Abstract
AIM: To study the composition of liver inflammatory infiltrate in biopsy material from patients chronically infected with hepatotropic viruses and to evaluate the correlation of inflammatory infiltrate with hepatitis B virus (HBV) and hepatitis C virus (HCV) viral antigen expression in chronic B and C hepatitis.
METHODS: The phenotype of inflammatory cells was evaluated by the EnVision system, using a panel of monoclonal antibodies. HBV and HCV antigens were detected with the use of monoclonal anti-HBs, polyclonal anti-HBc and anti-HCV antibodies, respectively.
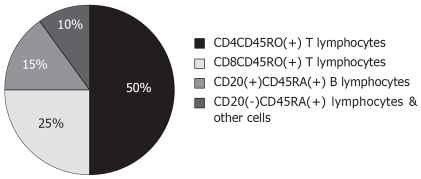
RESULTS: The cellular composition of liver inflammatory infiltrate was similar in the patients with B and C hepatitis: ~50%-60% of cells were T helper lymphocytes. Approximately 25% were T cytotoxic lymphocytes; B lymphocytes comprised 15% of inflammatory infiltrate; other cells, including NK, totalled 10%. Expression of HLA antigens paralleled inflammatory activity. Portal lymphadenoplasia was found more often in hepatitis C (54.5%) than in hepatitis B (30.6%). Expression of HBcAg was found more often in chronic B hepatitis of moderate or severe activity. Overall inflammatory activity in HBV-infected cases did not correlate with the intensity of HBsAg expression in hepatocytes. Inflammatory infiltrates accompanied the focal expression of HCV antigens. A direct correlation between antigen expression and inflammatory reaction in situ was noted more often in hepatitis C than B.
CONCLUSION: Irrespective of the etiology and activity of hepatitis, components of the inflammatory infiltrate in liver were similar. Overall inflammatory activity did not correlate with the expression of HBsAg and HCVAg; HBcAg expression, however, accompanied chronic hepatitis B of moderate and severe activity.
Keywords: Chronic B and C hepatitis, Inflammatory infiltrate, Lymphoid follicle, Lymphadenoplasia
INTRODUCTION
A cytopathic effect is absent in hepatocytes during viral replication, which is a characteristic feature of infection with hepatotropic viruses. Necrosis of hepatocytes is considered to be a result of cellular immunity reactions directed against viral antigens on the surface of these cells[1,2]. It is assumed that immunological mechanisms, insufficient for the full eradication of viruses, are responsible for liver damage and extrahepatic manifestations of infection[3]. Extrahepatic manifestations of hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, i.e. glomerulonephritis and vasculitis caused by the deposition of immune complexes, are connected with the humoral immunity directed at viral antigens[4–6].
Analysis of the etiopathogenic phenomena of chronic viral hepatitis takes into account two basic factors that influence the course and resolution of infection. The first, and of paramount importance, is a viral factor. It has been demonstrated that mutations in the HBV genome[7], superinfection or coinfection with hepatitis D virus (HDV)[8], infection with HCV 1b genotype, and appearance of HCV quasispecies worsen the disease prognosis[9]. However, the second factor of great importance is the host factor and virus to host interaction[10,11].
Inflammatory infiltrate present in a needle biopsy specimen is evidence of immunological processes in situ, as a reaction to viral protein expression. Host attempts to eliminate viruses lead to damage and subsequent necrosis of hepatocytes[12–14]. Damage to infected hepatocytes is a result of immune reactions aimed at eliminating infection. Therefore, necrosis of hepatocytes and mononuclear cell reaction are key features of such a reaction[15]. The intensity of the immune reaction in liver tissue depends both on the immunological status of the host and immunogenicity of HBV or HCV proteins expressed in the liver.
The aim of this study was to evaluate the type of immune reaction (by immunophenotyping the inflammatory infiltrate components) and the relationship between viral antigen expression and inflammation. The pattern of immunological response may bring the insight into etiopathogenesis of chronic viral hepatitis and mechanisms of immune reactions in situ.
MATERIALS AND METHODS
Liver biopsy taken from 217 patients of Hepatology Clinic, Institute of Infectious and Parasitic Diseases, chronically infected with HBV or HCV were evaluated histopathologically. All patients were diagnosed on the basis of plasma presence of HBsAg, anti-HBc, HBeAg, anti-HBe and anti-HCV by the immunoenzymatic method (Abbott, Chicago, USA) and presence of HBV DNA, HCV RNA by PCR and RT-PCR, respectively. Out of 217, there were 137 patients with chronic hepatitis C (mean age 41; range 9-73 years), 72 patients with chronic hepatitis B (mean age 29.7; range 7-79 years) and 8 patients with mixed etiology of hepatitis: HBV/HCV or HBV/hepatitis D viroid (HDV) (mean age 33.8; 21-44 years). Out of 217 liver biopsies, we chose at random 20 biopsies of chronic type B hepatitis and 20 biopsies of chronic type C hepatitis. The main criterion was the amount of frozen tissue and tissue in paraffin block.
Examination of needle liver biopsy specimens
Specimens taken by a blind biopsy with 1.6 mm needle were received fresh on gauze rinsed with PBS. Tissue material > 15 mm in length was divided into 3 pieces with a sterile surgical blade. One fragment, ca. 5 mm in length, was frozen at -80°C in petroleum ether cooled with acetone and dry ice. The frozen tissue was stored at -40°C for further use. The second fragment, 2 mm long, was frozen and stored at -65°C until homogenization, extraction of nucleic acids and testing by PCR or RT-PCR. The third fragment, at least 15 mm long, was fixed in 4% buffered formalin and routinely processed in paraffin. Serial slides 4 microns thick were stained with H&E, impregnated with silver by the Gomori method for reticulin fibres and stained by chromotrope 2R and aniline blue for collagen fibres.
Liver disease was diagnosed according to generally accepted criteria[16,17]. Examinations of inflammatory activity and the stage of fibrosis of chronic hepatitis were performed according to criteria proposed by international experts[18]. All histological features were finally scored using Histological Activity Index (HAI) using eighteen points scale to assess the grade of the disease (inflammatory activity in the lobules and portal tracts, piecemeal necrosis and bridging necrosis) as: minimal: 1-3 points; mild: 4-8 points; moderate: 9-12 points; severe: 13-18 points. The stage of the disease was assessed using five points scale as: 0-no fibrosis, normal connective tissue; 1-portal fibrosis, fibrous portal expansion; 2-periportal fibrosis, periportal or rare portal-portal septa; 3-septal fibrosis, fibrous septa with architectural distortion; 4-cirrhosis.
Analysis of the cellular composition of inflammatory infiltrate in liver tissue was performed on the frozen sections. A total of 40 tissue samples, five specimens each of minimal, mild, moderate and severe hepatitis B and C, were examined. The number of samples studied by immunohistochemisty was determined by the size of frozen material available for extensive evaluation. Five liver specimens diagnosed by histopathology as normal (liver biopsy performed for other reasons than hepatitis, e.g. Gilbert’s syndrome), served as controls. The evaluation was performed not by calculating cells, but by approximation of proportion.
The phenotype of inflammatory cells was evaluated by the EnVision system (anti-mouse globulins, DAKO, DakoCytomation, 2600 Glostrup, Denmark) using monoclonal antibodies listed below: (1) Anti CD45RO (UCHL-1 clone)-activated T cells, memory cells of both CD4 and CD8 subpopulations; (2) Anti-CD45RO (OPD-4 clone)-CD4 memory cells; (3) Anti-CD8-cytotoxic CD8 lymphocytes; (4) Anti-CD45 RA (clone 4KB5)-most B lymphocytes and a small subpopulation of T naive lymphocytes; (5) Anti-CD20 (L26 clone)-B lymphocytes; (6) Anti-CD35 (follicular dendritic cells); (7) Anti-CD56 (part of NK cells); (8) Anti-CD68 (macrophages); (9) Anti-HLA (class I); anti-HLA DR (class II α-chain); anti-HLA DP, DQ, DR (class II β-chain).
Serial sections of frozen liver biopsy fragments were dried at 22°C and fixed in acetone, 5 min [for HBV and HDV, cluster of differentiation (CD) antigens, HLA classI& II molecules and adhesion molecules]; or in acetone, 5 min, followed by chloroform, 5 min (for HCV antigens).
The expression patterns of HBV, HCV and HDV antigens were investigated in order to prove the viral etiology of hepatitis. HBV antigens were detected in frozen sections by the indirect immunoperoxidase method with the use of monoclonal anti-HBs antibodies (Dako) and rabbit polyclonal anti-HBc antibodies (immunization with HBcAg coding HBV fragment synthesized in Escherichia coli). HCV antigens were detected with the use of human FITC-labeled polyclonal anti-HCV antibodies[19,20], followed by monoclonal anti-fluorescein antibodies and finally by anti-mouse globulin antibodies labeled with peroxidase (EnVision system, Dako). In cases in which HCVAg was not detected in the first attempt of staining, serial sections were examined. The immunomorphological search for viral antigens expression was performed on frozen, and additionally, if technically possible, on paraffin sections.
The specificity of the methods applied in this study was ascertained by negative staining results when a primary antibody was omitted, or replaced by animal or human sera that did not contain antibodies against antigens of the viruses examined.
RESULTS
Etiology
Chronic hepatitis C was confirmed histologically in 137 cases and chronic hepatitis B in 72 cases. There were also 8 patients with mixed etiology of HBV/HCV and HBV/HDV hepatitis.
The phenotype of cells in the inflammatory infiltrate
Cellular composition of the inflammatory infiltrate was the same both in patients with hepatitis B and hepatitis C (Figure 1). The same proportion was also found in the normal liver (a few mononuclear cells were scattered in sinusoids and single mononuclear cells in the portal tracts).
Figure 1.
Approximate composition (%) of inflammatory infiltrate in chronic hepatitis B and C.
Lymphocytes of helper-inducer phenotype (CD4+CD45RO+) made up 50%-60% of cells. CD4 lymphocytes were localized within lobules, as well as in portal tracts. They were the main component of the inflammatory infiltrate in areas of spotty necrosis within lobules, and in the foci of piecemeal necrosis. Lymphocytes of the CD8 phenotype constituted 20%-25% of the inflammatory infiltrate. In the normal liver they were present mainly in the sinusoids and as single cells in portal tracts. They made up a significant, but not the only, element of spotty and piecemeal necrosis. In some areas they constituted almost 100% of cells. In the portal tracts they were localized mainly in the peripheral part of this structure.
Altogether, CD45RO+ cells, i.e. activated T memory cells, constituted 75%-80% of the inflammatory infiltrate. In chronic hepatitis B or C of any activity, CD45RO+ T lymphocytes were found around foci of spotty necrosis within lobules, in portal tracts, and in sinusoids.
Taking into account the number of activated T lymphocytes (CD45RO+) (almost 80% of all the inflammatory cells) and percentage of T helper and T suppressor lymphocytes (~50% and ~25%, respectively), it may be presumed that about 5%-10% of T lymphocytes express both CD4 and CD8 phenotypes.
About 20% of the infiltrating cells consisted of CD45RA+ phenotype cells: B lymphocytes and a small subpopulation of virgin T lymphocytes. The number of CD45RA+ lymphocytes rose together with the increasing activity of hepatitis. CD20+ B lymphocytes constituted 5%-10% of the inflammatory infiltrate. In normal liver, single B cells were present only in the sinusoids. The number of B lymphocytes increased parallel to inflammatory activity, but the proportion of the cellular composition of inflammatory infiltrate remained constant.
The relative composition of CD68+ macrophages and CD56+ NK cells in the infiltrate was also evaluated. In normal liver, macrophages were found mainly as single cells in sinusoids and in the portal tracts. The number of macrophages increased in sinusoids and portal tracts in chronic hepatitis B and C. Single CD68+ cells were found in lymphoid follicles (mainly secondary) and in germinal centers. Cells of the CD56+ phenotype made up only a small percentage (1%-2%) of the inflammatory infiltrate.
HLA classIantigens were detected in the form of tiny, granular deposits on sinusoidal cells of normal liver. They were present as tiny foci of a weak membranous pattern. In chronic hepatitis B and C the sinusoidal pattern of HLA classImolecule expression was also observed, irrespective of inflammatory activity. This was accompanied by the expression of these molecules on inflammatory and sinusoidal cells. The intensity of HLA classIexpression in sinusoids was much stronger in comparison with the normal liver, not only because of their sinusoidal expression, but also because of their presence on inflammatory cells.
Expression of HLA class II molecules: DR α-chain and DP, DQ, DR β-chain in normal liver was detected on sinusoidal cells and in the form of granular deposits of a sinusoidal pattern. Single mononuclear cells in sinusoids and portal tracts also showed α- and β-chains of HLA class II expression. HLA class II molecule expression in chronic hepatitis B and C was detected as a sinusoidal pattern, and on most inflammatory cells in the portal tracts, in foci of piecemeal and spotty necrosis and in areas of bridging necrosis. Cells of lymphoid follicles in portal tracts showed a strong expression of these molecules. In most cases, HLA class II molecule expression on the surface of a hepatocyte was not observed.
The formation of lymphoid follicles in chronic B and C hepatitis
Lymphoid follicles in portal tracts were present in both hepatitis B and C.
The dense clusters of mononuclear cells (follicles) were considered as primary (without germinal centers) or secondary (with germinal centers). Secondary follicles were quite rare-they were seen in 3 cases of chronic B and in 5 cases of chronic C hepatitis. Liver lymphadenoplasia was seen more often in hepatitis C: 75 patients (54.7%) than in hepatitis B: 22 patients (30.6%); additionally, it was observed in 1 case of hepatitis B + D.
The occurrence of lymphoid follicles in chronic hepatitis B and C correlated with inflammatory activity. The phenotype of cells composing lymphoid follicles was also analyzed. Primary follicles were formed by B lymphocytes (CD20+), T lymphocytes (CD4CD45RA+ and CD4CD45RO+) of both naive and memory type, and CD8+ T lymphocytes. Macrophages (CD68) and NK cells (CD56) were scarce.
Secondary follicles contained germinal centers with B (CD20+) and with T (CD4CD45RO+) lymphocytes. Lymphoid follicles cells showed expression of histocompatibility antigens (HLA-II and HLA-I). We found also expression of HCV antigens inside germinal centers in the portal tract (Figure 2A). Outside follicles, there was a mixture of mononuclear cells, including T cells (also CD8+), macrophages, NK cells and scarce plasma cells.
Figure 2.
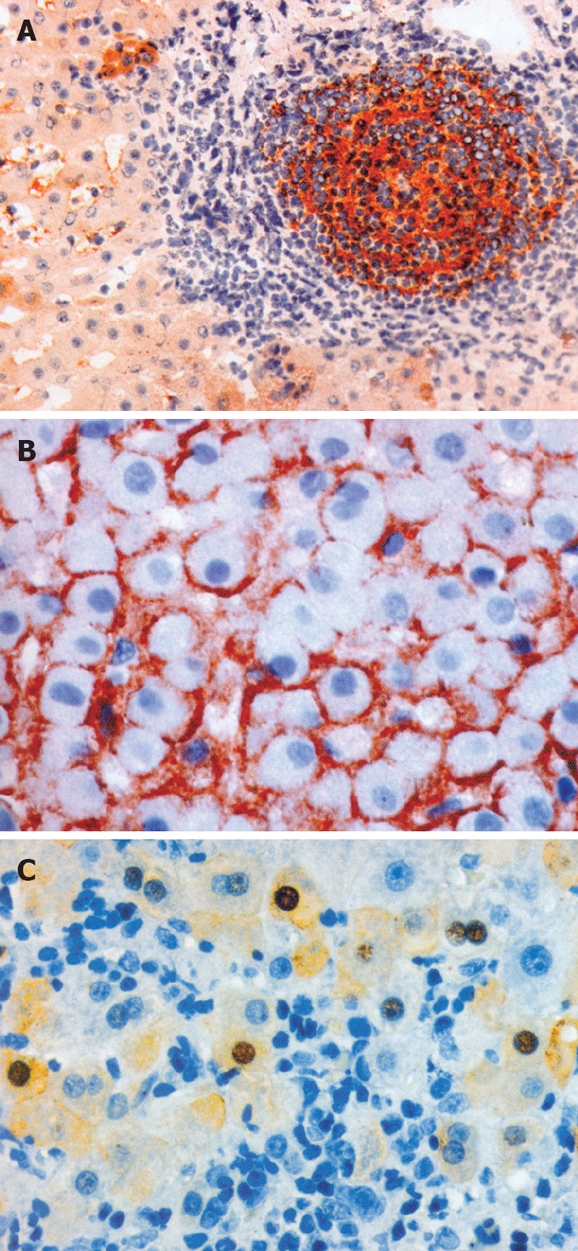
A: Expression of HCV antigens in the lymph follicle in the portal tract and in the peripheral hepatocyte (EnVision, × 200); B: Honeycomb pattern of HBsAg expression. No inflammatory reaction (EnVision, × 400); C: Inflammatory infiltrates around hepatocytes containing HBcAg: nuclear and cytoplasmatic expression of HBcAg (EnVision, × 400).
The correlation of inflammatory infiltrate with HBV viral antigens expression in chronic B hepatitis
HBs antigen in liver tissue presented as cytoplasmic droplets, ‘festones’, cytoplasmic embeddings and as a honeycomb pattern. Although HBsAg expression usually did not correlate with the presence of inflammatory infiltrate (Figure 2B), in some cases an inflammatory response was present in the vicinity of hepatocytes with HBsAg expression. Intense and diffuse HBsAg expression was not accompanied by any inflammation, but the inflammatory reaction was related to focal and weak HBsAg expression. No strong correlation was noted between the pattern of HBsAg expression (droplets, festones, honeycomb pattern) and inflammatory activity. A negative correlation, however, was found between the intensity of HBsAg expression localized on membranes of hepatocytes (honeycomb pattern) and the activity of the inflammatory process.
In 43 cases (59.7%), HBcAg was detected in nuclei and/or cytoplasm of hepatocytes as well as on cellular membranes (Figure 2C). Table 1 presents expression of viral antigens in liver. HBcAg presence in relation to overall inflammatory activity was found in 2 patients (4.7%) with chronic hepatitis of minimal activity, 24 (55.8%) with hepatitis of mild activity, 12 patients (28%) with moderate activity, and in 5 patients (11.6%) with severe activity.
Table 1.
Expression of viral antigens in the liver (total number of patients 217)
| Patients | HBsAg | HBcAg | HCVAg | HDVAg |
| Patients with chronic hepatitis B (n = 72) | 69 (95.83%) | 43 (59.72%) | - | - |
| Patients with chronic hepatitis C (n = 137) | - | - | 67 (48.9%) | - |
| Patients with chronic hepatitis of mixed etiology (n = 8) | 8 (100%) | 4 (50%) | 4 (50%) | 1 (12.5%) |
In 29 patients (40.3%) HBcAg was not detected. Among those 29 patients, in 15 (51.7%) there was minimal inflammatory activity, in 12 (41.4%) mild activity, while 1 case (3.5%) each of moderate and severe activity was found. To summarize, HBcAg was found predominantly in patients with chronic hepatitis of moderate and severe activity; only in 2 out of 19 such cases (10.5%) expression of HBcAg was not found. However, localization of HBcAg in the nuclei or cytoplasm of hepatocytes did not correlate directly with the inflammatory infiltrate at those sites. There were foci where inflammatory cells surrounded hepatocytes containing HBcAg, but there was no direct relation between the pattern of HBcAg expression and the presence or lack of inflammatory infiltrate. In addition, no correlation was found between the pattern of HBV antigen expression and the type of hepatocyte necrosis.
The correlation of inflammatory infiltrate with HCV viral antigens expression in chronic C hepatitis
HCV antigen (HCVAg) was detected in 67/137 patients (49%), and exclusively in the cytoplasm of hepatocytes as granules or amorphous deposits. There was a correlation between HCV antigen expression and inflammatory infiltrates within lobules and in areas of piecemeal necrosis. Furthermore, a positive correlation between the number of hepatocytes containing HCVAg deposits and inflammatory activity was found. A particularly strong expression of HCVAg was detected in hepatocellular carcinoma cells in a patient with liver cirrhosis.
A comparison was made between the inflammatory activity of chronic C hepatitis with or without HCVAg expression in the liver. It was observed that overall inflammatory activity did not correlate with the presence of HCVAg, partly because of the fact that detection of this antigen was dependent on the amount of sections investigated.
DISCUSSION
The analysis of inflammatory infiltrate phenotype in this study showed that the proportions of mononuclear cell composition were similar, irrespective of the etiology and activity of chronic hepatitis. The most numerous were CD45RO+ lymphocytes-they made up 75% of all inflammatory cells. B lymphocytes constituted 15% of inflammatory infiltrate and other cells (including NK cells) 10%. The CD4/CD8 ratio was > 1.5.
The results presented in this study are in accordance with those obtained by Volpes et al in 17 biopsy specimens from chronic hepatitis B and chronic hepatitis C and 5 specimens from acute hepatitis B and acute hepatitis C[21]. In all these cases, CD45RO+ lymphocytes dominated in inflammatory infiltrate. Analysis of the relative composition of inflammatory infiltrate of chronic hepatitis C in children by Wozniakowska-Gesicka established that CD45RO+ comprised 66%-75% and CD8+ lymphocytes up to 33% of inflammatory cells. B lymphocytes (CD20+) made up 10%-33% of the infiltrate. The results of the present study correspond with those obtained as Woźniakowska-Gesicka et al reported[22].
It is assumed that CD4+ T lymphocytes recognize antigens that are presented by antigen-presenting cells, APC (macrophages, dendritic cells, B lymphocytes) with HLA class II molecules and thus take part in the local inflammatory reaction[23]. The majority of liver infiltrating cells were of the CD45RO+CD4+ phenotype. Furthermore, the proportion of T helper to T suppressor cells in liver tissue was ~2.0. This reflects the pathophysiological conditions of the inflammatory process in the liver. Considering the results of this study, a conclusion can be drawn that proportions of cells in normal and in hepatitis liver are fairly stable. Lukomska et al showed that cytotoxic lymphocytes in liver sinusoids derive from peripheral blood, and their number increases during inflammation due to in situ divisions[24]. This may suggest that the increase of other cellular elements during inflammation may reflect the same kind of divisions at inflammatory sites. CD4 effector cells coordinate the immune response by recognition of foreign antigens, but also by secretion of cytokines which act on other cells[25].
The recognition of foreign antigens by effector cells is crucial for any attempts to eliminate viruses. We have compared the intensity of expression and localization of HLA classIand II molecules with different degrees of activity of hepatitis. HLA classImolecules appeared on the surface of hepatocytes both in the normal liver and in chronic hepatitis. The sinusoidal pattern and very delicate focal membraneous expression on the surface of a few hepatocytes were seen in the normal liver. A more intensive expression was observed in cases of hepatitis. The simultaneous expression of viral antigens and HLA molecules on the surface of hepatocytes may certainly indicate the presence of viral antigens, but such expression was not always accompanied by inflammatory infiltrate. This may reflect the status of local tolerance to these antigens. Different intensities of HLA molecules were observed in serial staining of specimens, while the difference in specimen thickness did not exceed 0.5 μm. The other technical parameters, such as incubation time with antibodies or reaction time, did not differ by more than a few seconds. Altogether, localization of the molecule expression was repeatable, and this allowed us to draw conclusions.
A strong association was found between HLA classIexpression and HBsAg presence on the surface of hepatocytes in the foci of piecemeal necrosis, with accompanying CD8+ lymphocyte infiltrate. Senaldi et al studied isolated hepatocytes from the livers of children with suspected hepatitis and found HLA classIexpression on the surface of 85%-100% hepatocytes[26]. Later, Fiore et al showed HLA classIexpression on the hepatocytes of all chronic hepatitis C (CHC) cases studied, but found no association between the intensity of expression of these molecules and inflammatory activity[27]. Van den Oord et al proved that the pattern of expression of HLA classImolecules was independent of inflammatory activity, but in areas of spotty necrosis the expression had a focal membranous pattern[28]. The results of our study fit the data obtained by the last two authors. The expression patterns of HBV and HCV antigens and the activity of hepatitis showed only a partial association in the current study.
In this study, a granular pattern of HLA class II molecule expression on the surface of sinusoidal cells (macrophages, endothelial cells and most inflammatory cells) was found. HLA class II molecules were also found on cells comprising lymphoid aggregates in portal tracts and, less frequently, on the surface of follicular dendritic cell extensions. In a few cases, small groups of hepatocytes with the membranous type of HLA class II molecule expression were found. In most cases in this study, no expression of HLA-DR on hepatocytes was found, a finding that does not support the presumed role of hepatocytes as antigen presenting cells in the inflammatory process[28–30]. Using double immunohistochemical staining, Liang et al similarly did not find any association between HBcAg and HLA class II molecule expression[31].
Infection with HBV and HCV caused inflammation in liver tissue and formation of lymphoid follicles in the liver. Lymphoid follicles were found more often in chronic hepatitis C (~55%) than in chronic hepatitis B (~31%).
Immunomorphological analysis of lymphoid follicles in chronic hepatitis B and C supports the hypothesis that they arise in portal tracts as a result of chronic local antigen stimulation. Taking into account the fact that lymphadenoplasia is considered as a humoral T-dependent B-cell reaction in chronic local antigenic stimulation, we may conclude that in many cases of chronic hepatitis a local humoral reaction takes place. This may influence the course of disease.
The degree of inflammatory activity did not correlate with the intensity of HBsAg expression in hepatocytes in this study. However, an inverse relationship between the degree of diffuse membranous HBsAg expression and inflammatory local response in chronic hepatitis B (CHB) of minimal and mild activity was established. Nuclear and/or cytoplasmic HBcAg expression in hepatocytes was frequently found in CHB of moderate and severe activity. There was no positive association between the degree of inflammation and the expression of HCV antigens in CHC. However, in comparison to CHB, focal expression of HCV antigens was frequently accompanied by inflammatory infiltrate. The lack of a close relationship between inflammatory cellular reaction and viral antigen expression may suggest that viral antigens are not the only components that take part in the inflammatory reactions in situ.
The mechanism of focal tolerance to viral antigens in the liver observed in this study-lack of cellular reaction in spite of intense and diffuse viral antigen expression-is unclear. A small number of CD56(+) NK cells may indicate the impairment of self vs non-self recognition, and may influence immunological response and elimination of the virus. In chronic inflammatory reaction, secondary immune response is more specific and therefore more efficient than natural immunity. The presence of lymphoid follicles in some portal tract reflects T-cell dependent B-cell reaction found in e.g. autoimmune inflammatory diseases, self perpetuating processes. In chronic viral hepatitis we observe both cellular and humoral reactions in situ. Detailed analysis of cellular reaction in the liver and of the topographic relations of inflammatory cells and viral antigen expression could contribute significantly towards a better understanding of the immunopathogenesis of chronic hepatitis.
COMMENTS
Background
Infection with hepatotropic viruses such as hepatitis C virus (HCV) and hepatitis B virus (HBV) can result in chronic infection, which leads to liver cirrhosis and hepatocellular carcinoma. Immunological mechanisms against viral infections are responsible for damage and necrosis of hepatocyte. Inflammatory infiltrate present in a needle biopsy specimen is evidence of immunological processes in situ, as a reaction to viral protein expression. The aim of this study was to evaluate the relationship between viral antigen expression and inflammation. The pattern of immunological response may bring the insight into etiopathogenesis of chronic viral hepatitis and mechanisms of immune reactions in situ.
Research frontiers
The aim of this study was to determine the composition of liver inflammatory infiltrate in biopsy material from patients chronically infected with hepatotropic viruses and to evaluate the correlation of inflammatory infiltrate with HBV and HCV viral antigen expression in specimens of minimal, mild, moderate and severe chronic B and C hepatitis. Irrespective of the etiology and activity of hepatitis, components of the inflammatory infiltrate in liver were the same.
Innovations and breakthroughs
The correlation between HBV antigens and HCVAg expression in liver and inflammatory infiltrate was investigated. Overall inflammatory activity did not correlate with the expression of HBsAg and HCVAg; however, HBcAg expression accompanied chronic hepatitis B of moderate and severe activity. The study was undertaken on a large amount of clinicopathological material consisting of 217 biopsy specimens (137 from hepatitis C, 72 from hepatitis B and 8 from mixed etiology). The innovative parts of this study were findings of HLA class I, HLA class II (connected with the antigen presentation), CD4 and CD8, and B lymphocytes in the vicinity of infected hepathocytes. T lymphocytes CD4 and CD8 were most often of CD 45 RO+, phenotype of the memory type.
Application
The study offers precise immunohistochemical/ histopathological diagnosis of liver biopsy. It can bring along many mixed infections, detected not so often before. This offers the clinician the unique opportunity to compare the results of treatment with the previous picture.
Peer review
This study described the composition of liver inflammatory infiltrate in biopsy materials and the correlation of inflammatory infiltrate with HBV and HCV viral antigen expression. The use of many liver biopsy samples is a strength point of the manuscript. The authors concluded that the components of inflammatory infiltrates in the liver were similar irrespective of the etiology and activity of hepatitis. This observation seems interesting for understanding the immunopathogenesis of viral hepatitis.
Acknowledgments
The authors gratefully acknowledge the expert technical assistance of Ms. Agata Kozera, Ms. Barbara Bartocha, Ms. Wanda Szymanek-Stos and Ms. Helena Rachubik.
Supported by Grants PCZ 009/19 and PBZ-KBN 119/P05/2005 from the Committee for Scientific Research, Poland
Peer reviewers: Dirk Hartwig, PhD, Department of Central Laboratory, Universitytsklinikum Schleswig-Holstein Campus Lübeck, Ratzeburger Allee 160, Lübeck D-23538, Germany; Michael A Zimmerman, PhD, Division of Transplant Surgery, University of Colorado Health Sciences Center, 1635 N. Ursula St, PO Box 6510, Aurora 80045, Uruguay; Satoshi Yamagiwa, PhD, Division of Gastroenterology and Hepatology, Niigata University Graduate School of Medical and Dental Sciences, 757 Asahimachi-dori 1, Chuo-ku, Niigata 951-8510, Japan
S- Editor Li DL L- Editor Li M E- Editor Ma WH
References
- 1.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Agrati C, D’Offizi G, Narciso P, Abrignani S, Ippolito G, Colizzi V, Poccia F. Vdelta1 T lymphocytes expressing a Th1 phenotype are the major gammadelta T cell subset infiltrating the liver of HCV-infected persons. Mol Med. 2001;7:11–19. [PMC free article] [PubMed] [Google Scholar]
- 3.Cerny A, Chisari FV. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology. 1999;30:595–601. doi: 10.1002/hep.510300312. [DOI] [PubMed] [Google Scholar]
- 4.Galossi A, Guarisco R, Bellis L, Puoti C. Extrahepatic manifestations of chronic HCV infection. J Gastrointestin Liver Dis. 2007;16:65–73. [PubMed] [Google Scholar]
- 5.Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB. Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach. Dig Liver Dis. 2007;39:2–17. doi: 10.1016/j.dld.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Naicker S, Fabian J, Naidoo S, Wadee S, Paget G, Goetsch S. Infection and glomerulonephritis. Semin Immunopathol. 2007;29:397–414. doi: 10.1007/s00281-007-0088-x. [DOI] [PubMed] [Google Scholar]
- 7.Glebe D, Urban S. Viral and cellular determinants involved in hepadnaviral entry. World J Gastroenterol. 2007;13:22–38. doi: 10.3748/wjg.v13.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh TH, Liu CJ, Chen DS, Chen PJ. Natural course and treatment of hepatitis D virus infection. J Formos Med Assoc. 2006;105:869–881. doi: 10.1016/S0929-6646(09)60172-8. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez-Hernandez N, Torres-Puente M, Bracho MA, Garcia-Robles I, Ortega E, del Olmo J, Carnicer F, Gonzalez-Candelas F, Moya A. Epidemic dynamics of two coexisting hepatitis C virus subtypes. J Gen Virol. 2007;88:123–133. doi: 10.1099/vir.0.82277-0. [DOI] [PubMed] [Google Scholar]
- 10.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 11.Pol S, Mallet VO. Improving anti-hepatitis C virus therapy. Expert Opin Biol Ther. 2006;6:923–933. doi: 10.1517/14712598.6.9.923. [DOI] [PubMed] [Google Scholar]
- 12.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 15.Chu CM, Liaw YF. Intrahepatic distribution of hepatitis B surface and core antigens in chronic hepatitis B virus infection. Hepatocyte with cytoplasmic/membranous hepatitis B core antigen as a possible target for immune hepatocytolysis. Gastroenterology. 1987;92:220–225. doi: 10.1016/0016-5085(87)90863-8. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi L, Spichtin HP, Gudat F. Chronic hepatitis. In: MacSween RNM, Anthony PP, Scheuer PJ, editors. Pathology of the Liver. Edinburgh: Churchill Livingstone; 1987. pp. 310–343. [Google Scholar]
- 17.Scheuer PJ, Lefkowitch JH. Liver Biopsy Interpretation. Laboratory Techniques. London: W.B. Saunders Company Ltd; 1994. pp. 10–15. [Google Scholar]
- 18.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 19.Krawczynski K, Beach MJ, Bradley DW, Kuo G, di Bisceglie AM, Houghton M, Reyes GR, Kim JP, Choo QL, Alter MJ. Hepatitis C virus antigen in hepatocytes: immunomorphologic detection and identification. Gastroenterology. 1992;103:622–629. doi: 10.1016/0016-5085(92)90856-t. [DOI] [PubMed] [Google Scholar]
- 20.Ballardini G, Groff P, Giostra F, Francesconi R, Miniero R, Ghetti S, Zauli D, Bianchi FB. Hepatocellular codistribution of c100, c33, c22, and NS5 hepatitis C virus antigens detected by using immunopurified polyclonal spontaneous human antibodies. Hepatology. 1995;21:730–734. [PubMed] [Google Scholar]
- 21.Volpes R, van den Oord JJ, Desmet VJ. Memory T cells represent the predominant lymphocyte subset in acute and chronic liver inflammation. Hepatology. 1991;13:826–829. [PubMed] [Google Scholar]
- 22.Wozniakowska-Gesicka T, Wisniewska-Ligier M, Kaluzynski A, Turant M. Morphological and immunological features of liver inflammatory infiltrate in children with chronic hepatitis C. Pol J Pathol. 2002;53:117–122. [PubMed] [Google Scholar]
- 23.Jakobisiak M. Presentation of antigens to T lymphocytes (in Polish) In: Golab J, Jakóbisiak M, Lasek W, editors. Immunology (in Polish) Warsaw: PWN; 2002. pp. 157–175. [Google Scholar]
- 24.Lukomska B, Pienkowska B, Andrzejewski W, Olszewski WL. Liver sinusoidal cytotoxic cells are recruited from blood and divide locally. J Hepatol. 1991;12:332–335. doi: 10.1016/0168-8278(91)90836-z. [DOI] [PubMed] [Google Scholar]
- 25.Rehermann B, Chisari FV. Hepatitis B virus: molecular biology and immunopathology-experimental and clinical features. In: Wilson RA, editor. Viral hepatitis. Diagnosis, treatment, prevention. New York: Marcel Dekker, Inc; 1997. pp. 85–118. [Google Scholar]
- 26.Senaldi G, Lobo-Yeo A, Mowat AP, Mieli-Vergani G, Vergani D. Class I and class II major histocompatibility complex antigens on hepatocytes: importance of the method of detection and expression in histologically normal and diseased livers. J Clin Pathol. 1991;44:107–114. doi: 10.1136/jcp.44.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiore G, Angarano I, Caccetta L, Serrone M, Jirillo E, Schiraldi O, Antonaci S. In-situ immunophenotyping study of hepatic-infiltrating cytotoxic cells in chronic active hepatitis C. Eur J Gastroenterol Hepatol. 1997;9:491–496. doi: 10.1097/00042737-199705000-00015. [DOI] [PubMed] [Google Scholar]
- 28.van den Oord JJ, de Vos R, Desmet VJ. In situ distribution of major histocompatibility complex products and viral antigens in chronic hepatitis B virus infection: evidence that HBc-containing hepatocytes may express HLA-DR antigens. Hepatology. 1986;6:981–989. doi: 10.1002/hep.1840060529. [DOI] [PubMed] [Google Scholar]
- 29.Desmet VJ. Immunopathology of chronic viral hepatitis. Hepatogastroenterology. 1991;38:14–21. [PubMed] [Google Scholar]
- 30.Volpes R, van den Oord JJ, Desmet VJ. Can hepatocytes serve as ‘activated’ immunomodulating cells in the immune response? J Hepatol. 1992;16:228–240. doi: 10.1016/s0168-8278(05)80121-7. [DOI] [PubMed] [Google Scholar]
- 31.Liang YR. [HLA-DR expression on hepatic cells in hepatitis B] Zhonghua Bing Li Xue Za Zhi. 1992;21:212–214. [PubMed] [Google Scholar]