contains no explanation or discussion of the causes of excess reports of infection among people randomly assigned to receive hypnotics. New studies should be performed to determine if more-frequent reports of infection among those randomly assigned to receive hypnotics are associated with objective evidence of more-frequent infection in hypnotics groups, as contrasted with placebo groups.
It seems unlikely that any causal pathway would be attributable to an increase in sleep produced by the hypnotics, since the difference in total sleep time between hypnotic and placebo groups was much less than an hour in most of the studies. A possible role of neurotransmitters and sympathetic tone leading to mucosal engorgement was briefly discussed in regard to 1 case of chronic sinusitis associated with the use of zolpidem.23 One might also speculate that hypnotics impair immune surveillance, but we are unaware that hypnotic effects on the human immune system have been evaluated directly. Benzodiazepines such as diazepam have complex effects on the immune system and may impair the response to infection.24–26 An excess of cancers was observed in trials of the same drugs, using data derived from some of the same studies.27 A suppression of immune surveillance might explain both an excess of incident cancer and an excess of infections, but this is only speculation.
Another possibility is that these sedative drugs impair the clearing of oral secretions during sleep, especially during the hours of peak drug concentrations. Swallowing of saliva is strongly inhibited in deep sleep,28 so inhibition might also occur when short-acting hypnotics reach their peak blood concentrations. Hypnotics might likewise impair clearing of gastroesophageal reflux. It has been shown that benzodiazepine use is a strong predictor of increased heartburn during sleep, presumably because benzodiazepine agonists might relax the lower esophageal sphincter and increase reflux events during sleep.29,30 The resulting aspiration, with acid fluid or vapor irritation of the bronchial or nasal mucosa and sinuses, might lead to excess inflammation and infection.31,32 An authoritative review found a substantial number of papers relating gastroesophageal reflux to rhinitis and sinusitis, but the quality of the evidence was considered limited.33 We know of no direct studies testing for reflux with the hypnotics studied. Also, there appeared to be an increase in urinary tract infections, which would be difficult to explain by hypnotic effects on gastroesophageal regurgitation.
We hope that the manufacturers, government health research agencies, and private organizations funding biomedical research will work to establish the scientific basis of these infection reports associated with the use of hypnotics. Meanwhile, clinicians, patients receiving hypnotics, and the public should be made aware that use of these 4 hypnotics possibly confers a risk of infection.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGEMENTS
Supported by Scripps Clinic Academic Affairs. Preparation of this manuscript did not receive industry support. It analyzes industry-supported premarketing and off-label clinical trials of 4 hypnotics done by other research groups, as well as postmarketing trials. Farhad F. Shadan, MD, PhD, provided helpful comments on this manuscript. James A. Koziol, PhD, provided statistical consultation. We thank an anonymous reviewer for suggesting the simulation.
REFERENCES
- 1.Saul S. Some sleeping pill users range far beyond bed. [Accessed July 24, 2008];New York Times. 2006 Mar 8; at http://www.nytimes.com/2006/03/08/business/08ambien.html?scp=1&sq=ambien%20IMS&st=cse.
- 2.Kripke DF. Risks of chronic hypnotic use. In: Lader M, Cardinali DP, Pandi-Perumal SR, editors. Sleep and Sleep Disorders: a Neuropsychopharmacological Approach. Georgetown, TX: Landes Bioscience; 2004. Available at http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=eurekah.chapter.28842. [Google Scholar]
- 3.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 4.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–50. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LUNESTA™ (eszopiclone) TABLETS 1 mg, 2 mg, 3 mg. [Accessed July 24, 2008];2007 at http://www.fda.gov/cder/foi/label/2004/021476lbl.pdf.
- 7.Kripke DF. Editorial: Who should sponsor sleep disorders pharmaceutical trials? J Clin Sleep Med. 2007;3:671–3. [PMC free article] [PubMed] [Google Scholar]
- 8.Drugs@FDA. [Accessed August 14, 2008];2006 at http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 9.Krystal AD, Erman M, Zammit GK, Soubrane C, Roth T. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31:79–90. doi: 10.1093/sleep/31.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Nordic Cochrane Center. Copenhagen: Cochrane Collaboration; 2008. Review Manager 5.0.14. [Google Scholar]
- 11.Center for Drug Evaluation and Research Approval Package for: Application Number 21-476: Medical Review(s) [Accessed August 14, 2008];2004 at http://www.fda.gov/cder/foi/nda/2004/021476_Lunesta_medr.PDF.
- 12.Soares CN, Joffe H, Rubens R, Caron J, Roth T, Cohen L. Eszopiclone in patients with insomnia during perimenopause and early postmenopause: a randomized controlled trial. Obstet Gynecol. 2006;108:1402–10. doi: 10.1097/01.AOG.0000245449.97365.97. [DOI] [PubMed] [Google Scholar]
- 13.Walsh JK, Krystal AD, Amato DA, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep. 2007;30:959–68. doi: 10.1093/sleep/30.8.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–60. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Center for Drug Evaluation and Research Application Number 21-782: Medical Review. [Accessed December 13, 2007];2005 :8–9. at http://www.fda.gov/cder/foi/nda/2005/021782s000_Rozerem_medr.pdf.
- 16.Zammit G, Erman M, Wang-Weigand S, Sainati S, Zhang J, Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med. 2007;3:495–504. [PMC free article] [PubMed] [Google Scholar]
- 17.Center for Drug Evaluation and Research Application Number: 020859: Medical Review(s), Part 6. [Accessed July 21, 2008];1999 at http://www.fda.gov/cder/foi/nda/99/20859_Sonata_medr_P6.pf.
- 18.Ambien CR Prescribing Information. New York, NY: Sanofi-Synthelabo; 2005. [Accessed October 7, 2008]. at http://products.sanofi-aventis.us/ambien_cr/ambienCR.html. Also available as Prescribing Information New Ambien CR (Zolpidem Tartrate Extended Release). Sanofi Aventis; 2005 (Color pamphlet) [Google Scholar]
- 19.Dorsey CM, Lee KA, Scharf MB. Effect of zolpidem on sleep in women with perimenopausal and postmenopausal insomnia: a 4-week, randomized, multi-center, double-blind, placebo-controlled study. Clin Ther. 2004;26:1578–86. doi: 10.1016/j.clinthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Ambien Prescribing Information. [Accessed September 22, 2008];2008 at http://products.sanofi-aventis.us/ambien/ambien.pdf.
- 21.Elie R, Ruther E, Farr I, Emilien G, Salinas E. Sleep latency is shortened during 4 weeks of treatment with zaleplon, a novel nonbenzodiazepine hypnotic. Zaleplon Clinical Study Group. J Clin Psychiatry. 1999;60:536–44. doi: 10.4088/jcp.v60n0806. [DOI] [PubMed] [Google Scholar]
- 22.Center for Drug Evaluation and Research Application Number: 020859: Medical Review(s), Part 6, Table 8.1.5.3.1. [Accessed July 21, 2008];1999 :22. at http://www.fda.gov/cder/foi/nda/99/20859_Sonata_medr_P6.pdf.
- 23.Malloy KM, Ketrick J, Pribitkin EA. Zolpidem tartrate use as contributory factor in sinus disease. Sleep. 2006;29:849–50. doi: 10.1093/sleep/29.6.849. [DOI] [PubMed] [Google Scholar]
- 24.Zavala F. Benzodiazepines, anxiety and immunity. Pharmacol Ther. 1997;75:199–216. doi: 10.1016/s0163-7258(97)00055-7. [DOI] [PubMed] [Google Scholar]
- 25.Massoco C, Palermo-Neto J. Effects of midazolam on equine innate immune response: a flow cytometric study. Vet Immunol Immunopathol. 2003;95:11–9. doi: 10.1016/s0165-2427(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 26.Galdiero F, Bentivoglio C, Nuzzo I, et al. Effects of benzodiazepines on immunodeficiency and resistance in mice. Life Sci. 1995;57:2413–23. doi: 10.1016/0024-3205(95)02199-0. [DOI] [PubMed] [Google Scholar]
- 27.Kripke DF. Possibility that certain hypnotics might cause cancer in skin. J Sleep Res. 2008;7:245–50. doi: 10.1111/j.1365-2869.2008.00685.x. [DOI] [PubMed] [Google Scholar]
- 28.Sato K, Nakashima T. Human adult deglutition during sleep. Ann Otol Rhinol Laryngol. 2006;115:334–9. doi: 10.1177/000348940611500503. [DOI] [PubMed] [Google Scholar]
- 29.Fass R, Quan SF, O'Connor GT, Ervin A, Iber C. Predictors of heartburn during sleep in a large prospective cohort study. Chest. 2005;127:1658–66. doi: 10.1378/chest.127.5.1658. [DOI] [PubMed] [Google Scholar]
- 30.Hall AW, Moossa AR, Clark J, Cooley GR, Skinner DB. The effects of premedication drugs on the lower oesophageal high pressure zone and reflux status of rhesus monkeys and man. Gut. 1975;16:347–52. doi: 10.1136/gut.16.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbero GJ. Gastroesophageal reflux and upper airway disease. Otolaryngol Clin North Am. 1996;29:27–38. [PubMed] [Google Scholar]
- 32.Phipps CD, Wood WE, Gibson WS, Cochran WJ. Gastroesophageal reflux contributing to chronic sinus disease in children—a prospective analysis. Arch Otolaryngol Head Neck Surg. 2000;126:831–6. doi: 10.1001/archotol.126.7.831. [DOI] [PubMed] [Google Scholar]
- 33.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps. Rhinology. 2007;(Suppl 20):1–136. [PubMed] [Google Scholar]