Abstract
Capsule endoscopy has been shown to detect small bowel inflammatory changes better than any other imaging modality. Selection criteria have been optimized to increase the yield of capsule endoscopy in patients suspected to have Crohn’s disease. Capsule endoscopy allows for earlier diagnosis of Crohn’s disease of the small bowel and improved diagnosis of colitis in patients where it is unclear if they suffer from Crohn’s or ulcerative colitis. A test capsule is available to assess for small bowel strictures and thus avoid capsule retention. A common language has been developed and a new scoring index will be added to capsule software. It is envisioned that the manner in which we treat Crohn's disease in the future will change, based on earlier diagnosis and treatment aimed at mucosal healing rather than symptom improvement.
Keywords: Capsule endoscopy, Crohn’s disease, Capsule scoring index
INTRODUCTION
Capsule endoscopy (CE) was initially marketed in 2001 and in short time, this procedure gained a reputation for providing state-of-the-art imaging of the small intestine. It is recommended as the third test used in the investigation of obscure gastrointestinal bleeding after colonoscopy and upper endoscopy[1]. Capsule endoscopy has also been shown to be of use in patients with suspected Crohn’s disease. Many papers have been published on the utility of capsule endoscopy for the diagnosis of Crohn’s disease in cases of both suspected and known illness[2–5]. In a pooled data analysis, CE had a miss rate for ulcers of 0.5%[6]. A meta-analysis of eleven studies including 223 patients, comparing CE to other imaging modalities of the small bowel for inflammatory bowel disease established that CE has an incremental diagnostic yield of 25%-40% over other modalities such as barium studies and CT scanning (Table 1)[7]. This ability to visualize ulcers and other subtle inflammatory lesions led to the use of this technology to study the adverse effects of non-steroidal anti-inflammatory drugs (NSAID) on the small intestine[8]. The International Conference on Capsule Endoscopy (ICCE) consensus statement concluded that capsule endoscopy identifies small-bowel mucosal lesions not seen with other imaging modalities and may therefore play an important diagnostic role in the evaluation and monitoring of patients with known or suspected Crohn’s disease[9]. Secondly, they concluded that capsule endoscopy may have a unique role in assessing mucosal healing after medical therapy, for assessing early postoperative recurrence and in guiding therapy, and finally the consensus statement concluded that capsule endoscopy may identify sub-clinical markers in asymptomatic family members and contribute to the understanding of the natural history inflammatory bowel disease (IBD).
Table 1.
The incremental yield of capsule endoscopy over other testing (Treister et al[7])
| Total yield CE (%) | Total yield other modality (%) | % IY for CE (95% CI) | |
| Small bowel series | 66 | 24 | 42 (0.30-0.54) |
| Ileoscopy | 61 | 46 | 15 (0.02-0.27) |
| CT enterography | 75 | 37 | 38 (0.23-0.54) |
| Push enteroscopy | 51 | 7 | 44 (0.31-0.57) |
| Small bowel MRI | 60 | 40 | 20 (0.41-0.81) |
SUSPECTED CROHN’S DISEASE
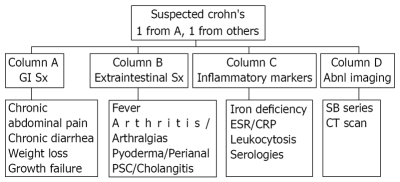
With capsule endoscopy able to identify mucosal changes before other technologies, it is often used in patients with suspected Crohn’s disease. This is a group that previously had not been formally defined. Suspicion of Crohn’s disease was previously left to the discretion of the treating physician and usually was considered when a patient had either abdominal pain or persistent diarrhea. Yields of capsule endoscopy are low when performed in patients with abdominal pain alone[10] and in patients with abdominal pain and diarrhea alone[11]. When other criteria are added this yield increases. The addition of a sign or symptom of inflammation increases the yield of capsule endoscopy. In the CEDAP-Plus study of 50 patients with suspected Crohn’s disease, signs of inflammation included elevated erthrocyte sedimentation rate, elevated C-reactive protein, thrombocytosis and leukocytosis and one of these markers increased the yield of capsule endoscopy with an odds ration of 3.2[12]. The landmark paper by Fireman enrolled patients with abdominal pain, diarrhea, anemia, and weight loss. These patients had had symptoms for an average of 6.3 years and all had normal colonoscopies, upper endoscopies and small bowel series. Crohn’s disease was diagnosed in 12 of the 17 by capsule endoscopy. It is clear that selective criteria were needed. The first consensus statement of the ICCE addressed the proper selection of patients and the group of suspected Crohn’s disease was defined[9]. More recently, the ICCE convened in part to expand their definition of patients who should be considered as being suspect for Crohn’s disease[13]. An algorithm was formulated (Figure 1). Patients should be considered for capsule endoscopy to diagnose or exclude the diagnosis of Crohn’s disease if they had symptoms plus either extraintestinal manifestations, inflammatory markers or abnormal imaging studies.
Figure 1.
Criteria for suspected Crohn’s disease (Mergener et al[13]).
INDETERMINATE COLITIS
Another emerging use of capsule endoscopy in the field of inflammatory bowel disease is in patients with indeterminate colitis. Colonoscopic and pathologic criteria cannot differentiate Crohn’s from ulcerative colitis in 10%-15% of colitic cases[14]. Proper identification of the disease state is important especially when choosing a surgical intervention. A few studies have examined the potential role of CE to rule out small bowel lesions suggestive of Crohn’s disease in the setting of indeterminate colitis. Mow reported finding small bowel lesions in 40% of indeterminate cases[4]. Manoury reported 30 patients with indeterminate colitis and negative serologies in whom CE identified 5 cases with Crohn’s[15]. This problem is especially difficult in children where the diagnosis cannot be assured in up to 30% of cases[16]. Use of capsule endoscopy in children has increased. Studies show that swallowing is possible in almost all cases, and in small children, the capsule is placed endoscopically[17].
RETENTION
Despite the ability to identify ulcers where no other technology could, there have been limitations to the application of this new technology. Fear of retention is one. Having a capsule retained in the small bowel remains a major concern for physicians performing capsule endoscopy since it could possibly lead to surgery in a patient who may otherwise have been treated medically for the same illness. This has been felt to be true especially for patients with Crohn’s disease or NSAID enteropathy. The ICCE consensus statement on capsule retention reported a 1.5% risk of retention when capsule endoscopy is performed in the setting of suspected Crohn’s disease[18]. Cheifetz reported retention in 13% of exams performed in the setting of previously known Crohn’s disease[19].
The ICCE consensus statement defined capsule retention as having a capsule endoscope remain in the digestive tract for a minimum of two weeks[18]. Retention was also defined as the capsule permanently remaining in the bowel lumen unless extracted by endoscopic or surgical methods or if passed as a result of medical therapy. There is no data on the success of medical therapies for retention such as initiating a course of steroids or infliximab, stopping NSAIDs, or using prokinetics or cathartics to aid in passage of the capsule. There is no time limit to institute management for capsule removal and capsules have stayed in patients asymptomatically for over 3 years.
It is up to the physician and patient together to decide the best management for capsule retention. The choice of surgical, endoscopic, or medical management once capsule retention has been diagnosed depends on the cause of the retention, the indication for the exam in the first place, and the extent of previous treatment. If retention occurs behind a tumor or mass, surgical intervention is typically pursued quickly. If retention occurs behind a Crohn’s stricture and the patient has had pronounced bleeding, again surgical intervention may prove the most efficacious method of not only removing the capsule but also dealing with the cause of hemorrhage. This is equally true for retention in a patient with known Crohn’s disease and recurrent symptoms but without documented disease by any other method and failure to respond to medical therapy prior to the capsule exam. Those with known Crohn’s disease who have already maximized their treatment with biologics and steroids for ongoing symptoms are not likely to improve without surgical intervention. Finally, for the patient in whom bleeding is not pronounced or in whom prior disease was only suspected but not treated, capsule retention behind a NSAID or Crohn’s stricture can be managed with double balloon enteroscopy[20]. This technique allows for capsule retrieval and then the patient can be treated medically for their underlying illness.
In an effort to avoid such situations, a dissolving test capsule called a patency capsule has been developed[21]. This capsule is the same size as a video capsule. It is constructed of cellophane with wax plugs at either end and it contains lactose mixed with 10% barium to make it radio-opaque. The wax plugs have holes that allow sucus entericus to dissolve the lactose and thus collapse the capsule into its various parts. A 2 mm × 10 mm radiotag inside the capsule allows the patient to be scanned externally to see if it is present in the body. Typically the patency capsule is swallowed by the patient and he or she is scanned 30 h after ingestion. At 30 h the capsule begins to dissolve. Thirty-eight percent of patency capsules are dissolved by 35 h and all are dissolved by 36-72 h.
CAPSULE SCORING INDEX
The other major limitation to the adoption of this technology has been the lack of standardization when describing small bowel inflammatory lesions, in terms of their extent and severity. Specifically, no one language for findings has been developed, and no severity scale of mucosal disease activity or even a threshold for disease diagnosis has been agreed upon. There are many clinical scoring indices for Crohn’s disease including the Crohn’s disease activity index (CDAI), the Harvey-Bradshaw and van Hees indices among others[22]. Since prior to CE, there has been no good direct measure of mucosal disease activity in the small intestine, these indices are based on clinical symptoms and some laboratory parameters. There is high interobserver variability in these scores due to their subjective nature[23]. Capsule endoscopy has provided the ability to detect mucosal inflammatory change of the small intestine often missed by other techniques. Interpretation and comparison of previous reports on the yield of CE have been limited due to the lack of a standardized and validated scoring index. The landmark papers by Fireman and Eliakim describing the yield of CE in suspected Crohn’s disease did not outline the findings necessary to make the diagnosis[2,24]. Goldstein, who compared the effect of naproxen versus celecoxib on the small intestine, counted the number of mucosal breaks to measure adverse drug effects[8]. Mow used the cut off of three ulcers of any size to establish a diagnosis of Crohn’s disease[4]. Fidder defined a positive capsule study for Crohn’s disease as four or more ulcers, erosions, or a region with clear exudate and mucosal hyperemia and edema[25]. The meta-analysis of CE in the setting of Crohn’s disease recognized this lack of a uniform method of categorizing findings at capsule endoscopy[7].
A scoring index has been developed to assess mucosal inflammatory disease in the small bowel detected by CE and this will be included in the next version of capsule endoscopy software (Table 2)[26]. This scoring index is based on three capsule endoscopic variables: villous appearance, ulceration and stenosis (Figure 2). In addition, each variable is assessed by other parameters including size and extent of the change. The changes in villous appearance and ulceration are assessed by tertiles, dividing the small bowel transit time into three equal time allotments. The stenosis evaluation is done for one entire study. The endoscopic variables have been specifically defined. Villous appearance is defined as edema where villous width is equal or greater than villous height. Villous appearance is based on mucosa distinct and separated from an ulcer rather than contiguous to a mucosal break. Ulcerations are defined as mucosal breaks with white or yellow bases surrounded by red or pink collars. Ulcer size is based on the entire lesion including its surrounding collar and is measured according to the percentage of the capsule image occupied by the ulcerated lesion. Ulcer size is based on the largest ulcer seen in each tertile. The number of lesions was defined as single, few (2-7 lesions) or multiple (8 or more lesions). The index was created in four separate steps. First, as outlined above, the characteristics of inflammatory change in the small bowel were identified. The terminology used accepted structured language developed for capsule endoscopy[27]. Second, blinded readers graded the presence or absence of each parameter on de-identified videos prospectively and also graded a perceived global assessment of overall severity of the findings. Third, the individual parameters and their descriptors were ranked in order of severity. In the fourth step, values for each parameter were created using the descent gradient method, a mathematic method to optimize numbers assigned to a rank order of variables. The premise was to assure that a final numerical score reflected the global assessment and that the global assessment agreed with the ranking of finding severity.
Table 2.
Parameters and weightings for the capsule endoscopy scoring index (Gralnek et al[26])
| Parameters | Number | Longitudinal extent | Descriptors | |
| First tertile | Villous appearance | Normal: 0 | Short segment: 8 | Single: 1 |
| Edematous: 1 | Long segment: 12 | Patchy: 14 | ||
| Whole tertile: 20 | Diffuse: 17 | |||
| Ulcer | None: 0 | Short segment: 5 | < 1/4: 9 | |
| Single: 3 | Long segment: 10 | 1/4-1/2: 12 | ||
| Few: 5 | Whole tertile: 15 | > 1/2: 18 | ||
| Multiple: 10 | ||||
| Second tertile | Villous appearance | Normal: 0 | Short segment: 8 | Single: 1 |
| Edematous: 1 | Long segment: 12 | Patchy: 14 | ||
| Whole tertile: 20 | Diffuse: 17 | |||
| Ulcer | None: 0 | Short segment: 5 | < 1/4: 9 | |
| Single: 3 | Long segment: 10 | 1/4-1/2: 12 | ||
| Few: 5 | Whole tertile: 15 | > 1/2: 18 | ||
| Multiple: 10 | ||||
| Third tertile | Villous appearance | Normal: 0 | Short segment: 8 | Single: 1 |
| Edematous: 1 | Long segment: 12 | Patchy: 14 | ||
| Whole tertile: 20 | Diffuse: 17 | |||
| Ulcer | None: 0 | Short segment: 5 | < 1/4: 9 | |
| Single: 3 | Long segment: 10 | 1/4-1/2: 12 | ||
| Few: 5 | Whole tertile: 15 | > 1/2: 18 | ||
| Multiple: 10 | ||||
| Stenosis-Rated for Whole Study | Stenosis | None: 0 | Ulcerated: 24 | Traversed: 7 |
| Single: 14 | Non-Ulcerated: 2 | Not traversed: 10 | ||
| Multiple: 20 |
Figure 2.
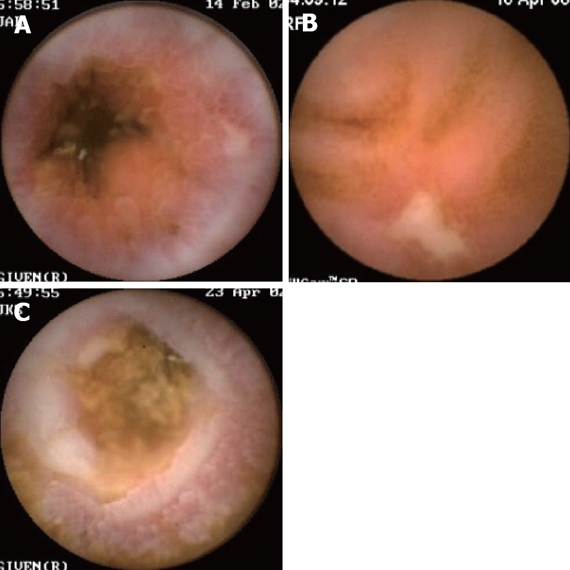
Examples of endoscopic findings of Crohn’s disease at capsule endoscopy. A: Edema; B: Ulceration; C: Stricturing.
The score provides a common language to quantify mucosal changes associated with any inflammatory process. The index does not diagnose or measure a disease; it measures mucosal change. In addition, this scoring index does not have the discriminatory ability to differentiate these illnesses. At the same time however, the index could be used for a number of different purposes including differentiating normal small bowel from disease states. This scoring index may be able to help establish the diagnosis of small bowel Crohn’s disease when combined with other clinical signs/symptoms including patient history, clinical presentation and laboratory values. The index could also be potentially used to measure and document mucosal healing in response to therapy. The CE scoring index can provide one more point of evaluation along with other patient-level data to assist in determining appropriate patient management. Finally, the score could be a standardized method of communication both for treating physicians and for research purposes when assessing therapies and outcomes of patient’s with small bowel Crohn’s disease.
THE FUTURE OF CAPSULE ENDOSCOPY IN IBD
Capsule endoscopy has the opportunity to propel a coming paradigm shift in the treatment of Crohn’s disease. It is clear that capsule endoscopy identifies the earliest inflammatory changes in the small bowel. At the same time, the average time from the onset of a patient’s symptoms until diagnosis historically lags an average of 35 mo[28]. Thus capsule endoscopy has the opportunity to diagnose Crohn’s disease earlier than ever before. What remains unclear is if early diagnosis provides patient benefit. Does earlier diagnosis and thus earlier intervention change the natural history of the disease? This is not known, though studies in children with fistulous disease had greater response to therapy the earlier they were diagnosed[29]. Thus it is theorized that early diagnosis will bring earlier treatment and thus improved outcomes. Another paradigm shift in the making is the method of assessing disease activity. Previously, physicians have used patient symptoms to guide treatment. Unfortunately placebo response rates by symptoms average 18% (0%-50%)[30]. Remission has been defined as symptom improvement typically using the CDAI. But remission does not correlate with mucosal healing. In Rutgeerts’ trial of 75 patients treated with infliximab, 67% of healed patients were in symptom remission, while 56% of remission patients did not heal[31]. Prior to capsule endoscopy there was no reliable method to determine the extent or severity of the disease in the small bowel though colonic evaluation has been available. It has been proposed that in the future, patient management decisions may be based on measures of mucosal healing rather than symptom response[32]. A variety of tools are available to assess overall disease activity including fecal and serum biomarkers, endoscopy, radiology, and capsule endoscopy.
In summary, capsule endoscopy has been shown to detect small bowel inflammatory changes better then any other imaging modality. A common language has been developed. It is envisioned that the manner in which we treat Crohn’s disease in the future will change, based on earlier diagnosis and treatment aimed at mucosal healing rather than symptom improvement.
Peer reviewer: Mario Guslandi, Professor, Department of Gastroenterology, S: Raffaele University Hospital, S: Raffaele University Hospitalvia Olgettina 60, Milano 20132, Italy
S- Editor Zhong XY L- Editor Lalor PF E- Editor Yin DH
References
- 1.Raju GS, Gerson L, Das A, Lewis B. American Gastroen-terological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1694–1696. doi: 10.1053/j.gastro.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Fireman Z, Mahajna E, Broide E, Shapiro M, Fich L, Sternberg A, Kopelman Y, Scapa E. Diagnosing small bowel Crohn's disease with wireless capsule endoscopy. Gut. 2003;52:390–392. doi: 10.1136/gut.52.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrerias J, Caunedo A, Rodriguez-Tellez M, Pellicer F, Herrerias JM Jr. Capsule endoscopy in patients with suspected Crohn's disease and negative endoscopy. Endoscopy. 2003;35:564–568. doi: 10.1055/s-2003-40241. [DOI] [PubMed] [Google Scholar]
- 4.Mow WS, Lo SK, Targan SR, Dubinsky MC, Treyzon L, Abreu-Martin MT, Papadakis KA, Vasiliauskas EA. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2:31–40. doi: 10.1016/s1542-3565(03)00289-1. [DOI] [PubMed] [Google Scholar]
- 5.Ge ZZ, Hu YB, Xiao SD. Capsule endoscopy in diagnosis of small bowel Crohn's disease. World J Gastroenterol. 2004;10:1349–1352. doi: 10.3748/wjg.v10.i9.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis BS, Eisen GM, Friedman S. A pooled analysis to evaluate results of capsule endoscopy trials. Endoscopy. 2005;37:960–965. doi: 10.1055/s-2005-870353. [DOI] [PubMed] [Google Scholar]
- 7.Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's disease. Am J Gastroenterol. 2006;101:954–964. doi: 10.1111/j.1572-0241.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 9.Kornbluth A, Colombel JF, Leighton JA, Loftus E. ICCE consensus for inflammatory bowel disease. Endoscopy. 2005;37:1051–1054. doi: 10.1055/s-2005-870315. [DOI] [PubMed] [Google Scholar]
- 10.Spada C, Pirozzi GA, Riccioni ME, Iacopini F, Marchese M, Costamagna G. Capsule endoscopy in patients with chronic abdominal pain. Dig Liver Dis. 2006;38:696–698. doi: 10.1016/j.dld.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Fry LC, Carey EJ, Shiff AD, Heigh RI, Sharma VK, Post JK, Hentz JG, Fleischer DE, Leighton JA. The yield of capsule endoscopy in patients with abdominal pain or diarrhea. Endoscopy. 2006;38:498–502. doi: 10.1055/s-2006-925340. [DOI] [PubMed] [Google Scholar]
- 12.May A, Manner H, Schneider M, Ipsen A, Ell C. Prospective multicenter trial of capsule endoscopy in patients with chronic abdominal pain, diarrhea and other signs and symptoms (CEDAP-Plus Study) Endoscopy. 2007;39:606–612. doi: 10.1055/s-2007-966640. [DOI] [PubMed] [Google Scholar]
- 13.Mergener K, Ponchon T, Gralnek I, Pennazio M, Gay G, Selby W, Seidman EG, Cellier C, Murray J, de Franchis R, et al. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy. 2007;39:895–909. doi: 10.1055/s-2007-966930. [DOI] [PubMed] [Google Scholar]
- 14.Guindi M, Riddell RH. Indeterminate colitis. J Clin Pathol. 2004;57:1233–1244. doi: 10.1136/jcp.2003.015214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maunoury V, Savoye G, Bourreille A, Bouhnik Y, Jarry M, Sacher-Huvelin S, Ben Soussan E, Lerebours E, Galmiche JP, Colombel JF. Value of wireless capsule endoscopy in patients with indeterminate colitis (inflammatory bowel disease type unclassified) Inflamm Bowel Dis. 2007;13:152–155. doi: 10.1002/ibd.20060. [DOI] [PubMed] [Google Scholar]
- 16.Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD, Griffiths AM, Jevon GP, Higuchi LM, Hyams JS, et al. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn's and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44:653–674. doi: 10.1097/MPG.0b013e31805563f3. [DOI] [PubMed] [Google Scholar]
- 17.Ge ZZ, Chen HY, Gao YJ, Gu JL, Hu YB, Xiao SD. Clinical application of wireless capsule endoscopy in pediatric patients for suspected small bowel diseases. Eur J Pediatr. 2007;166:825–829. doi: 10.1007/s00431-006-0331-9. [DOI] [PubMed] [Google Scholar]
- 18.Cave D, Legnani P, de Franchis R, Lewis BS. ICCE consensus for capsule retention. Endoscopy. 2005;37:1065–1067. doi: 10.1055/s-2005-870264. [DOI] [PubMed] [Google Scholar]
- 19.Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, Lichtiger S, Lewis BS. The risk of retention of the capsule endoscope in patients with known or suspected Crohn's disease. Am J Gastroenterol. 2006;101:2218–2222. doi: 10.1111/j.1572-0241.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka S, Mitsui K, Shirakawa K, Tatsuguchi A, Nakamura T, Hayashi Y, Sakamoto C, Terano A. Successful retrieval of video capsule endoscopy retained at ileal stenosis of Crohn's disease using double-balloon endoscopy. J Gastroenterol Hepatol. 2006;21:922–923. doi: 10.1111/j.1440-1746.2006.04120.x. [DOI] [PubMed] [Google Scholar]
- 21.Spada C, Shah SK, Riccioni ME, Spera G, Marchese M, Iacopini F, Familiari P, Costamagna G. Video capsule endoscopy in patients with known or suspected small bowel stricture previously tested with the dissolving patency capsule. J Clin Gastroenterol. 2007;41:576–582. doi: 10.1097/01.mcg.0000225633.14663.64. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen LG, Fredholm L, Hyltoft Petersen P, Hey H, Munkholm P, Brandslund I. How accurate are clinical activity indices for scoring of disease activity in inflammatory bowel disease (IBD)? Clin Chem Lab Med. 2005;43:403–411. doi: 10.1515/CCLM.2005.073. [DOI] [PubMed] [Google Scholar]
- 23.Sostegni R, Daperno M, Scaglione N, Lavagna A, Rocca R, Pera A. Review article: Crohn's disease: monitoring disease activity. Aliment Pharmacol Ther. 2003;17 Suppl 2:11–17. doi: 10.1046/j.1365-2036.17.s2.17.x. [DOI] [PubMed] [Google Scholar]
- 24.Eliakim R, Fischer D, Suissa A, Yassin K, Katz D, Guttman N, Migdal M. Wireless capsule video endoscopy is a superior diagnostic tool in comparison to barium follow-through and computerized tomography in patients with suspected Crohn's disease. Eur J Gastroenterol Hepatol. 2003;15:363–367. doi: 10.1097/00042737-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Fidder HH, Nadler M, Lahat A, Lahav M, Bardan E, Avidan B, Bar-Meir S. The utility of capsule endoscopy in the diagnosis of Crohn's disease based on patient's symptoms. J Clin Gastroenterol. 2007;41:384–387. doi: 10.1097/01.mcg.0000225621.02094.8a. [DOI] [PubMed] [Google Scholar]
- 26.Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146–154. doi: 10.1111/j.1365-2036.2007.03556.x. [DOI] [PubMed] [Google Scholar]
- 27.Delvaux M, Friedman S, Keuchel M, Hagenmuller F, Weinstein M, Cave D, de Franchis R, Gay G, Korman LY. Structured terminology for capsule endoscopy: results of retrospective testing and validation in 766 small-bowel investigations. Endoscopy. 2005;37:945–950. doi: 10.1055/s-2005-870266. [DOI] [PubMed] [Google Scholar]
- 28.Mekhjian HS, Switz DM, Melnyk CS, Rankin GB, Brooks RK. Clinical features and natural history of Crohn's disease. Gastroenterology. 1979;77:898–906. [PubMed] [Google Scholar]
- 29.Lionetti P, Bronzini F, Salvestrini C, Bascietto C, Canani RB, De Angelis GL, Guariso G, Martelossi S, Papadatou B, Barabino A. Response to infliximab is related to disease duration in paediatric Crohn's disease. Aliment Pharmacol Ther. 2003;18:425–431. doi: 10.1046/j.1365-2036.2003.01672.x. [DOI] [PubMed] [Google Scholar]
- 30.Su C, Lichtenstein GR, Krok K, Brensinger CM, Lewis JD. A meta-analysis of the placebo rates of remission and response in clinical trials of active Crohn's disease. Gastroenterology. 2004;126:1257–1269. doi: 10.1053/j.gastro.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 31.Rutgeerts P, Diamond RH, Bala M, Olson A, Lichtenstein GR, Bao W, Patel K, Wolf DC, Safdi M, Colombel JF, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc. 2006;63:433–442; quiz 464. doi: 10.1016/j.gie.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Loftus EV Jr. Clinical perspectives in Crohn's disease. Objective measures of disease activity: alternatives to symptom indices. Rev Gastroenterol Disord. 2007;7 Suppl 2:S8–S16. [PubMed] [Google Scholar]