Abstract
Cholangiocarcinomas arise from the epithelial cells of the bile ducts and are associated with poor prognosis. Despite new diagnostic approaches, the definite diagnosis of this malignancy continues to be challenging. Cholangiocarcinomas often grow longitudinally along the bile duct rather than in a radial direction. Thus, large tumor masses are frequently absent and imaging techniques, including ultrasound, CT, and MRI have only limited sensitivity. Tissue collection during endoscopic (ERCP) and/or percutaneous transhepatic (PTC) procedures are usually used to confirm a definitive diagnosis of cholangiocarcinoma. However, forceps biopsy and brush cytology provide positive results for malignancy in about only 50% of patients. Percutaneous and peroral cholangioscopy using fiber-optic techniques were therefore developed for direct visualization of the biliary tree, yielding additional information about endoscopic appearance and tumor extension, as well as a guided biopsy acquistion. Finally, endoscopic ultrasonography (EUS) complements endoscopic and percutaneous approaches and may provide a tissue diagnosis of tumors in the biliary region through fine-needle aspiration. In the future, new techniques allowing for early detection, including molecular markers, should be developed to improve the diagnostic sensitivity in this increasing tumor entity.
Keywords: Diagnosis, Brush cytology, Forceps biopsy, Cholangiocarcinoma
INTRODUCTION
Cholangiocarcinomas are topographically categorized as intrahepatic or extrahepatic carcinomas. Extrahepatic cholangiocarcinomas are further subdivided into hilar, middle and distal carcinomas. The most common type of hilar cholangiocarcinoma is classified into 4 stages according to the bismuth classification[1]. Surgery is the only curative treatment in patients with cholangiocarcinoma. The results are more favourable for patients with early-stage disease. Therefore, a reliable diagnostic procedure is of great importance for these patients. However, confirmation of cholangiocarcinoma can be very difficult because of a wide spectrum of alternative diagnoses, including other carcinomas, metastasis and benign biliary strictures. Therefore, multidisciplinary investigative approaches are needed to overcome this problem. Cholangiocarcinomas often grow longitudinally along the bile duct rather than in a radial direction away from the bile duct. Consequently, imaging techniques including ultrasound, CT, and MRI are of limited sensitivity for the detection of cholangiocarcinoma[2]. Biliary tissue collection during endoscopic procedures is widely used for distinction between benign and malignant strictures and provides the only definitive diagnosis that can be used for establishing therapeutic strategies. To obtain tissue samples, brush cytology and/or forceps biopsy were routinely performed in patients with suspected malignant biliary strictures.
BIOCHEMICAL INVESTIGATIONS
Obstructive jaundice is typically associated with an increase of serum bilirubin, alkaline phosphatase and gamma-glutamyl transpeptidase. These laboratory parameters are unspecific and do not allow a distinction between benign and malignant bile duct strictures. The most widely studied tumor markers are carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA). Both tumor markers may be elevated in cholangiocarcinoma[3–5]. However, CA19-9 and CEA are not specific for cholangiocarcinoma. CA19-9 is also raised in pancreatic cancer, colorectal cancer, gastric cancer, and gynaecological malignancies[6]. Additionally, CA19-9 may be elevated in patients with acute cholangitis[7]. In a series of patients without primary sclerosing cholangitis, the sensitivity of a serum CA19-9 level of more than 100 U/mL in diagnosing cholangiocarcinoma was 53%[3]. Furthermore, the authors reported in patients with unresectable cholangiocarcinoma a significantly greater mean CA19-9 concentration compared to patients with resectable cholangiocarcinoma. Recently, John et al[8] reported that sensitivity and specificity were 67.5% and 86.8%, respectively, when using a cut-off value of 100 U/mL. In another report that included 37 patients with primary sclerosing cholangitis, a serum CA19-9 concentration above 100 U/mL sensitivity was 89% and specificity was 86% for the diagnosis of cholangiocarcinoma[4]. CEA also has unsatisfactory diagnostic sensitivity and specificity for cholangiocarcinoma[9]. In conclusion, the diagnostic value of tumor markers in cholangiocarcinoma is limited. However, CA19-9 is useful in following the effect of treatment and to detect disease recurrence.
IMAGING
Ultrasonography
Patients suffering from jaundice usually undergo transabdominal ultrasonography to evaluate the bile duct diameter and hepatic parenchyma. Furthermore, gallstones can be excluded. In most patients cholangiocarcinomas are not directly detectable, but indirect signs are visible in the majority of patients. Distal lesions cause dilation of both intrahepatic and extrahepatic bile ducts, whereas proximal lesions only cause dilation of intrahepatic bile ducts. The localization of the bile duct lesion can be suggested if there is an abrupt change in ductal diameter. The diagnostic accuracy of ultrasonography was investigated in 429 patients with obstructive jaundice. In this series ultrasonography demonstrated ductal obstruction in 89%, and the sensitivity for localizing the site of obstruction was 94%[10]. The sensitivity and specificity of ultrasonography depends on tumor localization, the quality of the equipment and the experience of the investigator[11]. Ultrasound findings are limited in patients with liver cirrhosis and primary sclerosing cholangitis due to a lack of visible dilated bile ducts. Doppler ultrasonography provides information on hepatic and portal vessel patency. Recent studies reported that contrast enhanced ultrasonography provides sensitive and specific criteria for the differentiation between malignant and benign liver lesions[12–15]. Preliminary data for cholangiocarcinoma suggest a behavior that is not dissimilar to metastatic lesions[14,16]. However, the limited number of cases in the reported series does not allow conclusive considerations for cholangiocarcinoma. Therefore, further studies with appropriate numbers of patients are needed.
Computed tomography
Computed tomography (CT) is a commonly used approach for the detection and staging of cholangiocarcinoma. The radiological findings depend on localization and morphology of the tumor. CT scan permits identification of bile duct dilatation as well as assessment of lymph node, liver parenchyma, vascular encasement and metastasis[17]. Additionally, computed tomography is useful for detecting the presence of liver atrophy. Dilatation of bile ducts combined with atrophy suggests the obstruction of the portal vein[18]. However, conventional computed tomography is limited in the ability to estimate the extent of cholangiocarcinoma and resectability. Tillich et al[17] reported a series of 29 patients with hilar cholangiocarcinoma who underwent multiphasic helical CT, including arterial and portal venous phase. In these patients resectability was correctly predicted in only 60%. In another series, Yamashita et al[19] reported only 59% sensitivity in identifying a primary lesion by using contrast-enhanced computed tomography. Recently, the accuracy of preoperative high-resolution computed tomography to determine resectability in patients with hilar cholangiocarcinoma was evaluated[20]. In this series negative and positive predictive values of high-resolution computed tomography to determine resectability were 92% and 85%, respectively. Thus, only new CT scanning techniques should be taken into account since radiological procedures have had a considerable improvement in the last years.
Magnetic resonance imaging and magnetic resonance cholangiopancreaticography
In recent years, magnetic resonance imaging (MRI), especially in combination with magnetic resonance cholangiopancreaticography (MRCP) has improved diagnosing cholangiocarcinoma and determining resectability[21–23]. Magnetic resonance imaging can assess the local tumor extension, lymph nodes, metastasis and liver parenchyma. It is important to use sequences with thin-slice thickness (3-4 mm) that provide sufficient signal to obtain good quality images and are sufficiently thin to detect subtle abnormalities. At present, good quality MRI in the hands of experienced centers, can be an excellent imaging approach for the diagnosis and staging of cholangiocarcinoma[24]. Moreover, magnetic resonance angiography (MRA) provides good assessment for infiltration of blood vessels. Magnetic resonance cholangiography can provide a three-dimensional reconstruction of the biliary tree without injection of intravenous and biliary contrast fluid. Therefore, the risk for cholangitis is reduced[21], and additionally there is no risk for contrast induced nephropathy. MRCP allows the assessment of bile ducts above and below a total obstruction. Therefore, MRCP should be considered for planning the treatment of patients suffering from cholangiocarcinoma. Zidi et al[25] reported a correct malignant hilar tumor stage using MRCP in 78% of the investigated patients. Furthermore, in this series an underestimated tumor extension was reported in 22%[25]. Biliary stent placement and percutaneous drainage results in mild inflammation of bile duct walls, which appears as an increased gadolinium enhancement with an appearance indistinguishable from the superficial spread of cholangiocarcinoma. To avoid this problem MRI and MRCP should be performed before endoscopic stenting and percutaneous transhepatic drainage[23].
Positron emission tomography (PET)
Several studies reported intensive accumulation of nucleotide tracer 18-fluorodeoxyglucose (FDG) in cholangiocarcinoma[26–28]. PET scanning with focal FDG accumulation permits visualization of cholangiocarcinomas. PET scan can detect cholangiocarcinomas as small as 1 cm[29,30]. FDG-PET is of value for staging of bile duct cancers, especially for discovering distant metastasis and malignant lymph nodes. In one series, PET led to a change of therapeutic management in 30% of patients suffering from cholangiocarcinoma because of detection of primary unsuspected metastases[26]. The limitation of FDG-PET is false positive results in patients with biliary tract infections, primary sclerosing cholangitis, and biliary stenting via endoscopic retrograde cholangiography (ERC) and PTBD[26,31]. The diagnostic sensitivity can be increased by using 18-fluorodeoxyglucose (FDG) in combination with CT scanning (FDG-PET/CT). Reinhardt et al[28] evaluated the effectiveness of this new dual-modality technique for noninvasive differentiation of extrahepatic bile duct strictures. This series included 14 patients with histological proven cholangiocarcinoma and 8 patients with benign bile duct strictures. In this series, all patients with cholangiocarcinoma presented with focal increased tracer uptake compared to patients with benign bile duct stricture. Overall, our experience is that 18F-FDG PET/CT does not provide high accuracy for noninvasive detection of perihilar cholangiocarcinoma in extrahepatic bile duct strictures, which may be mainly due to the small size of the tumors.
ENDOSCOPIC APPROACHES
Endoscopic retrograde cholangiography
Retrograde injection of contrast fluid into the biliary tract allows the assessment of localization and morphology of bile duct strictures. Malignancy is suggested when there are findings of asymmetric, irregular strictures. Moreover, resectability can be evaluated. However, the differentiation in benign and malignant bile duct stricture may be difficult. Park et al[32] identified 20 out of 27 malignant bile duct strictures using ERC alone. In this series diagnostic sensitivity and specificity for endoscopic retrograde cholangiography was 74% and 70%, respectively. Other authors have reported similar results for detecting malignant bile duct strictures by direct cholangiography[33]. Compared to non-invasive imaging techniques, endoscopic retrograde cholangiography allows tissue collection for cytological and histological investigation. Additionally, ERC allows biliary stent implantation for palliative treatment in irresectable tumors.
Percutaneous transhepatic cholangiography (PTC)
In patients with difficult bile duct access percutaneous transhepatic approaches offer a valuable alternative for bile duct access. The effectiveness of this procedure in diagnostic and therapy of complex biliary obstruction has been well documented[34,35]. Because percutaneous transhepatic bile duct access is an invasive technique, potential complications including bleeding, cholangitis, biliary leakage, duodenal perforation and death can occur. In previous series, procedure related death ranging from 0.6% to 5.6% was reported[36–39]. Therefore, endoscopic retrograde cholangiography is usually favoured above percutaneous transhepatic cholangiography. Percutaneous transhepatic approaches also allow tissue collection and biliary drainage.
Cholangioscopy
Cholangioscopy using fiber-optic techniques provide direct visualization of the biliary tree. Differentiation between benign and malignant bile duct stricture using a cholangioscope has not been well defined. However, typical signs for malignancy including mucosal ulcerations, irregular mucosa and asymmetric stricture may be visible. Moreover, cholangioscopic guided forceps biopsy and brush cytology may enhance the diagnostic accuracy of tissue diagnosis. The most common approach is percutaneous transhepatic cholangioscopy. Another possibility is to perform peroral transpapillary cholangioscopy using a mother baby endoscope. Fukuda et al[40] evaluated the utility of peroral cholangioscopy for distinguishing malignant from benign biliary disease. The authors identified 22 out of 38 malignant bile duct strictures using ERC in combination with tissue sampling. The addition of peroral cholangioscopy correctly identified all 38 malignant strictures in this series.
Intraductal ultrasonography
Intraductal ultrasonography (IDUS) is a promising imaging modality for the evaluation of a variety of biliary disorders[41,42]. Intraductal ultrasonography does not provide definite diagnoses. However, the characterization of biliary structures provided by IDUS can be used in combination with other diagnostic approaches to develop appropriate therapeutic strategies. Intraductal ultrasonography can provide the local staging to select patients with cholangiocarcinoma who benefit from surgical resection[43–46]. Recently, Stavropoulos et al[47] reported that intraductal ultrasonography increased the accuracy of ERCP in distinguishing between benign and malignant strictures from 58% to 90%. This high rate of diagnostic accuracy using intraductal ultrasonography has been confirmed by others[48,49].
EUS guided fine-needle aspiration
Endoscopic ultrasonography (EUS) complements the role of endoscopic and percutaneous transhepatic approaches and may provide a tissue diagnosis through fine-needle aspiration (FNA). The yield of EUS-FNA in patients with suspected cholangiocarcinoma was evaluated by Eloubeidi et al[50]. The authors reported a diagnostic sensitivity of 86%. However, another group reported lower rates of diagnostic sensitivity (45%) for detection of bile duct lesions by using ultrasound guided fine needle aspiration[51]. EUS-FNA may represent an alternative approach in the diagnosis of cholangiocarcinoma, especially in patients with negative brush cytology and forceps biopsy findings. One of the major limitations of endoscopic brush cytology from bile duct strictures is the poor quality of cytologic samples. Therefore, negative cytological results do not permit reliable exclusion of malignancy.
Brush cytology and forceps biopsy
Tissue collection during endoscopic and/or percuta-neous transhepatic procedures are the most common techniques for providing a definitive diagnosis of cholangiocarcinoma[52]. Brush cytology, first described in 1975, is the most common tissue sampling technique in patients with suspected bile duct strictures[53]. It is generally safe, requires little time, and is technically easier compared to forceps biopsy. The sensitivity of brush cytology for diagnosis of malignant biliary strictures ranges from 30% to 60% in most published series[54–56]. Tissue samples for histological investigation can be obtained from biliary strictures by using forceps. This technique is more time consuming than brushing and is less widely used, but it provides a sample of subepithelial stroma. In patients with malignant biliary stricture the overall cancer detection rate of forceps biopsy is often higher than forbrush cytology, ranging from 43% to 81%[57–59]. In these published series, the sensitivity of brush cytology and forceps biopsy was evaluated in a heterogeneous patient group with several malignant bile duct strictures. Recently, the diagnostic sensitivity of transpapillary brush cytology and forceps biopsy was evaluated in patients with hilar cholangiocarcinomas[60]. In this series, the sensitivity of transpapillary brush cytology was 41.4% and the sensitivity of forceps biopsy was 53.4%. In combined approaches the diagnostic sensitivity increased to only 60.3%.
Fluorescence in situ hybridization (FISH)
Recently, investigators have attempted to improve diagnostic assessment with an advanced cytological technique for the detection of malignant pancreaticobiliary strictures[61]. Fluorescence in situ hybridization (FISH) has been shown to increase the sensitivity for the diagnosis of malignant pancreaticobiliary strictures compared to conventional cytology. Kipp et al[62] used a multitarget FISH probe set which has previously shown high impact in monitoring recurrent urothelial carcinoma[63]. This advanced technique identifies malignant cells by detecting aneusomy and deletion of the locus 9p21. By applying this technique for brush cytology and bile aspirate specimens in 131 patients with bile duct strictures (including 71 with primary sclerosing cholangitis, FISH analysis showed sensitivity of 35% and specificity of 91%. When patients with primary sclerosing cholangitis were excluded, sensitivity for malignancy detection by FISH was 16%[64]. This indicates that probe sets specific for biliary neoplasms will be required for higher sensitivity. However, not all malignant tumors present aneusomy or aneuploidy. In the biliary tract, the percentage of cancers displaying aneuploidy has been estimated to be approximately 80%[65].
CONCLUSION
Figure 1 demonstrates the diagnostic algorithm used in our hospital for patients with suspected extrahepatic bile duct obstruction. Cholangiocarcinomas are still difficult to diagnose. In the future we need better early detection methods including molecular markers and improved histological techniques. Furthermore, new imaging and endoscopic techniques should be developed to improve the diagnostic accuracy and tumor extension.
Figure 1.
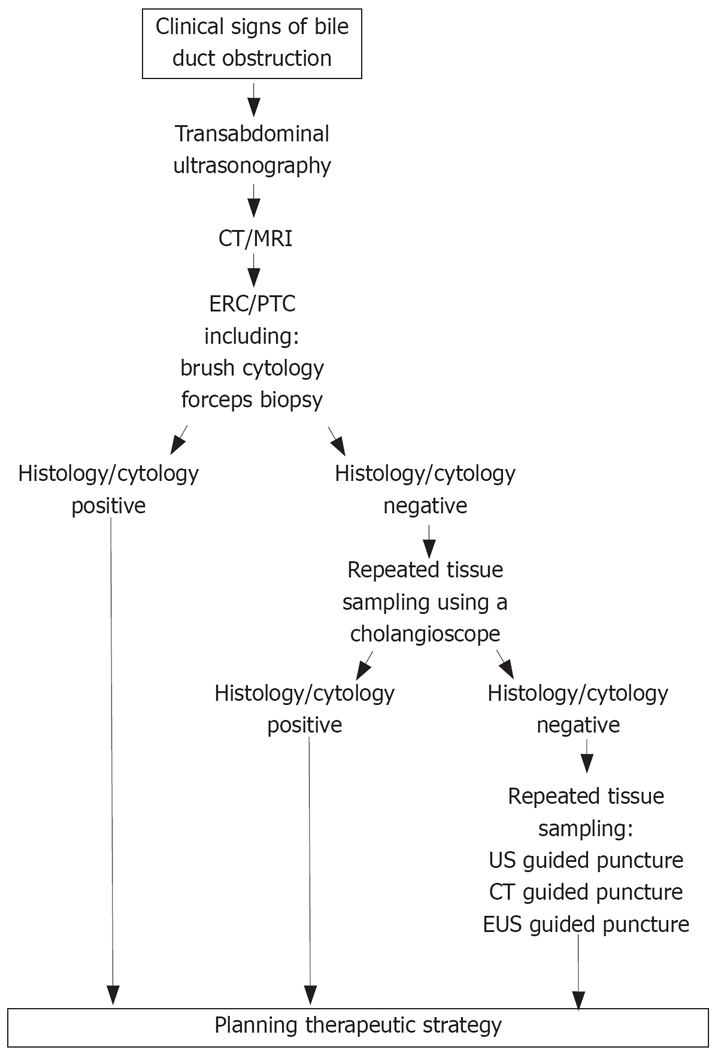
The diagnostic algorithm in patients with suspected extrahepatic bile duct obstruction.
Peer reviewers: James M Millis, Professor, University of Chicago, Section of Transplantation, MC 5027, 5841 S. Maryland Avenue, Chicago, IL 60637, United States; Wei Tang, MD, EngD, Assistant Professor, H-B-P Surgery Division, Artificial Organ and Transplantation Division, Department of Surgery, Graduate School of Medicine, The University of Tokyo, Tokyo 113-8655, Japan
S- Editor Zhong XY L- Editor Roberts SE E- Editor Zhang WB
References
- 1.Bismuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg. 1988;12:39–47. doi: 10.1007/BF01658484. [DOI] [PubMed] [Google Scholar]
- 2.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167–179. doi: 10.1159/000077991. [DOI] [PubMed] [Google Scholar]
- 3.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 4.Nichols JC, Gores GJ, LaRusso NF, Wiesner RH, Nagorney DM, Ritts RE Jr. Diagnostic role of serum CA 19-9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clin Proc. 1993;68:874–879. doi: 10.1016/s0025-6196(12)60696-x. [DOI] [PubMed] [Google Scholar]
- 5.Nakeeb A, Lipsett PA, Lillemoe KD, Fox-Talbot MK, Coleman J, Cameron JL, Pitt HA. Biliary carcinoembryonic antigen levels are a marker for cholangiocarcinoma. Am J Surg. 1996;171:147–152; discussion 152-153. doi: 10.1016/S0002-9610(99)80090-7. [DOI] [PubMed] [Google Scholar]
- 6.Lamerz R. Role of tumour markers, cytogenetics. Ann Oncol. 1999;10 Suppl 4:145–149. [PubMed] [Google Scholar]
- 7.Albert MB, Steinberg WM, Henry JP. Elevated serum levels of tumor marker CA19-9 in acute cholangitis. Dig Dis Sci. 1988;33:1223–1225. doi: 10.1007/BF01536670. [DOI] [PubMed] [Google Scholar]
- 8.John AR, Haghighi KS, Taniere P, Esmat ME, Tan YM, Bramhall SR. Is a raised CA 19-9 level diagnostic for a cholangiocarcinoma in patients with no history of sclerosing cholangitis ? Dig Surg. 2006;23:319–324. doi: 10.1159/000098014. [DOI] [PubMed] [Google Scholar]
- 9.Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004;24:139–154. doi: 10.1055/s-2004-828891. [DOI] [PubMed] [Google Scholar]
- 10.Sharma MP, Ahuja V. Aetiological spectrum of obstructive jaundice and diagnostic ability of ultrasonography: a clinician's perspective. Trop Gastroenterol. 1999;20:167–169. [PubMed] [Google Scholar]
- 11.Robledo R, Muro A, Prieto ML. Extrahepatic bile duct carcinoma: US characteristics and accuracy in demonstration of tumors. Radiology. 1996;198:869–873. doi: 10.1148/radiology.198.3.8628885. [DOI] [PubMed] [Google Scholar]
- 12.Nicolau C, Vilana R, Catala V, Bianchi L, Gilabert R, Garcia A, Bru C. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol. 2006;186:158–167. doi: 10.2214/AJR.04.1009. [DOI] [PubMed] [Google Scholar]
- 13.Bartolotta TV, Taibbi A, Galia M, Runza G, Matranga D, Midiri M, Lagalla R. Characterization of hypoechoic focal hepatic lesions in patients with fatty liver: diagnostic performance and confidence of contrast-enhanced ultrasound. Eur Radiol. 2007;17:650–661. doi: 10.1007/s00330-006-0432-x. [DOI] [PubMed] [Google Scholar]
- 14.Celli N, Gaiani S, Piscaglia F, Zironi G, Camaggi V, Leoni S, Righini R, Bolondi L. Characterization of liver lesions by real-time contrast-enhanced ultrasonography. Eur J Gastroenterol Hepatol. 2007;19:3–14. doi: 10.1097/01.meg.0000250585.53608.3c. [DOI] [PubMed] [Google Scholar]
- 15.Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound. 2006;34:261–272. doi: 10.1002/jcu.20234. [DOI] [PubMed] [Google Scholar]
- 16.Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, Zheng YL, Liang JY. Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med. 2006;25:23–33. doi: 10.7863/jum.2006.25.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Tillich M, Mischinger HJ, Preisegger KH, Rabl H, Szolar DH. Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1998;171:651–658. doi: 10.2214/ajr.171.3.9725291. [DOI] [PubMed] [Google Scholar]
- 18.Hann LE, Getrajdman GI, Brown KT, Bach AM, Teitcher JB, Fong Y, Blumgart LH. Hepatic lobar atrophy: association with ipsilateral portal vein obstruction. AJR Am J Roentgenol. 1996;167:1017–1021. doi: 10.2214/ajr.167.4.8819404. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita Y, Takahashi M, Kanazawa S, Charnsangavej C, Wallace S. Parenchymal changes of the liver in cholangiocarcinoma: CT evaluation. Gastrointest Radiol. 1992;17:161–166. doi: 10.1007/BF01888536. [DOI] [PubMed] [Google Scholar]
- 20.Aloia TA, Charnsangavej C, Faria S, Ribero D, Abdalla EK, Vauthey JN, Curley SA. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am J Surg. 2007;193:702–706. doi: 10.1016/j.amjsurg.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Manfredi R, Barbaro B, Masselli G, Vecchioli A, Marano P. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24:155–164. doi: 10.1055/s-2004-828892. [DOI] [PubMed] [Google Scholar]
- 22.Manfredi R, Masselli G, Maresca G, Brizi MG, Vecchioli A, Marano P. MR imaging and MRCP of hilar cholangiocarcinoma. Abdom Imaging. 2003;28:319–325. doi: 10.1007/s00261-002-0047-x. [DOI] [PubMed] [Google Scholar]
- 23.Masselli G, Gualdi G. Hilar cholangiocarcinoma: MRI/MRCP in staging and treatment planning. Abdom Imaging. 2008;33:444–451. doi: 10.1007/s00261-007-9281-6. [DOI] [PubMed] [Google Scholar]
- 24.Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Suppl 6:VI1–VI9. doi: 10.1136/gut.51.suppl_6.vi1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zidi SH, Prat F, Le Guen O, Rondeau Y, Pelletier G. Performance characteristics of magnetic resonance cholangiography in the staging of malignant hilar strictures. Gut. 2000;46:103–106. doi: 10.1136/gut.46.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–97. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Lee JD, Yang WI, Park YN, Kim KS, Choi JS, Yun M, Ko D, Kim TS, Cho AE, Kim HM, et al. Different glucose uptake and glycolytic mechanisms between hepatocellular carcinoma and intrahepatic mass-forming cholangiocarcinoma with increased (18)F-FDG uptake. J Nucl Med. 2005;46:1753–1759. [PubMed] [Google Scholar]
- 28.Reinhardt MJ, Strunk H, Gerhardt T, Roedel R, Jaeger U, Bucerius J, Sauerbruch T, Biersack HJ, Dumoulin FL. Detection of Klatskin's tumor in extrahepatic bile duct strictures using delayed 18F-FDG PET/CT: preliminary results for 22 patient studies. J Nucl Med. 2005;46:1158–1163. [PubMed] [Google Scholar]
- 29.Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK Jr, Pinson CW. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg. 1998;133:510–515; discussion 515-516. doi: 10.1001/archsurg.133.5.510. [DOI] [PubMed] [Google Scholar]
- 30.Kim YJ, Yun M, Lee WJ, Kim KS, Lee JD. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur J Nucl Med Mol Imaging. 2003;30:1467–1472. doi: 10.1007/s00259-003-1297-8. [DOI] [PubMed] [Google Scholar]
- 31.Wakabayashi H, Akamoto S, Yachida S, Okano K, Izuishi K, Nishiyama Y, Maeta H. Significance of fluorodeoxyglucose PET imaging in the diagnosis of malignancies in patients with biliary stricture. Eur J Surg Oncol. 2005;31:1175–1179. doi: 10.1016/j.ejso.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Park MS, Kim TK, Kim KW, Park SW, Lee JK, Kim JS, Lee JH, Kim KA, Kim AY, Kim PN, et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004;233:234–240. doi: 10.1148/radiol.2331031446. [DOI] [PubMed] [Google Scholar]
- 33.Rosch T, Meining A, Fruhmorgen S, Zillinger C, Schusdziarra V, Hellerhoff K, Classen M, Helmberger H. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc. 2002;55:870–876. doi: 10.1067/mge.2002.124206. [DOI] [PubMed] [Google Scholar]
- 34.Zuidema GD, Cameron JL, Sitzmann JV, Kadir S, Smith GW, Kaufman SL, White RI Jr. Percutaneous transhepatic management of complex biliary problems. Ann Surg. 1983;197:584–593. doi: 10.1097/00000658-198305000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington DP, Barth KH, Maddrey WC, Kaufman SL, Cameron JL. Percutaneously placed biliary stents in the management of malignant biliary obstruction. Dig Dis Sci. 1979;24:849–857. doi: 10.1007/BF01324901. [DOI] [PubMed] [Google Scholar]
- 36.Mueller PR, van Sonnenberg E, Ferrucci JT Jr. Percutaneous biliary drainage: technical and catheter-related problems in 200 procedures. AJR Am J Roentgenol. 1982;138:17–23. doi: 10.2214/ajr.138.1.17. [DOI] [PubMed] [Google Scholar]
- 37.Yee AC, Ho CS. Complications of percutaneous biliary drainage: benign vs malignant diseases. AJR Am J Roentgenol. 1987;148:1207–1209. doi: 10.2214/ajr.148.6.1207. [DOI] [PubMed] [Google Scholar]
- 38.Clark RA, Mitchell SE, Colley DP, Alexander E. Percutaneous catheter biliary decompression. AJR Am J Roentgenol. 1981;137:503–509. doi: 10.2214/ajr.137.3.503. [DOI] [PubMed] [Google Scholar]
- 39.Carrasco CH, Zornoza J, Bechtel WJ. Malignant biliary obstruction: complications of percutaneous biliary drainage. Radiology. 1984;152:343–346. doi: 10.1148/radiology.152.2.6739796. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda Y, Tsuyuguchi T, Sakai Y, Tsuchiya S, Saisyo H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc. 2005;62:374–382. doi: 10.1016/j.gie.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 41.Tamada K, Inui K, Menzel J. Intraductal ultrasonography of the bile duct system. Endoscopy. 2001;33:878–885. doi: 10.1055/s-2004-814512. [DOI] [PubMed] [Google Scholar]
- 42.Levy MJ, Vazquez-Sequeiros E, Wiersema MJ. Evaluation of the pancreaticobiliary ductal systems by intraductal US. Gastrointest Endosc. 2002;55:397–408. doi: 10.1067/mge.2002.121878. [DOI] [PubMed] [Google Scholar]
- 43.Tamada K, Ido K, Ueno N, Kimura K, Ichiyama M, Tomiyama T. Preoperative staging of extrahepatic bile duct cancer with intraductal ultrasonography. Am J Gastroenterol. 1995;90:239–246. [PubMed] [Google Scholar]
- 44.Tamada K, Ido K, Ueno N, Ichiyama M, Tomiyama T, Nishizono T, Wada S, Noda T, Tano S, Aizawa T. Assessment of portal vein invasion by bile duct cancer using intraductal ultrasonography. Endoscopy. 1995;27:573–578. doi: 10.1055/s-2007-1005760. [DOI] [PubMed] [Google Scholar]
- 45.Tamada K, Ido K, Ueno N, Ichiyama M, Tomiyama T, Nishizono T, Wada S, Noda T, Tano S, Aizawa T. Assessment of hepatic artery invasion by bile duct cancer using intraductal ultrasonography. Endoscopy. 1995;27:579–583. doi: 10.1055/s-2007-1005761. [DOI] [PubMed] [Google Scholar]
- 46.Tamada K, Nagai H, Yasuda Y, Tomiyama T, Ohashi A, Wada S, Kanai N, Satoh Y, Ido K, Sugano K. Transpapillary intraductal US prior to biliary drainage in the assessment of longitudinal spread of extrahepatic bile duct carcinoma. Gastrointest Endosc. 2001;53:300–307. doi: 10.1016/s0016-5107(01)70402-6. [DOI] [PubMed] [Google Scholar]
- 47.Stavropoulos S, Larghi A, Verna E, Battezzati P, Stevens P. Intraductal ultrasound for the evaluation of patients with biliary strictures and no abdominal mass on computed tomography. Endoscopy. 2005;37:715–721. doi: 10.1055/s-2005-870132. [DOI] [PubMed] [Google Scholar]
- 48.Tamada K, Tomiyama T, Wada S, Ohashi A, Satoh Y, Ido K, Sugano K. Endoscopic transpapillary bile duct biopsy with the combination of intraductal ultrasonography in the diagnosis of biliary strictures. Gut. 2002;50:326–331. doi: 10.1136/gut.50.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vazquez-Sequeiros E, Baron TH, Clain JE, Gostout CJ, Norton ID, Petersen BT, Levy MJ, Jondal ML, Wiersema MJ. Evaluation of indeterminate bile duct strictures by intraductal US. Gastrointest Endosc. 2002;56:372–379. [PubMed] [Google Scholar]
- 50.Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, Syed SA, Vickers SM, Mel Wilcox C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209–213. doi: 10.1016/s1542-3565(04)00005-9. [DOI] [PubMed] [Google Scholar]
- 51.Byrne MF, Gerke H, Mitchell RM, Stiffler HL, McGrath K, Branch MS, Baillie J, Jowell PS. Yield of endoscopic ultrasound-guided fine-needle aspiration of bile duct lesions. Endoscopy. 2004;36:715–719. doi: 10.1055/s-2004-825657. [DOI] [PubMed] [Google Scholar]
- 52.Brugge WR. Endoscopic techniques to diagnose and manage biliary tumors. J Clin Oncol. 2005;23:4561–4565. doi: 10.1200/JCO.2005.19.729. [DOI] [PubMed] [Google Scholar]
- 53.Osnes M, Serck-Hanssen A, Myren J. Endoscopic retrograde brush cytology (ERBC) of the biliary and pancreatic ducts. Scand J Gastroenterol. 1975;10:829–831. [PubMed] [Google Scholar]
- 54.Jailwala J, Fogel EL, Sherman S, Gottlieb K, Flueckiger J, Bucksot LG, Lehman GA. Triple-tissue sampling at ERCP in malignant biliary obstruction. Gastrointest Endosc. 2000;51:383–390. doi: 10.1016/s0016-5107(00)70435-4. [DOI] [PubMed] [Google Scholar]
- 55.Mansfield JC, Griffin SM, Wadehra V, Matthewson K. A prospective evaluation of cytology from biliary strictures. Gut. 1997;40:671–677. doi: 10.1136/gut.40.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macken E, Drijkoningen M, Van Aken E, Van Steenbergen W. Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg. 2000;63:254–259. [PubMed] [Google Scholar]
- 57.Ponchon T, Gagnon P, Berger F, Labadie M, Liaras A, Chavaillon A, Bory R. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc. 1995;42:565–572. doi: 10.1016/s0016-5107(95)70012-9. [DOI] [PubMed] [Google Scholar]
- 58.Pugliese V, Conio M, Nicolo G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995;42:520–526. doi: 10.1016/s0016-5107(95)70004-8. [DOI] [PubMed] [Google Scholar]
- 59.Kubota Y, Yamaguchi T, Tani K, Takaoka M, Fujimura K, Ogura M, Yamamoto S, Mizuno T, Inoue K. Anatomical variation of pancreatobiliary ducts in biliary stone diseases. Abdom Imaging. 1993;18:145–149. doi: 10.1007/BF00198052. [DOI] [PubMed] [Google Scholar]
- 60.Weber A, von Weyhern C, Fend F, Schneider J, Neu B, Meining A, Weidenbach H, Schmid RM, Prinz C. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J Gastroenterol. 2008;14:1097–1101. doi: 10.3748/wjg.14.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kipp BR, Stadheim LM, Halling SA, Pochron NL, Harmsen S, Nagorney DM, Sebo TJ, Therneau TM, Gores GJ, de Groen PC, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675–1681. doi: 10.1111/j.1572-0241.2004.30281.x. [DOI] [PubMed] [Google Scholar]
- 63.Zellweger T, Benz G, Cathomas G, Mihatsch MJ, Sulser T, Gasser TC, Bubendorf L. Multi-target fluorescence in situ hybridization in bladder washings for prediction of recurrent bladder cancer. Int J Cancer. 2006;119:1660–1665. doi: 10.1002/ijc.21704. [DOI] [PubMed] [Google Scholar]
- 64.Wamsteker EJ, Anderson MA. Fluorescence in situ hybridization for the detection of malignant bile duct strictures: has FISH found a new pond? Am J Gastroenterol. 2004;99:1682–1683. doi: 10.1111/j.1572-0241.2004.40744.x. [DOI] [PubMed] [Google Scholar]
- 65.Bergquist A, Tribukait B, Glaumann H, Broome U. Can DNA cytometry be used for evaluation of malignancy and premalignancy in bile duct strictures in primary sclerosing cholangitis? J Hepatol. 2000;33:873–877. doi: 10.1016/s0168-8278(00)80117-8. [DOI] [PubMed] [Google Scholar]


