Abstract
Video capsule endoscopy is an attractive and patient-friendly tool that provides high quality images of the small bowel. Obscure gastrointestinal bleeding is the primary and most evaluated indication to capsule endoscopy; however, indications are expanding and a small number of preliminary reports have been presented concerning the role of video capsule endoscopy in the diagnosis of celiac disease. The purpose of this review is to update the current knowledge and to hypothesize on future perspectives of the use of video capsule endoscopy in patients with celiac disease.
Keywords: Capsule endoscopy, Celiac disease, Diagnosis of celiac disease, Celiac disease complications
INTRODUCTION
Celiac disease is a gluten-dependent enteropathy, characterized by chronic small bowel inflammation and mucosal atrophy. Up to a few years ago, celiac disease was considered a rare pathology. Today, its prevalence has been estimated ranging between 0.2% and 1% in the United States and European population[1]. Although celiac disease was believed to be a pediatric syndrome, it is now recognized mainly as an adult disease that involves multiple organs. Abdominal pain, diarrhea, growth failure and malabsorption are its typical clinical presentation. However, the increased interest for this pathology over the last 2 decades allowed diagnosing celiac disease also in those with the silent or “atypical” form. These patients may present vague and subclinical manifestations such as dyspeptic symptoms or esophageal reflux, irritable bowel syndrome, polyneuropathy or iron deficiency anemia[2]. The disease can also be totally asymptomatic and the diagnosis can be accidental. The diagnosis of celiac disease is made by demonstrating the characteristic histopathological changes on intestinal biopsy obtained by esophagogastroduodenoscopy. Villous atrophy on a duodenal biopsy represents the internationally accepted gold standard diagnostic test for celiac disease. Serological tests include antigliadin, antiendomysium and anti-tissue transglutaminase antibodies. These tests are highly reliable for the diagnosis of celiac disease and represent a cheap and non-invasive method to identify patients who will be referred to endoscopy, specifically with the purpose of obtaining duodenal biopsy. The positive and negative predictive value of combining the measurement of antibodies from tissue transglutaminase and antiendomysium has been reported to be greater than 96%[3].
Capsule endoscopy is a well tolerated, minimally invasive method for the visualization of the entire small bowel and is currently used to evaluate patients with obscure bleeding, inflammatory bowel diseases, suspected small bowel tumours, polyposis syndromes, nonsteroidal anti-inflammatory drug injury and complicated celiac disease[4]. Video capsule endoscopy is provided with an 8-fold magnification capacity lens optical system that allows a magnification similar to that of dissection microscopy[5] and is therefore able to assess the small bowel villous structure. For this reason, video capsule endoscopy could offer an alternative to duodenal biopsy in patients who are unable or unwilling to undergo esophagogastroduodenoscopy. This article reviews the current knowledge and future prospects of the use of video capsule endoscopy in patients affected by celiac disease.
CRITERIA FOR DIAGNOSIS OF CELIAC DISEASE
The first step in pursuing a diagnosis for celiac disease is a serological test. The immunoglobulin A (IgA) anti-human tissue transglutaminase (t-TG) and IgA endomysial antibody immunofluorescence (EMA) are the best tests available. Although these tests are highly sensitive and specific, small bowel biopsy remains the standard for the diagnosis of celiac disease. Demonstration of hyperplastic villous atrophy of the small bowel and clinical remission when a gluten-free diet is followed represent the diagnostic tests for celiac disease[6].
ENDOSCOPIC SIGNS IN CELIAC DISEASE
Four endoscopic markers suggestive of villous atrophy have been described in celiac disease: loss or reduction in duodenal Kerkring’s folds, mosaic mucosal pattern, scalloped configuration of duodenal folds and micronodular pattern of the mucosa[7,8]. These markers should serve as a tool to assist endoscopists in deciding when small bowel biopsies may be indicated. Sensitivity of these markers for the diagnosis of celiac disease has been reported to be between 47% and 100%[8–10]. Endoscopic markers overall have a wide range in sensitivity mainly because of their absence when lesser degrees of villous atrophy are present. Therefore, endoscopic evaluation without biopsies is not considered sensitive enough for a diagnosis and is considered inadequate to confirm or to exclude celiac disease[11]. However, when endoscopic signs are present, they have a high specificity, ranging between 92% and 100%[2,10,12]. For this reason, investigators should be aware of the importance of such markers detected during endoscopy. Recognition of endoscopic signs of celiac disease could help to select patients for biopsy and avoid delays in the diagnosis of the disease, preventing long term complications. Recently, endoscopic approaches for the evaluation of duodenal villous abnormalities have been proposed allowing a direct visualization of the duodenal villous structure during routine upper endoscopy. Endoscopic visualization of intestinal villous pattern with the “immersion technique” provides a sensitivity, specificity, positive predictive value and negative predictive value of 85%, 99%, 99% and 90%, respectively[11,13]. Moreover, it has been demonstrated that the new high-magnification and high-resolution endoscopes are able to better evaluate the presence or absence of villous pattern. Sensitivity, specificity, positive predictive value and negative predictive value of endoscopic magnification (× 2) for detection of total villous atrophy were all 100%. Similar results were obtained combining the “immersion technique” with magnification[14]. Therefore, theoretically, in patients with suspected celiac disease (EMA+ and/or t-TG antibodies+) using these new endoscopic approaches, the biopsy sampling of the small bowel could be avoided when a flat duodenal mucosa is observed and should be reserved only for those patients in whom villi are observed at endoscopy[11].
CAPSULE ENDOSCOPY
Capsule endoscopy has become an important tool in the investigation of patients with small bowel diseases. Preliminary reports suggest that capsule endoscopy could represent an attractive and non-invasive diagnostic tool in patients with suspected celiac disease[15–19]. It can also be used in patients with known celiac disease for monitoring small bowel healing; in patients with alarming symptoms despite a closely followed gluten-free diet and in long-term surveillance to detect malignancies[20]. The diagnosis of celiac disease is still based on the recognition of villous atrophy on duodenal biopsy; however duodenal biopsy cannot be considered an optimal gold standard. The major limits are represented by the need to perform an upper gastrointestinal (GI) endoscopy which represents an invasive procedure; the difficulty to obtain proper oriented tissue samples; the occurrence of patchy mucosal lesions that can be missed by the biopsy; and the limited portion of gut examined with the risk of loosing the diagnosis of complications, such as enteropathy-associated T-cell lymphoma and ulcerative jejunoileitis. Capsule endoscopy is provided with certain features that may overcome some of the limits of traditional upper GI endoscopy. Capsule endoscopy is a non-invasive, painless and well accepted procedure. The capsule has the magnification power of a dissecting microscope: the optical system has an 8-folds magnification power, therefore the villi can be easily observed (Figure 1). The test is performed without air inflation, with the optical dome of capsule endosocopy close to the mucosa, improving the assessment of the villous architecture. Finally, it allows the visualization of the entire small bowel, providing an estimation of the extent of small bowel involvement and facilitating the diagnosis of complications.
Figure 1.
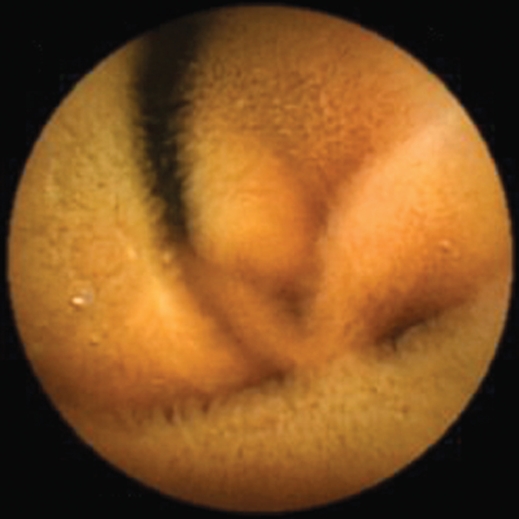
Normal endoscopic pattern at video capsule endoscopy. Villous pattern can be easily observed.
In a preliminary study, Petroniene et al[16] compared 10 celiac patients with histologically proven villous atrophy (Marsh III) with 10 control patients with normal histology, showing that capsule endoscopy has an excellent accuracy in identifying villous atrophy. When compared to upper GI endoscopy, capsule endoscopy reported an identical specificity (100%); a tendency towards a better sensitivity (70% vs 60%); and a positive predictive value and negative predictive value of 100% and 77%, respectively. These data were confirmed by recent studies[17,19]. Hopper et al[17] showed that 17 out of 20 patients with celiac disease had villous atrophy also detected by capsule endoscopy. In this paper, the sensitivity, specificity, positive predictive value and negative predictive value for capsule endoscopy in recognising villous atrophy were 85%, 100%, 100% and 88.9%, respectively. Upper GI endoscopy detected endoscopic markers consistent with celiac disease in 16 out of 20 celiac patients with a sensitivity, specificity, positive predictive value and negative predictive value of 80%, 100%, 100% and 85.7%, respectively. Capsule was more sensitive than conventional endoscopy in identifying endoscopic markers, but the difference observed did not achieve statistical significance. In the largest presented series, Rondonotti et al[19] reported on 43 consecutive patients with signs and/or symptoms suggestive of celiac disease and positive serological markers who underwent upper GI endoscopy and video capsule endoscopy. 87.5% of patients who had characteristic histological changes were diagnosed with celiac disease by capsule endoscopy. Overall, capsule endoscopy was reported to have a sensitivity of 87.5%, a specificity of 90.9%, a positive predictive value of 96.5%, a negative predictive value of 71.4% and positive and negative likelihood ratios of 9.6 and 0.14, respectively. Capsule endoscopy appeared highly performing in patients with Marsh III lesions, as it is able to correctly diagnose 89% of patients with this type of histological change.
Such promising data are not confirmed in the series presented by Biagi et al[5]. In this series, the authors classified the mucosal appearance as seen at capsule endoscopy as normal, hypotrophic and atrophic and evaluated whether there was a correlation between the degree of villous atrophy at the histology and capsule endoscopy results. Video capsule findings regarding the degree of small bowel mucosal atrophy showed only a moderate agreement with the histologic pattern, with a high sensitivity (range, 90.5%-95.2%), but a low specificity (63.6%). Positive predictive value was 100% and negative predictive value ranged between 77.8% and 87.5%.
Overall, capsule endoscopy seems to be able to recognize the endoscopic markers of celiac disease described in the literature[15,16,21]. Scalloping, mosaicism, micronodularity and reduction of folds (Figure 2) can all be seen by capsule endoscopy[16,19,22]. In addition to general mucosal pattern, capsule endoscopy can easily recognize finger-like villi (Figure 1)[16,17]. Table 1 shows the literature data on sensitivity, specificity, positive predictive value and negative predictive value of capsule endoscopy in celiac disease. Capsule endoscopy has a high sensitivity (range, 70%-95.2%), even better than that of upper GI endoscopy, an overall high specificity (range, 63.6%-100%) and high positive predictive value and negative predictive value, respectively between 96.5%-100% and 71.4%-88.9%. This means that when an atrophic pattern is observed by capsule endoscopy, patients have a very high probability to have celiac disease. However, the relatively low negative predictive value suggests that a normal capsule endoscopy pattern can not exclude definitively villous atrophy.
Figure 2.
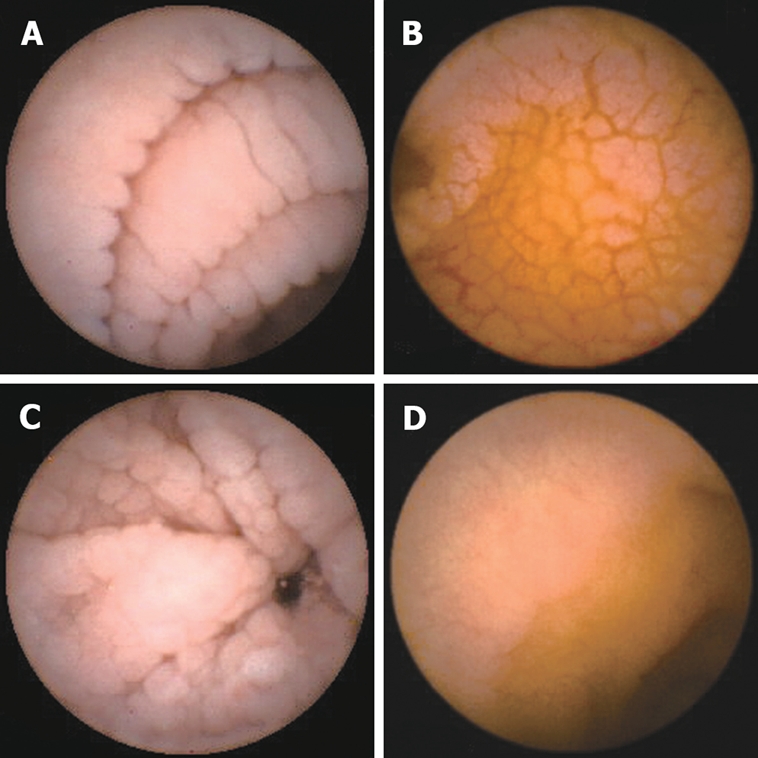
Endoscopic markers of celiac disease at capsule endoscopy: scalloping (A), mosaic pattern (B), micronodularity (C), and reduction of folds (D).
Table 1.
Sensitivity, specificity, PPVs and NPVs of capsule endoscopy in celiac disease from referenced articles (%)
The accuracy obtained by capsule endoscopy is similar to that of magnification endoscopy or of endoscopy with the “immersion technique”[11,13], however capsule endoscopy has the great advantage of being a non-invasive technique and to visualize the entire small bowel[15]. Overall, interobserver agreement was moderate to high in the detection of villous atrophy and ranged between 0.41 and 0.87[16,19]. The interobserver agreement is an important factor in assessing the accuracy of the tests employing subjective visual evaluation. Agreement between investigators for the diagnosis of specific endoscopic markers is different: while it is low for erosions (κ, 0.27-0.72) and reduction in the number or loss of duodenal folds (κ = 0.41 and 0.59, respectively), it is good for mosaic pattern (κ = 0.76) and scalloped folds (κ between 0.65 and 0.85)[5,16,19]. The lack of an overall high degree of agreement between investigators could mean that the correct visualization of villous atrophy is difficult even for physicians with long-term experience in capsule endoscopy. It has been demonstrated that the interobserver agreement varies significantly between investigators ranging from poor to perfect (κ, 0.26-1.0). The agreement is perfect between experts who have extensive experience with capsule endoscopy[16]. Familiarity with capsule endoscopy may be an important factor affecting the accuracy of the procedure and training sessions are needed for those endoscopists who are interested in capsule endoscopy. In fact, with physicians more experienced in evaluating patients with suspected or known celiac disease, an overall improvement in the interobserver agreement may be expected[19].
Capsule endoscopy also provides information on how much bowel is involved by the celiac disease, allowing the visualization of portions of the small bowel not accessible by other traditional endoscopic methods. Although precise evaluation of the extent of the affected bowel is not possible, it is, however, possible to determine whether the disease is confined to the duodenum, to the proximal part of the jejunum or whether it extends throughout the whole of the small bowel. Rondonotti et al[19] showed that 66.6% of patients had an extension of the mucosal changes seen at capsule endoscopy beyond the proximal small bowel, and 11.1% had lesions that involved the small bowel entirely. The significance of the extent of involvement of the small bowel in patients with celiac disease is still unclear. However, available data suggest that there is a trend in correlation between the severity of symptoms and the degree of small bowel involvement[16,19]. This could be an interesting and important topic for future research. Moreover, evaluation of the entire small bowel may reveal mucosal changes undetected by traditional endoscopy in case of a “patchy” distribution (Figure 3). Rondonotti et al[19] reported of a patient who had a normal mucosa at upper GI endoscopy and scalloping of folds in the distal part of duodenum at capsule endoscopy. Authors argued that the normal duodenal histology in this patient was a sampling error and the capsule findings were compatible with a “patchy” distribution of mucosal changes.
Figure 3.
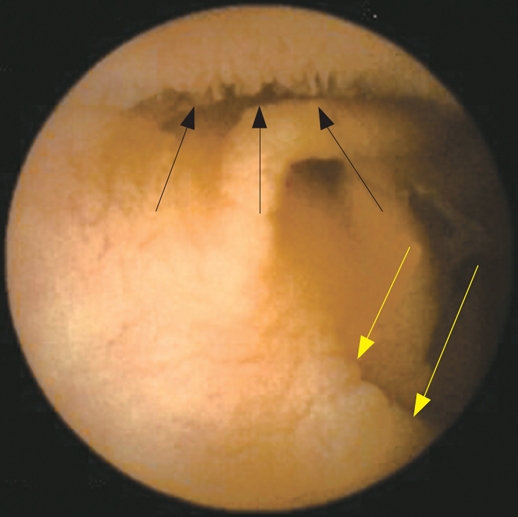
“Patchy” atrophy detected by capsule endoscopy. Capsule endoscopy shows a normal villous pattern in the upper part of the image (black arrows) and villous atrophy in the lower part (yellow arrows).
Celiac disease may be complicated by a variety of pathologies, including small bowel adenocarcinoma, intestinal T-cell lymphoma and ulcerative jejunitis. These complications are often not identifiable by conventional imaging modalities as they are located beyond the site reachable by traditional endoscopy. Capsule endoscopy has been reported to be able to demonstrate intussusception, ulcerative jejunoileitis, lymphoma and adenocarcinoma in patients with celiac disease[23–27]. In a series of 47 celiac patients with a high risk of complication (persistent unexplained abdominal pain, weight loss, history of small bowel neoplasia, long-standing celiac disease, positive faecal occult blood test or iron deficiency anaemia unresponsive to iron supplementation), lesions were detected in about 50% of cases[20]. These data support the role of capsule endoscopy in patients who have complicated disease, who present alarm symptoms or who do not respond to a gluten-free diet[28]. In this group of patients, capsule endoscopy should be promptly performed as it avoids several unnecessary diagnostic tests, it permits to visualize small bowel complications that generally are diagnosed late, and it allows initiation of specific therapies.
Unsuspected celiac disease can also be diagnosed during capsule endoscopy performed for other indications including abdominal pain, gastrointestinal bleeding, and dyspepsia[22,29,30]. In these cases, findings evocative for celiac disease should suggest the performance of additional testing to rule out celiac disease. For this reason, recognition of endoscopic markers for celiac disease and villous atrophy is mandatory for physicians who perform capsule endoscopy.
Data presented in the literature are interesting and they make us optimistic about the role of capsule endoscopy in the evaluation of patients with celiac disease. However, some issues remain still open and need to be clarified. In all the studies presented, patients had a high pre-test probability (EMA and/or t-TG positive) of having celiac disease. This may provide an over estimation of the performance of capsule endoscopy in the detection of endoscopic markers and villous atrophy. A further limitation is represented by patients with less severe histological changes (Marsh I and II). In fact, it is demonstrated that capsule endoscopy is able to detect the majority of Marsh III lesions, which are associated with evident mucosal abnormalities; however, it may not distinguish patients with Marsh I and II lesions as they may have a normal villous pattern. Furthermore, the role of capsule endoscopy in screening or surveillance for malignancies in patients with celiac disease should be clarified. It is unclear what group of patients should be screened or should undergo surveillance to detect small bowel malignancies[23]. Finally, it should be clarified whether capsule endoscopy could play a role in diagnosing celiac disease in patients with positive serologic tests and negative biopsies.
FUTURE PERSPECTIVES
Recently, new endoscopic approaches and technologies that allow a direct visualization of the duodenal villous structure with high accuracy have been proposed[11,13,31,32]. Using these new procedures, it is possible to detect the presence of the intestinal villi or to suggest their absence. These observations led the Authors to propose a new diagnostic approach to celiac disease avoiding the biopsy sampling. All these new methods still require “standard” endoscopy which is an invasive procedure, it is not well tolerated by patients and it only reaches the proximal part of the small bowel. Capsule endoscopy offers several advantages over standard endoscopy: it is a non-invasive procedure; it is well tolerated by patients; and it allows the visualization of the entire small bowel. Moreover, the optical system of the capsule allows an 8-fold magnification, providing a good visualization of small bowel villous pattern. Therefore, if a biopsy-avoiding approach using new endoscopic methods (such as the “immersion” technique or high-resolution and magnifying endoscope) seems reasonable, it appears more and more rational to obtain the same information recurring to a non-invasive procedure, that allows to explore all the small bowel. It is our feeling that in the next few years the diagnostic approach to celiac disease will change if larger studies will confirm the results published to date regarding the role of capsule endoscopy in celiac disease (Figure 4). “High risk” patients for celiac disease (positive specific antibodies)[3] will avoid traditional endoscopy and undergo capsule endoscopy directly; only those patients with a normal or suspected villous pattern at capsule endoscopy will undergo standard upper GI endoscopy with duodenal biopsy. Several studies are needed to confirm these new approaches.
Figure 4.
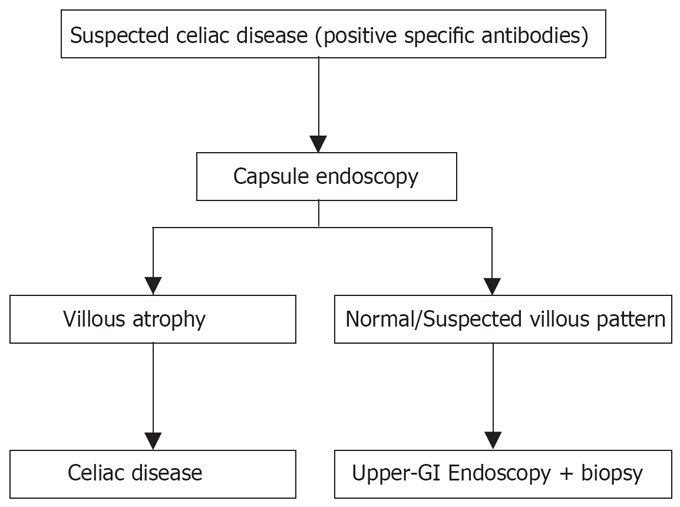
Possible algorithm in the diagnostic work-up of celiac disease.
CONCLUSION
The essential requirement for the diagnosis of celiac disease is the histopathologic demonstration of villous atrophy. For this reason, endoscopy plays a critical role as it permits to obtain duodenal specimens. Video capsule endoscopy provides good quality images of the small bowel mucosa, including well-defined villous pattern in patients with celiac disease. At present, capsule endoscopy may be an alternative to traditional endoscopy and duodenal biopsy in patients with suspected celiac disease who are unable or unwilling to undergo conventional upper GI endoscopy for confirmation of villous atrophy. Capsule endoscopy also provides information on the extent of the small bowel involved, but the meaning of this data is still unknown. Other potential indications for capsule endoscopy include complications related to celiac disease, refractory patients and long-term surveillance to detect malignancies. Further prospective studies are needed to confirm these preliminary results and to investigate if capsule endoscopy could represent a first line approach in patients with suspected celiac disease.
Peer reviewer: Dr. Simon S Campbell, MD, Depatment of Gastroenterology, Manchester Royal Infirmary, Oxford Road, Manchester, M12 9WL, United kingdom
S- Editor Zhong XY L- Editor Kremer M E- Editor Yin DH
References
- 1.Schapira M, Maisin JM, Ghilain JM, De Maeght S, Deltenre P, Henrion J. Epidemiology of coeliac disease. Acta Gastroenterol Belg. 2003;66:234–236. [PubMed] [Google Scholar]
- 2.Lee SK, Green PH. Endoscopy in celiac disease. Curr Opin Gastroenterol. 2005;21:589–594. doi: 10.1097/01.mog.0000174218.00333.19. [DOI] [PubMed] [Google Scholar]
- 3.Hopper AD, Cross SS, Hurlstone DP, McAlindon ME, Lobo AJ, Hadjivassiliou M, Sloan ME, Dixon S, Sanders DS. Pre-endoscopy serological testing for coeliac disease: evaluation of a clinical decision tool. BMJ. 2007;334:729. doi: 10.1136/bmj.39133.668681.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennazio M. Capsule endoscopy: where are we after 6 years of clinical use? Dig Liver Dis. 2006;38:867–878. doi: 10.1016/j.dld.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Biagi F, Rondonotti E, Campanella J, Villa F, Bianchi PI, Klersy C, De Franchis R, Corazza GR. Video capsule endoscopy and histology for small-bowel mucosa evaluation: a comparison performed by blinded observers. Clin Gastroenterol Hepatol. 2006;4:998–1003. doi: 10.1016/j.cgh.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Olds G, McLoughlin R, O'Morian C, Sivak MV Jr. Celiac disease for the endoscopist. Gastrointest Endosc. 2002;56:407–415. doi: 10.1016/s0016-5107(02)70047-3. [DOI] [PubMed] [Google Scholar]
- 7.Tursi A, Brandimarte G, Giorgetti GM, Gigliobianco A. Endoscopic features of celiac disease in adults and their correlation with age, histological damage, and clinical form of the disease. Endoscopy. 2002;34:787–792. doi: 10.1055/s-2002-34255. [DOI] [PubMed] [Google Scholar]
- 8.Brocchi E, Tomassetti P, Misitano B, Epifanio G, Corinaldesi R, Bonvicini F, Gasbarrini G, Corazza G. Endoscopic markers in adult coeliac disease. Dig Liver Dis. 2002;34:177–182. doi: 10.1016/s1590-8658(02)80190-6. [DOI] [PubMed] [Google Scholar]
- 9.Dickey W, Hughes D. Prevalence of celiac disease and its endoscopic markers among patients having routine upper gastrointestinal endoscopy. Am J Gastroenterol. 1999;94:2182–2186. doi: 10.1111/j.1572-0241.1999.01348.x. [DOI] [PubMed] [Google Scholar]
- 10.Oxentenko AS, Grisolano SW, Murray JA, Burgart LJ, Dierkhising RA, Alexander JA. The insensitivity of endoscopic markers in celiac disease. Am J Gastroenterol. 2002;97:933–938. doi: 10.1111/j.1572-0241.2002.05612.x. [DOI] [PubMed] [Google Scholar]
- 11.Cammarota G, Gasbarrini A, Gasbarrini G. No more biopsy in the diagnostic work-up of celiac disease. Gastrointest Endosc. 2005;62:119–121. doi: 10.1016/s0016-5107(05)01570-1. [DOI] [PubMed] [Google Scholar]
- 12.Bardella MT, Minoli G, Radaelli F, Quatrini M, Bianchi PA, Conte D. Reevaluation of duodenal endoscopic markers in the diagnosis of celiac disease. Gastrointest Endosc. 2000;51:714–716. doi: 10.1067/mge.2000.104653. [DOI] [PubMed] [Google Scholar]
- 13.Gasbarrini A, Ojetti V, Cuoco L, Cammarota G, Migneco A, Armuzzi A, Pola P, Gasbarrini G. Lack of endoscopic visualization of intestinal villi with the "immersion technique" in overt atrophic celiac disease. Gastrointest Endosc. 2003;57:348–351. doi: 10.1067/mge.2003.116. [DOI] [PubMed] [Google Scholar]
- 14.Cammarota G, Martino A, Pirozzi GA, Cianci R, Cremonini F, Zuccala G, Cuoco L, Ojetti V, Montalto M, Vecchio FM, et al. Direct visualization of intestinal villi by high-resolution magnifying upper endoscopy: a validation study. Gastrointest Endosc. 2004;60:732–738. doi: 10.1016/s0016-5107(04)02170-4. [DOI] [PubMed] [Google Scholar]
- 15.Petroniene R, Dubcenco E, Baker JP, Warren RE, Streutker CJ, Gardiner GW, Jeejeebhoy KN. Given capsule endoscopy in celiac disease. Gastrointest Endosc Clin N Am. 2004;14:115–127. doi: 10.1016/j.giec.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Petroniene R, Dubcenco E, Baker JP, Ottaway CA, Tang SJ, Zanati SA, Streutker CJ, Gardiner GW, Warren RE, Jeejeebhoy KN. Given capsule endoscopy in celiac disease: evaluation of diagnostic accuracy and interobserver agreement. Am J Gastroenterol. 2005;100:685–694. doi: 10.1111/j.1572-0241.2005.41069.x. [DOI] [PubMed] [Google Scholar]
- 17.Hopper AD, Sidhu R, Hurlstone DP, McAlindon ME, Sanders DS. Capsule endoscopy: an alternative to duodenal biopsy for the recognition of villous atrophy in coeliac disease? Dig Liver Dis. 2007;39:140–145. doi: 10.1016/j.dld.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Rondonotti E, de Franchis R. Diagnosing coeliac disease: is the videocapsule a suitable tool? Dig Liver Dis. 2007;39:145–147. doi: 10.1016/j.dld.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Rondonotti E, Spada C, Cave D, Pennazio M, Riccioni ME, De Vitis I, Schneider D, Sprujevnik T, Villa F, Langelier J, et al. Video capsule enteroscopy in the diagnosis of celiac disease: a multicenter study. Am J Gastroenterol. 2007;102:1624–1631. doi: 10.1111/j.1572-0241.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 20.Culliford A, Daly J, Diamond B, Rubin M, Green PH. The value of wireless capsule endoscopy in patients with complicated celiac disease. Gastrointest Endosc. 2005;62:55–61. doi: 10.1016/s0016-5107(05)01566-x. [DOI] [PubMed] [Google Scholar]
- 21.Kesari A, Bobba RK, Arsura EL. Video capsule endoscopy and celiac disease. Gastrointest Endosc. 2005;62:796–797. doi: 10.1016/j.gie.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Toth E, Ohlsson B, Ljungberg O, Thorlacius H. Celiac disease diagnosed using video capsule endoscopy in a patient with Crohn's disease. Endoscopy. 2006;38:548. doi: 10.1055/s-2006-925342. [DOI] [PubMed] [Google Scholar]
- 23.Green PH, Rubin M. Capsule endoscopy in celiac disease: diagnosis and management. Gastrointest Endosc Clin N Am. 2006;16:307–316. doi: 10.1016/j.giec.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Apostolopoulos P, Alexandrakis G, Giannakoulopoulou E, Kalantzis C, Papanikolaou IS, Markoglou C, Kalantzis N. M2A wireless capsule endoscopy for diagnosing ulcerative jejunoileitis complicating celiac disease. Endoscopy. 2004;36:247. doi: 10.1055/s-2004-814259. [DOI] [PubMed] [Google Scholar]
- 25.Joyce AM, Burns DL, Marcello PW, Tronic B, Scholz FJ. Capsule endoscopy findings in celiac disease associated enteropathy-type intestinal T-cell lymphoma. Endoscopy. 2005;37:594–596. doi: 10.1055/s-2005-861322. [DOI] [PubMed] [Google Scholar]
- 26.Rey JF, Ladas S, Alhassani A, Kuznetsov K. European Society of Gastrointestinal Endoscopy (ESGE). Video capsule endoscopy: update to guidelines (May 2006) Endoscopy. 2006;38:1047–1053. doi: 10.1055/s-2006-944874. [DOI] [PubMed] [Google Scholar]
- 27.Gay G, Delvaux M, Rey JF. The role of video capsule endoscopy in the diagnosis of digestive diseases: a review of current possibilities. Endoscopy. 2004;36:913–920. doi: 10.1055/s-2004-825868. [DOI] [PubMed] [Google Scholar]
- 28.Krauss N, Schuppan D. Monitoring nonresponsive patients who have celiac disease. Gastrointest Endosc Clin N Am. 2006;16:317–327. doi: 10.1016/j.giec.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Green PH, Shane E, Rotterdam H, Forde KA, Grossbard L. Significance of unsuspected celiac disease detected at endoscopy. Gastrointest Endosc. 2000;51:60–65. doi: 10.1016/s0016-5107(00)70389-0. [DOI] [PubMed] [Google Scholar]
- 30.Makins R, Blanshard C. Guidelines for capsule endoscopy: diagnoses will be missed. Aliment Pharmacol Ther. 2006;24:293–297. doi: 10.1111/j.1365-2036.2006.02991.x. [DOI] [PubMed] [Google Scholar]
- 31.Cammarota G, Martino A, Di Caro S, Cianci R, Lecca PG, Vecchio FM, Gasbarrini G. High-resolution magnifying upper endoscopy in a patient with patchy celiac disease. Dig Dis Sci. 2005;50:601–604. doi: 10.1007/s10620-005-2481-4. [DOI] [PubMed] [Google Scholar]
- 32.Cammarota G, Cesaro P, Martino A, Zuccala G, Cianci R, Nista E, Larocca LM, Vecchio FM, Gasbarrini A, Gasbarrini G. High accuracy and cost-effectiveness of a biopsy-avoiding endoscopic approach in diagnosing coeliac disease. Aliment Pharmacol Ther. 2006;23:61–69. doi: 10.1111/j.1365-2036.2006.02732.x. [DOI] [PubMed] [Google Scholar]


