Abstract
Achieving and maintaining effective suppression of serum testosterone levels in men treated with androgen ablation is one of the essential strategies in the management of prostate cancer. Historically, a serum testosterone below 50 ng/dL was considered to be the castrate level. Current data suggest that the new target for either surgical or chemical castration is a serum testosterone level of lower than 20 ng/dL in an attempt to maximize therapeutic outcomes. Testosterone breakthrough and the acute-on-chronic effects of administration of a luteinizing hormone-releasing hormone analogue may cause testosterone levels to periodically rise, sometimes to noncastrate levels. The goal of androgen ablation is to identify those agents that will most consistently achieve and maintain the lowest testosterone levels possible.
Key words: Prostate cancer, Androgen ablation, LHRH analogues, LHRH antagonists, Testosterone
The cornerstone of understanding the basic biology of prostate cancer relies upon the important discovery that prostate cancer is a hormonally responsive tumor. The current use of androgen ablation therapy in prostate cancer includes treatment based on serum prostate-specific antigen (PSA) only or local recurrence; neoadjuvant or adjuvant treatment of high-risk disease, usually in combination with radiation therapy; and treatment of patients with metastatic disease regardless of symptoms. The American Society of Clinical Oncology (ASCO) 2007 guidelines and National Comprehensive Cancer Network (NCCN) 2009 guidelines recommend either luteinizing hormone-releasing hormone (LHRH) agonists or bilateral orchiectomy as first-line therapy for men with advanced prostate cancer.1,2
Medical or chemical castration is almost exclusively performed by the use of injectable LHRH analogues, with a minor role for estrogen and limited experience with LHRH antagonists. Surgical castration through bilateral orchiectomy is infrequently used today.
Intermittent hormonal therapy (IHT) is being investigated as an alternative to continuous hormonal therapy with a potential for reduced morbidity and a delay of the progression to hormone-refractory disease.3 Although intermittent therapy may rely upon restoring a normal testosterone level, it is believed that the testosterone level should be as low as possible when the patient is on treatment, thus generating the lowest serum PSA level possible and likely improving outcome.4 Although the data on IHT are promising, trials reported thus far are relatively small and somewhat underpowered, and it is likely that its use will increase in the future as trials mature.
There is growing recognition that many men may not achieve acceptable levels of testosterone using androgen ablation. This has led to a renewed interest in the significance of the testosterone level in the modern era of prostate cancer management. Can we define the best castration therapy for prostate cancer? Is this the therapy that provides the lowest and most consistent levels of testosterone suppression? To quote Dr. Claude Schulman in a recent editorial: “less is more.”5
Pathophysiology of Prostate Cancer
Normal prostate cells and malignant prostate cancer cells at least initially rely on androgen stimulation via androgen receptors for growth and proliferation. Androgen withdrawal causes a retardation of prostate cell growth, thought to be from programmed cell death and ischemic injury from anoxia.6,7 Thus, manipulation of the hormonal milieu plays a role in the treatment of prostate cancer and often decreases morbidity and increases survival.8–10
Testosterone is not the cause of prostate cancer, but is considered essential for the growth of these tumors. There are many circulating androgenic compounds, including dihydrotestosterone (DHT) androstenedione, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulphate (DHEA-S). Many of these compounds are adrenal products that can be converted to the metabolically active DHT. However, over 90% of androgenic activity in the circulation is due to testosterone. Within the prostate cells, testosterone is converted into 5-α-dihydrotestosterone (DHT), by action of the enzyme 5-α reductase. As an intracellular androgen, DHT is approximately 10 times more powerful than testosterone. The production of the primary circulating androgen, testosterone, relies on the interplay of the hypothalamic-pituitary axis and the testes.11
In the normal adult male, androgen homeostasis is achieved through the pulsatile release of gonadotropin-releasing hormone (GnRH), also referred to as leuteinizing hormone-releasing hormone (LHRH), by the hypothalamus to the anterior pituitary gland about every 90 to 120 minutes. This interaction between GnRH and LH receptors in the pituitary gland promotes the release of LH into the systemic blood circulation, which in turn induces testosterone production by binding to receptors on Leydig cells in the testes. Negative feedback of GnRH is exerted by testosterone through androgen receptors on the hypothalamus and pituitary glands.
Pharmacotherapy of Prostate Cancer
At present, the primary approaches for the initial hormonal management of prostate cancer to reduce circulating serum levels of testosterone are estrogens, surgical orchiectomy, LHRH hormone agonists, and LHRH antagonists. Antiandrogens (steroidal and nonsteroidal) are sometimes used as initial treatment in some settings, but do not directly reduce circulating androgen levels.
Estrogen Therapy
Traditionally, estrogens such as diethylstilbestrol (DES) have been used to treat advanced prostate cancer. The mechanism includes inhibition of the release of LHRH from the hypothalamus with subsequent suppression of LH and, thus, testicular production of testosterone. There may also be a direct cytotoxic effect on the prostate cells themselves by estrogenic compounds. Serum testosterone level decreases in 1 to 2 weeks. Although DES is effective at reducing testicular production of testosterone, there are concerns over its safety (increased risk of cardiovascular and thrombotic events). Even with the recently reported benefit of estrogens in such areas as osteoporosis, it is not considered mainline therapy at present.12 DES is not manufactured in the United States, but is available from prescription compounding pharmacies.
Bilateral Orchiectomy
Bilateral removal of the testes is traditionally the gold standard for androgen ablation. The half-life of native testosterone is approximately 45 minutes. With bilateral orchiectomy, the time to nadir of testosterone is approximately 8.6 ±3.2 hours.13 In patients with symptomatic metastasis, significant improvement is seen in symptoms within 24 to 48 hours. Testosterone, on average, falls to 15 ng/dL (0.5 nmol/L).14 Orchiectomy is rarely performed today for several reasons. The procedure is irreversible, making the potential use of IHT impossible. It is also associated with significant psychologic impact.15 Subcapsular orchiectomy, with maintenance of the tunica albuginea and epididymidis, may provide psychologic benefit to some men who must undergo orchiectomy.
Antiandrogens
Although antiandrogens (androgen receptor blockers), such as bicalutamide, at a dose of up to 150 mg can be clinically beneficial in advanced prostate cancer, there are concerns over their use as monotherapy. Serum testosterone levels can increase due to central nervous system inhibition of testosterone signaling, although prostate cancer cellular receptors appear to be blocked. Increased cardiovascular mortality, possibly due to conduction abnormalities, has been an issue.16 These antiandrogen agents are not US Food and Drug Administration (FDA)-approved for monotherapy, although their role in the blockade of LHRH-induced flare is well established.1 Most often, nonsteroidal antiandrogens are administered for 2 weeks prior to beginning LHRH analogue therapy to reduce any adverse effects of hormonal surge. The 2 classes of antiandrogens are nonsteroidal (flutamide, nilutamide, and bicalutamide), and steroidal (cytoperone acetate), with the latter not available in the United States.
The recognition that very low levels of adrenal androgen production may result in prostate cancer progression despite testicular androgen ablation led to the concept of maximum androgen blockade (MAB). In MAB, antiandrogens are administered along with LHRH analogues long term. It has been postulated that the combined elimination of testicular and adrenal androgens may result in a survival advantage for prostate cancer patients, but clinical trials designed to examine this hypothesis have led to contradictory results.17,18
LHRH Agonists
LHRH agonists are decapeptides that exert a nonpulsatile, constant stimulation to the anterior pituitary gland, which in turn decreases LH and testosterone production. This class was originally developed as a new form of birth control.19 The binding affinity of synthesized LHRH analogues is significantly longer than the native peptide, hence removing the pulsatile signaling to the anterior pituitary gland. The receptor binding of native LHRH is about 6 minutes versus 3.5 hours for leuprolide and 5.5 hours for triptorelin.20 Increased binding affinity may be due to reduced susceptibility to enzymatic degradation compared with natural LHRH. Synthetic analogues, generated by only a single amino acid substitution at position 6, are generally 100 times more potent than natural LHRH. After treatment, LH release is transiently increased up to 2 weeks after the initial dose, referred to as the flare or hormonal surge. After this transient increase in LH and, thus, testosterone levels, the LH and follicle-stimulating hormone (FSH) production is downregulated and testosterone production is inhibited. The flare can be hazardous in some patients, such as those with increasing severity of bone pain from metastasis or ureteral or bladder outlet obstruction, or in whom neurologic compromise is imminent from metastatic disease of the spine. The initial use of a nonsteroidal antiandrogen such as bicalutamide can be effective in blocking the clinical flare.
LHRH analogues are found in a variety of formulations, and, depending on the medication, can be administered every 1 to 12 months. The currently available medications in the United States include different formulations of leuprolide, goserelin, histrelin, and triptorelin in a variety of dosing intervals ranging from monthly to yearly (Table 1). Associated side effects include hot flashes, decreased libido, erectile dysfunction, loss of bone mineral density, anemia, and mood changes.
Table 1.
LHRH Agonists and Antagonists for Primary Androgen Ablation for Prostate Cancer
| Medication | Class | Administration | Notes | |
| Abarelix (Plenaxis) | LHRH antagonist | Intramuscular injection every 2–4 weeks | Chance of anaphylaxis; no hormonal surge (no longer available in US for new patients) | |
| Buserelin (Suprefact) | LHRH agonist | SQ 500 µg (every 8 h × 7 days then 200 µg daily; | Not available in the US | |
| Depot 2 month: 6.3-mg implant every 8 weeks | ||||
| Depot 13 month: 9.45-my implant every 12 weeks | ||||
| Intranasal: 400 µg (200µg into each nostril) 3 times/day | ||||
| Degarelix (Firmagon) | LHRH antagonist | 120 mg IM; 2 doses initially, maintenance 80 mg IM every month | No hormonal surge Requires 2 injections first month | |
| Goserelin (Zoladex 3.6 my and 10.8 mg) | LHRH agonist | 3.6-mg implant SQ every month; 10.8-mg implant SQ every 3 months | Subcutaneous absorbable implant | |
| Histrelin implant (Vantas) | LHRH agonist | SQ implant; 50 mg every 12 months | Remove implant device at reinsertion | |
| Leuprolide (Lupron Depot | LHRH agonist | 7.5 mg SQ monthly (Depot) | Intramuscular injection | |
| Lupron Depot 3 month, | 22.5 mg IM every 3 months; | |||
| Lupron Depot 4 month) | 30 mg IM every 4 months | |||
| Leuprolide gel (Eligard 7.5 mg, | LHRH agonist | 7.5 mg SQ monthly; | Formulation requires refrigerated storage | |
| Eligard 22.5mg, | 22.5 mg SQ every 3 months; | |||
| Eligard 30 mg, | 30 mg SQ every 4 months; | |||
| Eligard 40 mg) | 45 mg SQ every 6 months | |||
| Leuprolide implant (Vaiadur) | LHRH agonist | SQ implant every 12 months (contains 65 mg leuprolide) | Remove implant device at reinsertion Off market for new patients in 2008 | |
| Triptorelin (Trelstar Depot, Tresltar LA) | LHRH agonist | 3.75 mg IM monthly (Depot) | Intramuscular injection | |
| 11.25 mg IM every 3 months (LA) |
IM, intramuscular; LHRH, luteinizing hormone-releasing hormone; SQ, subcutaneous.
Plenaxis®, Praecis Pharmaceuticals Inc., Cambridge, MA; Suprefact®, Sanofi-Aventis, Laval, Quebec, Canada; Firmagon®, Ferring Pharmaceuticals, Inc, Parsippany, NJ; Zoladex®, AstraZeneca Pharmaceuticals LP, Wilmington, DE; Lupron®, TAP Pharmaceuticals Inc, Lake Forest, IL; Eligard®, Sanofi-Aventis; Viadur™, Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ; Trelstar®, Watson Pharma Inc., Morristown, NJ.
LHRH Antagonists
Abarelix is an LHRH antagonist that directly inhibits binding onto the LH receptor in the anterior pituitary gland. Unlike LHRH analogues, there is no hormonal surge. This drug was taken off the US market due to financial and safety concerns, but may still be used in men who were treated before May 2005. It is available in several countries outside of the United States. This medication was limited by a chance of anaphylaxis and the possibility of an increased QT interval.21 The latest LHRH antagonist is degarelix, widely available in Europe and recently approved in the United States. It does not appear to have the adverse safety profile that was attributed to abarelix. With the recent release in the United States of a commercially available LHRH antagonist, the current role in the management of prostate cancer remains to be clearly defined.22
LHRH Escape
The prescribing guidelines for all FDA-approved LHRH agonists and antagonists recommend monitoring testosterone levels to ensure that castrate level is maintained. It is not guaranteed that patients initiated on LHRH therapy will maintain testosterone suppression at all time points. Although the overwhelming majority of prostate cancer patients during treatment with LHRH analogues achieve serum testosterone values within the castrate level, individual patients may fail to reach this therapeutic goal.23
Testosterone escape is defined as a single serum testosterone value rising above 50 ng/dL at any point while under treatment with LHRH analogue therapy. Furthermore, it is recognized that some men may experience surges in testosterone during long-term treatment upon readministration of the agonist drug, described as the acute-on-chronic effect.24 The mechanism is similar to the initial flare reaction with the first treatment (a transient stimulation of LH production by the LHRH agonist). Testosterone surges can also be seen at any time during treatment, referred to as a break-through response.
LHRH agonists are associated with acute-on-chronic effect in 4% to 10% of patients treated with standard LHRH therapy.15,25 Up to 23% of men on goserelin escaped from the castrate level and overall breakthroughs are reported in the literature in 2% to 13% of patients on LHRH agonists overall.26–28 About 2% to 17% of patients fail to achieve a serum testosterone level lower than 50 ng/dL and about 13% to 38% of patients fail to achieve a serum testosterone level lower than 20 ng/dL, as reviewed by Tombal and Berges29 and based on reports of leuprolide acetate depot formulations and goserelin implants.
Other explanations are possible for the occasional failure of an LHRH analogue to achieve the desired effect of serum testosterone. One recent discovery is polymorphisms in LH accounting for variable responses to LHRH analogues in women that will need to be confirmed in men.30 Furthermore, obesity and an association with higher prostate cancer mortality has been noted. Despite lower pretreatment serum testosterone levels, obese men have higher total and free testosterone levels during leuprolide treatment than men with a normal body mass index (BMI). These differences may contribute to the association between obesity and increased prostate cancer mortality.31 Additional concerns have been raised about dosing LHRH analogues in obese men. The issue of BMI having an effect on the depot preparation of LHRH analogues has been reported by several investigators who have studied different doses of leuprolide with respect to body weight.32 In a review of the TAX 327 clinical trial of Taxotere® (docetaxel; Sanofi-Aventis, Bridgewater, NJ) versus mitoxantrone for hormone-resistant prostate cancer, higher testosterone levels among obese men suggested incomplete gonadal suppression with current androgen ablation therapies.33 Tombal and Berges25 noted that 1 patient in their leuprolide gel study experienced a testosterone breakthrough. They noted that the patient was markedly obese based on BMI.25 Dosing of LHRH analogues in the obese man is deserving of increased attention as obesity is a documented adverse risk factor for prostate cancer outcomes.
Optimum Testosterone Level in the Treatment of Prostate Cancer
Normal serum testosterone ranges (which may vary slightly by laboratory) are 300 to 1000 ng/dL (10.4–34.7 nmol/L) for men aged 17 years and older. Due to intra-assay variability, a deviation of about 7% should be accounted for when interpreting testosterone values. A total serum testosterone level (free + protein bound) of lower than 200 ng/dL (6.9 nmol/L) (American Association of Clinical Endocrinologists) or lower than 300 ng/dL (10 nmol/L) (FDA) is associated with hypogonadism and warrants further workup in an otherwise normal adult.34 Free testosterone (adult male range, 8.8–27 pg/mL) is sometimes used in the evaluation of hypogonadism as elevated or decreased sex hormone-binding globulin (SHBG) changes the bioavailability of the free form (metabolically active) of testosterone. As an example, obesity is characterized by a reduced total testosterone with normal free testosterone due to reduced protein binding. Serum SHBG concentrations increase with age. With increasing age, less of the total testosterone is free or biologically active, as SHBG binds testosterone with high affinity.35
There is clearly no defined answer to the optimum level of testosterone that should be achieved in the treatment of prostate cancer. Traditional definitions are based on the so-called castrate levels of testosterone. However, what the castrate level actually is depends on the therapeutic intervention: less than 20 ng/dL (0.69 nmol/L) has been routinely reported for surgical orchiectomy and less than 50 ng/dL (1.735 nmol/L) has been reported with LHRH therapy.
An expert consensus meeting was held in 2005 in San Antonio, Texas, and a similar session took place during the Sixth International Consultation on New Developments in Prostate Cancer and Prostate Diseases in Paris, France, in June 2005, to discuss definitions regarding optimal testosterone control in prostate cancer.36 The experts agreed that the term castration is misleading in the case of LHRH agonists, as it means surgical removal of the testes by bilateral orchiectomy. They noted that bilateral orchiectomy should be used as a benchmark for introducing the appropriate testosterone level that needs to be achieved with LHRH agonists. As most patients will achieve and maintain a serum testosterone level of lower than 20 ng/dL after bilateral orchiectomy, the experts agreed that this level should be used for defining chemical castration. Furthermore, it was agreed that a serum testosterone level over 50 ng/dL during LHRH analogue therapy is clinically relevant and could have implications on treatment outcome.
Another group who convened a similar roundtable of experts concluded that “assessment of patients receiving LHRH agonists should be based on PSA levels rather than serum testosterone levels, although levels of serum testosterone similar to those obtained after orchiectomy still need to be achieved.”37
Several other authors have suggested that the castrate testosterone level needs to be redefined based on modern testosterone assay techniques.14,36,38,39 Based on the most contemporary literature, the current castrate level is defined as lower than 50 ng/dL. The older assay technique for the determination of serum testosterone was known as the double isotope derivative dilution method. This traditional assay was prone to error with lower testosterone levels. Current techniques use the chemoluminescent assay that is reported to be more robust at lower testosterone levels.40 Using chemiluminescent techniques, testosterone values of 16 ng/dL (0.55 nmol/L) and 15 ng/dL (0.5 nmol/L) were reported after bilateral orchiectomy.14,41 A general consensus now exists that testosterone levels achieved and maintained with LHRH agonist therapy should be equivalent to surgical castration.25,36
Currently unknown is the absolute minimal level of testosterone necessary to effectively prevent prostate cancer growth and progression. The issue of optimum testosterone levels and androgen suppression escape has been recently evaluated by Morote.39 He and his colleagues performed a study of 73 men with nonmetastatic prostate cancer with serial PSA and testosterone measurements treated with LHRH androgen suppression. They were stratified into tertiles (testosterone < 20, 20–50, and > 50 ng/dL). The best outcomes in preventing androgen- independent prostate cancer progression occurred at a breakpoint of 32 ng/dL. Breakthrough increases greater than this threshold predicted a lower survival free of androgen-independent progression. There was a 137-month versus an 88-month difference in progression to androgenindependent prostate cancer favoring the lower mean testosterone level. Men who maintained a serum PSA level of lower than 20 ng/dL had a mean PSA progression-free survival of 106 months versus 90 months for those with levels between 20 and 50 ng/mL and only 72 months if the mean serum PSA level was higher than 50 ng/mL. This study also suggested that maximal androgen blockade might benefit medically castrated cases of prostate cancer with breakthrough testosterone increases of higher than 50 ng/dL.
A similar concept has been reported by Perachino and associates.42 In a series of 162 men with metastatic prostate cancer, a multivariate approach defined the best prognostic model for survival based on 6-month testosterone levels of men treated with goserelin. Using the lower than 50 ng/dL medical castration threshold, 119 patients (73.5%) were considered responders after 6 months of goserelin therapy; using a threshold of lower than 20 ng/dL testosterone, only 46 patients (28.4%) were responders after 6 months. In considering multiple factors, serum testosterone level at 6 months correlates with patient survival; death risk is directly correlated not only to goserelin (P < .01) and to a basal PSA (P < .01), but also to a 6-month serum testosterone level (P = .0286). The lower the testosterone level after 6 months, the longer the survival.
Other Evidence to Support Lower Testosterone Levels and Improved Outcomes
The historic investigations known as the Veterans Administration Cooperative Urological Research Group (VACURG) studies formed a basis for the treatment of prostate cancer with DES before the availability of LHRH analogues. Due to higher death rates in the 5-mg DES treatment arm in VACURG I, lower DES doses were studied in VACURG II. Patients were randomized to 3 different dose ranges of DES (0.2 mg, 1 mg, or 5 mg) versus placebo.43 Men receiving 0.2 mg/day of DES had a significantly shorter overall survival than men receiving 5 mg/day. VACURG II showed some survival benefit for hormonal treatment when Kent and associates44 reported that 0.2 mg/day and 1 mg/day of DES failed to consistently suppress testosterone to castrate levels. These data suggest that ineffective androgen suppression may reduce survival in advanced prostate cancer.
Several studies have demonstrated that the addition of an antiandrogen to orchiectomy did not improve overall survival, whereas the addition of an antiandrogen to an LHRH analogue did.45–47 Although specific testosterone data are not available, it does suggest that ineffective or inconsistent testosterone suppression by LHRH analogues (masked by the addition of a nonsteroidal antiandrogen) might be an explanation.
Newer LHRH Analogues and Androgen Suppression
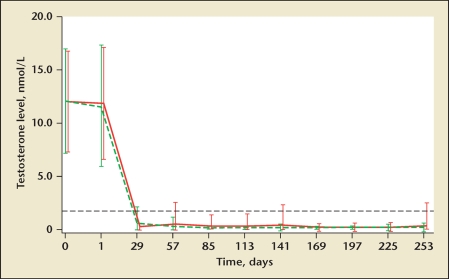
In a study that compared the efficacy of monthly administrations of the LHRH agonists triptorelin and leuprolide in men with advanced prostate cancer, researchers concluded that the 2 formulations were equivalent. However, further analysis of their findings demonstrated that the mean testosterone at 85 days was lower in the triptorelin than in the leuprolide acetate group, at 0.38 (0.1–13.8) nmol/L and 0.16 (0.1–0.7) nmol/L, respectively (based on SI metric units).48 During a 24-h period at 85 days, none of the patients in the triptorelin group but 3 in the leuprolide group had testosterone concentrations above castrate levels. These provocative data suggest that this formulation of triptorelin may result in lower mean testosterone levels than leuprolide (Figure 1). Similar observations of lower testosterone suppression have been made concerning the gel formulation of leuprolide.25
Figure 1.
Mean (SD) testosterone serum levels in men treated with triptorelin pamoate 3.75 mg (green dashed line) or leuprolide acetate 7.5 mg (red solid line) for 253 days. The black dashed line shows the castrate level of 1.735 nmol/L. Reproduced with permission from Heyns CF et al.48
Conclusions
Androgen ablation for prostate cancer can be associated with significant benefits in the appropriate patient. However, it is recognized that prostate cancer and all of its various treatments, hormonal and nonhormonal, can be associated with potential adverse impacts on quality of life.49 Along with the increasing recognition of the adverse consequences of androgen ablation therapy, effective strategies to manage these effects are being developed and refined.50 The risks and benefits of the type and duration of androgen ablation in an individual patient must be made carefully.
All LHRH analogue and antagonist manufacturers recommend that testosterone testing be conducted for patients receiving therapy with these agents. At a recent prostate cancer consensus meeting, 26% of attendees never measured serum testosterone levels and only 50% did so in the event of a PSA rise in their patients on LHRH analogue therapy.36 Only 21% measured serum testosterone levels at least once and only 3% always did.
As noted, a small but perhaps clinically significant number of patients fail to consistently suppress to castrate levels with LHRH analogues. Switching to another LHRH agent, as outlined in Table 1, can be attempted if this is the case. The addition of an antiandrogen can also be considered with surgical orchiectomy, a last option for failure to respond to medical castration.
Limited information exists on the absolute relationship between testosterone values and clinical outcomes. Several recent studies have highlighted the possibility that lower testosterone levels may be associated with improved outcomes, including increased time to the development of androgen-independent disease and overall survival. The studies by Morote and others strongly suggest that patients experiencing a breakthrough response during LHRH therapy have a reduced biochemical survival rate compared with those who did not experience testosterone breakthroughs. Future studies of androgen ablation should focus more intensely on testosterone and other circulating androgen levels as part of evaluating the effectiveness of treatments. Measurement of serum testosterone levels, in addition to serum PSA testing, should be strongly considered in clinical practice for those men on LHRH therapy, as well as those who have previously been on LHRH therapy for a period of time, to determine if and when their levels normalize.
Multiple expert panels and publications indicate that the new benchmark for serum testosterone levels for patients on androgen suppression should consistently be lower than 20 ng/dL, similar to that obtained with bilateral orchiectomy. Current and future pharmacologic agents used for androgen ablation should target these levels achieved by surgical orchiectomy to optimize the prostate cancer disease-specific outcomes.
Main Points.
Medical castration is almost exclusively performed by the use of injectable luteinizing hormone-releasing hormone (LHRH) analogues, with a minor role for estrogen and bilateral orchiectomy and limited experience with LHRH antagonists.
LHRH agonists are decapeptides that exert a nonpulsatile, constant stimulation to the anterior pituitary gland, which in turn decreases LH and testosterone production.
The prescribing guidelines for all US Food and Drug Administration-approved LHRH agonists and antagonists recommend monitoring testosterone levels to ensure that castrate level is maintained.
Currently unknown is the absolute minimum level of testosterone necessary to effectively prevent prostate cancer growth and progression. Multiple expert panels and publications indicate that the new benchmark for serum testosterone levels for patients on androgen suppression should consistently be lower than 20 ng/dL, similar to that obtained with bilateral orchiectomy.
Footnotes
Dr. Gomella is a consultant for Astra-Zeneca and Watson Pharmaceuticals.
References
- 1.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed June 4, 2009];Prostate Cancer V.2.2009. National Comprehensive Cancer Network Web site. http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf. Registration required.
- 3.Tunn U. The current status of intermittent androgen deprivation (IAD) therapy for prostate cancer: putting IAD under the spotlight. BJU Int. 2007;99(suppl 1):19–22. doi: 10.1111/j.1464-410X.2007.06596.x. discussion 23–24. [DOI] [PubMed] [Google Scholar]
- 4.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 5.Schulman CC. Hormone therapy for prostate cancer: less is more? Introduction and conclusions. Eur Urol Suppl. 2008;7:743–746. [Google Scholar]
- 6.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;167:948–951. discussion 952. [PubMed] [Google Scholar]
- 7.Buttyan R, Ghafar MA, Shabsigh A. The effects of androgen deprivation on the prostate gland: cell death mediated by vascular regression. Curr Opin Urol. 2000;10:415–420. doi: 10.1097/00042307-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 9.Walsh PC. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. J Urol. 1997;158:1623–1624. [PubMed] [Google Scholar]
- 10.Messing EM, Manola J, Yao J, et al. Eastern Cooperative Oncology Group study EST 3886 Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 11.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 12.Scherr D, Pitts WR , Jr, Vaughn ED., Jr Diethylstilbesterol revisited: androgen deprivation, osteoporosis and prostate cancer. J Urol. 2002;167:535–538. doi: 10.1016/S0022-5347(01)69080-3. [DOI] [PubMed] [Google Scholar]
- 13.Lin BJ, Chen KK, Chen MT, Chang LS. The time for serum testosterone to reach castrate level after bilateral orchiectomy or oral estrogen in the management of metastatic prostatic cancer. Urology. 1994;43:834–837. doi: 10.1016/0090-4295(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 14.Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021–1024. doi: 10.1016/s0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 15.Boccon-Gibod L. Optimising hormone therapy in advanced disease. Eur Urol Suppl. 2005;4(8):21–29. [Google Scholar]
- 16.Pinthus JH. AUA Update Series 2006. Vol. 25. Linthicum, MD: American Urological Association; 2006. Androgen deprivation therapy for prostate cancer; pp. 134–139. Lesson 15. [Google Scholar]
- 17.Bubley GJ. Is the flare phenomenon clinically significant? Urology. 2001;58(suppl 1):5–9. doi: 10.1016/s0090-4295(01)01235-3. [DOI] [PubMed] [Google Scholar]
- 18.Chodak G, Gomella L, Phung DH. Combined androgen blockade in advanced prostate cancer: looking back to move forward. Clin Genitourin Cancer. 2007;5:371–378. doi: 10.3816/cgc.2007.n.019. [DOI] [PubMed] [Google Scholar]
- 19.Schally AV, Coy DH, Arimura A. LH-RH agonists and antagonists. Int J Gynaecol Obstet. 1980;18:318–324. doi: 10.1002/j.1879-3479.1980.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 20.Coy DH, Labrie F, Savary M, et al. LH-releasing activity of potent LH-RH analogs in vitro. Biochem Biophys Res Commun. 1975;67:576–582. doi: 10.1016/0006-291x(75)90851-7. [DOI] [PubMed] [Google Scholar]
- 21.Huhtaniemi I, White R, McArdle CA, Persson BE. Will GnRH antagonists improve prostate cancer treatment? Trends Endocrinol Metab. 2009;20:43–50. doi: 10.1016/j.tem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 23.Yri OE, Bjoro T, Fossa SD. Failure to achieve castration levels in patients using leuprolide acetate in locally advanced prostate cancer. Eur Urol. 2006;49:54–58. doi: 10.1016/j.eururo.2005.09.009. discussion 58. [DOI] [PubMed] [Google Scholar]
- 24.Sharifi R, Browneller R Leuprolide Study Group, authors. Serum testosterone suppression and potential for agonistic stimulation during chronic treatment with monthly and 3-month depot formulations of leuprolide acetate for advanced prostate cancer. J Urol. 2002;168:1001–1004. doi: 10.1016/S0022-5347(05)64560-0. [DOI] [PubMed] [Google Scholar]
- 25.Tombal B, Berges R. How good do current LHRH agonists control testosterone? Can this be improved with Eligard®? Eur Urol Suppl. 2005;4(8):30–36. [Google Scholar]
- 26.Zinner NR, Bidair M, Centeno A, Tomera K. Similar frequency of testosterone surge after repeat injections of goserelin (Zoladex) 3.6 mg and 10.8 mg: results of a randomized open-label trial. Urology. 2004;64:1177–1181. doi: 10.1016/j.urology.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Fontana D, Mari M, Martinelli A, et al. 3-month formulation of goserelin acetate (‘Zoladex’ 10.8-mg depot) in advanced prostate cancer: results from an Italian open multicenter trial. Urol Int. 2003;70:316–320. doi: 10.1159/000070142. [DOI] [PubMed] [Google Scholar]
- 28.Khan MS, O’Brien A. An evaluation of pharmacokinetics and pharmacodynamics of leuprorelin acetate 3M-depot in patients with advanced and metastatic carcinoma of the prostate. Urol Int. 1998;60:33–40. doi: 10.1159/000030200. [DOI] [PubMed] [Google Scholar]
- 29.Tombal B, Berges R. Corrigendum to: “How good do current LHRH agonists control testosterone? Can this be improved with Eligard®?”. Eur Urol. 2006;49:937. [Eur Urol Suppl 4/8 (2005) 30–36] [Google Scholar]
- 30.Alviggi C, Clarizia R, Pettersson K, et al. Suboptimal response to GnRHa long protocol is associated with a common LH polymorphism. Reprod Biomed Online. 2009;18:9–14. doi: 10.1016/s1472-6483(10)60418-x. [DOI] [PubMed] [Google Scholar]
- 31.Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007;13:241–245. doi: 10.1158/1078-0432.CCR-06-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal N, Fletcher D, Ward J. Obesity and treatment of prostate cancer: what is the right dose of Lupron Depot? Clin Cancer Res. 2007;13:4027. doi: 10.1158/1078-0432.CCR-07-0788. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong AJ, Halabi S, de Wit R, et al. The relationship of body mass index and serum testosterone with disease outcomes in men with castration-resistant metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2009;12:88–93. doi: 10.1038/pcan.2008.36. [DOI] [PubMed] [Google Scholar]
- 34.Gomella LG, editor. 5-Minute Urology Consult. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; Testosterone levels. In press. [Google Scholar]
- 35.Miner MM, Sadovsky R. Evolving issues in male hypogonadism: evaluation, management, and related comorbidities. Cleve Clin J Med. 2007;74(suppl 3):S38–S46. doi: 10.3949/ccjm.74.suppl_3.s38. [DOI] [PubMed] [Google Scholar]
- 36.Zlotta A, Debruyne FMJ. Expert opinion on optimal testosterone control in prostate cancer. Eur Urol Suppl. 2005;4(8):37–41. [Google Scholar]
- 37.de Jong IJ, Eaton A, Bladou F. LHRH agonists in prostate cancer: frequency of treatment, serum testosterone measurement and castrate level: consensus opinion from a roundtable discussion. Curr Med Res Opin. 2007;23:1077–1080. doi: 10.1185/030079907x187973. [DOI] [PubMed] [Google Scholar]
- 38.Røhl HF, Beuke HP. Effect of orchidectomy on serum concentrations of testosterone and dihydrotestosterone in patients with prostatic cancer. Scand J Urol Nephrol. 1992;26:11–14. doi: 10.3109/00365599209180389. [DOI] [PubMed] [Google Scholar]
- 39.Morote J, Orsola A, Planas J, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178:1290–1295. doi: 10.1016/j.juro.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 40.Veldhuis JD, Liem AY, South S, et al. Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1995;80:3209–3222. doi: 10.1210/jcem.80.11.7593428. [DOI] [PubMed] [Google Scholar]
- 41.Oefelein MG, Cornum R. Failure to achieve castrate levels of testosterone during luteinizing hormone releasing hormone agonist therapy: the case for monitoring serum testosterone and a treatment decision algorithm. J Urol. 2000;164:726–729. doi: 10.1097/00005392-200009010-00025. [DOI] [PubMed] [Google Scholar]
- 42.Perachino M, Cavalli V, Monferrato C. Testosterone (T) level correlates with survival in pts with advanced prostate cancer (APC): the lower is really the better. J Urol. 2008;179:179–180. Abstract 512. [Google Scholar]
- 43.Byar DP. Proceedings: the Veterans Administration Cooperative Urological Research Group’s studies of cancer of the prostate. Cancer. 1973;32:1126–1130. doi: 10.1002/1097-0142(197311)32:5<1126::aid-cncr2820320518>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 44.Kent JR, Bischoff AJ, Arduino LJ, et al. Estrogen dosage and suppression of testosterone levels in patients with prostatic carcinoma. J Urol. 1973;109:858–860. doi: 10.1016/s0022-5347(17)60564-0. [DOI] [PubMed] [Google Scholar]
- 45.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 46.Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 47.Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists’ Collaborative Group. Lancet. 2000;355:1491–1498. [PubMed] [Google Scholar]
- 48.Heyns CF, Simonin MP, Grosgurin P, et al. Comparative efficacy of triptorelin pamoate and leuprolide acetate in men with advanced prostate cancer. BJU Int. 2003;92:226–231. doi: 10.1046/j.1464-410x.2003.04308.x. for the South African Triptorelin Study Group. [DOI] [PubMed] [Google Scholar]
- 49.Gomella LG, Johannes J, Trabulsi EJ. Current prostate cancer treatments: effect on quality of life. Urology. 2009;73(suppl):S28–S35. doi: 10.1016/j.urology.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Schwandt A, Garcia JA. Complications of androgen deprivation therapy in prostate cancer. Curr Opin Urol. 2009;19:322–326. doi: 10.1097/MOU.0b013e32832a082c. [DOI] [PubMed] [Google Scholar]