Abstract
AIM: To gain mechanistic insights into the role played by epidermal growth factor receptor (EGFR) in the regulation of vascular endothelial growth factors (VEGFs) in colorectal cancer (CRC).
METHODS: The impact of high-level expression of the growth factor receptors EGFR and VEGF receptor (VEGFR)3 and the VEGFR3 ligands VEGF-C and VEGF-D on disease progression and prognosis in human CRC was investigated in 108 patients using immunohistochemistry. Furthermore, the expression of the lymphangiogenic factors in response to the modulation of EGFR signalling by the EGFR-targeted monoclonal antibody cetuximab was investigated at the mRNA and protein level in human SW480 and SW620 CRC cell lines and a mouse xenograft model.
RESULTS: Human CRC specimens and cell lines displayed EGFR, VEGF-C and VEGF-D expression with varying intensities. VEGF-C expression was associated with histological grade. Strong expression of VEGF-D was significantly associated with lymph node metastases and linked to a trend for decreased survival in lymph node-positive patients. EGFR blockade with cetuximab resulted in a significant decrease of VEGF-D expression in vitro and in vivo.
CONCLUSION: In conclusion, the expression of VEGF-D in colorectal tumours is significantly associated with lymphatic involvement in CRC patients and such expression might be blocked effectively by cetuximab.
Keywords: Human colorectal cancer, Lymphangiogenesis, Vascular endothelial growth factor-C, Vascular endothelial growth factor-D, Epidermal growth factor receptor
INTRODUCTION
Globally, colorectal cancer (CRC) is one of the three most commonly diagnosed malignancies[1]. For patients with metastatic disease, systemic cytotoxic chemotherapy has been shown to clearly improve survival[2–5]. More recently, the addition of therapeutic antibodies including cetuximab[6–8] and bevacizumab[9,10] to such cytotoxic regimens has been shown to further improve outcomes in first- and second-line settings.
The mode of action of the new therapeutic antibody cetuximab is thought to be based primarily upon perturbation of epidermal growth factor receptor (EGFR)-ligand interactions[11]. Binding of cetuximab blocks EGFR-associated cellular signal transduction cascades, which govern processes such as tumour cell survival, proliferation, invasion and metastasis[12–16]. Since high-level expression of the EGFR gene has been associated with reduced survival in a range of malignancies, targeting growth factor receptor signalling cascades is a promising anticancer strategy[17–24]. Even more, an additional mode of action of cetuximab has been suggested which relates to tumour cell binding inducing an antibody-dependent cell-mediated cytotoxicity reaction[25,26].
However, even if a number of such molecular tumour characteristics appeared to be associated with the antitumor efficacy of EGFR-targeted agents[27], it remains a matter of debate as to whether the intensity or extent of immunohistochemical detection of EGFR expression in the tumour correlate with prognosis and response to EGFR-targeted agents[28,29].
The hypothesis that growth and spread of tumours are dependent on their vascular and lymphatic systems was proposed several decades ago[30]. Interest in this concept has recently been rekindled following the molecular identification of regulators of (lymph-) angiogenesis such as the vascular endothelial growth factor (VEGF) family, including the ligands VEGF-A, -B, -C and -D, and the VEGF receptors VEGFR1 (FLT1), VEGFR2 (KDR) and VEGFR3 (FLT4)[31] and their clinical utilization by recombinant strategies for targeting angiogenesis, such as the anti-VEGFA antibody, bevacizumab[9] and the decoy receptor, VEGF Trap[32]. Interestingly, VEGF-C and VEGF-D, signalling through VEGFR3, have been identified as key regulators of lymp-hangiogenesis[33–36]. Data from in vitro and murine tumour models further support the key role of VEGF-C and VEGF-D in malignancy. For many tumour types, clinical studies have revealed a correlation between VEGF-C, VEGF-D and VEGFR3 expression and lymphatic spread, tissue invasion or poor prognosis[37–41]. However, in other studies, clear associations were not identified[42,43] or low levels of VEGF-D were correlated with an increased risk of metastasis and reduced survival[44]. Similar data have also been reported for CRC. In one study, VEGF-C and VEGF-D expression correlated with the tumour invasion, lymphatic and venous involvement, lymph node metastasis and liver metastasis, and reduced survival time[45]. A second study also reported that high-grade VEGF-D expression was associated with lymphatic involvement and poor patient survival[46], while a third confirmed that VEGF-D expression correlated with the depth of tumour invasion, lymph node metastasis and reduced survival time[47]. However, in other analyses VEGF-D expression at the mRNA-level was reported to be downregulated in CRCs with lymphatic spread[48] and appeared to be lower at the leading edge of tumours in which lymphatic vessels were present[49].
Given that lymphangiogenesis is increasingly recognized as a critical component of tumourigenesis and that EGFR signalling, a key regulator of tumourigenesis in CRC, possibly acts to some extent through regulation of VEGF-C and VEGF-D expression, we evaluated the co-expression profiles of EGFR, VEGF-C and VEGF-D in human CRC specimens. Results were correlated with the patients' clinicopathological parameters and survival. Furthermore, in order to gain mechanistic insights into the role played by EGFR in the regulation of VEGF-D in colorectal cancer, we analyzed the effect of cetuximab in vitro and in vivo on the expression of VEGF-D in SW480 and SW620 human colon cancer cell and xenograft models of CRC. We thus showed that expression of VEGF-D is prognostically relevant in CRC and for the first time provided experimental evidence that EGFR-targeted antitumor therapy exerts its effect in part through suppressing lymphangiogenesis by downregulating VEGF-D.
MATERIALS AND METHODS
Tissue samples and patient characteristics
All tissues investigated in this study were obtained from patients (n = 108) who underwent colectomy between 1995 and 2003 at the Department of Abdominal Surgery, University Hospital Mainz, Germany. Written informed consent for experimental immunohistochemistry was obtained from all patients before analysis. Expression of EGFR was analyzed in all patients, with assessment of VEGF-C and VEGF-D conducted in 102 cases and 104 cases, respectively, because of limited availability of tumour material.
Patient age at the time of primary surgery ranged from 36.2 years to 83.1 years (63.6 ± 10.45 years). Seven patients were lost to follow up and were therefore censored at the time of last contact (34.86 ± 4.18 mo). Staging and diagnosis of CRC was assessed according to the World Health Organization classification and the TNM classification as set out by the International Union Against Cancer [Union International Contre le Cancer (UICC)]. After resection, patients were followed up every 6 mo. Patients with synchronous or metachronous metastasis underwent additional restaging every 3 mo during chemotherapy.
Immunohistochemical (IHC) staining
Formalin-fixed paraffin-embedded tissues of patients with CRC from the Department of Pathology, University Hospital Mainz, Germany, were used in this study. Tissue sections (4 μm) were cut from these blocks and used for IHC staining. All tissue sections were deparaffinized in xylene and rehydrated in a graded ethanol series.
Staining for EGFR was performed using the commercially available EGFR pharmDx kit (DakoCytomation, Carpinteria, CA, USA), which includes the pharmDx mouse anti- EGFR monoclonal antibody (clone 2-18C9), a negative control reagent (a mouse monoclonal antibody for an enzyme that is not expressed in mammalian tissue), and positive and negative control cancer cell preparations (CAMA-1 breast cancer and HT29 colon cancer cell lines). IHC staining was performed according to the manufacturer's instructions.
Staining for VEGF-C and VEGF-D was carried out following antigen retrieval. Sections were heated in citrate buffer and then cooled for 20 min. Endogenous peroxidase was blocked in 3% hydrogen peroxide in methanol for 15 min. To block nonspecific binding, prior to incubation with the primary antibody, tissue sections were incubated with serum-free DAKO Antigen-Block for 30 min. Primary antibodies specific for VEGF-C (sc-9047, Santa Cruz Biotechnology Inc. CA, USA) and VEGF-D (sc-13085, Santa Cruz Biotechnology Inc.) and were diluted 1:50 in DAKO ChemMate antibody diluent and sections were incubated for 16 h at 4°C (VEGF-C) or 2 h at room temperature (VEGF-D). After incubation with an anti-rabbit secondary antibody for 30 min, bound complex was visualized by using diaminobenzidine (ChemMateTM DAKO EnVisonTM Detection Kit). Sections were counterstained with Mayer's hematoxylin and mounted. Between all incubations, sections were washed in phosphate-buffered saline (PBS). Foetal kidney was used as a positive control. Negative controls were prepared by omitting primary antibody from the process (data not shown).
Evaluation of immunostaining
Immunostaining was independently evaluated by 4 authors who were blinded to patient outcome and all clinicopathological findings. EGFR-staining was interpreted according to standard parameters (EGFR pharmDx™ Interpretation Manual, DAKO). The staining intensities (Figure 1) were scored as negative, weak, moderate or strong. To unequivocally categorize cases into two groups, each sample was defined as EGFR-negative or EGFR-positive when < 1% or ≥ 1% of the tumour cells respectively showed an EGFR-immunospecific membranous brown staining.
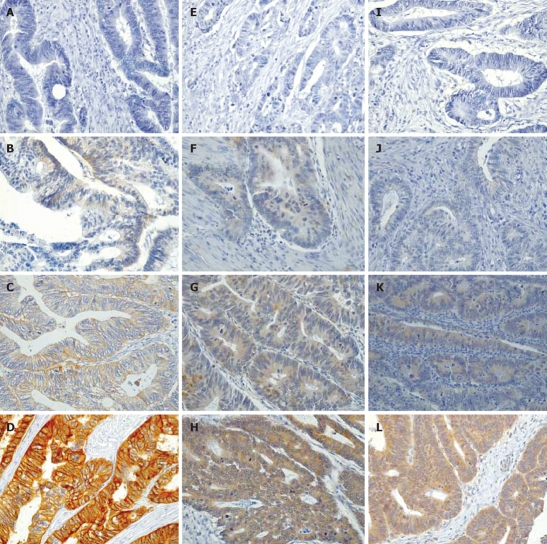
Figure 1.
Immunohistochemical analysis of the expression levels of EGFR (A-D), VEGF-C (E-H) and VEGF-D (I-L) in human colorectal cancer sections (× 400). A, E and I: No expression; B, F and J: Weak expression; C, G and K: Moderate expression; D, H and L: Strong expression.
Staining results for VEGF-C and VEGF-D were similarly classified by estimating both the staining intensity and the percentage of epithelial cells showing specific immunoreactivity. Staining intensities were not always homogeneous across individual tumour samples (as shown in Figure 1). All results showing more than 25% of tumour cells stained (with weak to strong positivity) were considered to represent biologically relevant levels of expression of these proteins and, for the purposes of statistical analysis, were counted as positive results.
Cell lines
The colon carcinoma cell lines SW480 and SW620 were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI 1640 media (Invitrogen, Carlsbad, CA, USA), supplemented with 10% foetal calf serum (FCS: PAA Laboratories, Pasching, Austria). Cells were cultured at 37°C in a 5% CO2 atmosphere and passaged routinely using Trypsin-ethylenediaminetetraacetic acid (PAA Laboratories) treatment.
Cetuximab
Clinical grade anti-EGFR monoclonal antibody cetuximab (Erbitux®) was supplied by Merck KGaA (Darmstadt) at a concentration of 2 mg/mL in a buffer consisting of 10 mmol/L sodium phosphate and 145 mmol/L sodium chloride at pH 7.
Stimulation assay
SW480 and SW620 cells were seeded at 3 × 105 cells/well in a 6-well tissue culture plate with media containing 10% FCS. After 24 h, cells were starved by incubation in media with a reduced FCS level (0.5%) for an additional 24 h. Afterwards, cells were incubated in medium plus 0.5% FCS supplemented either with 5 ng/mL epidermal growth factor (EGF, Sigma Chemical Co., St. Louis, MO, USA) for 20 min followed by 20 μg/mL cetuximab, or alternatively, the same medium supplemented with one or other of these substances. After culturing for another 24 h, monolayers were washed with icecold PBS, centrifuged and pellets were frozen for RNA isolation. The cell culture experiments were independently repeated at least two times.
RNA isolation and quantitative real time RT-PCR
RNA isolation was performed using the RNeasy Kit according to the manufacturer's recommendations (Qiagen, Hilden, Germany). Transcription of the housekeeping gene glycerinaldehyde-3-phosphate-dehydrogenase (GAPDH), VEGF-D and VEGFR3 was analyzed by a one-step reverse transcriptase-polymerase chain reaction (RT-PCR) using a LightCycler 2.0 system (Roche). RT-PCR was performed with 0.1 μg of RNA in a 35 cycle reaction (20 μL total volume; QuantiTect SYBRGreen RT-PCR, Qiagen) according to the recommendations of the manufacturer. All RT-PCR reactions were done in 2 replicates. All PCRs were established with an exponential phase efficiency of 2 to guarantee that the data were comparable. The evaluation of the expression of the target genes was performed relative to the expression of GAPDH. Control and test samples in the EGF/cetuximab and xenograft analyses were compared using the ΔΔCt approach (ΔΔCt=2^[(Ct target gene control - Ct GAPDH control) - (Ct target gene test condition - Ct GAPDH test condition)]. From this formula, for the test compared with the control condition, a ΔΔCt over 1 indicated an increase of a target gene expression and a ΔΔCt of less than 1 indicated a lower expression of the target gene relative to the housekeeping gene GAPDH.
RT-PCR primers used were: GAPDH, forward: 5'-CCCATCACCATCTTCCAGGAGCG-3' and reverse 5'-CATGCCAGTGAGCTTCCCGTTCA-3' (476 bp product); EGFR, forward: 5'-TCTCAGCAACATGTCGATGGA -3' and reverse 5'- GCACTGTATGCACTCAGAGTT-3' (92 bp product), VEGF-D, forward: 5'- GTATGGACTCTCGCTCAGCAT-3' and reverse: 5'- AGGCTCTCTTCATTGCAACAG-3' (225 bp product), VEGFR3, forward and reverse; QuantiTect Primer Assay: FLT4 (VEGFR3, 127 bp product, Qiagen). Cycle conditions of the one-step real time LightCycler RT-PCRs were as follows: for reverse transcription, 20 min at 50°C. The subsequent PCR reaction was characterized by: initial denaturation (15 min at 95°C) followed by the respective number of cycles (GAPDH: 25, VEGF-D: 35, VEGFR3: 40, EGFR: 35) of: denaturation (15 s at 94°C), annealing (25 s: GAPDH; 63°C, VEGF-D; 61°C, VEGFR3; 55°C, EGFR; 61°C) and elongation (35 s at 72°C). After the last cycle, a melting curve was plotted to confirm the amplification of a single specific RT-PCR product.
Western blotting
SW480 and SW620 cells were plated at 2.5 × 106 cells/well in 6 wells. Cells were harvested and lysed in RIPA buffer[50]. For EGFR, VEGF-D, VEGFR3 and α-tubulin analysis, 50 μg of cleared lysates were separated on 10.0% sodium dodecyl sulphatepolyacrylamide gels, blotted to nitrocellulose transfer membranes (Schleicher & Schuell) and blocked for 1 h in 5% non-fat dry milk, incubated with a specific primary antibody: antihuman EGFR sc-03, antihuman VEGF-D sc-13085, VEGFR3 sc-321 (Santa Cruz Biotechnology Inc: all antibodies were diluted 1:200 in 5% bovine serum albumin) or anti-α-tubulin (Sigma: diluted 1:10 000 in 5% non-fat dry milk). Detection of bound antibody was performed using a peroxidase-conjugated secondary goat anti-rabbit antibody (sc-2030; Santa Cruz Biotechnology Inc.) diluted 1:2000 in 5% non-fat dry milk and an ECL chemiluminescence detection kit (Perkin Elmer).
Animals
Female NOD/SCID -/- mice were purchased from the central animal facility (ZVTE, University of Mainz, Germany). The mice were maintained in a laminar airflow cabinet under pathogen-free conditions and used at 7-10 wk of age. Mice were housed in microisolator cages with laboratory chow.
Treatment of subcutaneous colorectal carcinoma xenografts
Colon carcinoma tumours were established as xenografts by injecting 1 × 107 SW480 cells, mixed in PBS : medium (1:1), subcutaneously into the left flank of eight NOD/SCID -/- mice. Ten days after cell injection, all mice bore a tumour with a minimum diameter of 4 mm. The mice were randomized into two groups of four animals. They were treated with either saline or cetuximab at 1 mg/dose every three days. Cetuximab and the saline placebo were administered intraperitoneally at a constant volume of 0.5 mL/injection. Treated animals were checked daily. After 5 wk of treatment, tumours were isolated and processed with a disperger to enhance subsequent RNA isolation with the RNeasy Kit (Qiagen, Hilden, Germany).
Statistical analysis
The association of staining intensity with clinicopa-thological patterns was assessed with the χ2 test and with the unpaired student t-test, where appropriate. Differences in migration were evaluated with the unpaired Student's t-test. Survival rates were visualized applying the Kaplan-Meier curves and log rank test. In vitro and in vivo real time gene expression medians were compared using Mann-Whitney U test. P < 0.05 was considered significant and P < 0.001 highly significant in all statistical analyses.
RESULTS
Patient profiles and tumour characteristics
Disease characteristics for the group of 108 patients selected are representative of CRC patient populations in industrialized countries, except for a lower percentage of T3 cancers (Table 1). The mean age of the patient cohort at the time of primary surgical intervention was 63.8 years (SD, 10.45 years). Tumour stage was distributed as follows: UICC stageIwas found in 14%, stage III in approximately a fifth and stages II, and IV, each in approximately a third of all patients (Table 1). The most common histopathological grading was pG2, which was reported for nearly 80% of cases. Approximately half of all patients had positive lymph node status. The UICC stage dependent survival rates after 3 years were 100% for stageI, 92% for stage II, 78% for stage III and 44% for stage IV, which were similar to those reported for other large CRC population series[51]. Expression was studied in specimens taken from all 108 CRC patients.
Table 1.
Baseline characteristics of the colorectal cancer patients (n = 108)
| n | % | |
| UICC-stage | ||
| I | 15 | 13.9 |
| II | 36 | 33.3 |
| III | 23 | 21.3 |
| IV | 34 | 31.5 |
| Grading | ||
| G 1 | 4 | 3.7 |
| G 2 | 85 | 78.7 |
| G 3 | 17 | 15.7 |
| G 4 | 2 | 1.9 |
| Lymph node metastasis | ||
| pN 0 | 53 | 49.1 |
| pN 1 | 21 | 19.4 |
| pN 2 | 34 | 31.5 |
Expression of EGFR, VEGF-C and VEGF-D in CRC specimens
The expression of EGFR, which could be assessed by IHC staining for all 108 samples, exhibited a predominantly membranous subcellular localization. In a few specimens, an additional weak cytoplasmic localization was found, which was not scored as indicative of positive staining for EGFR (Figure 1A-D). EGFR expression in CRC specimens was distributed as follows: in 59 cases (54.6%), the specimen showed no positive staining for EGFR, while in 49 patients (45.4%) the specimen showed a positive membranous staining, including 31 patients (28.7%) where staining was weakly positive and 9 patients (8.3%) each with moderately or strongly positive staining. No statistically significant correlation was identified between EGFR staining and the UICC stage, grading, tumour invasion or lymph node metastasis (Table 2). Comparison with the histopathological grading of the tumour cells however showed a trend towards the expression of EGFR in less well differentiated lesions. Thus, the prognostic value of IHC-determined EGFR expression status in relation to the prediction of poor survival[52–56] could not be confirmed in the current CRC population, again arguing to successfully integrate the anti-EGFR monoclonal antibody cetuximab into therapeutic regimens for patients whose tumours do not appear to express EGFR[57,58].
Table 2.
Correlation of expression levels of EGFR, VEGF-C and VEGF-D with tumour and patient characteristics
|
EGFR |
VEGF-C |
VEGF-D |
||||||||||
| Total | + ve | %1 | P | Total | + ve | %1 | P | Total | + ve | %1 | P | |
| Stage (UICC) | 0.197 | 0.220 | 0.048 | |||||||||
| I | 15 | 6 | 40.0 | 14 | 7 | 50.0 | 14 | 9 | 64.3 | |||
| II | 36 | 14 | 38.9 | 34 | 26 | 76.5 | 35 | 27 | 77.1 | |||
| III | 23 | 15 | 65.2 | 20 | 16 | 80.0 | 21 | 9 | 42.9 | |||
| IV | 34 | 14 | 41.2 | 34 | 25 | 73.5 | 34 | 25 | 73.5 | |||
| Differentiation grading | 0.063 | 0.030 | 0.066 | |||||||||
| Well | 4 | 0 | 0 | 4 | 1 | 25.0 | 4 | 1 | 25.0 | |||
| Not well | 104 | 49 | 47.1 | 98 | 73 | 74.5 | 100 | 69 | 69.0 | |||
| Tumour invasion (TNM) | 0.797 | 0.050 | 0.438 | |||||||||
| T1/T2 | 21 | 9 | 42.9 | 20 | 11 | 55.0 | 20 | 12 | 60.0 | |||
| T3/T4 | 87 | 40 | 46.0 | 82 | 63 | 76.8 | 84 | 58 | 69.0 | |||
| Lymph node metastases | 0.283 | 0.600 | 0.025 | |||||||||
| 1 to ≤ 6 | 37 | 20 | 54.1 | 35 | 27 | 77.1 | 36 | 18 | 50.0 | |||
| ≥ 7 | 18 | 9 | 50.0 | 17 | 13 | 76.5 | 17 | 14 | 82.4 | |||
| Age | 0.837 | 0.813 | 1.0 | |||||||||
| ≤ 60 | 35 | 15 | 42.9 | 33 | 25 | 75.8 | 33 | 22 | 66.7 | |||
| ≥ 61 | 73 | 34 | 43.6 | 69 | 49 | 71.0 | 71 | 48 | 67.6 | |||
Percentages relate to number of positive tumours out of total number of cases in the subclass.
The staining for VEGF-C and VEGF-D was predominantly cytoplasmic (Figure 1E-H and I-L, respectively). VEGF-C expression could be assessed in 102 specimens, with 74 specimens (68.5%) staining positive for VEGF-C and 28 specimens (25.9%) being negative. Twenty-five percent of the G1 tumours (n = 4) were positive, while 70.6% of G2 tumours, 70.6% of G3 tumours, and 50.0% of G4 graded tumours were positive for VEGF-C. Thus even if statistically significant (Table 2) but only 4 patients were G1, a clinical clear cut comparison of well versus not well differentiated tumours may not be done. However, another finding was the borderline significant correlation between tumour invasion and VEGF-C expression (P = 0.050). Only 11 (55.0%) of the pT1 and pT2 graded tumours were positive for VEGF-C compared with 63 (76.8%) of the tumours with deeper invasion. No statistically significant correlation was apparent with the other clinicopathological parameters including UICC stage and lymph node status (Table 2).
VEGF-D expression was analyzed in 104 available specimens, with 70 (64.8%) staining positive and 34 (31.5%) staining negative. Interestingly, VEGF-D staining was associated with UICC tumour stage (P = 0.048). Nine of 14 (64.3%) specimens from patients with UICC stageItumours stained positive, while 27 (77.1%) of the UICC stage II, 9 (42.9%) of the UICC stage III and 25 (73.5%) of the UICC stage IV tumours stained positive for VEGF-D. No significant association was found with several other parameters including grading, tumour invasion and pN status (Table 2). Again, the comparative analyses of the combined VEGF-C and VEGF-D co-expression with well versus not well differentiated tumours were statistically significant (P = 0.022), but possibly not clinically clear cut significant due to the low patient number analysed.
However, when VEGF-D staining was analyzed in relation to the number of tumour positive lymph nodes, there was a statistically significant overall association between the number of lymph node metastases and VEGF-D positivity of the primary tumour (P = 0.025). The patient cohort was stratified into patients with no lymph node metastases (n = 51), with up to 6 lymph node metastases (n = 36), and with more than 6 lymph node metastases (n = 17). Among those from patients with up to 6 lymph node metastases, 18 (50.0%) stained positive for VEGF-D, and among the patients with more than 6 lymph node metastases, 14 (82.4%) stained positive for VEGF-D.
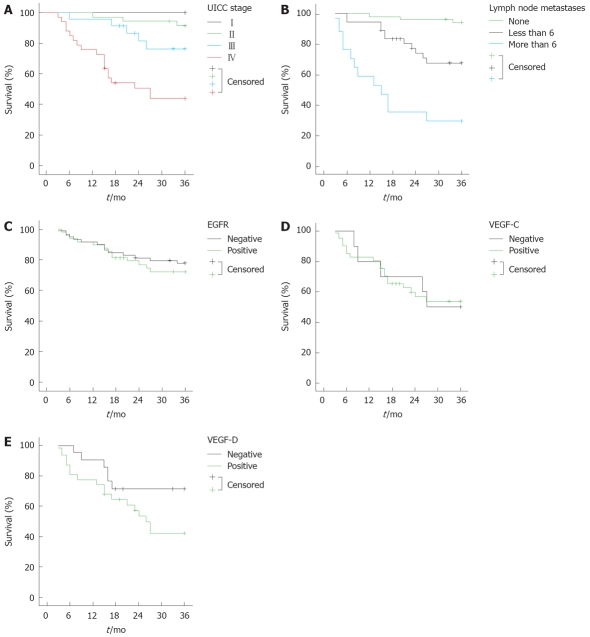
When VEGF-D staining was analyzed in relation to the survival of lymph node positive patients, there was a trend (P = 0.067) between the VEGF-D positive and negative groups (Figure 2). The patients with lymph node metastases whose tumours were VEGF-D positive had a 30% lower chance of survival at 36 mo in comparison to the patients with VEGF-D negative tumours. Kaplan Meier analysis also indicated highly statistically significant correlations between the number of lymph node metastases and UICC stage and overall survival (in both cases, log rank test P < 0.001; Figure 2). It was also noteworthy that in several specimens, VEGF-C and VEGF-D were strongly expressed at the tumour invasion front (data not shown). Consistently, the further comparison of VEGF-C and VEGF-D combined staining profiles in relation to survival, double VEGF-C and VEGF-D positive patients had a trend to an unfavorable 5 years prognosis compared to the negative group (log rank test P = 0.2), whereas triple EGFR, VEGF-C and VEGF-D positivity did not influence survival in these two cohorts (data not shown).
Figure 2.
Kaplan Meier survival analyses of colorectal cancer subgroups in relation to baseline disease characteristics and EGFR, VEGF-C or VEGF-D expression. A-C: Survival analysis for all patients in relation to UICC stage (P < 0.001), the number of metastases (P < 0.001) and EGFR staining (P = 0.546), respectively; D and E: The 3 years survival in patients with lymph node metastasis in relation, respectively, to the expression of VEGF-C (P = 0.967) and VEGF-D (P = 0.067).
EGFR, VEGF-C, VEGF-D and VEGFR3 expression in colorectal carcinoma cell lines
Real time RT-PCR assays and Western blotting were used to measure transcript and protein levels in experimental analyses. Whereas VEGF-D transcripts and protein were expressed at moderate levels in both SW480 and SW620 CRC cell lines, a similar analysis of expression of EGFR and VEGFR3 yielded varying results. EGFR mRNA and protein levels were high in SW480 and markedly lower in SW620 cells. Though mRNA levels of VEGFR3 were high in SW480 and moderate in SW620, the protein levels of VEGFR3 were also higher in SW480 than in SW620 cells (Figure 3).
Figure 3.
Quantitative EGFR, VEGFR3 and VEGF-D mRNA expression (A) and Western blot analysis (B) of untreated SW480 and SW620. The α-tubulin signal served as a control to confirm the loading of equivalent amounts of protein per track.
Effect of cetuximab in CRC cell lines and a xenograft model
In order to analyze the effect of cetuximab on VEGF-D and VEGFR3 transcription, SW480 cells were treated for 24 h with either EGF or cetuximab alone or a combination of these two agents. Incubation of the cells with EGF was associated with a marked relative increase in VEGF-D and VEGFR3 mRNA levels compared with control (0.5% FCS) values. Conversely, incubation with EGF and cetuximab simultaneously resulted in a highly statistically significant (P = 0.004) suppression of this inductive effect, for both genes (Figure 4A). There were similar highly statistically significant (P = 0.004) differences in the levels of VEGF-D and VEFR3 expression in the EGF versus cetuximab treated cells. Incubation with cetuximab alone also resulted in a decreased level of VEGF-D mRNA compared to the control. This effect was not seen for VEGFR3. In the EGFR negative cell line SW620 no such effects were detectable (data not shown).
Figure 4.
Effect of cetuximab on (A) the expression of VEGF-D and VEGFR3 in SW480 cells cultured in the presence and absence of EGF and (B) the in vivo levels of VEGF-D and VEGFR3 in a NOD/SCID mouse xenograft model.
In the in vivo xenograft model, treatment with either cetuximab or saline placebo was administered over a period of 28 d. After this period, the mRNA level of VEGF-D was highly statistically significantly (P = 0.004) reduced in the tumour tissue of mice which had received cetuximab compared with those that had received saline. No such effect could be detected for VEGFR3 (P = 0.577, Figure 4B).
DISCUSSION
A significant proportion of patients with advanced but non-metastatic CRC which has seemingly been curatively resected experience disease recurrence[52,53]. In order to identify high-risk patients at an early stage, it is important to understand the molecular mechanisms behind the behaviour of these tumour types[54,55]. Herein, activation of EGFR-mediated signalling cascades has been identified in promotion of cell proliferation, malignant transformation, angiogenesis and metastatic dissemination[54,56]. In addition, lymphangiogenesis, mediated through tumour-derived VEGF-C and VEGF-D, gained attention in relation to the facilitation of lymph node metastasis and tumour spread[37,39,40,57]. Recent data from clinical studies suggest that VEGF-C and/or VEGF-D expression in tumour tissue might be prognostic factors for lymphatic spread, tumour invasion and/or poor prognosis in a variety of cancers including gastric, colorectal, endometrial and breast[37,39,40,45,46].
To our knowledge, this is the first study analyzing concurrently the expression profiles of EGFR and the lymphangiogenic ligands VEGF-C and VEGF-D in a large series of human CRC specimens. With a relatively low patient numbers used for well differentiated tumors, the statistical correlations of this current study between the histopathological grading and VEGF-C, but not VEGF-D expression levels may only be assumed to hold true for a larger clinical setting. However most interestingly, the comparison of the expression profile of VEGF-D in the colorectal tumour series with clinicopathological parameters revealed a significant association between VEGF-D and the number of lymph node metastasis. Moreover, we show with a clear trend that patients in a pN+ setting and positive VEGF-D expression define a subgroup with shorter survival. These data are consistent with previous analyses in a range of other cancers. Furthermore, they are in agreement with the established clinical observation that lymphatic dissemination in particular the number of metastatic lymph nodes is closely related to the clinical outcome/prognosis of patients with CRC[59].
Signal transduction via the ligands VEGF-C and -D and VEGFR3 triggers lymphatic endothelial cell growth and migration[34–36]. Thus, it is noteworthy that in our specimens VEGF-C and VEGF-D were also strongly expressed at the tumour invasion front. We have previously shown that VEGFR3 is expressed in 67% of primary gastric cancers[60]. Data on animal models suggest that VEGF-C/VEGF-D/VEGFR3 signalling can promote tumour lymphangiogenesis and the metastatic spread of tumour cells[35,61–63]. Indeed, it has been suggested that primary tumours may prepare their future metastasis site by producing lymphangiogenic factors that mediate their efficient transport to the sentinel node[64]. Data from Jia et al suggested that VEGF-C expression may induce lymphangiogenesis in CRC and as a result, tumour cells could perhaps enter lymphatic vessels more easily[57,65]. These processes could be blocked by inhibition of VEGFR3 signalling by systemic delivery of a soluble VEGFR3-immunoglobulin fusion protein. However, lymph node metastasis was not suppressed if such treatment was started later, after tumour cells had already disseminated, suggesting that tumour cell entry into the lymphatic vessels is a key step during the metastatic process[66]. Consistently with these findings, our double VEGF-C and VEGF-D positive patients had unfavorable survival prognosis compared to the negative groups in the Kaplan-Meier analysis, again arguing for a large analysis of these markers in colorectal cancer patients with chemotherapy and/or cetuximab-based adjuvant therapy.
We further explored whether EGFR blockade influenced the expression of VEGF-D and VEGFR3 using in vitro and in vivo models. While treatment of the EGFR-expressing CRC cell line SW480 with EGF resulted in an increase in the transcript levels of both genes, there was a highly significant reduction in those induced mRNA levels when cetuximab was added. These data suggest that cetuximab, by blocking EGFR-associated signalling, might act as an inhibitor of VEGF-D expression, and consequently, of lymphangiogenesis. In contrast with an earlier report of another EGFR-specific antibody (ICR62) stimulating VEGF-D[67], cetuximab blocks additionally the production of pro-angiogenic factors such as VEGF and IL-8[12–14,68–70]. Although complete inhibition of VEGF-D expression was not achieved in our model systems the effect of cetuximab was nevertheless dramatic. In a therapeutic context, cetuximab, might also therefore induce a clinically-relevant reduction in tumour lymphangiogenesis. Given that the induction of lymphangiogenesis appears to be one of the early events in the progression of cancer[57], a therapy targeting such processes would have a clear role in (neo-)adjuvant chemopreventive settings. Further preclinical and clinical studies are therefore warranted to explore these issues.
In conclusion, immunohistochemical staining of VEGF-D coupled with the examination of lymph node status may aid in the definition of patient subpopulations with more aggressive tumours. A cetuximab-based adjuvant therapy might then improve overall survival in these patients. Still, the link between prognostic markers and response to a certain treatment remains elusive. However, the new data in the current paper suggest that the inhibition of VEGF-D signalling might contribute to the widely demonstrated clinical activity of cetuximab in the treatment of CRC.
COMMENTS
Background
Since the use of new biologic agents, such as epidermal growth factor receptor (EGFR)-targeted agents may improve our current therapeutic approaches for advanced human colorectal cancer (CRC) which seeds quite often metastatic tumor cells in the lymphatic glands, is of high interest to analyse prognostic and predictive expression markers for lymphangiogenic tumor spread, particularly in view of the possible modulation of these factors by the EGFR-targeted agents cetuximab and panitumumab.
Research frontiers
Our clinical human colon cancer specimens displayed these markers EGFR, vascular endothelial growth factor (VEGF)-C and VEGF-D expression. Strong expression levels of VEGF-D were associated with lymph node metastasis and linked to decreased survival in lymph node-positive colon cancer patients. In tumor models, the new antibody for EGFR blockade cetuximab resulted in a highly significant decrease of VEGF-D expression.
Innovations and breakthroughs
The lymphangiogenic marker VEGF-D thus associated with lymph node metastasis and is linked to a decreased survival in lymph node-positive patients. The EGFR blockade with cetuximab resulted in a significant decrease of VEGF-D expression, particularly favouring these EGFR-targeted agents as treatment options of lymph node-positive colorectal cancer.
Applications
Patients with advanced lymph node-positive colorectal cancer might better be selected as well as better be treated in the near future.
Peer review
Immunohistochemical staining of VEGF-D coupled with the examination of lymph node status may define patient subpopulations of with more aggressive colorectal cancers. Since the EGFR blockade with cetuximab resulted in a significant decrease of VEGF-D, EGFR-targeted agents may improve the overall survival, particularly as a new treatment option of lymph node-positive colorectal cancer.
Acknowledgments
The authors thank Merck KGaA, Darmstadt for an educational grant, as well as providing the EGFR DAKO kits and the EGFR antibody cetuximab for the experiments. The authors would also like to thank all colleagues and assistants of the animal core facility of Mainz University as well Dr. Andreas Teufel for administrative help with the xenograft models. The results of the manuscript are part of the MD and PhD theses of Christian Frings and Annett Mueller, respectively.
Peer reviewer: Peter L Lakatos, MD, PhD, Assistant Professor, 1st Department of Medicine, Semmelweis University, Koranyi S 2A, Budapest H1083, Hungary
S- Editor Zhong XY L- Editor Negro F E- Editor Ma WH
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 5.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 7.Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–2319. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Lang I, D'haens G, Moiseyenko V, Zaluski J, Folprecht G, Tejpar S, Kisker O, Stroh C, Rougier P. KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: The CRYSTAL experience. J Clin Oncol. 2008:266(18S) Part II of II, 1006s. Available from: URL: http://jco.ascopubs.org/search.dtl (Annual Meeting 2008)
- 9.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12:356–361. doi: 10.1634/theoncologist.12-3-356. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Rubin M, Fan Z, DeBlasio T, Soos T, Koff A, Mendelsohn J. Involvement of p27KIP1 in G1 arrest mediated by an anti-epidermal growth factor receptor monoclonal antibody. Oncogene. 1996;12:1397–1403. [PubMed] [Google Scholar]
- 13.Overholser JP, Prewett MC, Hooper AT, Waksal HW, Hicklin DJ. Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer. 2000;89:74–82. [PubMed] [Google Scholar]
- 14.Prewett M, Rothman M, Waksal H, Feldman M, Bander NH, Hicklin DJ. Mouse-human chimeric anti-epidermal growth factor receptor antibody C225 inhibits the growth of human renal cell carcinomaxenografts in nude mice. Clin Cancer Res. 1998;4:2957–2966. [PubMed] [Google Scholar]
- 15.Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995;95:1897–1905. doi: 10.1172/JCI117871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang SM, Li J, Harari PM. Molecular inhibition of angiogenesis and metastatic potential in human squamous cell carcinomas after epidermal growth factor receptor blockade. Mol Cancer Ther. 2002;1:507–514. [PubMed] [Google Scholar]
- 17.Faivre S, Djelloul S, Raymond E. New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors. Semin Oncol. 2006;33:407–420. doi: 10.1053/j.seminoncol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37 Suppl 4:S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 19.Reis-Filho JS, Pinheiro C, Lambros MB, Milanezi F, Carvalho S, Savage K, Simpson PT, Jones C, Swift S, Mackay A, et al. EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J Pathol. 2006;209:445–453. doi: 10.1002/path.2004. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch FR, Varella-Garcia M, Bunn PA Jr, Di Maria MV, Veve R, Bremmes RM, Baren AE, Zeng C, Franklin WA. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 21.Shia J, Klimstra DS, Li AR, Qin J, Saltz L, Teruya-Feldstein J, Akram M, Chung KY, Yao D, Paty PB, et al. Epidermal growth factor receptor expression and gene amplification in colorectal carcinoma: an immunohistochemical and chromogenic in situ hybridization study. Mod Pathol. 2005;18:1350–1356. doi: 10.1038/modpathol.3800417. [DOI] [PubMed] [Google Scholar]
- 22.Mrhalova M, Plzak J, Betka J, Kodet R. Epidermal growth factor receptor--its expression and copy numbers of EGFR gene in patients with head and neck squamous cell carcinomas. Neoplasma. 2005;52:338–343. [PubMed] [Google Scholar]
- 23.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 24.Lee JW, Soung YH, Kim SY, Nam HK, Park WS, Nam SW, Kim MS, Sun DI, Lee YS, Jang JJ, et al. Somatic mutations of EGFR gene in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11:2879–2882. doi: 10.1158/1078-0432.CCR-04-2029. [DOI] [PubMed] [Google Scholar]
- 25.Kurai J, Chikumi H, Hashimoto K, Yamaguchi K, Yamasaki A, Sako T, Touge H, Makino H, Takata M, Miyata M, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res. 2007;13:1552–1561. doi: 10.1158/1078-0432.CCR-06-1726. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Gordon M, Schultheis AM, Yang DY, Nagashima F, Azuma M, Chang HM, Borucka E, Lurje G, Sherrod AE, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25:3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 27.Khambata-Ford S, Garrett CR, Meropol NJ, Basik M, Harbison CT, Wu S, Wong TW, Huang X, Takimoto CH, Godwin AK, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol. 2007;25:3230–3237. doi: 10.1200/JCO.2006.10.5437. [DOI] [PubMed] [Google Scholar]
- 28.Dziadziuszko R, Hirsch FR, Varella-Garcia M, Bunn PA Jr. Selecting lung cancer patients for treatment with epidermal growth factor receptor tyrosine kinase inhibitors by immunohistochemistry and fluorescence in situ hybridization--why, when, and how? Clin Cancer Res. 2006;12:4409s–4415s. doi: 10.1158/1078-0432.CCR-06-0087. [DOI] [PubMed] [Google Scholar]
- 29.Dei Tos AP. The biology of epidermal growth factor receptor and its value as a prognostic/predictive factor. Int J Biol Markers. 2007;22:S3–S9. [PubMed] [Google Scholar]
- 30.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 31.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Tew WP, Colombo N, Ray-Coquard I, Oza A, del Campo J, Scambia G, Spriggs D. VEGF-Trap for patients (pts) with recurrent platinum-resistant epithelial ovarian cancer (EOC): Preliminary results of a randomized, multicenter phase II study. J Clin Oncol. 2007;25(18S):5508 (Abstract). [Google Scholar]
- 33.Pepper MS. Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res. 2001;7:462–468. [PubMed] [Google Scholar]
- 34.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 35.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci USA. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kukk E, Lymboussaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. VEGF-C receptor binding and pattern of expression with VEGFR-3 suggests a role in lymphatic vascular development. Development. 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Sakamoto A, Sakamoto T, Maruyama H, et al. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res. 2003;9:1361–1369. [PubMed] [Google Scholar]
- 38.Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, Emoto M, Umemoto M, Sakamoto T, Sato S, et al. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer. 2003;88:237–244. doi: 10.1038/sj.bjc.6600701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Prognostic significance of vascular endothelial growth factor D in breast carcinoma with long-term follow-up. Clin Cancer Res. 2003;9:716–721. [PubMed] [Google Scholar]
- 40.Juttner S, Wissmann C, Jons T, Vieth M, Hertel J, Gretschel S, Schlag PM, Kemmner W, Hocker M. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol. 2006;24:228–240. doi: 10.1200/JCO.2004.00.3467. [DOI] [PubMed] [Google Scholar]
- 41.Herrmann E, Eltze E, Bierer S, Kopke T, Gorge T, Neumann J, Hertle L, Wulfing C. VEGF-C, VEGF-D and Flt-4 in transitional bladder cancer: relationships to clinicopathological parameters and long-term survival. Anticancer Res. 2007;27:3127–3133. [PubMed] [Google Scholar]
- 42.Niki T, Iba S, Tokunou M, Yamada T, Matsuno Y, Hirohashi S. Expression of vascular endothelial growth factors A, B, C, and D and their relationships to lymph node status in lung adenocarcinoma. Clin Cancer Res. 2000;6:2431–2439. [PubMed] [Google Scholar]
- 43.Gunningham SP, Currie MJ, Han C, Robinson BA, Scott PA, Harris AL, Fox SB. The short form of the alternatively spliced flt-4 but not its ligand vascular endothelial growth factor C is related to lymph node metastasis in human breast cancers. Clin Cancer Res. 2000;6:4278–4286. [PubMed] [Google Scholar]
- 44.Maekawa S, Iwasaki A, Shirakusa T, Enatsu S, Kawakami T, Kuroki M, Kuroki M. Correlation between lymph node metastasis and the expression of VEGF-C, VEGF-D and VEGFR-3 in T1 lung adenocarcinoma. Anticancer Res. 2007;27:3735–3741. [PubMed] [Google Scholar]
- 45.Onogawa S, Kitadai Y, Tanaka S, Kuwai T, Kimura S, Chayama K. Expression of VEGF-C and VEGF-D at the invasive edge correlates with lymph node metastasis and prognosis of patients with colorectal carcinoma. Cancer Sci. 2004;95:32–39. doi: 10.1111/j.1349-7006.2004.tb03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White JD, Hewett PW, Kosuge D, McCulloch T, Enholm BC, Carmichael J, Murray JC. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002;62:1669–1675. [PubMed] [Google Scholar]
- 47.Hu WG, Li JW, Feng B, Beveridge M, Yue F, Lu AG, Ma JJ, Wang ML, Guo Y, Jin XL, et al. Vascular endothelial growth factors C and D represent novel prognostic markers in colorectal carcinoma using quantitative image analysis. Eur Surg Res. 2007;39:229–238. doi: 10.1159/000101855. [DOI] [PubMed] [Google Scholar]
- 48.Kawakami M, Furuhata T, Kimura Y, Yamaguchi K, Hata F, Sasaki K, Hirata K. Expression analysis of vascular endothelial growth factors and their relationships to lymph node metastasis in human colorectal cancer. J Exp Clin Cancer Res. 2003;22:229–237. [PubMed] [Google Scholar]
- 49.Duff SE, Jeziorska M, Kumar S, Haboubi N, Sherlock D, O'Dwyer ST, Jayson GC. Lymphatic vessel density, microvessel density and lymphangiogenic growth factor expression in colorectal cancer. Colorectal Dis. 2007;9:793–800. doi: 10.1111/j.1463-1318.2006.01199.x. [DOI] [PubMed] [Google Scholar]
- 50.Schulze-Bergkamen H, Fleischer B, Schuchmann M, Weber A, Weinmann A, Krammer PH, Galle PR. Suppression of Mcl-1 via RNA interference sensitizes human hepatocellular carcinoma cells towards apoptosis induction. BMC Cancer. 2006;6:232. doi: 10.1186/1471-2407-6-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreoni B, Chiappa A, Bertani E, Bellomi M, Orecchia R, Zampino M, Fazio N, Venturino M, Orsi F, Sonzogni A, et al. Surgical outcomes for colon and rectal cancer over a decade: results from a consecutive monocentric experience in 902 unselected patients. World J Surg Oncol. 2007;5:73. doi: 10.1186/1477-7819-5-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galizia G, Lieto E, Ferraraccio F, De Vita F, Castellano P, Orditura M, Imperatore V, La Mura A, La Manna G, Pinto M, et al. Prognostic significance of epidermal growth factor receptor expression in colon cancer patients undergoing curative surgery. Ann Surg Oncol. 2006;13:823–835. doi: 10.1245/ASO.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 53.Spano JP, Lagorce C, Atlan D, Milano G, Domont J, Benamouzig R, Attar A, Benichou J, Martin A, Morere JF, et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol. 2005;16:102–108. doi: 10.1093/annonc/mdi006. [DOI] [PubMed] [Google Scholar]
- 54.Cohen SJ, Cohen RB, Meropol NJ. Targeting signal transduction pathways in colorectal cancer--more than skin deep. J Clin Oncol. 2005;23:5374–5385. doi: 10.1200/JCO.2005.02.194. [DOI] [PubMed] [Google Scholar]
- 55.Tabernero J, Salazar R, Casado E, Martinelli E, Gomez P, Baselga J. Targeted therapy in advanced colon cancer: the role of new therapies. Ann Oncol. 2004;15 Suppl 4:iv55–iv62. doi: 10.1093/annonc/mdh905. [DOI] [PubMed] [Google Scholar]
- 56.Sebastian S, Settleman J, Reshkin SJ, Azzariti A, Bellizzi A, Paradiso A. The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim Biophys Acta. 2006;1766:120–139. doi: 10.1016/j.bbcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 57.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 58.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 59.Cserni G. Nodal staging of colorectal carcinomas and sentinel nodes. J Clin Pathol. 2003;56:327–335. doi: 10.1136/jcp.56.5.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drescher D, Moehler M, Gockel I, Frerichs K, Muller A, Dunschede F, Borschitz T, Biesterfeld S, Holtmann M, Wehler T, et al. Coexpression of receptor-tyrosine-kinases in gastric adenocarcinoma--a rationale for a molecular targeting strategy? World J Gastroenterol. 2007;13:3605–3609. doi: 10.3748/wjg.v13.i26.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 62.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–1891. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 64.Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. 2007;25:4298–4307. doi: 10.1200/JCO.2006.07.1092. [DOI] [PubMed] [Google Scholar]
- 65.Jia YT, Li ZX, He YT, Liang W, Yang HC, Ma HJ. Expression of vascular endothelial growth factor-C and the relationship between lymphangiogenesis and lymphatic metastasis in colorectal cancer. World J Gastroenterol. 2004;10:3261–3263. doi: 10.3748/wjg.v10.i22.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789–9798. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- 67.O-charoenrat P, Rhys-Evans P, Modjtahedi H, Eccles SA. Vascular endothelial growth factor family members are differentially regulated by c-erbB signaling in head and neck squamous carcinoma cells. Clin Exp Metastasis. 2000;18:155–161. doi: 10.1023/a:1006764100867. [DOI] [PubMed] [Google Scholar]
- 68.Hadari YR, Doody JF, Wang YF, Patel SN, Apblett RL, Loizos N, Pereira DS, Witte L, Bohlen P, Hicklin DJ, et al. 2004. The IgG1 monoclonal antibody cetuximab induces degradation of the epidermal growth factor receptor. ASCO Gastrointestinal Cancers Symposium, January 22-24, San Francisco, CA; p. 234 (Abstract). [Google Scholar]
- 69.Prewett M, Rockwell P, Rockwell RF, Giorgio NA, Mendelsohn J, Scher HI, Goldstein NI. The biologic effects of C225, a chimeric monoclonal antibody to the EGFR, on human prostate carcinoma. J Immunother Emphasis Tumor Immunol. 1996;19:419–427. doi: 10.1097/00002371-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 70.Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]