Summary
A comparison of risks for the secondary transmission of HIV between young HIV-infected women-who-have-sex-with-men (WSM) and men-who-have-sex-with-men (MSM) found that recent partner-specific sexual risk behaviors are high among both populations. However, differences in the specific behaviors between WSM and MSM support population-specific interventions to reduce the secondary transmission of HIV.
Background
Secondary transmission remains a significant concern among HIV-infected youth. Little is known, however, about how partner-specific sexual risk behaviors for the secondary transmission of HIV may differ between the two largest subgroups of HIV positive youth, women-who-have-sex-with-men (WSM) and men-who-have-sex-with-men (MSM),
Methods
During 2003-2004, a convenience sample of HIV-infected youth, 13-24 years of age, were recruited from 15 Adolescent Medicine Trials Network clinical sites. Approximately 10-15 youth were recruited at each site. Participants completed an ACASI survey including questions about sex partners in the past year. Cross-sectional data analyses, including bivariate and multivariable regressions using generalized estimating equations, were conducted during 2008 to compare recent partner-specific sexual risk behaviors between WSM and MSM.
Results
Of 409 participants, 91% (371) were included in this analysis, including 176 WSM and 195 MSM. Ninety-two percent (163 WSM, 177 MSM) provided information on characteristics of their sexual partners. There were significant differences between the two groups in recent partner-specific sexual risk behaviors including: lower rates of condom use at last sex among WSM (61% WSM vs. 78% MSM; p=0.0011); a larger proportion of the sex partners of MSM reported as concurrent (56% MSM vs. 36% WSM; p=0.0001); and greater use of hard drugs at last sex by MSM and/or their partner (18% MSM vs. 4% WSM; p=0.0008). When measuring risk as a composite measure of sexual risk behaviors known to be associated with HIV transmission, both groups had high rates of risky behaviors, 74.7% among young MSM compared to 68.1% of WSM.
Conclusions
These data suggest that recent partner-specific sexual risk behaviors for HIV transmission are high among young infected MSM and WSM. These findings suggest the need to offer interventions to reduce the secondary transmission of HIV to all HIV-positive youth in care. However, differences in risk behaviors between young MSM and WSM supports population-specific interventions.
Introduction
Youth experience disproportionately high rates of new HIV infections relative to other age groups. Current estimates in the United States suggest that one quarter of new infections are among youth 15 to 24 years of age.1,2 Secondary transmission remains a significant concern among HIV-infected youth for multiple reasons. Youth have a high prevalence of sexually transmitted infections (STIs) and many of these infections increase the likelihood of transmitting HIV.3,4 Youth compared to other age groups may live longer with HIV/AIDS, and thus may have more opportunity to transmit their infection.5 Perhaps more importantly, however, numerous studies suggest that many youth remain sexually active and continue to practice risky sexual behaviors even after diagnosis with HIV.6-13 All of these factors underscore the importance of youth-focused secondary HIV transmission prevention efforts.
Among youth there are two subgroups that suffer from high rates of HIV infection and are at-risk of transmitting infection – young African American heterosexual females or women-who-have-sex-with-men (WSM) and young men-who-have-sex-with-men (MSM).14,15,16 Little is known, however, about how the sexual risk behaviors of HIV-positive youth may differ by subgroup, i.e. WSM versus MSM. It is possible that young MSM and WSM are at similar risk for transmitting HIV but for different reasons, as found in adult populations.17 Understanding similarities and differences in secondary transmission behaviors of young MSM and WSM is essential to designing effective prevention interventions for HIV-positive youth.
The Connect to Protect (C2P) project, a part of the Adolescent Trials Network (ATN) for HIV/AIDS Interventions, presents a unique opportunity to determine whether young, HIV-positive WSM and MSM, both groups with large proportions of African Americans, have differing recent partner-specific sexual risk behaviors and if so, whether the differences suggest population-specific interventions to reduce the secondary HIV transmission.
Material and Methods
Connect to Protect: Partnerships for Youth Prevention Interventions
Funded by the National Institutes of Health, the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) is a multicenter collaborative network that was established in March 2001 to conduct biological, behavioral and clinical research among 12-24 year olds who are either HIV-infected or at-risk of acquiring HIV. To implement this research agenda, 15 clinical sites in urban communities were funded. This analysis was conducted using cross-sectional data collected as a part of one multi-phase prevention research initiative of the ATN entitled, Connect to Protect (C2P) Partnerships for Youth Prevention Interventions (see Ziff et al. (2006).18 All 15 sites obtained local IRB approval to conduct the research activities.19
Study Population & Procedures
During 2003-2004, a convenience sample of HIV-infected adolescents seeking care at each of the 15 ATN sites was recruited. Each site aimed to recruit 10 to 15 HIV-infected females and males each. In order to be eligible for the study, an HIV-infected adolescent needed to meet the following inclusion criteria: (1) acquired HIV-1 infection after the age of 9 years (a cut-off of 9 years was used to distinguish between behaviorally, versus prenatally, infected youth), (2) documented HIV-1 infection by a positive result on a licensed test, (3) self-reported being 12-24 years of age, (4) self-reported engaging in vaginal, anal, and/or oral sexual activity within the past 12 months, and (5) ability to understand and willingness to provide informed consent. The potential participant was also excluded if s/he was (1) visibly distraught and/or emotionally unstable, (2) visibly intoxicated or under the influence of psychoactive substances, and/or (3) presented clinically as acutely ill.
During initial meetings, staff provided a brief overview of the C2P initiative and asked if the potential participants would be interested in completing a 45-60 minute interview. If the potential participant was interested, C2P staff obtained verbal consent and administered an eligibility screening tool; if eligible, the individual was invited to participate in the interview. The interview was administered in a private area using Audio Computer-Assisted Self-Administered Interview (ACASI) technology.20
Sex Partner Elicitation & Study Variables
For this analysis, we classified participants as either women-who-have-sex-with-men (WSM) or men-who-have-ever-had-sex-with-men (MSM). Participants were classified as WSM if they self-reported their birth gender as female and their sexual orientation as heterosexual or bisexual. Participants were classified as MSM if they self-reported their birth gender as male and reported ever having sex with a man (note, MSM could include men who also had or have sex with women).
During the ACASI interview, index participants were asked about their sex partners, up to four sexual partners in the past year. To elicit information about sex partners, the index participant was initially asked to identify the two close social contacts (non-family), and to report if they had had sex with either of them in the past year. Index participants were also asked to report whether they had any other sex partners in the past year.
Participants were asked survey questions about themselves and their sex partner(s) including demographic characteristics and sexual history. Variables for the current analysis are described below.
Demographic variables: (1) age (index subject only), (2) Hispanic/Latino origin, (3) race categorized as black/African American, white, mixed race or other, and (4) ever homeless.
Lifetime sexual risk variables (index subject only): (1) lifetime number of sex partners, (2) ever had an STI, (3) ever had sex with someone who was HIV-infected, (4) ever had sex with someone who injects drugs (IDU) and (5) ever exchanged sex for drugs or money.
Current sexual risk variable (index subject only): number of sex partners in the past 3 months.
Recent partner-specific sexual risk variables: (1) condom use at last sex (oral, anal or vaginal); (2) sex partner made index have sex (oral, anal or vaginal) without a condom (i.e., forced sex); (3) the index reported sex partner concurrency, i.e. that the sex partner had other sex partners while in the relationship with the index subject; and (4) measured separately, use of alcohol, marijuana, or hard drugs (hard drugs were defined as “other drugs like cocaine, heroin or speed”) by the index and/or sex partner at last sex. In addition, a composite measure of recent partner-specific HIV transmission risk was created to identify whether or not the sexual partnership – index subject and each reported sex partner – represented a “risky” partnership for the secondary transmission of HIV. The composite measure included variables known to be associated with HIV transmission, specifically unprotected sex at last sex, forced sex without a condom, reported sex partner concurrency, and hard drug use by index or sex partner at last sex. Each included variable in the composite measure was coded dichotomously and a partnership was considered to be “risky” if any of the variables were positive.
Statistical Analyses
All statistical analyses were conducted in 2008 and generated using SAS version 9.1 (SAS Institute Inc., 2004). Means, standard deviations (SD), ranges and medians are reported for continuous-scaled characteristics and the number and frequency reported for categorical scaled characteristics. For the analysis of sex partner data, index participants were able to contribute information for more than one sex partner (up to four sex partners). This introduces potential correlation in the responses and violates the assumptions for the t-test and chi-square test. Therefore, a Generalized Estimating Equations (GEE) model, as implemented in SAS PROC GENMOD, was fit to the data to generate p-values for comparisons between WSM and MSM for the sex partner data with and without adjustment for covariates.21 The model allows for the possible correlation of responses concerning the characteristics of multiple sex partners of each index subject to be accounted for, yielding a valid p-value.
Results
Study Population
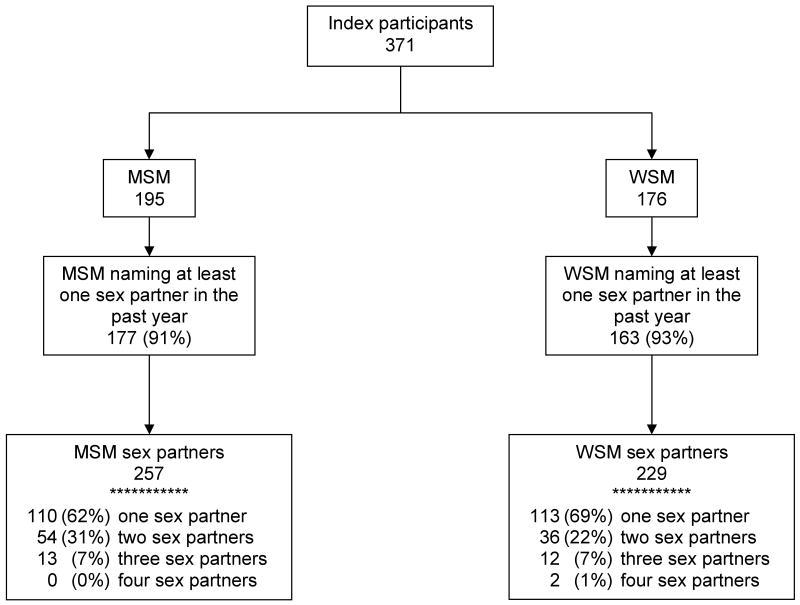
Of the 443 individuals (200 females; 241 males; 2 unknown birth gender) recruited to participate in this study, 6.8% (9 females; 21 males) were not eligible to participate and of the 413 eligible youth, 1% (2 females; 2 males) did not agree to participate. Of the remaining 409 participants, 189 self-reported their birth gender as female, of whom 93% (176) self-reported their sexual orientation as heterosexual or bisexual, and were defined as women-who-have-sex-with-men (WSM). Of the 409 participants, 218 self-reported their birth gender as male and of these, 89% (195) self-reported ever having sex with a man and were defined as men-who-have-sex-with-men (MSM). Thus the total sample size for this analysis was 371 (Figure 1).
Figure 1.
HIV-infected youth participants including men-who-have-ever-had-sex-with-men (MSM) and women-who-have-sex-with-men (WSM) and the numbers of sex partners reported in the past year, 2003-2004.
Each index subject could provide information for one to four sex partners within the past year; those index participants that did not identify a sex partner did not contribute information to the sex partner analysis. Among the 371 index participants included in this analysis, 91.6% (340/371) provided information on characteristics of sexual partners including 92.6% (163/176) of WSM and 90.8% (177/195) of MSM (Figure 1). The 163 WSM provided information on 229 sex partners with an average of 1.4 sex partners per index subject. The 177 MSM provided information on 257 sex partners with an average of 1.5 sex partners per index subject.
Characteristics of Index Participants
The demographic characteristics and lifetime and recent sexual risk behaviors of the index participants are presented overall and according to index subject group (MSM versus WSM) in Table 1. The average age of participants was 20.7 years for MSM and 20.6 years for WSM, with a range of 13 to 24 years. The majority of MSM, 54.6%, and WSM, 69.9%, were African American. Among MSM 37.4% were of Hispanic/Latino origin and among WSM 20.6% were Hispanic/Latino origin. Both subgroups reported high rates of ever having been homeless, including 44.6% of MSM and 35.8% of WSM.
Table 1.
Demographic characteristics and sexual risk behaviors stratified by men-who-have-ever-had-sex-with-men (MSM) and women-who-have-sex-with-men (WSM) recruited from a convenience sample of HIV-infected youth, ages 13-24 years, 2003-2004 (n=371).
| Index subject characteristics | MSM (n=195) |
WSM (n=176) |
|---|---|---|
| Demographics | ||
| Age, mean (SD) | 20.7 (2.2) | 20.6 (2.6) |
| Race, n (%) | ||
| African American | 106 (54.6) | 123 (69.9) |
| White | 24 (12.4) | 11 (6.3) |
| Mixed race | 35 (18.0) | 23 (13.1) |
| Other | 29 (15.0) | 19 (10.8) |
| Hispanic/Latino origin, n (%) | 73 (37.4) | 36 (20.6) |
| Ever been homeless, n (%) | 87 (44.6) | 63 (35.8) |
| Lifetime sexual risk behaviors | ||
| Number of sex partners in lifetime, | ||
| mean (SD) | 97.8 (251.8) | 20.3 (32.9) |
| range | 2–2500 | 1–200 |
| median | 25.0 | 8.0 |
| Ever had STI, n (%) | 147 (75.4) | 136 (77.3) |
| Ever had sex with someone who has HIV, n (%) | 93 (49.5) | 45 (26.0) |
| Ever had sex with someone who injects drugs (IDU), n (%) | 38 (19.8) | 18 (10.3) |
| Ever exchanged sex for drugs or money, n (%) | 58 (31.9) | 28 (16.6) |
| Current sexual risk behaviors | ||
| Number of sex partners in past 3 months, | ||
| mean (SD) | 5.1 (12.6) | 1.9 (3.5) |
| range | 0–120 | 0–30 |
| median | 2.0 | 1.0 |
The median number of reported sex partners in lifetime was 25 for MSM and 8 for WSM. About three quarters of MSM and WSM had ever had an STI. About one half of MSM and 26% of WSM reported ever having sex with someone HIV-positive. One fifth of MSM and 10.3% of WSM reported ever having sex with someone who injects drugs (IDU) and 31.9% of MSM and 16.6% of WSM reported ever exchanging sex for drugs or money. The median number of sex partners in the past three months was 2 for MSM and 1 for WSM.
Partner-specific Variables
To gain a better understanding of the partner-specific transmission risk among HIV-infected youth, we compared the partnerships of MSM and WSM, including demographics and recent partner-specific sexual risk behaviors (Table 2). The racial makeup of sex partners of MSM differed from that of WSM (p=0.015). A smaller proportion of sexual partners of MSM were of African American race compared to sexual partners of WSM (57.7% versus 68.0%), while a larger proportion of sexual partners of MSM were white compared to sexual partners of WSM (17.4% versus 4.8%). MSM and WSM were equally likely to have a partner of Hispanic/Latino origin.
Table 2.
Sex partner-specific demographics and sexual risk behaviors reported in the past year by men-who-have-ever-had-sex-with-men (MSM) and women-who-have-sex-with-men (WSM) participants recruited from a convenience sample of HIV-infected youth, ages 13-24 years, 2003-2004 (n=486).
| MSM (n = 257) |
WSM (n = 229) |
p-value* | |
|---|---|---|---|
| Sex partner demographics | |||
| Race, n (%) | |||
| African-American | 146 (57.7) | 155 (68.0) | 0.015 |
| White | 44 (17.4) | 11 (4.8) | |
| Mixed race | 31 (12.3) | 27 (11.8) | |
| Other | 32 (12.6) | 35 (15.4) | |
| Hispanic/Latino origin, n (%) | 66 (25.8) | 51 (22.4) | 0.46 |
| Recent partner-specific sexual risk behaviors | |||
| Condom use at last sex (oral, anal or vaginal), n (%) | 153 (77.7) | 116 (61.4) | <0.002 |
| Partner had other sex partner while index and he/she were in a sexual relationship, n (%) | 138 (56.1) | 80 (36.4) | <0.001 |
| Partner made index have sex (oral, anal or vaginal) without a condom: n (%) | |||
| Never happened | 212 (83.1) | 195 (85.9) | 0.41 |
| Happened one time | 12 (4.7) | 12 (5.3) | |
| Happened more than once but less than 5 times | 16 (6.3) | 9 (4.0) | |
| Happened 5 times or more | 15 (5.9) | 11 (4.8) | |
| At last sex, alcohol and drug use | |||
| Index had been using alcohol, n (%) | 64 (25.0) | 33 (14.5) | 0.024 |
| Index had been using marijuana, n (%) | 82 (32.0) | 49 (21.6) | 0.029 |
| Index had been using hard drugs like cocaine, heroin or speed, n (%) | 40 (15.6) | 7 (3.1) | <0.003 |
| Partner had been using alcohol, n (%) | 78 (30.5) | 56 (24.6) | 0.22 |
| Partner had been using marijuana, n (%) | 74 (28.9) | 71 (31.1) | 0.63 |
| Partner had been using hard drugs like cocaine, heroin or speed, n (%) | 37 (14.5) | 5 (2.2) | <0.001 |
| Overall index and/or partner had been using any alcohol or drug, n(%) | 56 (55.4) | 40 (37.7) | 0.014 |
| Composite measure of recent partner-specific HIV transmission risk, n (%) 1 | 177 (74.7) | 141 (68.1) | 0.16 |
P-values obtained using a GEE model that considers the correlation in responses from index participants who provide information on more than one sexual contact.
Defined by four measures including: (1) the index and sex partner did not used a condom at last sex; (2) the sex partner made the index have sex without a condom; (3) the index reported sex partner concurrency; and (4) the index or sex partner used hard drugs at the time of last sex.
A larger proportion of MSM than WSM indicated that they had used a condom at last sex with their sex partners (77.7% versus 61.4%, respectively; p=0.0011). A larger proportion of MSM than WSM reported sex partner concurrency (56.1% versus 36.4%; p=0.0001). The frequency that a partner ever made the index have sex without a condom did not differ between WSM and MSM (16.9% versus 14.4%; p=0.4). MSM were significantly more likely than WSM to have been using alcohol (25% versus 14.5%; p=0.0238), marijuana (32.0% versus 21.6%; p=0.0289) and hard drugs (like cocaine, heroin or speed) (15.6% versus 3.1%; p=0.0026) at the time of last sex. The partners of MSM and WSM were equally likely to have been using alcohol (30.5% versus 24.6%; p=0.22) and/or marijuana at last sex (28.9% versus 31.1%; p=0.63). However, a greater percentage of sex partners of MSM than of WSM used hard drugs at last sex (14.5% versus 2.2%; p=0.0003).
As defined by our composite measure of sexual risk behaviors known to be associated with HIV transmission, a larger proportion of partnerships of MSM (74.7%) than of partnerships of WSM (68.1%) were characterized as “risky” for secondary HIV transmission. The difference, however, was not statistically significant even after adjusting for index age, race and ethnicity (p=0.27).
Discussion
We examined the characteristics of a sample of HIV-positive youth and their partner-specific sexual risk behaviors in order to inform interventions to reduce the secondary transmission of HIV. Specifically, we were interested in whether there would be subgroup differences between WSM and MSM in recent partner-specific sexual risk behaviors that would suggest the need for population-specific interventions. We highlight a number of salient results and then suggest a number of important lessons for secondary prevention activities.
WSM and MSM in this study reported a high rate of recent partner-specific sexual risk behaviors. Both subgroups reported high rates of sex partner concurrency, 56.1% among MSM and 36.4% among WSM with significantly higher rates reported among MSM. Among MSM, this is similar to an estimate of index concurrency of 62% from an ongoing prospective study of HIV-negative MSM in Montreal22; although there have been few studies and none specifically among young MSM. These rates contrast greatly with an estimate of 14.4% index concurrency among all sexually-active adolescents in the National Longitudinal Study of Adolescent Health.23 Use of any drug (including alcohol, marijuana and other drugs like cocaine, heroin or speed) at last sex by index participants and/or their sexual partners was considerable and significantly different between the subgroups, 55.4% for MSM and 37.7% for WSM. These rates are considerably higher than those reported in 2007 by in-school youth in the Youth Behavioral Risk Survey (YRBS), of whom 27.5% of males and 17.7% of females reported any drug use at last sex.24 When measuring risk as a composite measure of sexual risk behaviors known to be associated with HIV transmission, we found considerable reports of risk behaviors among both groups with more partner-specific behavioral sexual risk reported among young MSM, 74.7%, compared to 68.1% of WSM, however, the difference was not statistically significant after adjusting for index age, race and ethnicity.
Overall, as in national data, it is noteworthy that youth of African American and Hispanic/Latino ancestry are disproportionately represented in our sample relative to the population of adolescents nationally. The convenience nature of the sampling for this study does not permit us to draw generalizable conclusions from this finding; however, it does suggest that prevention activities should be culturally sensitive. In addition, as both subgroups reported high rates of homelessness and exchange of sex for drugs or money, prevention activities should be aware of and potentially address the socioeconomic realities of affected youth as suggested in previous studies.13
Some population-specific lessons for secondary prevention for young HIV-positive MSM are suggested by our findings. Interventions that specifically address concurrency of partnerships and the high usage of alcohol, marijuana and other drugs in the context of sexual activities are particularly salient. The association between risky sexual behavior and drug use among MSM has been well-documented 10,25-27 although some recent evidence suggests that this relationship may vary by concordance of serostatus.28 Social marketing interventions that address the relationship of methamphetamine use to STI and HIV risk in San Francisco are examples of interventions that could be adapted to young MSM and particularly young MSM of color.29
Young HIV-infected WSM reported high rates of risky partnerships. Although compared to young MSM, WSM tended to have lower HIV transmission risk behaviors except for higher rates of condom non-use at last sex. These findings are similar to other STI studies examining sex partner selection patterns, some of which suggest that risky male sex partners may be key players in the acquisition of STIs and HIV among adolescent females.30-32 These findings, however, also suggest that young HIV-infected WSM are at considerable risk for the secondary transmission of HIV. Structural or community-level interventions which recognize the network position of young WSM and their sex partners, such as interventions that strive to encourage “safe” sexual partner selection patterns (“knowing your partner” interventions), may be appropriate for WSM to reduce STI acquisition and secondary transmission of HIV.
This study has a number of strengths. In order to properly characterize transmission risk, we used the reports by participants regarding multiple sex partners to generate partner-specific measures of transmission risk. This represents an improvement over previous studies as it allows for a better characterization of the totality of risk for secondary HIV transmission. Furthermore, the C2P ATN data provides a robust national sample of HIV positive youth, offering a unique opportunity to examine differences in sexual risk behaviors among two subgroups of HIV-positive youth.
The study also has several limitations. The study population represents HIV-infected youth who are currently enrolled in care, a population which may not be representative of all HIV-infected youth. In addition, participants were classified as MSM and/or WSM on the basis of self-reported data and included or excluded from this study on this basis which may have led to the inadvertent exclusion of individuals. This limitation may limit the generalizability of the results to all young HIV-positive MSM and WSM currently enrolled in care. However, we suspect that the use of an ACASI as our mode of data capture minimized the potential for study population bias.20 Another limitation is that all sex partner data represents information reported by the index regarding their sex partner, i.e., egocentric information, which requires that the index participant have knowledge and be willing to provide partner information. While this may limit the accuracy of the data gathered, there are significant challenges and limitations associated with locating and interviewing sex partners. An additional limitation is that we did not collect clinical information or conduct a medical chart review for this study. To the extent that the clinical status of individuals is related to partner-specific sexual risk behaviors this represents a limitation to our study.
If our findings are found to be applicable to all HIV-positive youth in care, our results suggest that the standard of care should include secondary prevention strategies specific to subpopulations of HIV-infected youth.1,33-34 These data suggest that recent partner-specific risks for HIV transmission are high among both young infected MSM and WSM. However, differences in specific risks support culturally-sensitive, population-specific interventions to reduce the secondary transmission of HIV.
Acknowledgments
This research was implemented during the first project period of the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN I). The ATN I was funded by the National Institutes of Health (grants U01 HD40506-01 and U01 HD40533) through the National Institute of Child Health and Human Development (Audrey Smith Rogers, Robert Nugent, Leslie Serchuck), with supplemental funding from the National Institutes on Drug Abuse (Nicolette Borek), Mental Health (Andrew Forsyth, Pim Brouwers) and Alcohol Abuse and Alcoholism (Kendall Bryant).
The following ATN I sites participated in this C2P study: University of South Florida: Patricia Emmanuel, Diane Straub, Shannon Cho, Georgette King, Mellita Mills, and Chodaesessie Morgan; Childrens Hospital of Los Angeles: Marvin Belzer, Miguel Martinez, Veronica Montenegro, Ana Quiran, Angele Santiago, and Gabriela Segura; Children's Hospital National Medical Center: Lawrence D'Angelo, William Barnes, Bendu Cooper, and Cassandra McFerson; the Children's Hospital of Philadelphia: Bret Rudy, Antonio Cardoso, and Marné Castillo; John H. Stroger Jr. Hospital and the CORE Center: Jaime Martinez, and Zephyr Beason; University of Puerto Rico: Irma Febo, Ileana Blasini, Ibrahim Ramos-Pomales, and Carmen Rivera-Torres; Montefiore Medical Center: Donna Futterman, Sharon S. Kim, Lissette Marrero, Stephen Stafford, and Carol Tobkes; Mount Sinai Medical Center: Linda Levin, Meg Jones, Christopher Moore, and Kelly Sykes; University of California at San Francisco: Barbara Moscicki, Colette Auerswald, and Kevin Sniecinski; Tulane University Health Sciences Center: Sue Ellen Abdalian, Lisa Doyle, Trimika Fernandez, and Sybil Schroeder; University of Maryland: Ligia Peralta, Bethany Griffin Deeds, Sandra Hipszer, Maria Metcalf, and Kalima Young; University of Miami School of Medicine: Lawrence Friedman, Angie Lee, Kenia Sanchez, Benjamin Quiles, and Shirleta Reid; Children's Diagnostic and Treatment Center: Ana Puga, Dianne Batchelder, Jamie Blood, Pam Ford, and Jessica Roy; Children's Hospital Boston: Cathryn Samples, Wanda Allen, Lisa Heughan, and Judith Palmer-Castor; University of California at San Diego: Stephen Spector, Rolando Viani, Stephanie Lehman, and Mauricio Perez.
The authors also acknowledge Connect to Protect's National Coordinating Center at Johns Hopkins School of Medicine and DePaul University's Quality Assurance Team, including staff members and consultants Nancy Willard, Suzanne Maman, Marizaida Sánchez-Cesáreo, Matthew Bowdy, Rachel Lynch, Audrey Bangi, Mimi Doll, Jason Johnson, Danish Meherally, Grisel Robles, and Leah Neubauer. We also thank the ATN Data and Operations Center (Westat Inc), including Jim Korelitz, Barbara Driver, Lori Perez, Rick Mitchell, Stephanie Sierkierka, and Dina Monte, and individuals from the ATN Coordinating Center at the University of Alabama, Birmingham, including Craig Wilson, Cindy Partlow, Marcia Berck, and Pam Gore. The authors also thank the ATN manuscript reviewers, including Mickey Lally, Cheri Boyer, Marcia Berck and George Siberry, for their constructive comments.
Funding: This work was supported with funding from the Eunice Shriver National Institute of Child Health and Human Development (U01 HD40506-01 and U01 HD40533) with supplemental funding from the National Institutes of Drug Abuse, Mental Health and Alcohol Abuse and Alcoholism and funding for the primary author from the National Institute of Drug Abuse (K01 5K01DA022298-02). The authors also thank the young men and women who participated in this study and the study field staff for their data collection efforts.
References
- 1.Rotheram-Borus MJ. Prevention of HIV among adolescents. Prev Sci. 2000;1(1):15–30. doi: 10.1023/a:1010071932238. [DOI] [PubMed] [Google Scholar]
- 2.Office of National AIDS Policy. Youth & HIV/AIDS: An American Agenda: A Report to the President. Washington, DC: Office of National AIDS Policy; 1996. [PubMed] [Google Scholar]
- 3.CDC. Sexually Transmitted Disease Surveillance, 2005. Atlanta, GA: U.S. Department of Health and Human Services; 2006. [Google Scholar]
- 4.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. HIV/AIDS Among Youth. Atlanta, GA: U.S. Department of Health and Human Services; 2006. pp. 1–5. [Google Scholar]
- 6.Murphy DA, Durako SJ, Moscicki AB, et al. No change in health risk behaviors over time among HIV infected adolescents in care: role of psychological distress. J Adolesc Health. 2001;29(3 Suppl):57–63. doi: 10.1016/s1054-139x(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 7.CDC. MMWR. Vol. 50. Atlanta, GA.: 2001. HIV incidence among young men who have sex with men - Seven U.S. cities, 1994-2000; pp. 440–444. [PubMed] [Google Scholar]
- 8.Kalichman SC, Kelly JA, Rompa D. Continued high-risk sex among HIV seropositive gay and bisexual men seeking HIV prevention services. Health Psychol. 1997;16(4):369–373. doi: 10.1037//0278-6133.16.4.369. [DOI] [PubMed] [Google Scholar]
- 9.Trent M, Chung S, Ellen JM, et al. New sexually transmitted infections among adolescent girls infected with HIV. Sex Transm Infect. 2007;83(6):468–469. doi: 10.1136/sti.2007.026161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morin SF, Steward WT, Charlebois ED, et al. Predicting HIV transmission risk among HIV-infected men who have sex with men: findings from the healthy living project. J Acquir Immune Defic Syndr. 2005;40(2):226–235. doi: 10.1097/01.qai.0000166375.16222.eb. [DOI] [PubMed] [Google Scholar]
- 11.Williams LA, Klausner JD, Whittington WL, et al. Elimination and reintroduction of primary and secondary syphilis. Am J Public Health. 1999;89(7):1093–1097. doi: 10.2105/ajph.89.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. Increases in unsafe sex and rectal gonorrhea among men who have sex with men--San Francisco, California, 1994-1997. MMWR. 1999;48(3):45–48. [PubMed] [Google Scholar]
- 13.Valleroy LA. HIV prevalence and associated risks in young men who have sex with men. JAMA. 2000;284(2):198–204. doi: 10.1001/jama.284.2.198. [DOI] [PubMed] [Google Scholar]
- 14.Espinoza L, Hall HI, Hardnett F, et al. Characteristics of persons with heterosexually acquired HIV infection, United States 1999-2004. Am J Public Health. 2007;97(1):144–149. doi: 10.2105/AJPH.2005.077461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CDC. HIV/AIDS Surveillance Report, 2006. Vol. 18. Atlanta, GA.: U.S. Department of Health and Human Services; 2008. pp. 1–55. [Google Scholar]
- 16.CDC. HIV prevalence, unrecognized infection, and HIV testing among men who have sex with men--five U.S. cities, June 2004-April 2005. MMWR. 2005;54(24):597–601. [PubMed] [Google Scholar]
- 17.Weinhardt LS, Kelly JA, Brondino MJ, et al. HIV transmission risk behavior among men and women living with HIV in 4 cities in the United States. J Acquir Immune Defic Syndr. 2004;36(5):1057–1066. doi: 10.1097/00126334-200408150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ziff MA, Harper GW, Chutuape KS, et al. Laying the foundation for Connect to Protect: a multi-site community mobilization intervention to reduce HIV/AIDS incidence and prevalence among urban youth. J Urban Health. 2006;83(3):506–522. doi: 10.1007/s11524-006-9036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeds BG, Castillo M, Beason Z, et al. Concepts and best practices: a case study of a common community-based participatory research HIV prevention protocol reviewed at 15 national sites. Journal of Empirical Research on Human Research Ethics. 2008 doi: 10.1525/jer.2008.3.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner CF, Ku L, Rogers SM, et al. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science. 1998;280(5365):867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 21.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 22.Vydykhan TN, Remis RS, Alary M, et al. Sexual partner concurrency among men who have sex with men (MSM) in Montreal. Tenth Annual Canadian Conference on HIV/AIDS Research.May 31, 2001. [Google Scholar]
- 23.Kelley SS, Borawski EA, Flocke SA, et al. The role of sequential and concurrent sexual relationships in the risk of sexually transmitted diseases among adolescents. J Adolesc Health. 2003;32(4):296–305. doi: 10.1016/s1054-139x(02)00710-3. [DOI] [PubMed] [Google Scholar]
- 24.CDC. Youth risk behavior surveillance--United States, 2007. MMWR. 2008;57(SS4):1–131. [PubMed] [Google Scholar]
- 25.Halkitis PN, Parsons JT, Stirratt MJ. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. J Homosex. 2001;41(2):17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- 26.Parsons JT, Vicioso K, Kutnick A, et al. Alcohol use and stigmatized sexual practices of HIV seropositive gay and bisexual men. Addict Behav. 2004;29(5):1045–1051. doi: 10.1016/j.addbeh.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Purcell DW, Parsons JT, Halkitis PN, et al. Substance use and sexual transmission risk behavior of HIV-positive men who have sex with men. J Subst Abuse. 2001;13(12):185–200. doi: 10.1016/s0899-3289(01)00072-4. [DOI] [PubMed] [Google Scholar]
- 28.Purcell DW, Moss S, Remien RH, et al. Illicit substance use, sexual risk, and HIV-positive gay and bisexual men: differences by serostatus of casual partners. AIDS. 2005;19 1:S37–S47. doi: 10.1097/01.aids.0000167350.00503.db. [DOI] [PubMed] [Google Scholar]
- 29.State of California Department of Health Services Office of AIDS. Prevention with positives: a guide to effective programs. California: California Department for Health Services; 2004. [Google Scholar]
- 30.Jennings JM, Luo RF, Lloyd LV, et al. Age-bridging among young, urban, heterosexual males with asymptomatic Chlamydia trachomatis. Sex Transm Infect. 2007;83(2):136–141. doi: 10.1136/sti.2006.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JK, Jennings JM, Ellen JM. Discordant sexual partnering: a study of high-risk adolescents in San Francisco. Sex Transm Dis. 2003;30(3):234–240. doi: 10.1097/00007435-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Auerswald CL, Muth SQ, Brown B, et al. Does partner selection contribute to sex differences in sexually transmitted infection rates among African American adolescents in San Francisco? Sex Transm Dis. 2006;33(8):480–484. doi: 10.1097/01.olq.0000204549.79603.d6. [DOI] [PubMed] [Google Scholar]
- 33.Institute of Medicine. No Time to Lose: Getting More from HIV Prevention. Washington, DC: National Academy of Sciences; 2001. [Google Scholar]
- 34.Collins C, Morin S, Shriver M, et al. Designing primary prevention for people living with HIV. San Francisco: AIDS Research Institute, University of California, San Francisco; 2000. [Google Scholar]