Abstract
This article presents the evolution of open radical retropubic prostatectomy (ORRP) into a minimally invasive procedure and reviews the literature to provide a legitimate comparison between ORRP and robotic-assisted laparoscopic radical retropubic prostatectomy (RALRP). The article is limited to manuscripts cited in the peer-reviewed literature, and an effort was made to identify those articles that fulfilled the highest level of medical evidence. In centers of excellence, ORRP is performed with no mortality, extraordinarily low technical and medical complications (1%), the rare need for blood transfusions, 1- to 2-day hospital stays, urinary catheters that are routinely removed in a week, the majority of men returning to work in 2 weeks, and up to 97% of men regaining urinary continence. Return of potency remains a challenge, especially for older men with marginal erections. RALRP is now the most common approach for the surgical removal of the malignant prostate. A critical review of the literature fails to support the marketing claims that RALRP is associated with shorter hospitalization, less pain, better cosmetics, shorter catheter time, lower transfusion rates, or improved continence and potency rates. The highest level of medical evidence suggests that RALRP may significantly compromise oncologic outcomes and that men undergoing this approach have higher regret rates than men undergoing ORRP.
Key words: Prostate cancer, Open radical retropubic prostatectomy, Robotic-assisted laparoscopic radical retropubic prostatectomy
Radical perineal prostatectomy was first advocated as a curative treatment for prostate cancer by H. H. Young in 1905.1 Because of significant operative and perioperative morbidity and mortality, and the small proportion of cancers diagnosed in their early stages, radical perineal prostatectomy was not widely performed, even by expert surgeons. It is of historical interest to note that Jewett, one of the leading radical perineal prostatectomists of his time, performed only 160 surgeries between 1951 and 1963.2
Millin described the radical retropubic prostatectomy in 19473; the advantages and disadvantages of the retropubic versus perineal prostatectomy were subsequently debated.4 The advocates of the perineal approach argued that the primary advantage of their minimally invasive procedure was less bleeding because the dissection was performed within the prostatic fascia, which avoided the dorsal venous complex. Both the diminished blood loss and the perineal incision contributed to a shorter hospitalization and more rapid recovery. Advocates of the retropubic approach argued the benefits of being able to perform a simultaneous pelvic lymphadenectomy, the opportunity to obtain wider surgical margins, and the fact that urologists were generally more familiar with and preferred the retropubic approach.
A description of the anatomy of the dorsal venous complex, along with a surgical technique to control this structure, greatly minimized the threat of major bleeding associated with the retropubic approach.5 The final blow for the perineal approach was the description by Walsh and Donker of the nerve-sparing radical retropubic prostatectomy that resulted in a greater likelihood of preserving erectile function.6 Although nerve-sparing radical perineal prostatectomy was eventually described, virtually all urologists had already abandoned this approach in favor of the retropubic approach.
Outcomes Following Radical Retropubic Prostatectomy in the 1990s
There were 2 concurrent developments responsible for the dramatic increase in the number of radical prostatectomies performed in the United States beginning in the 1980s. Prostate-specific antigen (PSA) screening greatly increased the detection of clinically localized prostate cancers that were amenable to curative intervention.7 The description of the anatomic nerve-sparing radical retropubic prostatectomy made the surgical option more attractive because of the marked reduction in surgical complications and improved quality-of-life outcomes.8
During the 1990s, several surgeons reported on their complication rates following radical retropubic prostatectomy.9–12 Overall, intraoperative and perioperative morbidity, reoperation rates, and the requirement for allogeneic blood transfusions were exceedingly low. Urinary continence was consistently restored in over 95% of cases.10–12 Despite the widespread acceptance of the nerve-sparing technique, potency rates remained problematic when outcomes were captured using validated self-administered instruments and the surgeon was not involved in data acquisition, entry, or retrieval.13,14
A Surgeon’s Quest to Improve Outcomes Following Radical Prostatectomy
To identify opportunities for improvement, in the early 1990s, this author critically examined his outcomes after having performed over 1000 open radical retropubic prostatectomies.15 Overall, intraoperative and perioperative complications and reoperation rates were exceedingly low, leaving little opportunity to improve pre- and postoperative management, surgical technique, and patient selection. However, the positive surgical margin (PSM) rate was 21%, the allogeneic blood transfusion rate was 11%, the anastomotic stricture rate was 12%, and the preservation of potency rate was 50%. This rigorous and objective self-assessment provided insights into potential improvements for surgical outcomes. The early 1990s ushered in an era where urologists embraced minimally invasive approaches to the management of many diseases, including benign prostatic hyperplasia (BPH), nephrolithiasis, and erectile dysfunction. It was in this spirit that this author questioned many tenets of postoperative management following radical prostatectomy such as advancing the diet, the timing of hospital discharge, the duration of catheter drainage, and the patient’s return to active employment and physical activities.
The highest priority was to reduce PSMs. There are 3 factors that likely contributed to the reduction of PSM rates from 21% to 8%.16 PSM rates are impacted by both the extent of the disease and surgical technique. Widespread PSA screening not only increased the detection rate of prostate cancer, but also resulted in a dramatic stage migration favoring the diagnosis of lower risk disease.17 Therefore, our lower PSM rate is explained in part by the stage migration attributable to PSA testing. In addition, lowering PSA thresholds for recommending biopsy provided another opportunity to detect smaller volume and less aggressive cancers.18 We also believe technical innovations are also responsible for our reduced PSM rates. Beginning in 1999, we routinely submitted circumferential excisional biopsies of the apical soft tissue margins for frozen section inspection with the intent of excising additional tissue if cancer was observed in the specimens.19 Approximately 7% of all apical soft tissue biopsies contained cancer. Only 4.7% of those cases with a PSM limited to the excised apical soft tissue developed a biochemical recurrence at 5 years, suggesting that this maneuver likely had a favorable impact on disease control.20 Prior to 2001, decisions regarding preservation of the neurovascular bundles were made on a case-by-case basis. There is little doubt that preservation of the neurovascular bundles in cases with established extracapsular extension may compromise cancer control. Therefore, in 2001, we developed and used an algorithm to guide our decision to perform nerve-sparing surgery based on risk factors for extracapsular extension.21
Another challenge was to decrease the risk of allogeneic blood transfusions. Since 1996, we have routinely administered preoperative erythrocyte stimulating proteins (ESPs) as a means to increase the production of red cell volume.22 Our studies have shown that ESPs raise the hematocrit on average 4 percentage points,23 which exceeds the endogenous erythrogenic response associated with autologous blood donation.24 We have also reported that the risk of any intraoperative or perioperative thromboembolic or cardiovascular complication in a consecutive series of 1095 cases of radical retropubic prostatectomy was 0.45% for those men receiving preoperative ESPs.24 This is the lowest rate reported in the literature and confirms the cardiovascular safety of this blood management strategy. We attribute the reduction of our allogeneic blood transfusion rate of 4.6%16 primarily to the use of ESPs.
We were quite surprised that our anastomotic stricture rate was 12%.25 Our observation that men who developed anastomotic strictures had a greater tendency to heal their surgical incision with a keloid suggested that in some men the formation of an anastomotic stricture was not due to technical issues, but simply a propensity toward hypertrophic healing of the anastomosis.26 Nevertheless, anastomotic strictures did occur in cases without associated keloid formation, suggesting that some strictures were preventable. Since 2000, we have routinely performed cystograms to confirm the integrity of the anastomosis prior to catheter removal.27 In the 10% of cases exhibiting moderate or severe extravasation the urinary catheters were left indwelling until vesicourethral integrity was demonstrated.28 Arbitrarily removing the urinary catheter at 2 weeks in the presence of moderate or severe extravasation would likely have predisposed to stricture formation. Around this time, Catalona and colleagues10 reported that tightly reconstructing the bladder neck also contributed to stricture formation. Based on this report, we started calibrating our bladder neck reconstruction from 18 Fr to 24 Fr. We believe prolonged catheter draining in the presence of significant anastomotic extravasation and a more patulous bladder neck reconstruction contributed to reducing our stricture rate.
Preservation of potency rates reported in the literature for unselected cohorts range from 20% to 60%.29 There is no doubt that surgical experience and technique impact potency rates. The wide variability in reported potency rates has also been attributed to different definitions of potency, patient selection, and whether self-administered, validated questionnaires were used to assess potency.30 We were quite disappointed with our potency rate of 50%.11 We set out to improve potency rates by altering our surgical technique and identifying pharmacologic strategies to prevent damage to the corporal tissue resulting from iatrogenic neurapraxia. Our institution participated in the first randomized, double-blind study that provided compelling evidence that daily administration of phosphodiesterase type 5 (PDE5) inhibitors significantly improved potency rates.31 Since 1996, we have routinely recommended daily administration of a PDE5 inhibitor that is initiated at the time of catheter removal. In addition, in low-risk disease we perform a more aggressive nerve-sparing procedure. We believe the routine prophylactic administration of PDE5 inhibitors and a more aggressive nerve-sparing technique in selected cases account for the improvement in our overall potency rate to 59%.32 It is important to note that our 59% potency rate was achieved in the cohort of all men who were able to engage in sexual intercourse preoperatively, independent of age, quality of erections, prior use of PDE5 inhibitors, or nerve-sparing intent. A significant subset of our cohort had baseline erectile dysfunction. Restoration of potency was observed to be dependent upon age, baseline erectile function, preoperative use of PDE5 inhibitors, cardiovascular risk factors, history of diabetes, and nerve-sparing intent.32 Our potency rate approached 80% in younger men with no baseline erectile dysfunction undergoing a bilateral nerve-sparing procedure.33 To further improve post-prostatectomy potency, we now offer couples a rigorous rehabilitation protocol that includes the use of PDE5 inhibitors, intraurethral alprostadil, and a vacuum device beginning in the immediate postoperative period; penile injections are added 3 months following surgery based on the status of erectile function.
In the early 1990s, I questioned many tenets that were instilled in me during residency training, such as advancing the diet in the absence of flatus, removing the urinary catheter no earlier than 3 weeks, and restricting heavy physical activities for 6 weeks postoperatively.34 None of these practices were evidence based. Therefore, I began feeding my patients the night of surgery, discharging them on the first or second postoperative day, removing the urinary catheter in 1 week (providing there was no demonstrable extravasation on a postoperative day 7 cystogram), restricting driving an automobile for men taking narcotics for pain control, encouraging men to return to work as soon as they desired, and allowing men to resume unrestricted physical activity within 3 weeks. In essence, we transformed open radical retropubic prostatectomy into a minimally invasive surgical procedure simply by abandoning restrictive common practices that were not supported by medical evidence.
Opportunities for Improving Radical Prostatectomy
The robotic-assisted laparoscopic approach made its debut in 2000. Since then, this author has continuously re-examined personal outcomes to determine if this new technology would offer advantages other than as a tool to attract clinical volume (Table 1). Our medical center had just purchased a robotic surgical system at the request of our cardiothoracic surgeons. Interestingly, they quickly lost interest in robotics and abandoned the technology. The manufacturers of the system and some of the early adopters of this technology “promised” better potency, a faster recovery, better cosmetics, less pain, earlier removal of the catheter, less blood loss, and faster return to continence. There was not a shred of legitimate medical evidence to support any of these claims other than lower blood loss, which did not result in fewer transfusions or better functional outcomes.
Table 1.
Outcomes Following Open Radical Retropubic Prostatectomy Since 2000
| Outcome | Frequency or Percentage | Reference |
| Mortality (%) | 0 | 16 |
| Intraoperative injury (%) | 0.2 | 16 |
| Intestinal | 0 | |
| Obturator nerve | 0 | |
| Major vascular stricture | 0 | |
| Ureter | 0.2 | |
| Intraoperative injury requiring reoperation | 0.25 | 34 |
| Deep venous thrombosis/pulmonary embolus | 0.45 | 25 |
| Myocardial infarction | 0.55 | 25 |
| Allogeneic transfusion rate (%) | 4.6 | 16 |
| Mean length of hospital stay (days) | 2.1 | 16 |
| Mean length of hospital stay (2008) | 1.8 | Personal communication |
| Median time to return to work (days) | 14 | 36 |
| Positive surgical margins (%) | 8 | 16 |
| Anastomotic stricture total | 7.6 | 26 |
| Urinary continence | 97 | 37 |
| Potency | 59 | |
| Catheters removed ≤ postoperative day 8 | 89.3 | 29 |
In 2000, I was performing open radical retropubic prostatectomy through a 4-inch incision in 1 hour; the average length of hospital stay was 2 days, the transfusion rate was 4%, and urinary catheters were routinely removed in a week.16 In a prospective internal review board (IRB)-approved study, we reported that in a consecutive series of 547 men the median time to return to work and unrestricted activities was 2 weeks and 4 weeks, respectively (Table 2).35 Men were riding horses, competing in national track events, or preparing for marathons prior to 3 weeks postoperatively. Our reported continence rate, based on self-reported questionnaires, was 97%.36 In our hands, open radical retropubic prostatectomy had evolved into a “minimally invasive” procedure. Improving potency remained the only real and legitimate opportunity for robotics to improve outcomes following radical prostatectomy.
Table 2.
Return to Employment and Physical Activities Following Open Radical Retropubic Prostatectomy
| Work (Days) | |||
| Statistic | Part Time | Full Time | Activity (Days) |
| Mean | 17 | 25 | 34 |
| SD | 12.6 | 16.6 | 19.8 |
| Minimum | 1 | 3 | 2 |
| 25th percentile | 7 | 14 | 21 |
| Median | 14 | 21 | 30 |
| 75th percentile | 21 | 30 | 42 |
| Maximum | 84 | 112 | 120 |
Adapted from Sultan R et al.35
What Is the Appropriate Study Design for Comparing Open Radical Retropubic Prostatectomy With Robotic-Assisted Laparoscopic Retropubic Prostatectomy?
The ideal study design for comparing open radical retropubic prostatectomy (ORRP) with robotic-assisted laparoscopic retropubic prostatectomy (RALRP) is a trial in which surgical candidates are randomized to these 2 surgical approaches, applying the same clinical pathways and methodology for assessing outcomes. It is unlikely this study will ever be conducted. A legitimate study design would be to compare these 2 procedures at the same institution using identical pathways and methodology for assessing outcomes. Ideally, the surgeons performing the procedures should be at the equivalent level of proficiency.37 It is totally inappropriate to compare ORRP performed by inexperienced surgeons to RALRP performed by highly experienced surgeons when there is no uniformity of clinical pathways.38
Prioritizing Outcomes
There are a host of clinical outcomes that merit comparison between these 2 surgical procedures. It is imperative that the clinical relevance of these outcome measures be considered. Based upon a survey of men undergoing radical prostatectomy15 and a prospective longitudinal self-assessment study of satisfaction with the decision to undergo ORRP,39 along with my experiences managing almost 4000 men undergoing this procedure, I have categorized outcomes according to clinical relevance (Table 3).
Table 3.
Categorizing Outcomes Following Radical Retropubic Prostatectomy
| Highest Clinical Relevance |
| Mortality |
| Technical complications |
| Positive surgical margins |
| Biochemical recurrences |
| Continence |
| Potency |
| Transfusion rate |
| Cost |
| Anastomotic stricture |
| Lower Clinical Relevance |
| Pain |
| Length of stay |
| Length of incision |
| Blood loss |
| Return to work/activities |
The outcome of greatest importance is local disease control. Technical and medical complications, continence and potency status, and cost are also of high clinical relevance. The size of the incision, duration of the surgical procedure, length of hospital stay, immediate postoperative analgesic use, and time to return to work are of lower clinical relevance and have little impact on satisfaction rates.39
Unfortunately, there is a paucity of high-level medical evidence to make definite comparisons between the 2 procedures. The only legitimate claim supported by medical evidence is that RALRP is associated with less blood loss, which does not translate into significantly lower transfusion rates.40 The advantage related to lower blood loss is totally negated by the use of ESPs.23
A Comparison of Secondary Treatments, Anastomotic Strictures, and Complication Following ORRP Versus RALRP
One of the landmark publications comparing open versus laparoscopic-assisted radical prostatectomy (LRP) was recently published in the Journal of Clinical Oncology.41 The unique feature of this study was that ORRP and LRP cases were extracted from the Medicare database between the years 2003 and 2005, which eliminated potential date of surgery bias. The definitions for the various outcome assessments were also uniform. Based upon practice patterns during this time period, it is reasonable to assume the overwhelming majority of laparoscopic cases were performed with robotic assistance. Because the data were extracted directly from the Medicare database, there was no selection bias and, therefore, the study reflects standard of care. The outcomes were limited to Diagnosis Related Group codes that included salvage treatments (radiation therapy or androgen suppression therapy), anastomotic strictures, and complications. Length of stay was also examined. The findings of this landmark study are summarized in Table 4. In this study, disease control was ascertained by capturing the need for secondary cancer treatments (salvage radiation therapy or adjuvant hormonal therapy) within 1 year of surgery. Men undergoing LRP had almost a 400% greater likelihood of undergoing secondary treatment of presumed failure to control the disease. The second most important outcome examined was stricture rates. The rate of strictures was 40% higher in the men undergoing LRP. Unfortunately, complications were not categorized based upon severity. Complications were 40% higher in the men undergoing ORRP. Length of stay in this study was considerably longer in the ORRP group.
Table 4.
A Comparison of Open (ORRP) Versus Laparoscopic (LRP) Radical Retropubic Prostatectomy Based on the Medicare Database
| Outcome | ORRP | LRP | OR (95% CI) LRP vs ORRP |
| Salvage therapy (% pts) | 9.1 | 27.8 | 3.67 (2.81–4.81) |
| Anastomotic stricture | 1.4 (1.04–1.87) | ||
| LOS (days) | 4.4 | 1.4 | −2.99 (−3.45–−2.53) |
| Perioperative complications (%) | 36.4 | 29.8 | 0.73 (0.6–0.9) |
95% CI, 95% confidence interval; LOS, length of stay; pts, patients.
Data from Hu JC et al.41
The primary and most important goal of radical prostatectomy is to cure the disease. The dramatic increase in secondary salvage cancer treatments suggests that in clinical practice RALRP is failing to achieve the most important objective of treatment. It is difficult to reconcile the fact that RALRP has gained widespread acceptance in view of these very troubling outcomes.
Does RALRP Improve Quality-of-Life Outcomes?
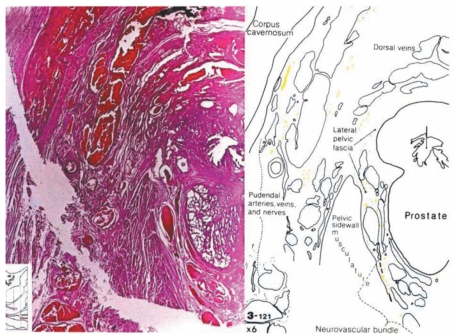
Many advocates of RALRP claim that magnification of the cavernous nerves facilitates intraoperative visualization and preservation of these structures during surgery. While a resident in urology at Johns Hopkins Medicine, I had the great fortune of working with my mentor, Patrick C. Walsh, MD, on a project that delineated the precise anatomic pathways of the cavernous nerves relative to pelvic structures. This project involved preparing over 7000 whole-mount step sections from an en bloc specimen removed from a male cadaver containing the penis, rectum, bladder, prostate, and pelvic side well. Every 10th section was stained, and the key structures were identified and 3-dimensionally reconstructed.42 Based on this detailed anatomic study, it is readily apparent that the cavernous nerves are so minute that magnification provided by a robotic surgical system would not allow visualization of these structures (Figure 1). Magnification may aid in visualizing the neurovascular bundle but not the individual branches of the cavernous nerve. The composite neurovascular bundle can be seen without magnification. Open surgeons who feel magnification is useful can simply wear magnification loops instead of investing in a $2 million surgical system.
Figure 1.
Cross-section of the prostate demonstrating the size of the cavernous nerves mid-gland. Reproduced with permission from Lepor H et al.42
Rojas-Cruz and Mulhall delivered a podium presentation at the 2007 national American Urological Association meeting refuting unsupported Internet Web site claims about the benefits of RALRP related to potency.43 These investigators concluded that the majority of robotic Web sites stated RALRP had erectile function outcomes comparable to open prostatectomy, despite the absence of scientific data to support these claims. This misinformation is giving patients who are considering radical prostatectomy unrealistic expectations.
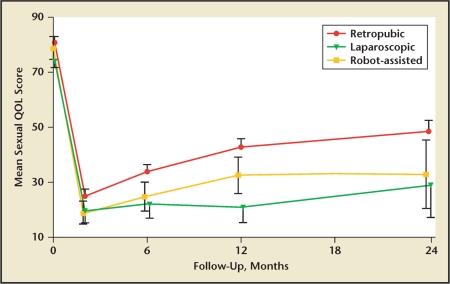
The study with the highest level of medical evidence comparing potency following ORRP versus RALRP was supported by a grant from the National Institutes of Health.44 A total of 602 men undergoing ORRP, RALRP, or LRP at 8 academic centers completed the UCLA Prostate Cancer Index at baseline and at several time points postoperatively. The potency outcomes are presented in Figure 2. Although the composite sexual function scores were greatest in the ORRP group, the differences among the 3 groups were not statistically significant.
Figure 2.
Comparison of potency rates following open radical retropubic prostatectomy, robotic-assisted laparoscopic retropubic prostatectomy, and laparoscopic retropubic prostatectomy. QOL, quality of life. Reproduced with permission from Wagner AA et al.44
Schwab and colleagues compared quality-of-life outcomes following ORRP versus RALRP performed at Eastern Virginia Medical College using the same self-assessment instrument.45 Again, although the sexual function scores were greatest in the ORRP group, the differences were not statistically significant. There were also no differences in the continence scores.
In a recent randomized study, daily intraurethral alprostadil was compared with daily sildenafil for penile rehabilitation.46 The men randomized into this study from New York University Langone Medical Center (NYULMC) underwent ORRP and those from Georgetown University Hospital underwent RALRP .... The mean International Index of Erectile Function (IIEF) scores and the single question capturing erectile function at 1 year were not significantly different between the 2 groups, providing further evidence that the robotic approach does not favor preservation of potency.
Are Satisfaction Rates Higher Following ORRP Versus RALRP?
Ideally, a comparison of open versus robotic radical retropubic prostatectomy should use the same outcome instrument and the assessments should be conducted concurrently and uniformly. A Likert-scale instrument designed to capture both satisfaction and regret rates was mailed to all men who had undergone open or robotic radical retropubic prostatectomy at Duke Medical Center between the years 2000 and 2007.47 Six hundred fifteen men returned the questionnaire. The corrected satisfaction rates were 440% higher and the regret rates were 302% lower for men who had undergone ORRP compared with RALRP. The authors, who were open and robotic surgeons, concluded that the higher dissatisfaction rates associated with the robot were likely explained by unrealistic expectations.
We have recently examined satisfaction rates for a cohort of 1662 men enrolled in our IRB-approved NYULMC Longitudinal Radical Prostatectomy Database.45 Using a Likert scale, self-assessment of satisfaction with decision to undergo ORRP was ascertained at 3 months, 6 months, 1 year, 2 years, 4 years, and 7 years following surgery. Satisfaction rates at all time points were 93. Using a multivariate analysis, long-term satisfaction was independently influenced only by disease control, continence status, and potency status. Other endpoints, categorized in Table 1 as low impact endpoints, did not influence long-term satisfaction rates.
RALRP: Is It Minimally Invasive?
RALRP requires 4 1-inch incisions to introduce the surgical instruments and another infraumbilical incision is made to remove the surgical specimen. Depending on the size of the prostate, the total longitudinal length of the surgical incisions used to perform RALRP is between 6 and 8 inches. In our experience, ORRP with or without pelvic lymphadenectomy is routinely performed via a 4-inch lower midline incision. RALRP is typically performed via an intraperitoneal approach that requires insufflating the peritoneal cavity with air. ORRP is performed via an extraperitoneal approach that minimizes deleterious effects on bowel function. ORRP is performed via a midline incision that avoids trauma to the abdominal wall musculature. Finally, ORRP is typically performed with shorter anesthetic times. The RALRP technique for surgical removal of the prostate mimics the open approach. Based on the above, it could be concluded that the ORRP is the true minimally invasive procedure.
Nelson and colleagues48 compared length of hospital stay and Webster and colleagues49 compared postoperative pain for men undergoing ORRP versus RALRP subjected to similar clinical pathways and outcome scales. They observed no clinically or statistically significant differences between the 2 surgical approaches.
There has been no large, prospective, longitudinal study ascertaining when men return to work or unrestricted activities following RALRP. There is no reason why men undergoing RALRP should return to work or activities any sooner than following ORRP. The mean interval of time men return to employment and unrestricted physical activities in our series of ORRP was 2 and 4 weeks, respectively.35 It is unlikely that the robotic approach can achieve outcomes that are significantly better.
Catheterless RALRP has recently been reported.50 Instead of a urethral catheter, a small suprapubic catheter is placed intraoperatively. Although the suprapubic catheter obviously reduces penile discomfort, the authors did not capture the general inconvenience of a drainage bag. This short-term study also failed to report how often the small catheter required catheter irrigation due to clots. We have previously reported that men consider the urinary catheter a significant impediment to their recovery,27 which justifies efforts for avoidance or early removal of the urinary catheter. We previously reported our experience removing the urinary catheter in the 80% of men who exhibited no extravasation on a postoperative day 3 or 4 cystogram.51 The limitation for catheter removal at 3 or 4 days was acute urinary retention in over 20% of cases requiring catheter replacement. Therefore, we concluded the benefits of early removal were not justified. We have observed in men undergoing catheterless robotic surgery a high rate of catheter clotting (H. Lepor, personal observation). The long-term implication of a nonstented anastomosis is also unproven. It is important to realize that a catheterless ORRP can also be performed. At the moment, based on our experience with unscheduled visits to irrigate suprapubic catheters and the unknown risks of nonstented anastomosis, we are reluctant at our institution to offer this to men undergoing open or robotic radical retropubic prostatectomies.
Conclusions
The factors predicting long-term satisfaction following ORRP are limited to biochemical recurrence, continence, and potency.39 In 2000, I predicted that RALRP would not improve any of these clinically important outcomes. It was also my impression that the robotic approach would not even prove to be “minimally invasive” when compared with ORRP. Medical evidence has proved these observations.
The most recent evidence suggests that RALRP may even be a step backward. Salvage treatments for failed cancer control are significantly higher,41 as are dissatisfaction and regret rates.47 The one outcome that open surgeons need to improve is potency, and every legitimate comparative trial fails to show the benefit of the robotic approach.42–46
Do experienced robotic surgeons achieve excellent results? The answer is yes. However, so do open surgeons.
Many years ago I stated that RALRP must be superior to ORRP due to its learning curve, increased cost, and unknown disease control. It appears that the robotic procedure has not yet achieved superiority over the open procedure.
It is possible that future innovations will ultimately render the robotic procedure superior to open radical retropubic prostatectomy. We are not there today.
Despite the lack of credible outcomes data showing superiority, it is testimony to the marketing ability of the manufacturers of this technology that over 70% of radical prostatectomies today are performed via the robotic approach.
Main Points.
There were 2 concurrent developments that were responsible for the dramatic increase in the number of radical prostatectomies performed in the United States: prostate-specific antigen screening that increased the detection of clinically localized disease and the advent of anatomical nerve-sparing radical retropubic prostatectomy.
The robotic-assisted laparoscopic approach to radical retropubic prostatectomy made its debut in 2000. Improving potency was the only opportunity for robotics to improve outcomes following radical prostatectomy.
The clinical outcomes of greatest importance when comparing open radical retropubic prostatectomy (ORRP) and robotic-assisted laparoscopic radical retropubic prostatectomy (RALRP) are local disease control, technical and medical complications, continence and potency status, and cost.
Quality-of-life outcomes in studies comparing ORRP and RALRP are not statistically significant. Salvage treatments for failed cancer control and dissatisfaction and regret rates are higher in patients undergoing RALRP.
References
- 1.Young HH. The early diagnosis and radical cure of carcinoma of the prostate: a study of 40 cases and presentation of a radical operation which was carried out in four cases. Johns Hopkins Hosp Bull. 1905;16:315–321. [Google Scholar]
- 2.Elder JS, Jewett HJ, Walsh PC. Radical perineal prostatectomy for clinical stage B2 carcinoma of the prostate. J Urol. 1982;127:704–706. doi: 10.1016/s0022-5347(17)54005-7. [DOI] [PubMed] [Google Scholar]
- 3.Millin T. Retropubic Urinary Surgery. London: Livingstone; 1947. [Google Scholar]
- 4.Walsh PC, Lepor H. The role of radical prostatectomy in the management of prostatic cancer. Cancer. 1987;60(3 suppl):526–537. doi: 10.1002/1097-0142(19870801)60:3+<526::aid-cncr2820601515>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Reiner WG, Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorini’s plexus during radical retropubic surgery. J Urol. 1979;121:198–200. doi: 10.1016/s0022-5347(17)56718-x. [DOI] [PubMed] [Google Scholar]
- 6.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 7.Catalona WJ, Richie JP, Ahmann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–1290. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 8.Walsh PC, Partin AW, Epstein JI. Cancer control and quality of life following anatomical radical retropubic prostatectomy: results at 10 years. J Urol. 1994;152:1831–1836. doi: 10.1016/s0022-5347(17)32396-0. [DOI] [PubMed] [Google Scholar]
- 9.Shekarriz B, Upadhyay J, Wood DP. Intraoperative, perioperative, and long-term complications of radical prostatectomy. Urol Clin North Am. 2001;28:639–653. doi: 10.1016/s0094-0143(05)70168-3. [DOI] [PubMed] [Google Scholar]
- 10.Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. J Urol. 1999;162:433–438. [PubMed] [Google Scholar]
- 11.Lepor H, Nieder AM, Ferrandino MN. The intraoperative and postoperative complications of radical retropubic prostatectomy in a consecutive series of 1,000 cases. J Urol. 2001;166:1729–1733. [PubMed] [Google Scholar]
- 12.Steiner MS, Morton RA, Walsh PC. Impact of anatomical radical prostatectomy on urinary continence. J Urol. 1991;145:512–514. doi: 10.1016/s0022-5347(17)38382-9. discussion 514–515. [DOI] [PubMed] [Google Scholar]
- 13.Penson DF. The effect of erectile dysfunction on quality of life following treatment for localized prostate cancer. Rev Urol. 2001;3:113–119. [PMC free article] [PubMed] [Google Scholar]
- 14.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 15.Lepor H. Radical prostatectomy: status and opportunities for improving outcomes. Cancer Invest. 2004;22:435–444. doi: 10.1081/cnv-200029074. [DOI] [PubMed] [Google Scholar]
- 16.Lepor H, Kaci L. Contemporary evaluation of operative parameters and complications related to open radical retropubic prostatectomy. Urology. 2003;62:702–706. doi: 10.1016/s0090-4295(03)00515-6. [DOI] [PubMed] [Google Scholar]
- 17.Han M, Partin AW, Piantadosi S, et al. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001;166:416–419. [PubMed] [Google Scholar]
- 18.Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/mL and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997;277:1452–1455. [PubMed] [Google Scholar]
- 19.Shah O, Melamed J, Lepor H. Analysis of apical soft tissue margins during radical retropubic prostatectomy. J Urol. 2001;165:1943–1948. doi: 10.1097/00005392-200106000-00023. discussion 1948–1949. [DOI] [PubMed] [Google Scholar]
- 20.Godoy G, Tarren BU, Lepor H. Is the apical soft tissue margin a better predictor of biochemical recurrence than the surgical specimen? Urol Oncol. In press. [DOI] [PubMed]
- 21.Shah O, Robbins DA, Melamed J, Lepor H. The New York University nerve sparing algorithm decreases the rate of positive surgical margins following radical retropubic prostatectomy. J Urol. 2003;169:2147–2159. doi: 10.1097/01.ju.0000057496.49676.5a. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblum N, Levine MA, Handler T, Lepor H. The role of preoperative epoetin alfa in men undergoing radical retropubic prostatectomy. J Urol. 2000;163:829–833. [PubMed] [Google Scholar]
- 23.Nieder AM, Rosenblum N, Lepor H. Comparison of two different doses of preoperative recombinant erythropoietin in men undergoing radical retropubic prostatectomy. Urology. 2001;57:737–741. doi: 10.1016/s0090-4295(00)01056-6. [DOI] [PubMed] [Google Scholar]
- 24.Lipkin M, Lepor H. The preoperative use of erythropoietin stimulating proteins prior to radical prostatectomy is not associated with increased cardiovascular or thromboembolic morbidity or mortality. Urology. In press. [DOI] [PubMed]
- 25.Huang G, Lepor H. Factors predisposing to the development of anastomotic strictures in a singlesurgeon series of radical retropubic prostatectomies. BJU Int. 2006;97:255–258. doi: 10.1111/j.1464-410X.2005.05908.x. [DOI] [PubMed] [Google Scholar]
- 26.Park R, Martin S, Goldberg JD, Lepor H. Anastomotic strictures following radical prostatectomy: insights into incidence, effectiveness of intervention, effect on continence, and factors predisposing to occurrence. Urology. 2001;57:742–746. doi: 10.1016/s0090-4295(00)01048-7. [DOI] [PubMed] [Google Scholar]
- 27.Lepor H, Nieder AM, Frainman MC. Early removal of urinary catheter after radical retropubic prostatectomy is both feasible and desirable. Urology. 2001;58:425–429. doi: 10.1016/s0090-4295(01)01218-3. [DOI] [PubMed] [Google Scholar]
- 28.Lepor H, Kozirovsky M, Laze J, Telegrafi S. Transabdominal sonocystography: a novel technique to assess vesicourethral extravasation following radical prostatectomy. J Urol. 2008;180:2459–2462. doi: 10.1016/j.juro.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 29.McCullough AR. Prevention and management of erectile dysfunction following radical prostatectomy. Urol Clin North Am. 2001;28:613–627. doi: 10.1016/s0094-0143(05)70166-x. [DOI] [PubMed] [Google Scholar]
- 30.Mulhall JP. Defining and reporting erectile function outcomes after radical prostatectomy: challenges and misconceptions. J Urol. 2009;181:462–471. doi: 10.1016/j.juro.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 31.Padma-Nathan H, McCullough AR, Levine LA, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20:479–486. doi: 10.1038/ijir.2008.33. [DOI] [PubMed] [Google Scholar]
- 32.Marien T, Sankin A, Lepor H. Factors predicting preservation of erectile function in men undergoing open radical retropubic prostatectomy. J Urol. 2009;181:1817–1822. doi: 10.1016/j.juro.2008.11.105. [DOI] [PubMed] [Google Scholar]
- 33.Twiss C, Slova D, Lepor H. Outcomes for men younger than 50 years undergoing radical prostatectomy. Urology. 2005;66:141–146. doi: 10.1016/j.urology.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 34.Lepor H. Open versus laparoscopic radical prostatectomy. Rev Urol. 2005;7:115–127. [PMC free article] [PubMed] [Google Scholar]
- 35.Sultan R, Slova D, Thiel RP, Lepor H. Time to return to work and physical activities following open radical retropubic prostatectomy. J Urol. 2006;176:1420–1423. doi: 10.1016/j.juro.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Lepor H, Kaci L, Xue X. Continence following radical retropubic prostatectomy using self-reporting instruments. J Urol. 2004;171:1212–1215. doi: 10.1097/01.ju.0000110631.81774.9c. [DOI] [PubMed] [Google Scholar]
- 37.Kaufman MR, Baumgartner RG, Anderson LW, et al. The evidence-based pathway for perioperative management of open and robotically assisted laparoscopic radical prostatectomy. BJU Int. 2007;99:1103–1108. doi: 10.1111/j.1464-410X.2007.06777.x. [DOI] [PubMed] [Google Scholar]
- 38.Tewari A, Srivasatava A, Menon M. Members of the VIP Team. A prospective comparison of radical retropubic robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92:205–210. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 39.Abraham N, Makarov DV, Laze J, Lepor H. Longitudinal and prospective self assessment of satisfaction up to 7 years after open retropubic radical prostatectomy. J Urol. 2009;181:670. Abstract 1857. [Google Scholar]
- 40.Smith JA, Jr, Herrell SD. Robotic-assisted laparoscopic prostatectomy: do minimally invasive approaches offer significant advantages? J Clin Oncol. 2005;23:8170–8175. doi: 10.1200/JCO.2005.03.1963. [DOI] [PubMed] [Google Scholar]
- 41.Hu JC, Wang Q, Pashos CL, et al. Utilization and outcomes of minimally invasive radical prostatectomy. J Clin Oncol. 2008;26:2278–2284. doi: 10.1200/JCO.2007.13.4528. [DOI] [PubMed] [Google Scholar]
- 42.Lepor H, Gregerman M, Crosby R, et al. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol. 1985;133:207–212. doi: 10.1016/s0022-5347(17)48885-9. [DOI] [PubMed] [Google Scholar]
- 43.Rojas-Cruz C, Mulhall JP. Sexual health misinformation on robotic prostatectomy websites. J Urol. 2007;177:342. Abstract 1034. [Google Scholar]
- 44.Wagner AA, Wei JT, Dunn RL, et al. Patient-reported outcomes after retropubic, laparoscopic, or robotic-assisted prostatectomy: results from a prospective, multi-center study. J Urol. 2007;177:184. Abstract 552. [Google Scholar]
- 45.Schwab CW, II, Fabrizio MD, Given RW, et al. Prospective longitudinal comparison of health related quality of life in patients undergoing treatment for localized prostate cancer: an evaluation of three surgical treatment modalities from a single institution. J Urol. 2007;177:7. Abstract 19. [Google Scholar]
- 46.Lepor H, McCullough A, Engel JD. Renewing intimacy: advances in treating erectile dysfunction postprostatectomy. Rev Urol. 2008;10:245–253. [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeck FR, Krupski TL, Sun L, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54:785–793. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 48.Nelson B, Kaufman M, Broughton G, et al. Comparison of length of hospital stay between radical retropubic prostatectomy and robotic assisted laparoscopic prostatectomy. J Urol. 2007;177:929–931. doi: 10.1016/j.juro.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 49.Webster TM, Herrell SD, Chang SS, et al. Robotic assisted laparoscopic radical prostatectomy versus retropubic radical prostatectomy: a prospective assessment of postoperative pain. J Urol. 2005;174:912–914. doi: 10.1097/01.ju.0000169455.25510.ff. discussion 914. [DOI] [PubMed] [Google Scholar]
- 50.Tan GY, Goel RK, Kaouk JH, Tewari AK. Technological advances in robotic-assisted laparoscopic surgery. Urol Clin North Am. 2009;36:237–249. doi: 10.1016/j.ucl.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 51.Patel R, Lepor H. Removal of the urinary catheter on postoperative days 3 or 4 following radical retropubic prostatectomy. Urology. 2003;61:156–160. doi: 10.1016/s0090-4295(02)02105-2. [DOI] [PubMed] [Google Scholar]