Abstract
Background
New technologies are available to reduce or prevent retained surgical sponges (RSS), but their relative cost-effectiveness are unknown. We developed an empirically-calibrated decision-analytic model comparing standard counting against alternative strategies: universal or selective X-ray, bar-coded sponges (BCS), and radiofrequency-tagged (RF) sponges.
Methods
Key model parameters were obtained from field observations during a randomized-controlled BCS trial (N=298), an observational study of RSS (N=191,168), and clinical experience with BCS (N~60,000). Since no comparable data exist for RF, we modeled its performance under two alternative assumptions. Only incremental sponge-tracking costs, excluding those common to all strategies, were considered. Main outcomes were RSS incidence and cost-effectiveness ratios for each strategy, from the institutional decision-maker’s perspective.
Results
Standard counting detects 82% of RSS. Bar-coding prevents at least 97.5% for an additional $95,000 per RSS averted. If RF is as effective as bar-coding, it would cost $720,000 per additional RSS averted (versus standard counting). Universal X-ray and selective X-ray for high-risk operations are more costly, but less effective than BCS—$1.1–1.4 million per RSS event prevented. In sensitivity analyses, results were robust over the plausible range of effectiveness assumptions, but sensitive to cost.
Conclusions
Using currently available data, this analysis provides a useful model for comparing the relative cost-effectiveness of existing sponge-tracking strategies. Selecting the best method for an institution will depend on its priorities: ease-of-use, cost-reduction or ensuring RSS are truly “never-events”. Given medical and liability costs exceeding $200,000 per incident, novel technologies can substantially reduce the incidence of RSS, at acceptable cost.
Introduction
Despite increasing attention to patient safety in the operating room (OR), inadvertently retained sponges and instruments remain a serious preventable complication in surgery. The National Quality Forum includes retained equipment among its list of reportable “never-events” that are expected never to occur to patients in U.S. hospitals.1 The Joint Commission on the Accreditation of Healthcare Organizations considers them sentinel events requiring immediate investigation and response.2 The Centers for Medicare and Medicaid Services recently announced its decision to deny reimbursement to hospitals for the costs associated with objects left in patients during surgery.3
Unfortunately, strategies to prevent retained equipment have relied solely on the diligence of the surgical team to track the myriad of sponge products and other surgical equipment introduced to the operative field. Standard protocols require two members of the surgical team to concurrently count every item as it is introduced into the sterile field, and again at the end of the operation, and to perform radiographs if they cannot account for all items.4 Surgeons are further encouraged to perform a thorough wound exploration before completion of the procedure, regardless of the outcome of counts.5–7 Although these protocols are labor-intensive—they occupy as much as 14% of the operative time8—failure to follow the guidelines is rarely the problem. Instead, the weak link in the count process has been the deception of a falsely correct count—72% to 88% of cases of retained surgical equipment occur in operations with correct counts.9–12 In these cases, a manual counting error allows the team to believe all items have been identified when in fact something has been misplaced in the patient. Such errors are especially common in high-risk situations, such as obese patients, emergencies and operations with an unexpected change in procedure.7–9, 13 Some institutions have adopted a practice of standard, immediate postoperative high-resolution radiographic screening for all operations involving a body cavity,10 but this strategy is extremely costly14 and it could still miss some objects, especially sponges5, 15 and needles.16, 17 Selective use for high-risk operations has been suggested as an alternative,9, 14, 18, 19 and high-resolution images do improve detection, compared with portable intraoperative films.10 Yet, there have been no studies to evaluate the potential added value and cost of such an approach.
Prevalent strategies to eliminate this problem have been undermined by their intrinsic susceptibility to human error,5, 8, 20, 21 provoking calls for technological adjuncts to counting.5, 18, 19, 22–25 Recently, surgical sponges have been a primary target for innovation among both surgical safety researchers and medical device manufacturers, because they represent the majority of retained objects.9, 10, 26, 27 There are currently three technologies approved by the U.S. Food and Drug Administration—bar-coded (BCS) sponges (Safety-Sponge™ System, SurgiCount Medical, Temecula, California), radiofrequency (RF) tagged sponges (RF Surgical Detection System™, RF Surgical Systems, Bellevue, Washington), and passive radiofrequency identification (RFID) tagged sponges (SmartSponge™, ClearCount Medical Solutions, Pittsburgh, Pennsylvania). Proof-of-principle studies have been published demonstrating detection of RF/RFID-tagged sponges in surgical incisions,22, 28 but controlled clinical trial data exist only for the BCS technology.25 A central difficulty in this field is that clinical trials would require randomization of well over 100,000 patients to reliably detect a significant reduction in actual retained surgical sponge (RSS) events.
As an alternative approach, decision-analytic simulation offers a viable opportunity to compare proposed strategies, estimate their relative cost-effectiveness, and facilitate the integration of new research findings. In this analysis, therefore, we modeled the key determinants of the risk of RSS, and applied all available published data, as well as accrued experience from institutions using these technologies, to the estimation of model parameters. The result is a generalizable schema by which analyses of relative cost-effectiveness of strategies to prevent RSS may easily be updated as new data or technologies become available.
Methods
We used standard decision trees to model the predicted incidence of RSS and incremental costs for each prevention strategy. (The full decision trees are included for reviewers in the Appendix). All analyses were conducted in TreeAge Pro 2007 (TreeAge Software, Williamstown, Massachusetts).
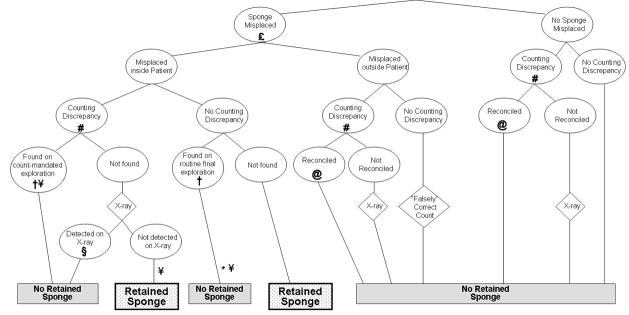
A schematic model of key OR sponge-tracking events is shown in Figure 1. Strategies incur incremental costs both from the cost of sponges (relative to standard radio-opaque sponges) and other equipment (such as bar-code readers for the BCS system and detection wands and console for the RF system), as well as from any X-rays that must be performed. Benefits are measured as the proportion of cases resulting in RSS. There is no cost assigned to falsely correct counts or unreconciled discrepancies if they do not result in a RSS.
FIGURE 1.
Structure of the sponge-tracking model in a standard counting protocol.
Each pair of yes/no arrows represents a chance node in the decision model. Chance probabilities for each strategy were derived from previous research and from algebraic back-computation from epidemiologic data. Costs are accrued both from the intrinsic cost of the technologies employed and from any X-rays incurred as a result of unreconciled discrepancies. In accordance with a previously described taxonomy32, we consider discrepancies to be instances in which a subsequent count does not agree with a previous one (this definition differs from that of Egorova et al.29 who refer to unreconciled incorrect final counts as discrepancies). Misplaced sponges are those unintentionally lost in the OR, either on the floor, in the trash, in the drapes, or elsewhere. Misplaced sponges may or may not be subsequently found. Those misplaced within the patient’s body cavity may be found either before the patient leaves the operating room (a “near miss”) or post-operatively (an RSS adverse event).
Universal use of X-ray decreases the RSS rate primarily by detection of most sponges that are left in the patient, in the absence of a discrepancy (*) For the selective mandatory radiography strategy, obesity is assumed to decrease the likelihood of discovering a sponge misplaced in the patient (†) and decrease the sensitivity of X-rays (§). Emergencies and unexpected procedure changes are assumed to increase the likelihood of misplacing a sponge (£). The bar-coded sponge (BCS) system affects the RSS rate by increasing detection of incidents of misplaced sponges (#). Radiofrequency-tagged (RF) sponges would be expected to increase reconciliation of discrepancies (@) and increase discovery of sponges misplaced in the patient (¥). The RFID system is expected to have effects common to both BCS and RF.
For the base case, we estimated model probabilities on a per-operation basis for an average-risk inpatient operation. Model input parameters were estimated using primary data from several observational and epidemiologic studies of retained sponges and instruments (see Table 1). For the base case estimation of RSS incidence under standard counting practices, we used data from a comprehensive four-year year study of nearly 200,000 operations performed in an institution that routinely screens all patients undergoing operations involving a body cavity with high-resolution postoperative survey radiography.10, 24 They found that RSS occur in approximately one per 8312 operations.10 Unlike previous studies, which identified cases from incidence reports,12, 29 administrative databases,30, 31 or malpractice claims,9 this study10 provides the closest overall estimate of RSS incidence, because it includes both clinically-evident cases and those detected by high-resolution X-ray that might otherwise have escaped timely detection.
TABLE 1.
Base case natural history assumptions.
| Description | Value | Source |
|---|---|---|
| Incidence of retained surgical sponges | 0.0001203 | Cima10 |
|
| ||
| Probability of a misplaced sponge in an average operation | 0.0873 | Computed from data in Greenberg32 and Cima10 |
|
| ||
| Proportion of misplaced sponges that are misplaced in the patient | 0.0909 | Greenberg25 |
|
| ||
| Frequency of counting discrepancies | 0.0811 | Greenberg25, 32 |
|
| ||
| Positive predictive value of counting discrepancy | 0.9167 | Greenberg25, 32 |
|
| ||
| Negative predictive value for counting discrepancy | 0.9859 | Computed from data in Greenberg32, Cima10, Forgue33 |
|
| ||
| Proportion of discrepancies that are reconciled | 0.9167 | Greenberg25, 32 |
|
| ||
| Probability of discovering a sponge misplaced in a patient during wound exploration when: | Computed from data in Greenberg32, Cima10, Forgue33 | |
| There is a counting discrepancy | 0.9651 | |
| There is not a counting discrepancy | 0.9160 | |
|
| ||
| Sensitivity of intraoperative radiographs | 0.91 | Revesz15 |
|
| ||
| Specificity of intraoperative radiographs | 0.895 | Revesz15 |
Estimates of the likelihood of discrepancies and reconciliation, and of misplaced and retained sponges were derived from field observations.25, 32 The sensitivity ( and specificity (0.91 and 0.895, respectively) of X-ray were taken from Revesz et al., who studied radiologists’ and surgeons’ ability to detect sponges placed within surgical incisions in cadavers.15 When inputs were not otherwise available from such studies, we computed conditional probabilities through calibration methods (e.g. maximum likelihood techniques) and/or algebraic back-calculation (ie. computation of missing intervening values in a decision tree when initial probabilities and final proportions are available).
Treatment Strategies
(1) No sponge tracking
Operations are performed with non-radiopaque sponges. Care is taken to remove all packs and other equipment, but no sponge-counting or other active surveillance techniques are used. We computed the predicted incidence of RSS in this strategy through back-calculation from epidemiologic analyses—using the known incidence of RSS with standard counting and the previously observed frequency of misplacing a sponge in the incision, we solved for the likelihood that a misplaced sponge would be discovered incidentally and then computed a predicted RSS incidence in the absence of counts.9, 10, 29 This computed prediction matched empirical reports of historical incidence data.33 The results of this simulation are used only as a baseline from which to evaluate the effect of standard counting procedures. All subsequent strategies are compared with the standard counting practice.
(2) Standard counting protocol
Operations are performed with radiopaque sponges. All recommended counting practices4 are used, wound exploration is performed at the end of the procedure,5 and all unreconciled discrepancies are investigated with intraoperative radiography before final closure. Unobserved model parameters (for example, the likelihood of a misplaced sponge in the absence of a counting discrepancy) were derived by calibrating these values to generate model predictions for RSS incidence that most closely match the observed estimates from epidemiologic data.10
(3) Universal radiography without counting
Operations are performed as in (1), except that intraoperative X-ray is performed before final closure in all operations.
(4) Universal radiography, added to standard counting
Operations are performed as in (2), except that intraoperative X-ray is performed before final closure in all operations.
(5) Selective mandatory radiography for high-risk operations
Operations are performed as in (2), except that intraoperative X-ray is performed before final closure for obese patients (with body mass index greater than thirty-five), emergency operations, and when there has been an unplanned change in procedure.9
For this scenario, we assumed that obesity increases RSS risk both by decreasing the likelihood that a sponge misplaced in the patient will be discovered before closure,9, 13 and by decreasing the sensitivity of intraoperative radiography to detecting a retained sponge.34 We assumed that emergencies and procedure changes increase RSS risk by increasing the likelihood that OR teams will misplace a sponge.9 We estimated the proportion of operations belonging to each high-risk group from the National Surgical Quality Improvement Program35 cohorts at our institutions. Finally, we calibrated the missing parameter estimates to conform to the reported odds ratios for each high-risk category.9
(6) Bar-coded sponge system
All sponges contain a unique data-matrix symbology tag annealed to the gauze. Sponges are scanned with a bar-code reader as they are added to the sterile field, and again as they are removed. Wound exploration is performed before closure in all operations, and unreconciled discrepancies prompt intraoperative X-ray. Model parameters were estimated from a randomized-controlled trial of the bar-coding system,25 and from clinical experience with the system (Rick Bertran, personal communication).
(7) RF-tagged sponge system
Operations are performed as in (2), including all aspects of the standard counting procedure, except that sponges containing a micro RF tag are used. Regardless of the outcome of the standard counting protocol, the count is followed by a complete scan of the patient with an RF detection wand, according to procedures described by the product’s distributor (Medline Industries Inc., Mundelein, Illinois). In the absence of obtainable effectiveness data, we computed what the RF system’s cost per incident prevented would be across a range of assumptions, varying the projected incidence of RSS from equivalence with bar-coding to 100 percent prevention of RSS.
(8) RFID system
We were unable to obtain cost data about the RFID system from either the manufacturer or surgical administrators, because no institution had fully implemented the system at the time of writing. Thus, we could not compute meaningful cost-effectiveness ratios for RFID, and do not include it explicitly in the analyses to follow.
Cost Estimates
The base case cost estimates are shown in Table 2. Cost data for plain radiography were obtained from published studies.9, 29 The incremental costs of the novel technologies were obtained from OR managers at academic institutions that have implemented the systems. Bar-coded sponge costs were obtained from University of California, San Francisco Medical Center (J. Bennan, personal communication) and RF sponge system costs were obtained from the Hospital of the University of Pennsylvania (L.R. Kaiser MD, personal communication). In both cases, OR administrators considered the incremental additional costs of the sponges, as well as the amortized costs of associated equipment purchases and any other upfront capital costs for implementation.
TABLE 2.
Cost estimates.
| Strategy | Base price* | % needing intraoperative X-ray † | Total cost | Source |
|---|---|---|---|---|
| Standard counting | Reference | 0.6%¥ | $0.80 § | Greenberg et al. |
| Universal X-ray | $125 | 100.0% | $125.00 | Gawande et al.; Egorova et al. |
| Selective X-ray | $0 | 24.3%@ | $30.36 | Gawande et al.; Revesz et al. |
| Bar-coded sponge system | $9 | 1.3%¥ | $10.60 # | Personal communication (see note below) |
| RF-tagged sponge system | $75.18 | 0.1%¥ | $75.31 £ | Personal communication (see note below) |
Base costs were calculated using a comparison to the reference strategy of standard counting—the current standard of care
Predictions based on data from observational trials,25, 32 calibrated to epidemiologic incidence data.10
The included cost of standard counting derives from the predicted rate of intraoperative radiographs,
The predicted rates of intraoperative radiographs were obtained from the model, and validated by comparison with both published rates,25, 32 and the observed rate in one large academic institution (M. Kwaan, Brigham and Women’s Hospital, personal communication).
The proportion of patients expected to require x-ray was derived by back-calculation from the observed odds ratios for each high-risk group, and calibrated to the frequencies in our institutions’ NSQIP cohorts.
J. Bennan, UCSF, personal communication.
L.R. Kaiser, University of Pennsylvania personal communication.
We make a simplifying assumption that lifetime induced and indirect costs from a societal perspective are unnecessarily broad for this inquiry, even though society-level approaches are often recommended.36 We do so for three reasons: (i) RSS are considered a “never-event” to be eliminated,1, 2 so it is not essential to compare cost-effectiveness of strategies to prevent RSS against other health priorities—RSS events themselves serve as a common denominator, apart from their broader sequelae; (ii) hospitals are the principal decision-makers regarding whether to implement these strategies, so an institutional perspective on cost (considering only the direct incremental costs of the strategy, while keeping in mind the potential savings from avoidance of non-reimbursable acute surgical care for RSS and resultant litigation) most closely represents the decision of interest37; and (iii) hospital costs for medical and surgical management of RSS may not be reimbursed,3 and the costs of implementing prevention strategies are unlikely to be borne at the societal level, by either patients or third-party payers. Because future costs and health outcomes are not considered in this analysis, discounting is not required.
We have not included any potential costs associated with time in the OR spent counting. Counting is principally performed in parallel with operative procedures,8 and because most operational costs in the OR are fixed (rather than variable),38 additional costs and/or savings accrue only when additional operations may be added into a daily schedule, and the threshold at which changes in operative time affect revenues for surgical volume is quite high.39 Because there is a steep learning curve with implementation of new technologies, and differences in operative time associated with these adjuncts have generally been small, this threshold is unlikely to be reached (J. Bennan, UCSF, personal communication; A. Kollengode, personal communication; see also Greenberg et al.25).
Cost-Effectiveness Analyses
We computed cost-effectiveness in two ways. First, because some decision-makers are interested in comparing each strategy only against current practice, we computed relative cost-effectiveness ratios (RCER), comparing each strategy’s cost and predicted incidence of RSS with those of standard counting. Second, for decision-makers interested in comparisons between the strategies, we performed incremental cost effectiveness analyses that compare each strategy’s cost and predicted incidence with that of the next-most effective strategy. For this analysis, we ranked strategies in order of increasing cost. Any strategy that was more costly but not more beneficial was considered dominated. The incremental cost-effectiveness ratio (ICER) is then computed for each remaining strategy by dividing additional cost by additional benefit, as compared with the next-most costly non-dominated strategy.
Sensitivity Analyses
Because there is uncertainty in our effectiveness estimates for the sponge-tracking technologies, we computed cost-effectiveness ratios across a range of efficacy estimates, up to and including the circumstance in which they completely eliminate RSS. To evaluate the effect of variability in cost estimates for the technologies, we also evaluated the sensitivity of our estimates to differences in cost.
Results
Base Case
The expected costs, number of RSS events prevented, RCER and ICER for each of the eight strategies are presented in Table 3. If no active tracking were performed, the incidence of RSS would be approximately 67 per 100,000 operations. Standard counting protocols alone are predicted to prevent 82% of these, resulting in a baseline estimated incidence rate of 12 per 100,000, which calibrates well with published epidemiologic data.10, 29 All strategies that included mandatory radiographs—whether applied universally or only in high-risk operations—were dominated by the bar-coded sponge strategy, because of its lower cost and greater benefit. The RCER for each of the X-ray strategies exceeded $1,000,000 per RSS prevented.
TABLE 3.
Relative and incremental cost-effectiveness of strategies to prevent retained surgical sponges.
| Strategy | Total Cost | Incidence (per 100000) | Percent of retained sponges averted | RCER | ICER |
|---|---|---|---|---|---|
| No tracking | $0.00 | 67 * | Reference | — | — |
| Count | $0.80 | 12 † | 82.0% | Reference | $1,500 |
| Selective XR | $30.31 | 9.4 § | 85.8% | $1,100,000 | Dominated |
| Xray only | $125.00 | 6.0 § | 91.0% | $2,100,000 | Dominated |
| Universal XR | $125.00 | 3.0 § | 95.5% | $1,400,000 | Dominated |
| BCS | $10.60 | 1.7 £ | 97.5% | $95,000 | $95,000 |
| RF model 1* | $75.31 | 1.7 ¥ | 97.5% | $720,000 | Dominated |
| RF model 2 † | $75.31 | 0 ¥ | 100% | $620,000 | $3,900,000 |
Computed from calibrated model, validated by comparison with historical data from Forgue and Aimes.33
Derived from Cima et al.10
Computed from calibrated model.
Derived from reported clinical experience to date (R. Bertran, personal communication).
RF Model 1 assumes that the incidence of RSS with RF is equivalent to bar-coding.
RF Model 2 assumes that the incidence of RSS with RF is null.
Overall effectiveness estimates for BCS were derived from recorded clinical experience to date—at least 60,000 operations have been completed without a RSS event, so we conservatively assumed that that the RSS incidence with BCS would be no more than 1 in 60,000 (or 1.7 per 100,000) in the base case. At this level, the RCER and ICER for the bar-coded sponge strategy was approximately $95,000 per RSS event prevented, compared with standard counting.
If the overall effectiveness of the RF system were the same as BCS (i.e. the incidence of RSS was the same; see Table 2, RF Model 1), RF would be dominated, because of higher cost with no increase in benefit. Its RCER would be $720,000 per RSS prevented. If, at the other extreme, RF were able to prevent 100 percent of RSS (see Table 2, RF Model 2), the RCER compared with standard counting would be $620,000 per RSS event prevented, but the ICER under this assumption would be $3.9 million per RSS, when compared with BCS (the next-best non-dominated strategy).
Sensitivity Analyses
We evaluated three alternative scenarios, whose results are shown in Table 4. First, if we assume that the BCS system increases detection of discrepancies at the rate observed in the randomized trial,25 but does not otherwise improve the rate at which missing sponges are recovered of missing sponges (in the base case, we found by back-calculation that the likelihood of discovery of a missing sponge is greater when discrepancies occur with BCS than counting), both the RCER and ICER for BCS would increase to $140,000, but its position relative to other non-dominated strategies would not change. Next, if there were no decrement in X-ray sensitivity in obese patients, performance of the selective X-ray strategy improves slightly, but does not alter the conclusions from the base case. And third, even as the sensitivity of intraoperative radiographs approaches 100%, the selective X-ray strategy is still strongly dominated by BCS, and the universal X-ray strategy remains extremely costly, with an RCER of $1.3 million compared with counting, and an ICER of $27 million compared with BCS.
TABLE 4.
Sensitivity Analyses
| Scenario | Strategy | Total Cost | Incidence (per 100000) | RCER | ICER |
|---|---|---|---|---|---|
| Bar-coded sponge system increases discrepancy rate without additionally improving detection of missing sponges | No tracking | $0.00 | 67 | — | — |
| Count | $0.80 | 12 | Reference | $1,500 | |
| Selective XR | $30.31 | 9.4 | $1,100,000 | Dominated | |
| BCS | $10.60 | 4.9 | $140,000 | $140,000 | |
| Universal XR | $125.00 | 3.0 | $1,400,000 | Dominated | |
| RF model 2 | $75.31 | 0 | $620,000 | $1,30,000 | |
| Radiographs in the obese are no less sensitive than those in non-obese | No tracking | $0.00 | 67 | — | — |
| Count | $0.80 | 12 | Reference | $1,500 | |
| Selective XR | $30.31 | 9.0 | $970,000 | Dominated | |
| BCS | $10.60 | 1.7 | $95,000 | $95,000 | |
| RF model 2 | $75.31 | 0 | $620,000 | $4,500,000 | |
| Sensitivity of intraoperative radiographs is 100% | No tracking | $0.00 | 67 | — | — |
| Count | $0.80 | 9.9 | Reference | $1,400 | |
| Selective XR | $30.31 | 5.6 | $690,000 | Dominated | |
| BCS | $10.60 | 0.4 | $100,000 | $100,000 | |
| Universal XR | $125.00 | 0 | $1,300,000 | $27,000,000 |
Discussion
Retained sponges remain a persistent and dreaded occurrence in surgery. Unfortunately, more than just diligence of highly-sensitized surgical personnel will be required if they are ever truly to be a “never-event”. Standard counting protocols already detect more than 80 percent of RSS that would otherwise occur. Still sponges are left behind in approximately 12 per 100,000 operations,10 stimulating increasing interest in strategies to augment the sponge count. Because RSS are already rare, however, the incremental benefit from even the most effective technological adjuncts will be small, and could only be observed in a clinical trial with tens of thousands of patients.18, 19, 23 Thus, aside from isolated proof-of-principle trials for certain devices,22, 28 there is no real clinical evidence to date that quantifies the effectiveness of any of these technologies in operational use. The one clinical trial in this field focused instead on the rate of counting discrepancies as a proxy for the detection of missing sponges.25
The goal of this study was to provide quantitative estimates from which decision-makers may evaluate the various interventions that have been proposed. Through decision-analytic modeling, we illustrate that in order for a sponge tracking strategy to be cost-effective, it must come very close to eliminating RSS altogether, while keeping its incremental costs quite low. From an institutional standpoint, the costs incurred from a RSS event include the direct medical costs—the average Medicare payment for admissions with retained foreign bodies exceeded $60,000—and the costs of resulting litigation—averaging $150,000 at a large malpractice insurer in Massachusetts (W. Berry MD, personal communication), but potentially much higher elsewhere in the U.S. To be cost-saving, when compared with these expected losses, a strategy that completely eliminated RSS would still need to cost less than $26 per operation.
In this analysis, we find that universal X-ray strategies are prohibitively costly for the prevention of RSS. Even if the sensitivity of intraoperative radiographs were perfect, and surgeons could completely eliminate RSS, this achievement would come at a cost of more than $1.3 million per RSS event prevented. Several authors have suggested that X-rays could be selectively employed for high-risk operations,9, 14, 18, 19 but even under the most favorable circumstances, even this narrower strategy costs $1 million for every RSS it prevents. On the other hand, unlike the BCS and RF strategies, X-rays have the capacity to detect retained instruments and needles,10 so some institutions may find marginally more value in the strategy than is reflected in this model, which only considers its effect on sponges.
Our effectiveness estimates for each of the new technologies are crude and somewhat uncertain, because little direct clinical evidence exists to inform our estimates. However, the conclusions were robust across a wide range of plausible effectiveness estimates. With an already low baseline incidence of RSS, the cost-effectiveness ratios are driven mainly by the per-operation cost of each technology. For these estimates, we relied on expert opinions from large academic institutions that have implemented the systems in their operating rooms, and have real operational experience with the total costs involved in their use.
Because of its low cost per operation, the BCS system is predicted to be cost effective across a full range of plausible effectiveness estimates, with an ICER between $95,000 and $140,000, when compared with standard counting procedures. In comparison, at its currently reported cost, the ICER for the RF system exceeds $3 million, when compared with BCS.
Each hospital’s choice of strategy will depend on its resources, its priorities, and its perception of the consequences of RSS. Those more highly motivated to ensure that RSS are “never-events”—either because of the regulatory environment, or fear of bad publicity and an interest in protecting their reputation—may have higher willingness to pay to prevent RSS event. For some decision makers, local factors related to ease-of-use, workability, and/or the preferences of influential OR personnel—characteristics not considered in our analysis—will undoubtedly affect the relative attractiveness of the available strategies. Likewise, some may be interested in a broader perspective, that considers costs to society (such as lost productivity, uncovered medical costs, or utility-adjusted outcomes), to payers (including incurred hospital charges), or to providers and other OR personnel (operative times and efficiency). Some large hospitals might value indirect costs or savings from implementation, such as operative time and efficiency, personnel training and documentation costs, while low-volume community hospitals might find the fixed startup costs of technology acquisition more burdensome.
Nevertheless, this study provides critical quantitative information that will inform institutional decision-makers who are now presented with several new options to address the problem of retained surgical sponges. With increasing pressure from regulatory agencies and the public to make this a “never-event”, many institutions are now seeking out such information. In the future, as hospitals acquire experience with the actual costs and real-world effectiveness of new technologies and strategies, this decision-analytic model may be modified and updated to provide ongoing decision support for surgeons and hospitals seeking to eliminate RSS in their operating rooms.
Acknowledgments
Funding: Dr. Regenbogen was supported by Kirschstein National Research Service Award T32-HS000020 from the Agency for Healthcare Research and Quality.
Abbreviations
- BCS
bar-coded sponge
- ICER
incremental cost-effectiveness ratio
- OR
operating room
- RSS
retained surgical sponge
- RCER
relative cost-effectiveness ratio
- RF
radiofrequency
- RFID
radiofrequency identification
Footnotes
Disclosures: Drs. Greenberg and Gawande have received research grant support from SurgiCount Medical, Temecula, California. The funding agencies were not involved in the conduct, analysis, or composition of this study, and were not given the opportunity to approve or edit the manuscript prior to submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Serious Reportable Events in Healthcare. A Consensus Report. Washington, DC: National Quality Forum; 2002. Mar, [Google Scholar]
- 2.Sentinel Event Policy and Procedures. Oakbrook Terrace, IL: The Joint Commission; 2007. Jul, [Google Scholar]
- 3.Medicare Program: Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2008 Rates. Fed Regist. 2007;72(162):47179–228. [PubMed] [Google Scholar]
- 4.Recommended practices for sponge, sharp and instrument counts. Denver, CO: Association of Perioperative Registered Nurses; 2004. [DOI] [PubMed] [Google Scholar]
- 5.Gibbs VC, Coakley FD, Reines HD. Preventable errors in the operating room: retained foreign bodies after surgery--Part I. Current problems in surgery. 2007;44(5):281–337. doi: 10.1067/j.cpsurg.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Botet del Castillo FX, Lopez S, Reyes G, et al. Diagnosis of retained abdominal gauze swabs. Br J Surg. 1995;82(2):227–8. doi: 10.1002/bjs.1800820232. [DOI] [PubMed] [Google Scholar]
- 7.Bani-Hani KE, Gharaibeh KA, Yaghan RJ. Retained surgical sponges (gossypiboma) Asian journal of surgery/Asian Surgical Association. 2005;28(2):109–15. doi: 10.1016/s1015-9584(09)60273-6. [DOI] [PubMed] [Google Scholar]
- 8.Christian CK, Gustafson ML, Roth EM, et al. A prospective study of patient safety in the operating room. Surgery. 2006;139(2):159–73. doi: 10.1016/j.surg.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Gawande AA, Studdert DM, Orav EJ, Brennan TA, Zinner MJ. Risk factors for retained instruments and sponges after surgery. N Engl J Med. 2003;348(3):229–35. doi: 10.1056/NEJMsa021721. [DOI] [PubMed] [Google Scholar]
- 10.Cima RR, Kollengode A, Garnatz J, Storsveen A, Weisbrod C, Deschamps C. Incidence and characteristics of potential and actual retained foreign object events in surgical patients. J Am Coll Surg. 2008;207(1):80–7. doi: 10.1016/j.jamcollsurg.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser CW, Friedman S, Spurling KP, Slowick T, Kaiser HA. The retained surgical sponge. Ann Surg. 1996;224(1):79–84. doi: 10.1097/00000658-199607000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lincourt AE, Harrell A, Cristiano J, Sechrist C, Kercher K, Heniford BT. Retained foreign bodies after surgery. The Journal of surgical research. 2007;138(2):170–4. doi: 10.1016/j.jss.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen NT, Wilson SE, Wolfe BM. Rationale for laparoscopic gastric bypass. J Am Coll Surg. 2005;200(4):621–9. doi: 10.1016/j.jamcollsurg.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Devgan L, Waters H, Pronovost PJ, Makary MA. A cost analysis of intra-operative X-ray screening for retained surgical foreign bodies. The Journal of surgical research. 2007;137(2):186. [Google Scholar]
- 15.Revesz G, Siddiqi TS, Buchheit WA, Bonitatibus M. Detection of retained surgical sponges. Radiology. 1983;149(2):411–3. doi: 10.1148/radiology.149.2.6622683. [DOI] [PubMed] [Google Scholar]
- 16.Ponrartana S, Coakley FV, Yeh BM, et al. Accuracy of plain abdominal radiographs in the detection of retained surgical needles in the peritoneal cavity. Ann Surg. 2008;247(1):8–12. doi: 10.1097/SLA.0b013e31812eeca5. [DOI] [PubMed] [Google Scholar]
- 17.Macilquham MD, Riley RG, Grossberg P. Identifying lost surgical needles using radiographic techniques. AORN journal. 2003;78(1):73–8. doi: 10.1016/s0001-2092(06)61347-1. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg CC, Gawande AA. Beyond counting: current evidence on the problem of retaining foreign bodies in surgery? Ann Surg. 2008;247(1):19–20. doi: 10.1097/SLA.0b013e318160c194. [DOI] [PubMed] [Google Scholar]
- 19.Berkowitz S, Marshall H, Charles A. Retained intra-abdominal surgical instruments: time to use nascent technology? The American surgeon. 2007;73(11):1083–5. [PubMed] [Google Scholar]
- 20.Dierks MM, Christian CK, Roth EM, Sheridan TB. Healthcare safety: the impact of disabling “safety” protocols. IEEE Transactions. 2004;34(6):693–8. [Google Scholar]
- 21.Beyea SC. Counting instruments and sponges. AORN journal. 2003;78(2):290, 3–4. doi: 10.1016/s0001-2092(06)60778-3. [DOI] [PubMed] [Google Scholar]
- 22.Fabian CE. Electronic tagging of surgical sponges to prevent their accidental retention. Surgery. 2005;137(3):298–301. doi: 10.1016/j.surg.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Rattner DW. Invited commentary: Making patient safety automatic. Surgery. 2005;137(3):302–3. doi: 10.1016/j.surg.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Sarr MG. Editor’s note: Retained foreign bodies--why do we still allow them to occur? Surgery. 2005;137(3):304–5. doi: 10.1016/j.surg.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg CC, Diaz-Flores R, Lipsitz SR, et al. Bar-coding surgical sponges to improve safety: a randomized controlled trial. Ann Surg. 2008;247(4):612–6. doi: 10.1097/SLA.0b013e3181656cd5. [DOI] [PubMed] [Google Scholar]
- 26.Schonleben K, Strobel A, Schonleben F, Hoffmann A. [Retained foreign bodies from the surgical point of view] Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen. 2007;78(1):7–12. doi: 10.1007/s00104-006-1271-7. [DOI] [PubMed] [Google Scholar]
- 27.Lincourt AE, Harrell A, Cristiano J, Sechrist C, Kercher K, Todd Heniford B. Retained Foreign Bodies After Surgery. J Surg Res. 2007 doi: 10.1016/j.jss.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Macario A, Morris D, Morris S. Initial clinical evaluation of a handheld device for detecting retained surgical gauze sponges using radiofrequency identification technology. Arch Surg. 2006;141(7):659–62. doi: 10.1001/archsurg.141.7.659. [DOI] [PubMed] [Google Scholar]
- 29.Egorova NN, Moskowitz A, Gelijns A, et al. Managing the prevention of retained surgical instruments: what is the value of counting? Ann Surg. 2008;247(1):13–8. doi: 10.1097/SLA.0b013e3180f633be. [DOI] [PubMed] [Google Scholar]
- 30.Romano PS, Geppert JJ, Davies S, Miller MR, Elixhauser A, McDonald KM. A national profile of patient safety in U.S. hospitals. Health affairs (Project Hope) 2003;22(2):154–66. doi: 10.1377/hlthaff.22.2.154. [DOI] [PubMed] [Google Scholar]
- 31.Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. Jama. 2003;290(14):1868–74. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg CC, Regenbogen SE, Lipsitz SR, Diaz-Flores R, Gawande AA. The frequency and significance of discrepancies in the surgical count. Ann Surg. 2008;248(2):337–41. doi: 10.1097/SLA.0b013e318181c9a3. [DOI] [PubMed] [Google Scholar]
- 33.Forgue E, Aimes A. Les “Pieges” de la Chirurgie. Paris: Masson et Cie; 1939. [Google Scholar]
- 34.Uppot RN, Sahani DV, Hahn PF, Gervais D, Mueller PR. Impact of obesity on medical imaging and image-guided intervention. Ajr. 2007;188(2):433–40. doi: 10.2214/AJR.06.0409. [DOI] [PubMed] [Google Scholar]
- 35.Khuri SF, Henderson WG, Daley J, et al. The patient safety in surgery study: background, study design, and patient populations. J Am Coll Surg. 2007;204(6):1089–102. doi: 10.1016/j.jamcollsurg.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. Jama. 1996;276(16):1339–41. doi: 10.1001/jama.276.16.1339. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein MC, Fineberg HV, Elstein AS, et al. Clinical Decision Analysis. Philadelphia: W.B. Saunders; 1980. [Google Scholar]
- 38.Ward WJ, Spragens L, Smithson K. Building the business case for clinical quality. Healthcare Financial Management. 2006 December;:1–6. [PubMed] [Google Scholar]
- 39.Dexter F, Macario A. Decrease in case duration required to complete an additional case during regularly scheduled hours in an operating room suite: a computer simulation study. Anesth Analg. 1999;88(1):72–6. doi: 10.1097/00000539-199901000-00014. [DOI] [PubMed] [Google Scholar]