Abstract
Background
Inherited cardiac arrhythmia susceptibility contributes to sudden death during infancy and may contribute to perinatal and neonatal mortality, but the molecular basis of this risk and the relationship to genetic disorders presenting later in life is unclear. We studied the functional and pharmacological properties of a novel de novo cardiac sodium channel gene (SCN5A) mutation associated with an extremely severe perinatal presentation of long-QT syndrome in unrelated probands of different ethnicity.
Methods and Results
Two subjects exhibiting severe fetal and perinatal ventricular arrhythmias were screened for SCN5A mutations and the functional properties of a novel missense mutation (G1631D) were determined by whole-cell patch clamp recording. In vitro electrophysiological studies revealed a profound defect in sodium channel function characterized by ~10-fold slowing of inactivation, increased persistent current, slowing of recovery from inactivation, depolarized voltage dependence of activation and inactivation. Single channel recordings demonstrated increased frequency of late openings, prolonged mean open time and increased latency to first opening for the mutant. Subjects carrying this mutation responded clinically to the combination of mexiletine with propranolol and survived. Pharmacologically, the mutant exhibited 2-fold greater tonic and use-dependent mexiletine block than wildtype channels. The mutant also exhibited enhanced tonic (2.4-fold) and use-dependent block (~5-fold) by propranolol, and we observed additive effects of the two drugs on the mutant.
Conclusions
Our study demonstrates the molecular basis for a malignant perinatal presentation of long-QT syndrome, illustrates novel functional and pharmacological properties of SCN5A-G1631D which caused the disorder, and reveals therapeutic benefits of propranolol block of mutant sodium channels in this setting.
Keywords: sodium channel, SCN5A, arrhythmia, mexiletine, propranolol
INTRODUCTION
Sudden unexplained death due to cardiac arrhythmia may occur at any age. When death occurs during infancy for no apparent reason a diagnosis of the sudden infant death syndrome (SIDS) may be appropriate.1,2 Recent evidence suggests that 9–10% of SIDS victims carry germ line mutations in arrhythmia susceptibility genes such as those associated with the congenital long QT syndrome (LQTS).3 Anecdotally, ventricular arrhythmias occurring during the perinatal or neonatal periods are associated with a poor prognosis and a low survival rate.4–7 Whether cardiac arrhythmia susceptibility presenting in early life represents a biologically distinct disease is an unanswered question.
Mutations in SCN5A encoding the cardiac voltage-gated sodium channel NaV1.5 have been associated with a spectrum of increased sudden death risk extending from fetal life to adulthood. Recurrent third trimester fetal loss has been observed in the setting of occult SCN5A mutations.8 In older children and adults with LQTS of known genotype, only ~10% carry mutations in SCN5A,9–11 but the proportion of SCN5A mutations among SIDS victims with an LQTS gene defect approaches 50%.3 Further, among older children and adults with LQTS those individuals harboring SCN5A mutations exhibit a greater likelihood of severe symptoms including sudden death as compared to the majority of individuals who carry mutations in two potassium channel genes (KCNQ1, KCNH2).9,11 The higher proportion of SCN5A mutations among SIDS victims with known genotype as compared to older LQTS subjects might be explained by negative selection for more deleterious alleles. Support for this hypothesis requires evidence that mutations with greater functional consequences are responsible for more severe and earlier onset arrhythmia syndromes.
Here we present an extensive characterization of a novel SCN5A mutation that occurred de novo in unrelated and ethnically distinct newborns. In mutation carriers, life-threatening ventricular arrhythmias occurred within hours of birth. The mutation caused a profound degree of sodium channel dysfunction that was more severe than that observed for any previous SCN5A variant. Despite the extreme nature of the mutation and the associated dire clinical scenario, the subjects survived owing to prompt therapeutic interventions including treatment with the combination of mexiletine and propranolol, two drugs that exhibited enhanced and additive activity against the mutant allele. These observations illustrate the role of severe sodium channel mutations in a malignant perinatal variant of LQTS and successful use of combination pharmacotherapy to prevent perinatal mortality in this setting.
METHODS
Molecular Genetics
Informed consent for performing genetic studies was obtained using methods approved by the Ethics Review Board of IRCCS Fondazione Policlinico San Matteo (Pavia, Italy) or by the Institutional Research Board and Ethics Committee and the Committee on Genetic Analysis and Gene Therapy of the National Cardiovascular Center (Suita, Japan). Genomic DNA was isolated from whole blood and coding exons of SCN5A, KCNQ1, KCNH2, KCNE1 and KCNE2 were screened for genetic variants using previously described methods.12,13
Mutagenesis and Heterologous Expression of Na Channels
Mutations were engineered in a human heart sodium channel (NaV1.5) cDNA (hH1) using recombinant polymerase chain reaction. Final constructs were assembled in the mammalian expression plasmid pRc/CMV-hH1 and then sequenced to verify creation of the mutation and to exclude polymerase errors. Cells (tsA201) were transiently transfected with pRcCMV-hH1 or mutants using FuGene6 (Roche Diagnostics, Indianapolis, Inc.) combined with a bicistronic plasmid (pEGFP-IRES-hβ1) encoding enhanced green fluorescent protein and the human β1 subunit (hβ1) under the control of the CMV immediate early promoter. Additional methods are provided in an online supplement.
Statement of Responsibility
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Malignant perinatal arrhythmia associated with a novel SCN5A mutation
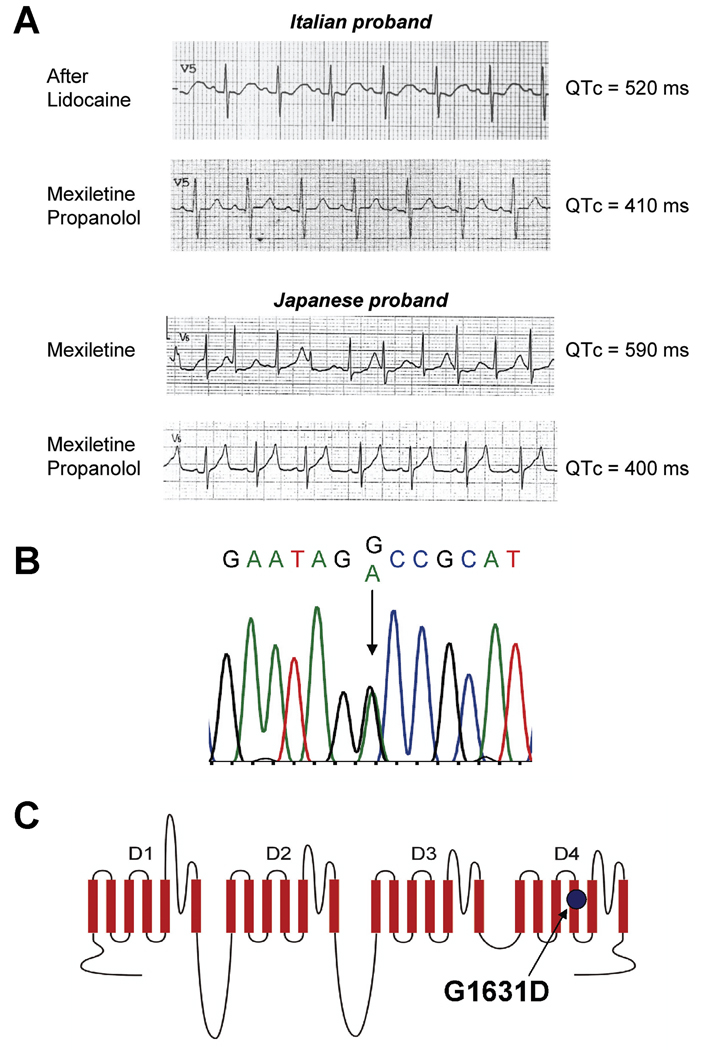
We identified a novel SCN5A mutation in two unrelated newborns that experienced life-threatening perinatal ventricular arrhythmias. The first subject was an Italian male delivered by emergency C-section at 32-weeks gestation for abnormal fetal heart rhythm. Initially, he appeared healthy (APGAR score 8) but then, within hours of his birth, developed polymorphic ventricular tachycardia with periods of bradycardia and frequent premature ventricular beats. Initial treatments with intravenous magnesium and isoproterenol were not effective, but administration of intravenous lidocaine suppressed ventricular arrhythmias and restored sinus rhythm revealing a prolonged QTc interval (520ms). Empiric treatment with propranolol (1.3 mg/kg/d) and mexiletine (11 mg/kg/d) controlled arrhythmias and normalized the QTc (410ms) (Fig. 1A). One month after discharge, the infant survived an episode of ventricular fibrillation. Ventricular arrhythmia was further controlled by rapid pacing (120 bpm) with increased dosages of propranolol (3 mg/kg/d) and mexiletine (16 mg/kg/d). During the following 12 months, the child exhibited no further ventricular arrhythmias but required recurrent hospitalizations for paroxysmal atrial flutter that was eventually controlled by ablation. The child has survived beyond age 26 months without further ventricular or atrial arrhythmias.
Figure 1.
Electrocardiographic responses to pharmacotherapy and genotype of probands. (A) Representative ECG traces (lead V5) showing responses to mexiletine and propranolol for the Italian and Japanese probands. Rate-corrected QT interval (QTc) measurements are indicated to the right of each tracing. (B) Sequencing electropherogram (Italian proband) illustrating heterozygosity for a G to A mutation corresponding to G1631D. (C) Location of G1631D in the predicted transmembrane topology of NaV1.5.
The second proband was a Japanese male delivered by emergency C-section at 34-weeks gestation because of ventricular arrhythmia (torsade de pointes, TdP) documented in utero by magnetocardiography. Initial APGAR scores were 8 and 9 but his QTc interval was 567ms and he had multiple episodes of TdP. Intravenous injection (3 mg/kg) followed by continuous infusion of mexiletine abolished TdP. He was discharged on oral mexiletine (20 mg/kg/day), but was re-admitted for treatment of recurrent TdP approximately 2 months later (QTc=590ms). Continuous infusion of mexiletine combined with oral mexiletine (serum drug concentration: 1.3–1.4µg/ml) considerably abbreviated the QTc (462–499ms), but did not completely suppress episodes of TdP. The addition of continuous infusion propranolol (0.5 mg/kg/day, serum drug concentration: 18.4–25.1 ng/ml) further shortened QTc (395–424ms) and fully suppressed ventricular arrhythmias. Finally, combination therapy with oral mexiletine and oral propranolol was effective in suppressing ventricular arrhythmias through age 8 months (Fig. 1A).
A novel SCN5A missense mutation (G1631D) was discovered in both probands (Fig. 1B). Family histories were negative for arrhythmia syndromes. Both sets of parents had normal ECGs and were mutation negative. Paternity testing demonstrated that the mutation was de novo in both cases. No other mutations were identified in SCN5A, KCNQ1, KCNH2, KCNE1 or KCNE2 in either proband.
Profound dysfunction of G1631D channels
The mutation results in substitution of a highly conserved glycine residue with a negatively charged glutamic acid in the S4 segment of domain 4 (D4/S4) (Fig. 1C). This residue is 100% conserved in all known voltage-gated sodium channel sequences from several diverse phyla. This structural domain in sodium channels participates as a component of the voltage-sensor important for activation and inactivation.14,15 Introduction of a negatively charged side group into this domain was predicted to have a significant functional effect. To test this hypothesis, we engineered G1631D in recombinant human NaV1.5 for heterologous expression and electrophysiological studies.
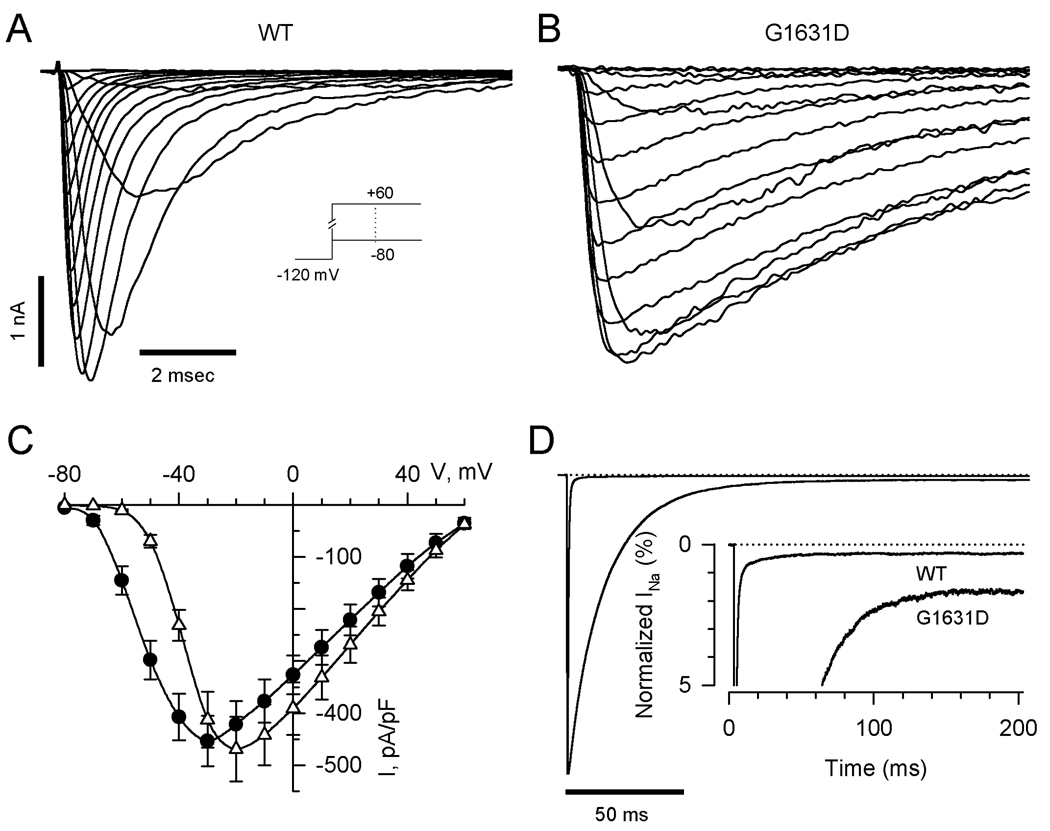
Figure 2 illustrates the general functional properties of wild-type (WT) and mutant NaV1.5 channels expressed heterologously in human tsA201 cells. Representative whole-cell current tracings demonstrate that the mutant exhibits a profound level of dysfunction characterized by substantial delays in activation and inactivation. Overall current density was similar between cells expressing WT or mutant channels but there was a positive shift in the peak current-voltage (I–V) relationship for the mutant (Fig. 2C). Mutant channels exhibited increased steady-state persistent current measured 200ms after the peak transient current (Fig. 2D; persistent current as % of peak current: WT, 0.31±0.04%, n=8; G1631D, 1.63±0.31%, n=9; P<0.001). Although increased persistent current is characteristic of SCN5A mutations associated with LQTS,16,17 no previously characterized mutation had such a profound inactivation defect.
Figure 2.
Whole-cell current recordings of WT and G1631D sodium channels. Representative sodium currents recorded from cells expressing WT (A) or G1631D (B) elicited by depolarizing steps from −80mV to +60mV in 10mV increments from holding potential −120mV. (C) Comparison of current-voltage relationships for WT (n=15) and G1631D (n=16). Current is normalized to cell capacitance to give sodium current density. (D) Increased TTX-sensitive persistent sodium currents for G1631D. Peak sodium currents were normalized. The zero-current level is indicated by a dotted line. The inset shows an expanded y-axis scaled to emphasize the relative proportion of persistent current for WT (n=8) and G1631D (n=9).
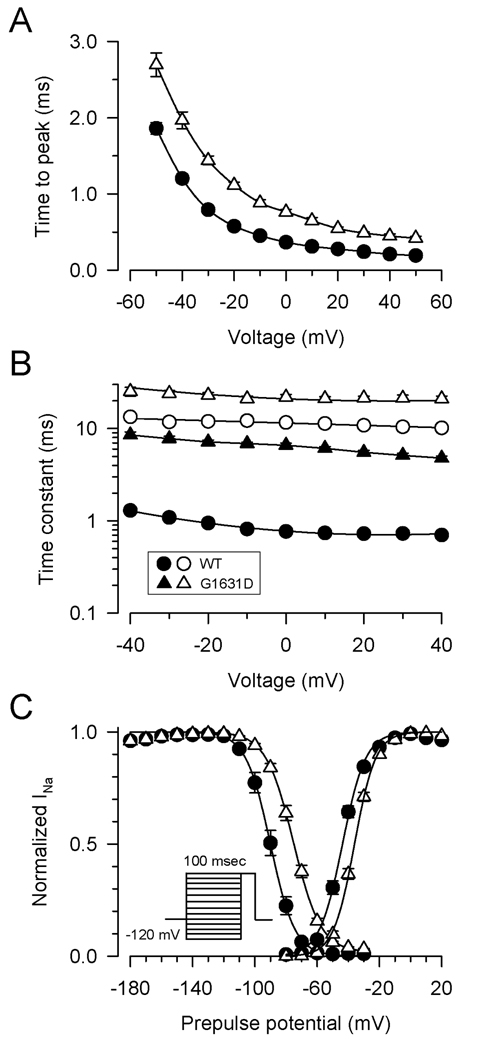
Figure 3 illustrates quantitative assessments of activation and inactivation. Mutant channels exhibited a global slowing of activation across the range of tested potentials as assessed by time to peak current (Fig. 3A). Similarly, G1631D exhibited a profound slowing of inactivation as illustrated by the voltage dependence of inactivation time constants (Fig. 3B). The degree of slowing of inactivation was approximately 10-fold compared to WT. The mutant also exhibited significant depolarizing shifts in the voltage dependence of activation (+12mV) and steady state inactivation (+14mV) (Fig. 3C,D; supplemental Table S1). These asymmetric depolarizing shifts in activation and steady state inactivation predict an increased window current defined as the overlap of these two curves (see Supplemental Fig. S1).
Figure 3.
Activation and inactivation of WT and mutant channels. (A) Time to peak activation in the voltage range of −60 to +50mV. Differences between WT (n=15) and G1631D (n=16) were significant at the P<0.001 level for all tested voltages. (B) Voltage dependence of inactivation time constants (same number of replicates as in A). Filled and open symbols indicate fast and slow component values, respectively. (C) Voltage dependence of activation and steady-state inactivation elicited by a 100ms conditioning pulse to various voltages (same number of replicates as in A).
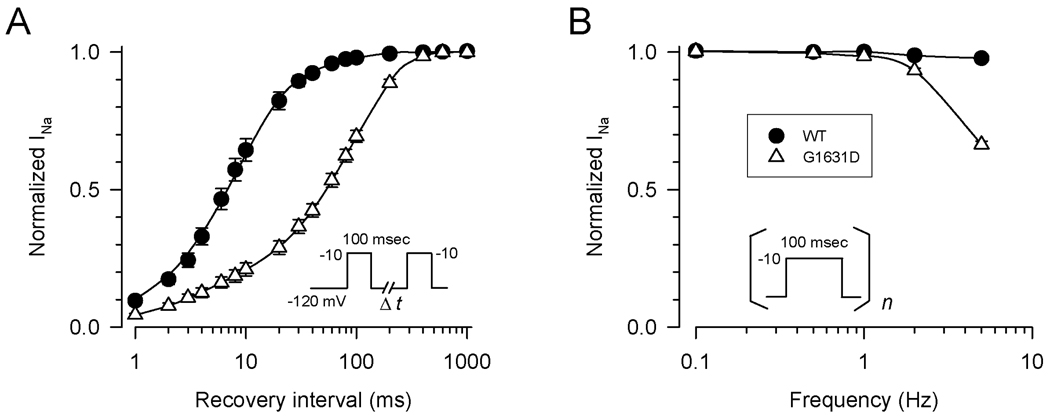
In Fig. 4A the time course of recovery from inactivation following a 100ms conditioning pulse illustrates that the mutant has profound slowing of recovery. This difference was explained by a larger slow component of recovery from inactivation as determined by double exponential fitting (see Supplemental Table S1). For WT channels, the majority of recovery occurs with a time constant of approximately 10ms. By contrast for G1631D channels, the predominant fraction of channels recover from inactivation with a time constant of approximately 100ms. The marked slowing of recovery from inactivation exhibited by G1631D correlated with a greater loss of channel availability during repetitive membrane depolarizations at frequencies exceeding 1Hz (Fig. 4B).
Figure 4.
Recovery from inactivation. (A) Time course of recovery from inactivation for WT (n=12) and G1631D (n=16) was elicited using the two-pulse protocol shown in the inset. Time constants and fractional amplitudes are given in Table S1. (B) Activity dependent loss of channel availability following trains of 100ms pulses to −10mV from a holding potential of −120mV applied at the frequency indicated (n=10–18 cells). Residual current following the 100th pulse was normalized to the first pulse current amplitude.
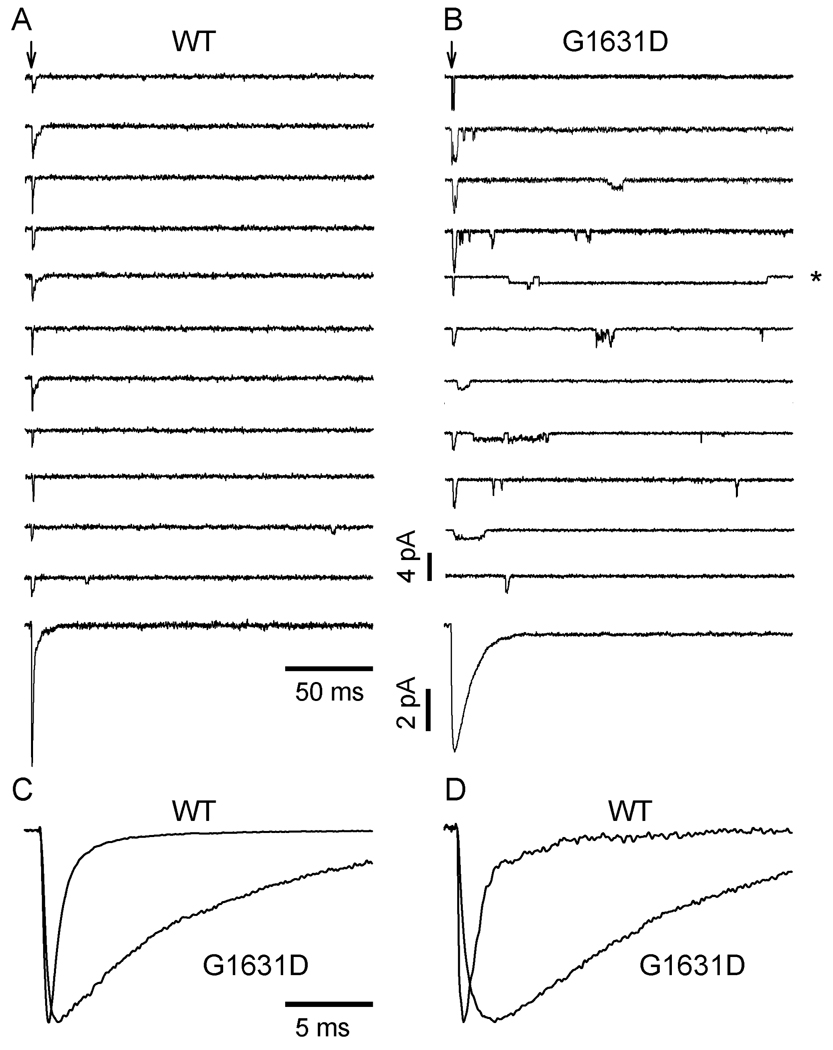
These profound gating abnormalities were correlated with aberrant single-channel events. Figure 5 illustrates representative single-channel recordings from cells expressing WT or mutant channels. Wild-type channels exhibited brief and transient openings clustered at the onset of the test depolarization. By contrast, the mutant exhibited a marked increase in probability of late reopenings and occasional prolonged openings (asterisk). Single channel conductance levels were similar for WT (24pS) and G1631D (25pS), but mutant channels exhibited significantly longer latency to first opening (WT: 0.59±0.03 ms; G1631D: 1.15±0.03 ms; n=3, P<0.001), increased mean open time (WT: 0.34±0.09 ms; G1631D: 0.98±0.03 ms; n=3, P=0.029), and increased NPo (WT: 0.14±0.02; G1631D: 0.22±0.03; n=3, P=0.042) when assessed at a test pulse of −20 mV. Ensemble averaged currents derived from single-channel records closely resemble those obtained from whole-cell recordings. These findings collectively indicate that G1631D causes a fundamental defect in channel activation and inactivation associated with dramatic clinical consequences.
Figure 5.
Single-channel properties of WT and G1631D channels. Sodium channel activities recorded at −20mV from a multichannel outside-out patch excised from a cell expressing WT (A) or G1631D (B). Vertical arrows indicate the onset of patch depolarization from −120mV to −20mV. Lower traces show the ensemble averaged current obtained from 100 consecutive traces for WT and G1631D, respectively. (C) and (D) are comparisons of normalized and superimposed current traces of WT and G1631D at test potential of −20mV from whole-cell recordings (C) and single-channel recordings (D).
Enhanced mexiletine sensitivity of G1631D channels
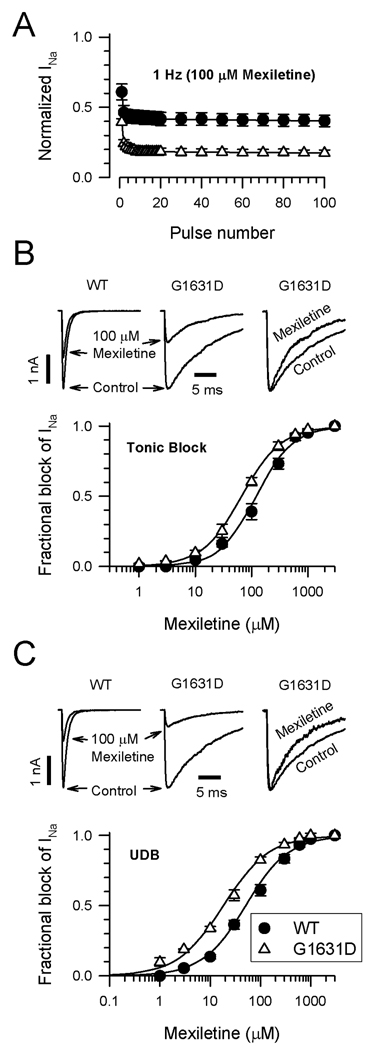
Despite the profound nature of the sodium channel dysfunction caused by G1631D, both probands survived likely because of prompt intervention including pharmacological treatments. We compared the effect of mexiletine on WT and mutant channels. Figure 6A illustrates the responses of WT and G1631D to repetitive membrane depolarizations delivered at a frequency of 1Hz in the presence of mexiletine (100µM). Both channels exhibited an initial drop in channel availability followed by further use-dependent loss of activity, but the effect is substantially greater for G1631D suggesting that the mutant has enhanced mexiletine sensitivity. Concentration-response relationships for tonic (Fig. 6B) and use-dependent (Fig. 6C) mexiletine block of WT and G1631D supported this hypothesis. Mexiletine block of WT channels exhibited EC50 values of 120.9µM and 50.9µM for tonic and use-dependent block, respectively. By contrast, G1631D was 1.8-fold and 2.8-fold more sensitive to tonic (EC50 66.7µM) and use-dependent (EC50 18.3µM) mexiletine block, respectively. Further, mexiletine induced a hyperpolarizing shift in steady-state inactivation of mutant channels such that this property became more similar to WT channels (G1631D V½: no drug, −74.8±1.1 mV, n=16; 3µM mexiletine, −85.5±1.3mV, n=9, P<0.001). By contrast, the same drug concentration has no significant effect on steady-state inactivation of WT channels (WT V½: no drug, −89.3±1.1mV, n=16; 3µM mexiletine, −86.6±3.2mV, n=6, NS). Mexiletine also had moderate effects on the kinetics of G1631D inactivation (Fig. 6B,C) illustrated by significant reductions in the time constants for inactivation, and significantly reduced the level of persistent current (no drug: 1.63±0.31%, n=9; 10µM mexiletine, 0.54±0.06%, n=8; P=0.0098).
Figure 6.
Effects of mexiletine on WT and G1631D. (A) Mexiletine (100 µM) block of WT (n=8) and G1631D (n=8) during a 1Hz train of depolarizing pulses to −10mV from a holding potential of −120mV. (B) Tonic mexiletine block of WT and G1631D. Upper traces (left, middle) illustrate the effects of 100µM mexiletine during a single depolarizing voltage step to −10mV. Normalized traces (right) recorded in the absence (control) or presence of drug illustrate the effect of mexiletine on the inactivation time course. The plot illustrates the concentration-response relationships for tonic block by mexiletine (each data point represents the mean of 4–12 cells). (C) Use-dependent mexiletine block of WT and G1631D. Upper traces (left, middle) illustrate the steady state effects of 100µM mexiletine during a 1Hz pulse train. Normalized traces (right) recorded in the absence (control) or presence of drug (100th pulse) illustrate the effect of mexiletine on the inactivation time course. Time constants in the absence of drug were: τ1=7.7±0.7ms, τ2=19.7±0.6ms, n=8; and in the presence of 100µM mexiletine: τ1=4.5±0.7ms, τ2=10.9±1.0ms, n=8 (P=0.0095 for τ1; P<0.0001 for τ2). The plot illustrates the concentration-response relationships for use-dependent block by mexiletine (each data point represents the mean of 4–12 cells). The lines in B and C were fit to the data according to the Hill equation.
Propranolol block of WT and G1631D channels
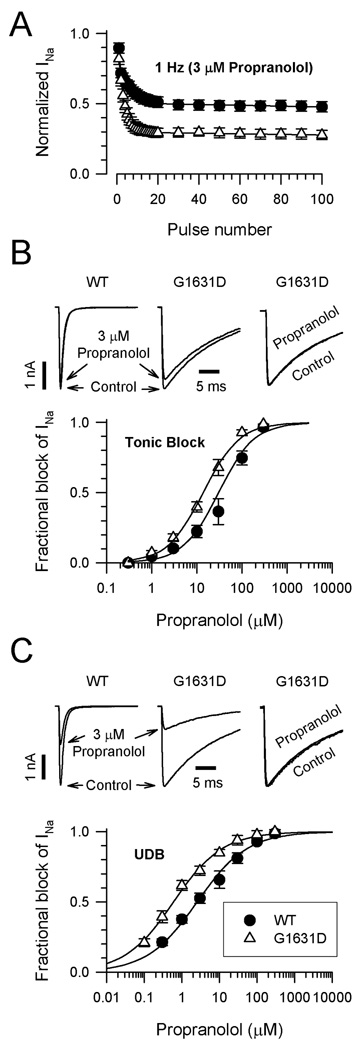
We also considered the role of propranolol in modulating mutant sodium channel behavior. Propranolol is a widely used β-adrenergic receptor antagonist, but early studies indicated that this drug also exhibits anti-arrhythmic (“membrane stabilizing”) properties at high serum concentrations possibly from effects on voltage-gated sodium channels.18,19 Figure 7A illustrates that both WT and G1631D channels are blocked by 3µM propranolol during repetitive stimulation (1Hz). Mutant channels exhibited a greater degree of steady-state block than WT channels under these conditions. Concentration-response curves demonstrated that propranolol exerts greater tonic (Fig. 7B) and use-dependent (Fig. 7C) block of G1631D than of WT channels. Propranolol use-dependent block was enhanced 5-fold by the mutation (EC50: WT, 3.0µM; G1631D, 0.6µM). The effect of propranolol, a racemic mixture, was not likely mediated through endogenous β-adrenergic receptors in the heterologous cell system because we observed similar blocking potency for R-(+)-propranolol which has no receptor antagonist properties (see Supplemental Fig. S2). Propranolol at a concentration similar to that observed in the Japanese proband (0.1µM) normalized steady-state inactivation of mutant channels (G1631D V½: no drug, −74.8±1.1mV, n=16; propranolol, −84.5±1.7mV, n=10, P<0.001) but had no effect on steady state inactivation of WT channels (WT V½: no drug, −89.3±1.1mV, n=16; propranolol, −88.8±0.9mV, n=5, NS). Propranolol did not affect the kinetics of inactivation for WT or mutant channels (Fig. 7B,C) or the level of persistent current observed for G1631D (no drug: 1.63±0.31, n=9; 1µM propranolol, 1.44±0.29, n=9; NS).
Figure 7.
Effects of propranolol on WT and G1631D. (A) Propranolol (3µM) block of WT (n=5) and G1631D (n=4) during a 1Hz train of depolarizing pulses to −10mV from a holding potential of −120mV. (B) Tonic propranolol block of WT and G1631D. Upper traces (left, middle) illustrate the effects of 3µM propranolol during a single depolarizing voltage step to −10mV. Normalized traces (right) recorded in the absence (control) or presence of drug illustrate the effect of propranolol on the inactivation time course. The plot illustrates the concentration-response relationships for tonic block by propranolol (each data point represents the mean of 4–11 cells). (C) Use-dependent propranolol block of WT and G1631D. Upper traces (left, middle) illustrate the steady state effects of 3µM propranolol during a 1Hz pulse train. Normalized traces (right) recorded in the absence (control) or presence of drug (100th pulse) illustrate the effect of propranolol on the inactivation time course. Time constants in the absence of drug were: τ1=7.8±0.5ms, τ2=19.5±0.7ms, n=4; and in the presence of 3µM propranolol: τ1=6.9±1.1ms, τ2=18.1±1.0ms n=4 (no significant differences in τ1 or τ2). The plot illustrates the concentration-response relationships for use-dependent block by propranolol (each data point represents the mean of 4–11 cells). The lines in B and C were fit to the data according to the Hill equation.
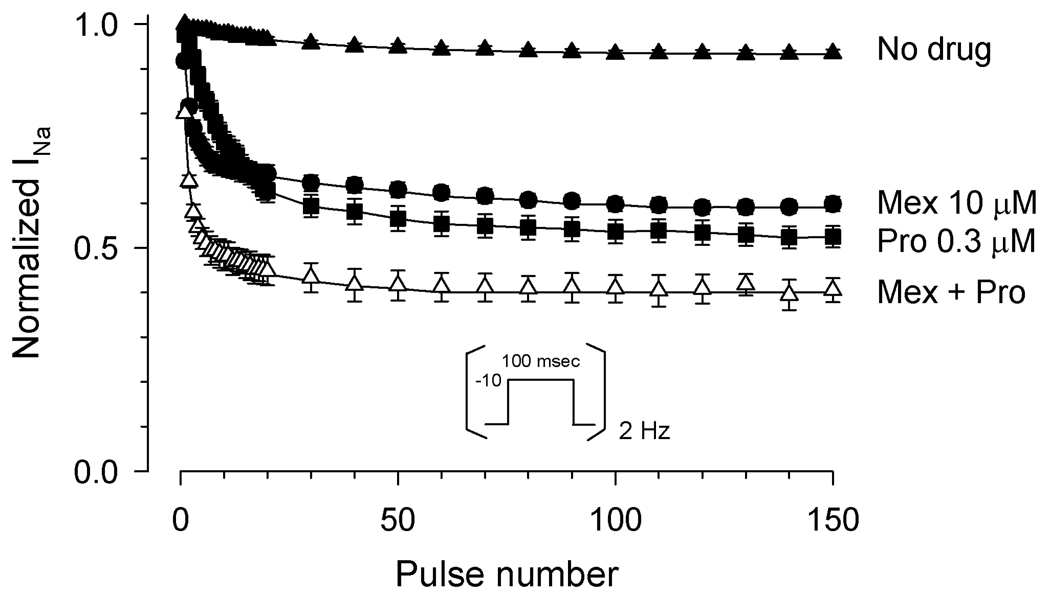
Because both probands responded clinically to the combination of mexiletine and propranolol, we tested the effects of both drugs together on G1631D channels. To more closely simulate the clinical conditions, we tested use-dependent block at 2Hz which was the approximate resting heart rate of the Japanese proband and the frequency of cardiac pacing in the Italian child. The combination of mexiletine (10µM) and propranolol (0.3µM) caused a substantial loss of channel availability during a 2Hz pulse train (Fig. 8) as compared with the drug-free condition. The level of channel inhibition observed for the combination of mexiletine and propranolol was greater than either drug applied alone indicating additive effects.
Figure 8.
Effects of mexiletine with or without propranolol on G1631D channels. Sodium current was measured sequentially during a 2Hz train of depolarizing pulses to −10mV from a holding potential of −120mV and values normalized to the current level following the initial pulse. Steady-state residual normalized sodium current following the 150th pulse was significantly lower for the combination of 10µM mexiletine with 0.3µM propranolol (fractional residual current, 0.4 ±0.03, n=3), as compared with either drug alone (mexiletine, 0.6±0.02, n=6, P=0.0039; propranolol, 0.5 ±0.03, n=6, P=0.041).
Discussion
During late fetal development through shortly after birth there is a vulnerable period when death occurs at a rate of 6–12/1000 live births per year,20 and congenital arrhythmia susceptibility may be a significant contributor to this problem.21,22 Life threatening cardiac arrhythmias during infancy and the perinatal period may go unnoticed owing to the lack of routine use of electrocardiographic monitoring of the fetus and newborn. Studies of two large series of autopsied SIDS victims demonstrated that up to 10% of SIDS cases may represent genetic disorders of congenital arrhythmia susceptibility such as the LQTS,3,23 short-QT syndrome24,25 and catecholaminergic polymorphic ventricular tachycardia.26 Understanding the genetic risks for perinatal mortality should promote efforts to identify and treat at-risk newborns.
Malignant perinatal variant of LQTS
The profoundly dysfunctional SCN5A mutation, G1631D, produced a clinical entity distinct from typical LQTS (LQT3 subtype). Clinically, subjects with typical LQTS first develop symptoms (syncope, cardiac arrest, sudden death) during late childhood, adolescence or early adulthood.9,27 Many mutation carriers may in fact be asymptomatic. The two probands we described appear to be affected by a much more severe and life-threatening process.
At the molecular level, most SCN5A mutations associated with LQTS cause a subtle gain-of-function defect characterized by increased persistent current.16,17 The markedly abnormal channel function we observed for G1631D including a 10-fold slowing of inactivation, substantial shifts in voltage dependence of activation and inactivation along with greatly impaired recovery from inactivation represent distinct molecular defects that distinguish this mutation from typical LQT3 alleles. Other SCN5A alleles may similarly predispose to early onset and severe perinatal arrhythmia syndromes,4,5,28,29 but the functional aberrations associated with most of these reported alleles resemble mutations found in older individuals.
Negative selection against SCN5A mutations
Mutations in SCN5A are represented disproportionately among SIDS victims who carry occult congenital arrhythmia susceptibility gene mutations as compared with older LQTS subjects. The lower proportion of SCN5A mutations among older children and young adults with LQTS as compared to the higher proportion in SIDS victims may be the result of negative selection against mutations in the sodium channel gene. Negative selection would cause an ascertainment bias for genotypes in living individuals in whom survival is favored when carrying mutations having less severe physiological consequences. In the case of SCN5A-G1631D, we assumed that without immediate treatment, this mutation would have been lethal. However, survival after successful treatment confounds the argument for negative selection.
Congenital arrhythmia susceptibility occurring in the perinatal and neonatal periods caused by SCN5A mutations appears biologically distinct from LQTS in older subjects. Carriers of certain SCN5A mutations may present with earlier onset and more severe congenital arrhythmia syndromes. An illustration of this idea is recurrent third-trimester fetal loss due to inheritance of an SCN5A mutation (R1623Q) from a mother who was mosaic for this deleterious allele.8 The R1623Q mutation, which affects a conserved residue in the D4/S4 segment nearby the location of G1631D, was originally identified in a Japanese child with a severe clinical presentation of LQTS30 and the molecular defect associated with this allele compromised inactivation to a greater extent than typical LQT3 mutations.31 Our observations regarding the severity of biophysical defects associated with G1631D also support the idea that earlier onset cardiac symptoms may sometimes correlate with a more severe molecular phenotype.
Genotype-specific pharmacological treatment
The clinical consequences of G1631D were perinatal arrhythmias successfully managed in part by pharmacotherapy with the combination of mexiletine and propranolol. Mexiletine as well as other sodium channel blockers have been proposed as gene-specific therapeutic agents in LQT3.32–34 In vitro studies have demonstrated the capability of these drugs to selectively suppress increased persistent current conducted by mutant channels29,35 and to normalize ventricular repolarization in animal models.36,37 One study suggested that certain biophysical properties of mutant NaV1.5 channels may be predictive of mexiletine responsiveness. Specifically, Ruan, et al., found that among 4 distinct SCN5A mutations, clinical benefit from mexiletine treatment was observed only in subjects carrying mutations that caused a hyperpolarizing shift in steady-state inactivation and this correlated with in vitro effects of the drug.38 However, this observation cannot be extrapolated to all SCN5A mutations as evidenced by the favorable response of G1631D to mexiletine both clinically and experimentally despite a depolarizing shift in steady-state inactivation (Fig. 3). Similarly, another recently reported SCN5A mutation (F1473C) was also associated with a favorable clinical response to high dose mexiletine despite having depolarized steady-state inactivation.29 Additional factors besides those emphasized by Ruan, et al.38 are likely to determine the clinical efficacy of mexiletine.
By contrast, use of β-blockers in the setting of SCN5A mutations has less certain benefits. Three studies have reported that β-blockers are generally less efficacious in LQT3 subjects, but the specific drug used varies considerably.9,39,40 For example, in the report by Priori et al. the specific β-blocker was known in 69% of cases and this was either propranolol or nadolol.40 As we have demonstrated in this study, propranolol may offer specific advantages in treating certain SCN5A mutations because of apparent local anesthetic-like properties of the drug.18,19 By contrast, we recently determined that nadolol has no activity against sodium channels (Wang, D.W., unpublished observations). The role of propranolol in treating individuals with SCN5A mutations warrants further study.
Combination pharmacotherapy in the two probands with G1631D may have uniquely contributed to their survival. In the Japanese newborn, mexiletine alone was not adequate to control ventricular arrhythmia despite shortening of the QT interval. The addition of propranolol to the treatment regimen conferred better arrhythmia control and survival. In the Italian proband, the co-administration of mexiletine with propranolol was efficacious, but this subject was also treated with ventricular pacing. Our study demonstrated additive effects of the two drugs at a pulsing frequency of 2Hz (Fig. 8). This observation suggested that a combination of mexiletine with propranolol in the setting of modest tachycardia were protective of ventricular arrhythmia caused by G1631D. We explain this effect by a combination of the intrinsic activity-dependent loss of channel availability observed for G1631D (Fig. 4B) with the use-dependent drug effects.
Supplementary Material
Acknowledgments
The authors thank Thomas H. Rhodes for providing technical support and Shuji Hashimoto in the Laboratory of Clinical Physiology, National Cardiovascular Center, for technical assistance for MCG recordings.
Funding Sources
This work was supported by a grant from the NIH (HL083374). Dr. Shimizu was supported by health sciences research grant (H18 - Research on Human Genome - 002) from the Ministry of Health, Labour and Welfare, Japan.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Byard RW, Krous HF. Sudden infant death syndrome: overview and update. Pediatr Dev Pathol. 2003;6:112–127. doi: 10.1007/s10024-002-0205-8. [DOI] [PubMed] [Google Scholar]
- 2.Rognum TO, Byard RW. In: Sudden infant death syndrome, etiology and epidemiology. Payne-James J, Byard RW, Corey TS, Henderson C, editors. Boston, Elsevier: Encyclopedia of Forensic and Legal Medicine; 2005. pp. 117–129. [Google Scholar]
- 3.Arnestad M, Crotti L, Rognum TO, Insolia R, Pedrazzini M, Ferrandi C, Vege A, Wang DW, Rhodes TE, George AL, Jr, Schwartz PJ. Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–367. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 4.Wedekind H, Smits JP, Schulze-Bahr E, Arnold R, Veldkamp MW, Bajanowski T, Borggrefe M, Brinkmann B, Warnecke I, Funke H, Bhuiyan ZA, Wilde AA, Breithardt G, Haverkamp W. De novo mutation in the SCN5A gene associated with early onset of sudden infant death. Circulation. 2001;104:1158–1164. doi: 10.1161/hc3501.095361. [DOI] [PubMed] [Google Scholar]
- 5.Chang CC, Acharfi S, Wu MH, Chiang FT, Wang JK, Sung TC, Chahine M. A novel SCN5A mutation manifests as a malignant form of long QT syndrome with perinatal onset of tachycardia/bradycardia. Cardiovasc Res. 2004;64:268–278. doi: 10.1016/j.cardiores.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Lupoglazoff JM, Denjoy I, Villain E, Fressart V, Simon F, Bozio A, Berthet M, Benammar N, Hainque B, Guicheney P. Long QT syndrome in neonates: conduction disorders associated with HERG mutations and sinus bradycardia with KCNQ1 mutations. J Am Coll Cardiol. 2004;43:826–830. doi: 10.1016/j.jacc.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Priori SG, Dumaine R, Napolitano C, Antzelevitch C, Stramba-Badiale M, Richard TA, Berti MR, Bloise R. A molecular link between the sudden infant death syndrome and the long-QT syndrome. N Engl J Med. 2000;343:262–267. doi: 10.1056/NEJM200007273430405. [DOI] [PubMed] [Google Scholar]
- 8.Miller TE, Estrella E, Myerburg RJ, Garcia dV, Moreno N, Rusconi P, Ahearn ME, Baumbach L, Kurlansky P, Wolff G, Bishopric NH. Recurrent third-trimester fetal loss and maternal mosaicism for long-QT syndrome. Circulation. 2004;109:3029–3034. doi: 10.1161/01.CIR.0000130666.81539.9E. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, Denjoy I, Guicheney P, Breithardt G, Keating MT, Towbin JA, Beggs AH, Brink P, Wilde AA, Toivonen L, Zareba W, Robinson JL, Timothy KW, Corfield V, Wattanasirichaigoon D, Corbett C, Haverkamp W, Schulze-Bahr E, Lehmann MH, Schwartz K, Coumel P, Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 10.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Priori SG, Schwartz PJ, Napolitano C, Bloise R, Ronchetti E, Grillo M, Vicentini A, Spazzolini C, Nastoli J, Bottelli G, Folli R, Cappelletti D. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 12.Crotti L, Lundquist AL, Insolia R, Pedrazzini M, Ferrandi C, De Ferrari GM, Vicentini A, Yang P, Roden DM, George AL, Jr, Schwartz PJ. KCNH2-K897T is a genetic modifier of latent congenital long-QT syndrome. Circulation. 2005;112:1251–1258. doi: 10.1161/CIRCULATIONAHA.105.549071. [DOI] [PubMed] [Google Scholar]
- 13.Yokokawa M, Noda T, Okamura H, Satomi K, Suyama K, Kurita T, Aihara N, Kamakura S, Shimizu W. Comparison of long-term follow-up of electrocardiographic features in Brugada syndrome between the SCN5A-positive probands and the SCN5A-negative probands. Am J Cardiol. 2007;100:649–655. doi: 10.1016/j.amjcard.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 14.Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiologic Rev. 1992;72:S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- 15.Chahine M, George AL, Jr, Zhou M, Ji S, Sun W, Barchi RL, Horn R. Sodium channel mutations in paramyotonia congenita uncouple inactivation from activation. Neuron. 1994;12:281–294. doi: 10.1016/0896-6273(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 16.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 17.Dumaine R, Wang Q, Keating MT, Hartmann HA, Schwartz PJ, Brown AM, Kirsch GE. Multiple mechanisms of Na+ channel-linked long-QT syndrome. Circ Res. 1996;78:916–924. doi: 10.1161/01.res.78.5.916. [DOI] [PubMed] [Google Scholar]
- 18.Dawson AK, Reele SB, Wood AJ, Duff HJ, Woosley RL, Smith RF. Electrophysiological effects of high-dose propranolol in dogs: evidence in vivo for effects not mediated by the beta adrenoceptor. J Pharmacol Exp Ther. 1984;229:91–97. [PubMed] [Google Scholar]
- 19.Duff HJ, Roden DM, Brorson L, Wood AJ, Dawson AK, Primm RK, Oates JA, Smith RF, Woosley RL. Electrophysiologic actions of high plasma concentrations of propranolol in human subjects. J Am Coll Cardiol. 1983;2:1134–1140. doi: 10.1016/s0735-1097(83)80340-4. [DOI] [PubMed] [Google Scholar]
- 20.Strasburger JF, Cheulkar B, Wichman HJ. Perinatal arrhythmias: diagnosis and management. Clin Perinatol. 2007;34:627–652. doi: 10.1016/j.clp.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berul CI. Neonatal long QT syndrome and sudden cardiac death. Prog Pediatr Cardiol. 2000;11:47–54. doi: 10.1016/s1058-9813(00)00035-7. [DOI] [PubMed] [Google Scholar]
- 22.Beinder E, Buheitel G, Hofbeck M. Are some cases of sudden intrauterine unexplained death due to the long QT syndrome? Prenat Diagn. 2003;23:1097–1098. doi: 10.1002/pd.702. [DOI] [PubMed] [Google Scholar]
- 23.Ackerman MJ, Siu BL, Sturner WQ, Tester DJ, Valdivia CR, Makielski JC, Towbin JA. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. JAMA. 2001;286:2264–2269. doi: 10.1001/jama.286.18.2264. [DOI] [PubMed] [Google Scholar]
- 24.Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C, Burashnikov E, Matsuo K, Wu YS, Guerchicoff A, Bianchi F, Giustetto C, Schimpf R, Brugada P, Antzelevitch C. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes TE, Abraham RA, Welch RC, Vanoye CG, Crotti L, Arnestad M, Insolia R, Pedrazzini M, Ferrandi C, Vege A, Rognum T, Roden DM, Schwartz PJ, George AL., Jr Cardiac potassium channel dysfunction in sudden infant death syndrome. J Mol Cell Cardiol. 2007;44:571–581. doi: 10.1016/j.yjmcc.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tester DJ, Spoon DB, Valdivia HH, Makielski JC, Ackerman MJ. Targeted mutational analysis of the RyR2-encoded cardiac ryanodine receptor in sudden unexplained death: a molecular autopsy of 49 medical examiner/coroner's cases. Mayo Clin Proc. 2004;79:1380–1384. doi: 10.4065/79.11.1380. [DOI] [PubMed] [Google Scholar]
- 27.Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med. 2008;358:169–176. doi: 10.1056/NEJMcp0706513. [DOI] [PubMed] [Google Scholar]
- 28.Schulze-Bahr E, Fenge H, Etzrodt D, Haverkamp W, Monnig G, Wedekind H, Breithardt G, Kehl HG. Long QT syndrome and life threatening arrhythmia in a newborn: molecular diagnosis and treatment response. Heart. 2004;90:13–16. doi: 10.1136/heart.90.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bankston JR, Yue M, Chung W, Spyres M, Pass RH, Silver E, Sampson KJ, Kass RS. A novel and lethal de novo LQT-3 mutation in a newborn with distinct molecular pharmacology and therapeutic response. PLoS ONE. 2007;2:e1258. doi: 10.1371/journal.pone.0001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamagishi H, Furutani M, Kamisago M, Morikawa Y, Kojima Y, Hino Y, Furutani Y, Kimura M, Imamura S, Takao A, Momma K, Matsuoka R. A de novo missense mutation (R1623Q) of the SCN5A gene in a Japanese girl with sporadic long QT sydrome. Hum Mutat. 1998;11:481. doi: 10.1002/(SICI)1098-1004(1998)11:6<481::AID-HUMU12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Kambouris NG, Nuss HB, Johns DC, Tomaselli GF, Marban E, Balser JR. Phenotypic characterization of a novel long-QT syndrome mutation (R1623Q) in the cardiac sodium channel. Circulation. 1998;97:640–644. doi: 10.1161/01.cir.97.7.640. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz PJ, Priori SG, Locati EH, Napolitano C, Cantu F, Towbin JA, Keating MT, Hammoude H, Brown AM, Chen LS. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–3386. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 33.Benhorin J, Taub R, Goldmit M, Kerem B, Kass RS, Windman I, Medina A. Effects of flecainide in patients with new SCN5A mutation: mutation-specific therapy for long-QT syndrome? Circulation. 2000;101:1698–1706. doi: 10.1161/01.cir.101.14.1698. [DOI] [PubMed] [Google Scholar]
- 34.Windle JR, Geletka RC, Moss AJ, Zareba W, Atkins DL. Normalization of ventricular repolarization with flecainide in long QT syndrome patients with SCN5A:DeltaKPQ mutation. Ann Noninvasive Electrocardiol. 2001;6:153–158. doi: 10.1111/j.1542-474X.2001.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang DW, Yazawa K, Makita N, George AL, Jr, Bennett PB. Targeting of long QT mutant sodium channels. J Clin Invest. 1997;99:1714–1720. doi: 10.1172/JCI119335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu W, Aiba T, Antzelevitch C. Specific therapy based on the genotype and cellular mechanism in inherited cardiac arrhythmias. Long QT syndrome and Brugada syndrome. Curr Pharm Des. 2005;11:1561–1572. doi: 10.2174/1381612053764823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade des pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- 38.Ruan Y, Liu N, Bloise R, Napolitano C, Priori SG. Gating properties of SCN5A mutations and the response to mexiletine in long-QT syndrome type 3 patients. Circulation. 2007;116:1137–1144. doi: 10.1161/CIRCULATIONAHA.107.707877. [DOI] [PubMed] [Google Scholar]
- 39.Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, Vincent GM, Locati EH, Priori SG, Napolitano C, Medina A, Zhang L, Robinson JL, Timothy K, Towbin JA, Andrews ML. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000;101:616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 40.Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, Nastoli J. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004;292:1341–1344. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.