Abstract
Background
Whereas prevalence of coronary heart disease (CHD) risk factors has declined over past decades in the United States, acute myocardial infarction (AMI) rates were steady. We hypothesized that this paradox is partly due to the advent of increasingly sensitive biomarkers for AMI diagnosis.
Methods and Results
In Framingham Heart Study participants over four decades, we compared incidence/survival rates of initial AMI diagnosis by electrocardiogram (AMI-ECG) irrespective of biomarkers, to those exclusively based on infarction biomarkers (AMI-marker). We employed Poisson regression to calculate annual incidence rates of first AMI over four decades (1960–69, 1970–79, 1980–89 and 1990–99), and compared rates of AMI-ECG with rates of AMI-marker. Cox proportional hazards was used to compare AMI case-fatality over four decades. In 9,824 persons (54% women; follow-up of 212,539 persons-years, aged 40–89 years) there were 941 AMIs including 639 AMI-ECG and 302 AMI-marker. From 1960 to 1999, rates of AMI-ECG declined by about fifty percent and rates of AMI-marker increased approximately two-fold. Crude 30-day, 1-year and five-year case fatality rates in 1960–69 and 1990–99, respectively, were 0.20 and 0.14; 0.24 and 0.21; and 0.45 and 0.41. Age- and sex-adjusted 30-day, 1-year and 5-year AMI case-fatality declined by 60% 1960 to 1999 (p-trend <0.001), with parallel declines noted following AMI-ECG and AMI-marker.
Conclusions
Over the past forty years, rates of AMI-ECG, have declined by fifty percent whereas rates of AMI diagnosed by biomarker have doubled. Our findings offer an explanation for apparent steady national AMI rates in the face of improvements in primary prevention.
Keywords: Myocardial infarction, temporal trends, biomarkers, electrocardiogram, diagnostic drift
Introduction
During the past four decades death rates from coronary heart disease (CHD) have declined by more than sixty percent. 1–4 Between nearly one half to upwards of three quarters of the decline in CHD mortality has been attributed to improvements in primary prevention and risk factor modification.5–10 Awareness, treatment and control of three key risk factors -- hypertension, hypercholesterolemia, and smoking have improved in recent decades. 1,11 Despite these improvements, hospitalization rates for acute myocardial infarction (AMI) have remained relatively stable over the past five decades. 1,4,12 The reasons for the paradoxical stability of AMI rates in the face of declining CHD risk factor prevalence are not clear.
Whereas electrocardiographic (ECG) criteria for AMI have not changed appreciably over the past fifty years, several different biomarkers of varying sensitivity and specificity have been introduced for the detection of AMI. Early on, serum glutamic oxalacetic transaminase (SGOT) and lactic dehydrogenase (LDH) were used in conjunction with clinical information to diagnose AMI. In more recent times, serum markers of myocardial cell damage including creatinine kinase (CPK), LDH isoenzymes, creatinine kinase-MB (CPK-MB), and troponin have been introduced sequentially to diagnose AMI, and have been firmly incorporated into international guidelines for AMI case definition.13 As compared with diagnosis based solely on history and ECG, AMI diagnosis based on serial biomarker measurements has substantially increased the detection of AMI cases.14–16
Previous investigations of United States trends in AMI incidence comparing different diagnostic criteria have been hospital-based and have encompassed limited time periods for their analysis.14,17 The Framingham Heart Study, which has over fifty years of physician-validated AMI data on a community-based cohort, offers a unique setting to study trends in AMI incidence and case-fatality rates based on the following AMI diagnostic criteria: A) AMI by ECG diagnosis (AMI-ECG) irrespective of biomarker elevation, which offers an unbiased assessment of long-term trends and B) AMI by biomarker diagnosis (AMI-marker) in the absence of diagnostic ECG changes, which reflects changing methods in clinical practice. The sum of these two mutually exclusive approaches represents total AMI.
We hypothesized a priori that rates of AMI-ECG have declined in the long term (consistent with improvements in CHD risk factors), while the rates of AMI-marker have increased (owing to greater biomarker sensitivity), resulting in a relatively steady rate of total AMI incidence over a forty year time interval. Accordingly, we analyzed forty year trends in incidence of first AMI and for two mutually exclusive AMI subgroups: A) AMI-ECG and B) AMI-marker. Such an approach may shed light on the paradoxical stability of national AMI rates in the setting of improvements in CHD risk factors and declining rates of CHD mortality.
Secondarily, we assessed time-period trends in mortality following AMI and its subcomponents, AMI-ECG, and AMI-marker. This analysis will help promote understanding of the relative effectiveness of secondary prevention efforts over time, when considered in conjunction with analyses of time-period changes in the incidence of initial AMI, which reflect advances in primary prevention.
Methods
The Framingham Heart Study is a community-based prospective observational study that began in 1948, enrolling 5209 men and women in an original study cohort.18 Original cohort members attended clinic examinations approximately every two years. In 1971, 5124 men and women enrolled into the Framingham Heart Study offspring cohort, which included the children and spouses of the children of the original cohort. Participant examinations for the offspring cohort occurred approximately every four to eight years; the design and methodology have been described elsewhere.19 This investigation included original and offspring cohort members.
We considered all original and offspring cohort members aged 40–89 years and free of AMI (recognized and unrecognized) at their first Framingham clinic examination in each decade of study (1960s, 1970s, 1980s, 1990s). Our final sample size consisted of 9,824 individuals. Each individual could enter the sample multiple times based on eligibility for time period and age group. For example, a participant aged 35 years in 1960 would not contribute follow-up time to the first time period until they turned 40 in 1965 thereafter contributing 5 years. That participant would contribute 5 years to 2nd time period and so on until the patient died or developed AMI. Similarly, a patient 75 years old in 1960 contributed at most 5 years to the last period. Participants provided written informed consent and the study protocol was approved by the Boston University Medical Center Institutional Review Board.
Risk Factor Assessment
At each routine clinic visit, participants underwent physical examination, 12-lead ECG, anthropometry, and laboratory assessment of vascular risk factors. Details regarding the ascertainment of risk factors have been previously described.19 Participants with systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥ 90 mm Hg (mean reading of two readings taken by an examining physician) or receiving medication for treatment of hypertension were defined as having hypertension. Plasma glucose and total cholesterol were measured. Diabetes was defined (throughout the study period) as fasting plasma glucose ≥126 mg/dL, a non-fasting glucose of ≥ 200 mg/dL, or treatment with either insulin or hypoglycemic agents. Participants were considered to be current smokers if they smoked on average at least one cigarette per day during the year prior to examination.
Serum biomarkers of myocardial infarction
Several serum biomarkers were used for AMI diagnosis during the study time period. Specific diagnostic biomarkers and the decades during which they were used for AMI diagnosis in the Framingham Heart Study included: SGOT (beginning in the mid-1950s), LDH (1960s), CPK (1970s), CPK-MB and LDH isoenzyme (1980s), and troponin (in the late 1990s). We did not use pre-specified cut-points to determine biomarker elevation since there was variability in assays used in the various hospitals from which medical records were collected. Thus, we considered a biomarker elevated if it exceeded the reference limit provided by the hospital laboratory report at the time of AMI hospitalization, according to the available medical record/chart.
Ascertainment of AMI and AMI Case-fatality
Framingham Heart Study participants are under continuous surveillance for CVD events and death. The surveillance process included the following: 1. A physician-administered questions about cardiovascular events during each routine follow-up Framingham Heart Study clinic visit and 2. A mailed health history update questionnaire (which prior to the late 1990’s consisted of a brief questionnaire for those who had not attending examinations and after the late 1990’s included detailed sections about interim cardiac events and hospitalizations). If a participant reported a possible interim event, all pertinent medical records were collected and reviewed by an events adjudication committee, consisting of three physicians who reviewed all available hospitalization records, physician office visit notes, and pathology reports.20 AMIs were diagnosed on the basis of 1) ischemic chest discomfort with diagnostic ECG changes (based on chart review) with or without diagnostic biomarker changes (AMI-ECG) or 2) ischemic chest discomfort with diagnostic serum biomarkers of infarction but without diagnostic ECG changes (AMI-marker). ECG criteria for AMI included development of pathologic Q-waves of ≥ 0.04 seconds, often accompanied by ST-elevation and followed by serial changes indicating a reversion of these ECG changes toward normal. We chose to exclude persons with unrecognized/silent AMI as it is impossible to determine the exact date of occurrence, which is assigned a midpoint between the last ECG without an abnormality and the first one manifesting Q-wave changes .
Case-fatality was assessed within 30-days, 1-year and 5-years. For 1-year and 5-year mortality, deaths occurring within the first 30-days were excluded from analysis. We did this in order to get a truer sense of how many “later” case fatalities occurred following AMI (since a large proportion of post- AMI deaths occur within 30 days of the index event as opposed to later). Furthermore, pathophysiologically “early” death from AMI is likely different from “later” deaths.
Statistical Methods
Prevalence rates and mean values (± standard deviations) of CVD risk factors were calculated for the study sample at the first examination cycle in each decade of study. We employed Poisson regression to calculate annual incidence rates of first AMI over four time periods (1960–69, 1970–79, 1980–89 and 1990–99), and compared rates of AMI-ECG with rates of AMI-marker. We tested for sex*age group, sex*time period, age group*time period, age group*AMI type, and time period*AMI type interactions for incidence rate trends; given multiple statistically significant p-values for these interactions, we present age- and sex-specific AMI incidence rates for each time period. Additionally, with small numbers of events for the oldest and youngest age groups, we provide trends in (log-transformed) event rates across the four time periods for age groups 50–59, 60–69 and 70–79 years, for men and women separately. We calculated tests of trend for overall AMI, AMI-ECG, and AMI-marker across time periods, with the 1960s serving as the referent decade (using a model accounting for the interactions listed above). We employed Cox proportional hazards models to calculate age- and sex-adjusted case-fatality curves and 30-day, 1-year and 5-year case-fatality rates following all AMI, AMI-ECG, and AMI-marker for each of the four time periods (1960–1969 serving as the as referent period). The follow-up period for case-fatality was until the end of 2006. The assumption of proportionality of hazards was satisfied over the 5-year follow-up period following AMI (p-value for time to death*period interaction >0.32 for overall AMI, AMI-ECG, and AMI-marker). A two-sided P value of less than 0.05 was considered to indicate statistical significance. All statistical analyses were performed with the use of the SAS statistical software (version 9.0).
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Characteristics of study sample
Study participant characteristics by decade are shown in Table 1. Among 9,824 participants, fifty-four percent were women; follow-up time was 212,539 persons-years. The mean age of participants at the start of each time period ranged from 53 years in the 1960s to 60 years in 1990s. Smoking rates, total cholesterol concentrations, and systolic and diastolic blood pressure decreased from 1960 to 1999.
Table 1.
Characteristics of Framingham Heart Study Participants at the Start of Each Time Period of Study, mean ± standard deviation, (%)
| 1960s | 1970s | 1980s | 1990s | |
|---|---|---|---|---|
|
Men (n) Women (n) |
1768 2366 |
2205 2687 |
2145 2662 |
2147 2632 |
| Mean age (yrs) | 53 ± 8 | 56 ± 10 | 60 ± 12 | 60 ± 13 |
| Women, % | (57) | (55) | (55) | (55) |
| Total Cholesterol, mg/dL | 248 ± 45 | 226 ± 43 | 221 ± 41 | 210 ± 39 |
| Systolic BP, mmHg | 136 ± 23 | 136 ± 22 | 133 ± 20 | 133 ± 21 |
| Diastolic BP, mmHg | 84 ± 12 | 82 ± 11 | 79 ±10 | 78 ± 10 |
| Body Mass Index, kg/m2 | 25.8 ± 4.1 | 26.5 ± 4.3 | 26.6 ± 4.4 | 27.0 ± 4.8 |
| Glucose, mg/dL | 82 ± 23 | 106 ± 18 | 95 ± 39 | 99 ± 30 |
| Hypertension % | (43) | (47) | (47) | (48) |
| Diabetes % | (4) | (8) | (10) | (8) |
| Smoking % | (53) | (28) | (30) | (35) |
Trends in overall AMI, AMI-ECG and AMI-marker rates
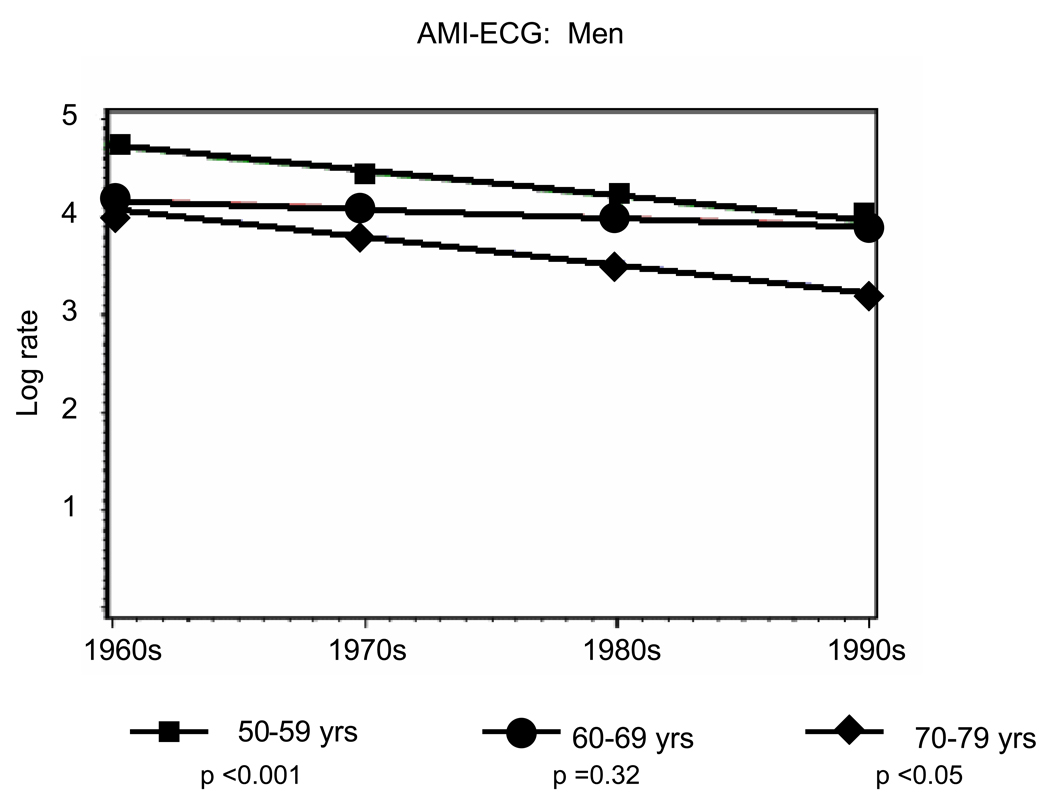
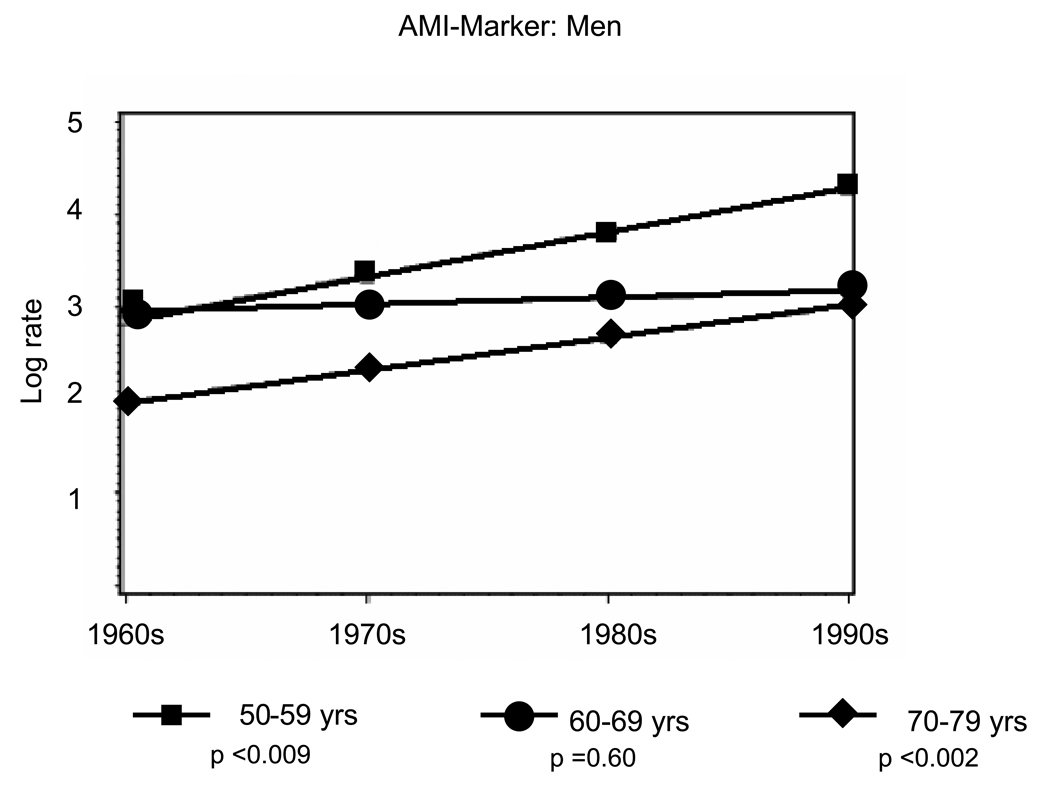
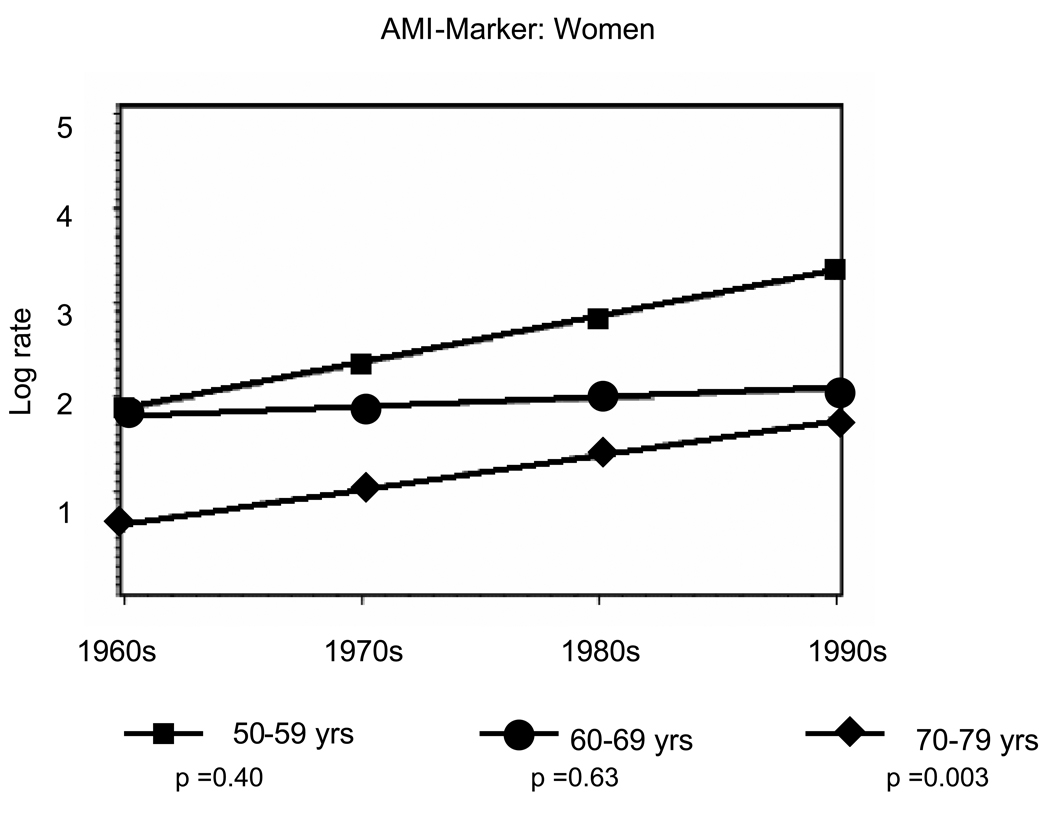
Overall, there were 941 first AMIs, including 639 (sixty-eight percent) AMI-ECG events and 302 (thirty-two percent) AMI-marker events. Age- and sex-specific incidence rate trends from the 1960s to the 1990s are presented in Table 2A (men) and Table 2B (women). Rates of AMI-ECG declined by about fifty percent and rates of AMI-marker doubled over the study period (Tables 2A and 2B, Figure 1 and Figure 2). Among men, statistically significant declines in AMI-ECG were noted in the age groups 50–59 (p trend <0.001) and 70–79 (p trend <0.05) (Figure 1, Panel A) and in women statistically significant declines in AMI-ECG were noted among those 70–79 years old (p trend <0.01) (Figure 1, Panel B). Among men, statistically significant increases in AMI-marker were noted in the age groups 50–59 and 70–79 (p trend for both <0.01) (Figure 2, Panel A) and in women statistically significant increases in AMI-marker were noted among those 70–79 years old (p trend <0.01) (Figure 1, Panel B). Trends for AMI-ECG and AMI-marker were largely flat for the age group 60–69 years.
Table 2.
| A: Decade-Specific Incidence Rates of Overall AMI, AMI-ECG and AMI-marker per 10,000 Person-Years Among Men | ||||
|---|---|---|---|---|
| 1960–69 | 1970–79 | 1980–89 | 1990–99 | |
| Overall AMI | ||||
| Age | ||||
| 40–49 | 43.80 | 22.05 | 29.43 | 24.71 |
| 50–59 | 62.62 | 53.86 | 59.75 | 39.78 |
| 69–69 | 74.05 | 84.33 | 97.90 | 55.11 |
| 70–79 | 152.17 | 83.84 | 135.56 | 117.36 |
| 80–89 | - | 98.09 | 129.90 | 166.03 |
| Events (n) | (130) | (143) | (190) | (144) |
| AMI-ECG | ||||
| Age | ||||
| 40–49 | 39.39 | 18.17 | 23.75 | 15.96 |
| 50–59 | 53.50 | 40.63 | 43.80 | 21.67 |
| 69–69 | 63.25 | 63.59 | 71.74 | 30.01 |
| 70–79 | 121.15 | 56.31 | 87.65 | 52.05 |
| 80–89 | - | 66.69 | 85.10 | 75.20 |
| Events (n) | (112) | (105) | (131) | (72) |
| AMI-marker | ||||
| Age | ||||
| 40–49 | 4.41 | 3.88 | 5.68 | 8.75 |
| 50–59 | 9.12 | 13.23 | 15.94 | 18.11 |
| 69–69 | 10.80 | 20.73 | 26.15 | 25.11 |
| 70–79 | 31.01 | 27.53 | 47.92 | 65.31 |
| 80–89 | - | 31.40 | 44.79 | 90.84 |
| Events (n) | (18) | (38) | (59) | (72) |
| Table 2B: Decade-Specific Incidence Rates of Overall AMI, AMI-ECG and AMI-marker per 10,000 Person-Years Among Women | ||||
|---|---|---|---|---|
| 1960–69 | 1970–79 | 1980–89 | 1990–99 | |
| Overall AMI | ||||
| Age | ||||
| 40–49 | 6.17 | 3.96 | 4.18 | 3.99 |
| 50–59 | 10.34 | 11.35 | 9.95 | 7.53 |
| 69–69 | 23.51 | 34.16 | 31.35 | 20.05 |
| 70–79 | 51.17 | 35.96 | 45.96 | 45.21 |
| 80–89 | - | 85.53 | 89.53 | 130.00 |
| Events (n) | (47) | (81) | (100) | (106) |
| AMI-ECG | ||||
| Age | ||||
| 40–49 | 5.55 | 3.26 | 3.37 | 2.57 |
| 50–59 | 8.84 | 8.56 | 7.29 | 4.10 |
| 69–69 | 20.09 | 25.76 | 22.97 | 10.91 |
| 70–79 | 40.74 | 24.15 | 29.72 | 20.05 |
| 80–89 | - | 58.15 | 58.66 | 58.88 |
| Events (n) | (38) | (59) | (72) | (50) |
| AMI-marker | ||||
| Age | ||||
| 40–49 | 0.62 | 0.70 | 0.81 | 1.4 |
| 50–59 | 1.51 | 2.79 | 2.66 | 3.43 |
| 69–69 | 3.43 | 8.40 | 8.37 | 9.13 |
| 70–79 | 10.43 | 11.81 | 16.25 | 25.16 |
| 80–89 | - | 27.38 | 30.87 | 71.13 |
| Events (n) | (9) | (22) | (28) | (56) |
122,560=person years of observation (men)
- no events for age group/ time period
89,979=person years of observation (women)
- no events for age group/ time period
Figure 1.
Panel A: Temporal trends for age-range specific incidence rates in AMI-ECG from 1960–1999 among Men
Panel B: Temporal trends for age-range specific incidence rates in AMI-ECG from 1960–1999 among Women
Figure 2.
Panel A: Temporal trends for age-range specific incidence rates in AMI-Marker from 1960–1999 among Men
Panel B: Temporal trends for age-range specific incidence rates in AMI-Marker from 1960–1999 among Women
Trends in 30-day, 1-year and 5-year AMI case-fatality
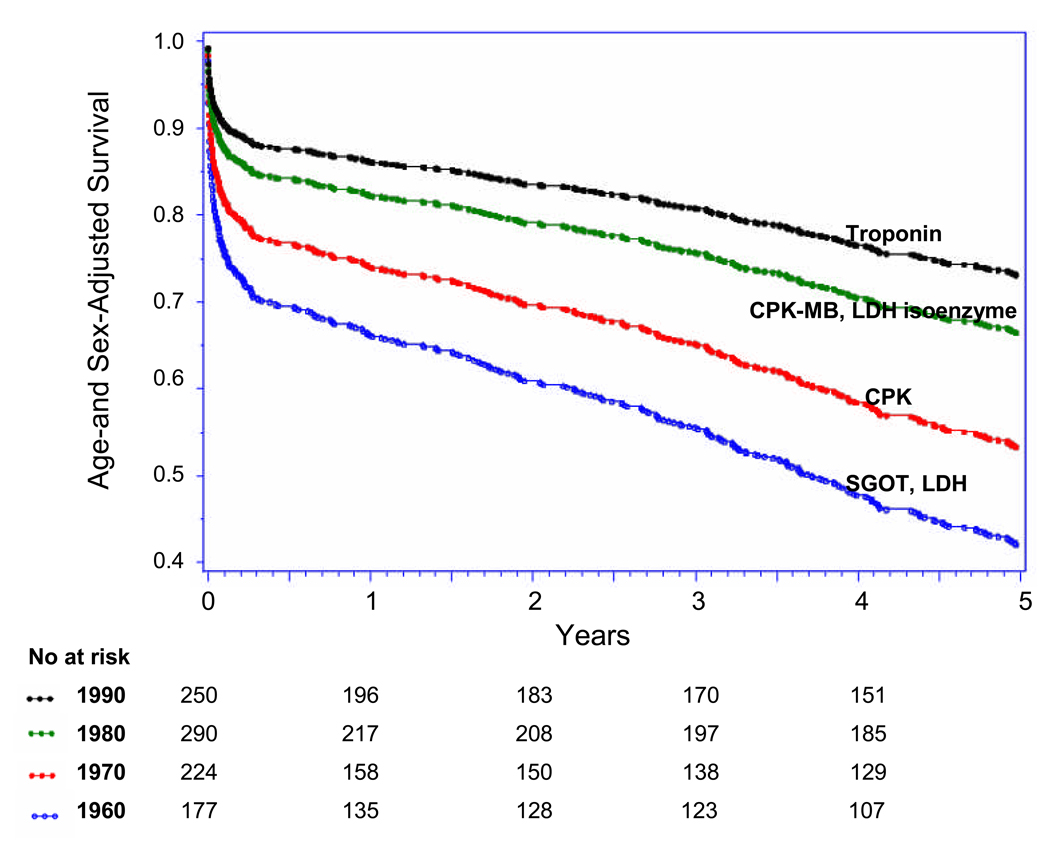
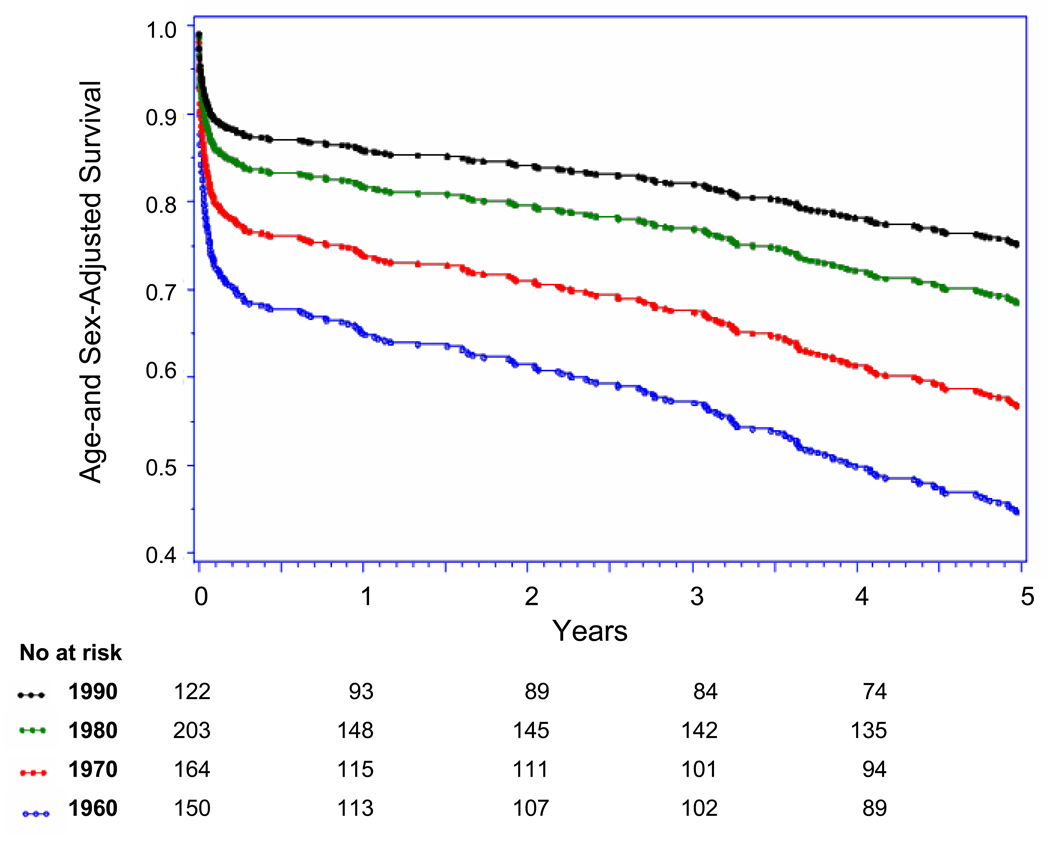
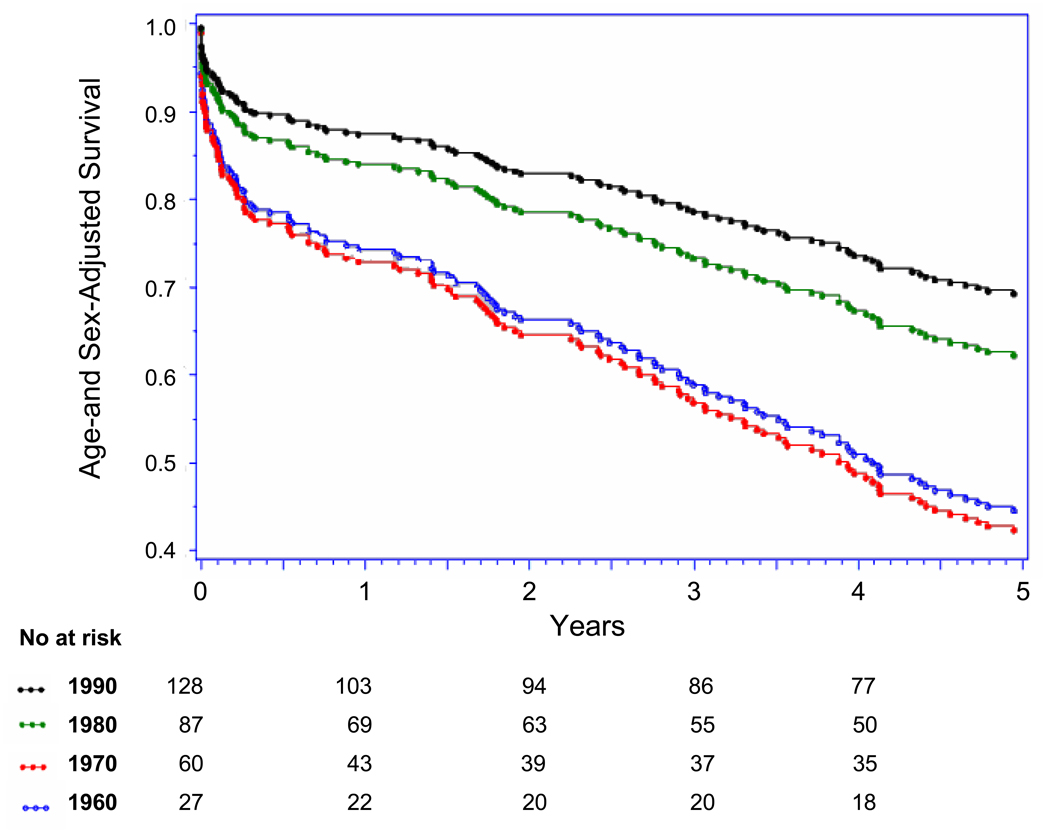
Five-year case-fatality rates following overall AMI decreased steadily from 1960 to 1999 (p-trend <0.001) (Table 3, Figure 3 Panel A). Trends in 5-year case-fatality rates following AMI-ECG and following AMI-marker mirrored overall 5-year AMI-case-fatality trends (Figure Panels B and C). Similarly, decreases were seen in 30-day and 1-year case-fatality following overall AMI, AMI-ECG, and AMI-marker. There was a particularly large shift toward decreased case-fatality between the 1970s and 1980s (Figure 3, Panel A–C).
Table 3.
Age-and Sex-Adjusted 30-day, 1-year and 5-Year Mortality Rates for Overall AMI, AMI-ECG and AMI-marker Among Framingham Heart Study Participants
| 30-day | |||||
|---|---|---|---|---|---|
| Outcome | 1960–1969 | 1970–1979 | 1980–1989 | 1990–1999 | Trend Test |
| All AMI | |||||
| Deaths (n) | (35) | (45) | (47) | (34) | |
| HR (95%)CI | Referent | 0.66 (0.42–1.05) | 0.45 (0.28–0.71) | 0.27 (0.16– 0.45) | P<.0001 |
| AMI-ECG | |||||
| Deaths (n) | (32) | (38) | (35) | (23) | |
| HR (95% CI) | Referent | 0.71(0.44–1.16) | 0.50 (0.30–0.82) | 0.38 (0.21–0.69) | P=0.0004 |
| AMI-marker | |||||
| Deaths (n) | (3) | (7) | (12) | (11) | |
| HR (95% CI) | Referent | 0.72 (0.18–2.81) | 0.46 (0.12–1.82) | 0.22 (0.06–0.89) | P=0.006 |
| 1-year | |||||
| Outcome | 1960–1969 | 1970–1979 | 1980–1989 | 1990–1999 | Trend Test |
| All AMI | |||||
| Deaths (n) | (42) | (66) | (73) | (54) | |
| HR (95% CI) | Referent | 0.83 (0.56–1.23) | 0.58 (0.39–0.87) | 0.35 (0.22–0.54) | P<.0001 |
| AMI-ECG | |||||
| Deaths (n) | (37) | (49) | (55) | (29) | |
| HR (95% CI) | Referent | 0.80 (0.52–1.25) | 0.68 (0.44–1.05) | 0.42 (0.25–0.71) | P<0.001 |
| AMI-marker | |||||
| Deaths (n) | (5) | (17) | (18) | (25) | |
| HR (95% CI) | Referent | 1.11 (0.41–3.04) | 0.43 (0.15–1.22) | 0.31 (0.11–0.87) | P<0.001 |
| 5-year | |||||
| Outcome | 1960–1969 | 1970–1979 | 1980–1989 | 1990–1999 | Trend Test |
| All AMI | |||||
| Deaths (n) | (80) | (104) | (111) | (104) | |
| HR (95% CI) | Referent | 0.73 ( 0.54–0.98) | 0.47 (0.35–0.64) | 0.36 (0.26–0.50) | P<0.006 |
| AMI-ECG | |||||
| Deaths (n) | (70) | (78) | (72) | (46) | |
| HR (95% CI) | Referent | 0.70 (0.50–0.98) | 0.47 (0.33–0.66) | 0.36 (0.24–0.53) | P<0.001 |
| AMI-marker | |||||
| Deaths (n) | (10) | (26) | (39) | (58) | |
| HR (95% CI) | Referent | 1.06 (0.51–2.21) | 0.59 (0.28–1.22) | 0.45 (0.22–0.93) | P=0.001 |
Figure 3.
Panel A: Up to Five-year Case Fatality Following Overall AMI by Decade
Panel B: Up to Five-year Case Fatality Following AMI-ECG by Decade
Panel C: Up to Five-year Case Fatality Following AMI-marker by Decade, (with major biomarker used during each decade)
Discussion
Principal findings
In a community-based cohort of 9,824 men and women followed for a four decade interval, we found that AMI-ECG rates declined about fifty percent with a concomitant two-fold increase in rates of AMI-marker. The 30-day, 1-year and 5-year case-fatality following overall AMI declined by fifty to seventy-five percent from 1960 to 1999, with parallel declines in case-fatality following both AMI-ECG and AMI-marker over this period. We conclude that national MI trend data may be biased by a diagnostic drift due to the advent of diagnostic biomarker tests for AMI; we were able to identify and quantify the possible magnitude of this effect within our study setting. These findings may serve to explain the paradoxical stability of AMI rates in the United States despite concomitant improvements in CHD risk factors.
Temporal Trends in AMI
Several epidemiologic studies conducted in United States have demonstrated steady rates of AMI from the 1970s to the 1990s,4,12,17,21 whereas data from the WHO-MONICA Project demonstrated modest declines in rates of AMI from 1985 to 1991.22 Data from the Worcester Heart Attack Study similarly demonstrated modest declines in the incidence of first AMI.23 Differences in study design and event ascertainment may have accounted for the differing results between these prior studies. In our study, particularly in men, overall AMI trends appear to be decreasing in a parallel fashion compared with AMI-ECG. In women, overall AMI rates were steady to decreased.
Defining AMI in population studies and clinical research is essential for accurate disease surveillance, clinical trial design and conduct, and for healthcare resource allocation.13,24,25 Several prior studies demonstrating trends in AMI rates have utilized international diagnostic codes (ICD) for hospital discharges to identify AMI cases 21,26–28 and may be subject to “diagnostic drift”.29 Specifically, diagnostic coding of AMI during hospitalizations may have increased due to changes in reimbursement practices and by the use of more sensitive biomarkers of infarction.24,29 Temporal-trend estimates of AMI based on ICD codes have shown steady rates 4,21 over the past several decades. In contrast, AMI-ECG rates in our study sample declined by about 50% from 1960 to 1999. AMI-ECG represents a relatively “unbiased” estimate of AMI that has not been influenced by the advent of increasingly sensitive biomarkers of infarction in recent decades.17 Not surprisingly, AMI-marker rates in our study increased over this same time period in a manner consistent with prior data in the WHO-MONICA Study, which showed higher AMI rates using biomarker-based definitions (troponin) as compared to ECG-based definitions.15 Similarly, an investigation in the Minnesota Heart Study demonstrated a fifty-percent increase in AMI detection in 1980 when CPK and CPK-MB information was added to the Minnesota Heart Study AMI diagnostic algorithm.14 Additional studies have mirrored these findings showing that troponin-influenced AMI diagnosis has increased the AMI detection rate as compared to AMI diagnosis based on CPK-MB and total CPK. 30,31 Our data extends these findings by demonstrating that biomarker-influenced AMI diagnosis has yielded a doubling in rates of AMI-marker over the forty year period spanning 1960 to 1999.
The proportion of overall AMI diagnosed by ECG (sixty-eight percent) was similar to figures reported in a prior report from the Minnesota Heart Survey. 17 That investigation concluded that incident AMI rates by ECG criteria were steady from 1975 to 1985 and declined from 1985 to 1995.17 We extend these findings by providing data from two additional decades of observation and our results demonstrate a fifty to sixty percent decline in AMI-ECG rates from 1960 to 1999. AMI-ECG likely represents a more advanced form of myocardial infarction -- declines demonstrated in out-of-hospital sudden cardiac death2,32–35 (attributable to improved primary prevention efforts)33 have likely contributed to some degree in declines in the incidence of AMI-ECG.
Another possible explanation for the decline in AMI-ECG and the relative rise in AMI-marker may have to do with decreases in time from onset of symptoms to hospital presentation and to treatment (data from the National Registry of Myocardial Infarction)36 which are thought to be due to public health education efforts and guideline implementation, which have collectively stressed the need to decrease “door-to-intervention” time for AMI.36 On the other hand, other studies of community-based individuals and clinical trial participants have shown no temporal declines in pre-hospital delay during AMI.37–39
Case fatality rates
Our finding that AMI case fatality declined from 1960 to 1999 is consistent with studies conducted in the United States 1,3,4,23 and in Europe 22 that demonstrated declines in overall AMI case-fatality over the past twenty to forty years. Several studies have demonstrated that out-of-hospital sudden cardiac death has declined substantially over the past several decades.2,32–35 We extend these findings by demonstrating that case-fatality following AMI-ECG and AMI-marker have both declined to a similar degree.
Prior studies have suggested that improvements in primary prevention account for forty to fifty percent of the reduction in CHD mortality in the Unites States from 1968 to 2000.6,10 Our finding of a fifty percent decline in incidence of first AMI using an AMI definition for which bias is inherently low (i.e. AMI-ECG) implies that primary prevention efforts also have influenced the incidence of AMI.
Strengths and Limitations
Four decades of physician-validated AMI and case-fatality data along with the ability to separate AMI diagnosed by ECG and AMI diagnosed by biomarkers are unique strengths of our investigation. Indeed, AMI diagnosis relying on ICD coding may have a sensitivity of only sixty percent when compared with physician validated AMI diagnosis.40 Our adjudication committee had access to simultaneous ECG and biomarker information and therefore the ECG adjudication could have been biased by knowledge of biomarker information. However, we believe that if such a bias was introduced it would have biased results toward a greater proportion of AMI-ECG over time. We could not separate the contribution of specific biomarkers to AMI diagnosis among AMI-marker cases. Our study sample is largely of white and of European descent; therefore our findings may not be applicable to other ethnic groups or other geographic regions. We had a relatively small number of events when gender, specific age groups, and four time periods are considered, possibly limiting our statistical power to detect differences. We had a limited number of subjects in each gender, age group, and time period. We did not provide confidence intervals for the trend analyses for the incidence rates of AMI-ECG and AMI-marker; and due to limited statistical power, we did not test the interaction term MI type with time period.
Implications of our findings
The diagnosis of AMI is ever-evolving and therefore it is a challenge to accurately characterize the “true” epidemiology of AMI. However, our data demonstrate that whereas rates of AMI diagnosed by ECG have declined, these were offset by rising rates of AMI diagnosed by biomarkers. Since the most sensitive biomarkers were not available (i.e. troponin) in the earlier study decades (1960s–1980s) AMI- marker earlier on may have been under-diagnosed. Regardless, the advent of increasingly sensitive biomarkers for AMI diagnosis has substantially influenced AMI detection rates in the United States over the past several decades.
Conclusions
Over the past forty years, rates of AMI diagnosed by ECG have declined by fifty percent and rates of AMI diagnosed by biomarkers have doubled, offering a possible explanation for apparent steady national rates of overall AMI in the face of improvements in primary prevention.
Acknowledgements
Dr. Levy had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Funding Sources:
Supported by National Institute of Health / National Heart, Lung, and Blood Institute, contract N01-HC-25195
Footnotes
Disclosures:
There are no conflicts of interest to disclose.
Clinical Implications:
Whereas prevalence of coronary heart disease risk factors has declined over past decades in the United States, acute myocardial infarction rates have been steady. The diagnosis of acute myocardial infarction is ever-evolving and therefore it is a challenge to accurately characterize the “true” epidemiology of AMI. Among Framingham Heart Study participants, we found that over the past forty years, rates of AMI-ECG, have declined by fifty percent whereas rates of AMI diagnosed by biomarker have doubled. The advent of increasingly sensitive biomarkers for AMI diagnosis has substantially influenced AMI detection rates in the United States over the past several decades. Our findings offer an explanation for apparent steady national AMI rates in the face of improvements in primary prevention.
Reference List
- 1.NHLBI Morbidity and Mortality Chart Book. 2004 [Google Scholar]
- 2.Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110:522–527. doi: 10.1161/01.CIR.0000136993.34344.41. [DOI] [PubMed] [Google Scholar]
- 3.Guidry UC, Evans JC, Larson MG, Wilson PW, Murabito JM, Levy D. Temporal trends in event rates after Q-wave myocardial infarction: the Framingham Heart Study. Circulation. 1999;100:2054–2059. doi: 10.1161/01.cir.100.20.2054. [DOI] [PubMed] [Google Scholar]
- 4.Rosamond WD, Chambless LE, Folsom AR, Cooper LS, Conwill DE, Clegg L, Wang CH, Heiss G. Trends in the incidence of myocardial infarction and in mortality due to coronary heart disease, 1987 to 1994. N.Engl.J Med. 1998;339:861–867. doi: 10.1056/NEJM199809243391301. [DOI] [PubMed] [Google Scholar]
- 5.Unal B, Critchley JA, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation. 2004;109:1101–1107. doi: 10.1161/01.CIR.0000118498.35499.B2. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N.Engl.J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 7.Laatikainen T, Critchley J, Vartiainen E, Salomaa V, Ketonen M, Capewell S. Explaining the decline in coronary heart disease mortality in Finland between 1982 and 1997. Am.J Epidemiol. 2005;162:764–773. doi: 10.1093/aje/kwi274. [DOI] [PubMed] [Google Scholar]
- 8.Beaglehole R. Medical management and the decline in mortality from coronary heart disease. Br.Med.J (Clin.Res.Ed) 1986;292:33–35. doi: 10.1136/bmj.292.6512.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capewell S, Morrison CE, McMurray JJ. Contribution of modern cardiovascular treatment and risk factor changes to the decline in coronary heart disease mortality in Scotland between 1975 and 1994. Heart. 1999;81:380–386. doi: 10.1136/hrt.81.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman L, Cook EF. The decline in ischemic heart disease mortality rates. An analysis of the comparative effects of medical interventions and changes in lifestyle. Ann.Intern.Med. 1984;101:825–836. doi: 10.7326/0003-4819-101-6-825. [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 12.Rosamond WD, Folsom AR, Chambless LE, Wang CH. Coronary heart disease trends in four United States communities. The Atherosclerosis Risk in Communities (ARIC) study 1987–1996. Int.J Epidemiol. 2001;30 Suppl 1:S17–S22. doi: 10.1093/ije/30.suppl_1.s17. [DOI] [PubMed] [Google Scholar]
- 13.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De CR, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 14.Burke GL, Edlavitch SA, Crow RS. The effects of diagnostic criteria on trends in coronary heart disease morbidity: the Minnesota Heart Survey. J Clin.Epidemiol. 1989;42:17–24. doi: 10.1016/0895-4356(89)90021-8. [DOI] [PubMed] [Google Scholar]
- 15.Salomaa V, Koukkunen H, Ketonen M, Immonen-Raiha P, Karja-Koskenkari P, Mustonen J, Lehto S, Torppa J, Lehtonen A, Tuomilehto J, Kesaniemi YA, Pyorala K. A new definition for myocardial infarction: what difference does it make? Eur.Heart J. 2005;26:1719–1725. doi: 10.1093/eurheartj/ehi185. [DOI] [PubMed] [Google Scholar]
- 16.Lee TH, Goldman L. Serum enzyme assays in the diagnosis of acute myocardial infarction. Recommendations based on a quantitative analysis. Ann.Intern.Med. 1986;105:221–233. doi: 10.7326/0003-4819-105-2-221. [DOI] [PubMed] [Google Scholar]
- 17.Crow RS, Hannan PJ, Jacobs DR, Jr, Lee SM, Blackburn H, Luepker RV. Eliminating diagnostic drift in the validation of acute in-hospital myocardial infarction--implication for documenting trends across 25 years: the Minnesota Heart Survey. Am.J Epidemiol. 2005;161:377–388. doi: 10.1093/aje/kwi048. [DOI] [PubMed] [Google Scholar]
- 18.Dawber TR, Kannel WB. An approach to longitudinal studies in a community: the Framingham Study. Ann.N.Y.Acad.Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP The Framingham offspring study. An investigation of coronary heart disease in families. Am.J.Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 20.Cupples LA, D'Agostino RB., Sr . Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Study, 30-year follow-up. In: Kannel WB, Wolf PA, Garrison RJ, editors. The Framingham Heart Study: an Epidemiologic Investigation of Cardiovascular Disease. Washington, DC: NIH Publication; 1987. pp. 87–203. [Google Scholar]
- 21.Fang J, Alderman MH. Dissociation of hospitalization and mortality trends for myocardial infarction in the United States from 1988 to 1997. Am.J Med. 2002;113:208–214. doi: 10.1016/s0002-9343(02)01172-5. [DOI] [PubMed] [Google Scholar]
- 22.Tunstall-Pedoe H, Kuulasmaa K, Mahonen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring trends and determinants in cardiovascular disease. Lancet. 1999;353:1547–1557. doi: 10.1016/s0140-6736(99)04021-0. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg RJ, Yarzebski J, Lessard D, Gore JM. A two-decades (1975 to 1995) long experience in the incidence, in-hospital and long-term case-fatality rates of acute myocardial infarction: a community-wide perspective. J.Am.Coll.Cardiol. 1999;33:1533–1539. doi: 10.1016/s0735-1097(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 24.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed]
- 25.Alpert JS. Will the real myocardial infarction please stand up? Clin.Chem. 2006;52:795–796. doi: 10.1373/clinchem.2006.068411. [DOI] [PubMed] [Google Scholar]
- 26.Arciero TJ, Jacobsen SJ, Reeder GS, Frye RL, Weston SA, Killian JM, Roger VV. Temporal trends in the incidence of coronary disease. Am.J Med. 2004;117:228–233. doi: 10.1016/j.amjmed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 27.McGovern PG, Pankow JS, Shahar E, Doliszny KM, Folsom AR, Blackburn H, Luepker RV The Minnesota Heart Survey Investigators. Recent trends in acute coronary heart disease--mortality, morbidity, medical care, and risk factors. N.Engl.J Med. 1996;334:884–890. doi: 10.1056/NEJM199604043341403. [DOI] [PubMed] [Google Scholar]
- 28.McGovern PG, Jacobs DR, Jr, Shahar E, Arnett DK, Folsom AR, Blackburn H, Luepker RV. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: the Minnesota heart survey. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 29.Assaf AR, Lapane KL, McKenney JL, Carleton RA. Possible influence of the prospective payment system on the assignment of discharge diagnoses for coronary heart disease. N.Engl.J Med. 1993;329:931–935. doi: 10.1056/NEJM199309233291307. [DOI] [PubMed] [Google Scholar]
- 30.Falahati A, Sharkey SW, Christensen D, McCoy M, Miller EA, Murakami MA, Apple FS. Implementation of serum cardiac troponin I as marker for detection of acute myocardial infarction. Am.Heart J. 1999;137:332–337. doi: 10.1053/hj.1999.v137.92412. [DOI] [PubMed] [Google Scholar]
- 31.Kontos MC, Fritz LM, Anderson FP, Tatum JL, Ornato JP, Jesse RL. Impact of the troponin standard on the prevalence of acute myocardial infarction. Am.Heart J. 2003;146:446–452. doi: 10.1016/S0002-8703(03)00245-X. [DOI] [PubMed] [Google Scholar]
- 32.Rosamond WD, Folsom AR, Chambless LE, Wang CH. Coronary heart disease trends in four United States communities. The Atherosclerosis Risk in Communities (ARIC) study 1987–1996. Int.J.Epidemiol. 2001;30 Suppl 1:S17–S22. doi: 10.1093/ije/30.suppl_1.s17. [DOI] [PubMed] [Google Scholar]
- 33.Goraya TY, Jacobsen SJ, Kottke TE, Frye RL, Weston SA, Roger VL. Coronary heart disease death and sudden cardiac death: a 20-year population-based study. Am.J.Epidemiol. 2003;157:763–770. doi: 10.1093/aje/kwg057. [DOI] [PubMed] [Google Scholar]
- 34.Rea TD, Crouthamel M, Eisenberg MS, Becker LJ, Lima AR. Temporal patterns in long-term survival after resuscitation from out-of-hospital cardiac arrest. Circulation. 2003;108:1196–1201. doi: 10.1161/01.CIR.0000087403.24467.A4. [DOI] [PubMed] [Google Scholar]
- 35.Bunch TJ, White RD, Friedman PA, Kottke TE, Wu LA, Packer DL. Trends in treated ventricular fibrillation out-of-hospital cardiac arrest: a 17-year population-based study. Heart Rhythm. 2004;1:255–259. doi: 10.1016/j.hrthm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Rogers WJ, Canto JG, Lambrew CT, Tiefenbrunn AJ, Kinkaid B, Shoultz DA, Frederick PD, Every N. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the US from 1990 through 1999: the National Registry of Myocardial Infarction 1, 2 and 3. J.Am.Coll.Cardiol. 2000;36:2056–2063. doi: 10.1016/s0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg RJ, Yarzebski J, Lessard D, Gore JM. Decade-long trends and factors associated with time to hospital presentation in patients with acute myocardial infarction: the Worcester Heart Attack study. Arch.Intern.Med. 2000;160:3217–3223. doi: 10.1001/archinte.160.21.3217. [DOI] [PubMed] [Google Scholar]
- 38.Gibler WB, Armstrong PW, Ohman EM, Weaver WD, Stebbins AL, Gore JM, Newby LK, Califf RM, Topol EJ. Persistence of delays in presentation and treatment for patients with acute myocardial infarction: The GUSTO-I and GUSTO-III experience. Ann.Emerg.Med. 2002;39:123–130. doi: 10.1067/mem.2002.121402. [DOI] [PubMed] [Google Scholar]
- 39.McGinn AP, Rosamond WD, Goff DC, Jr, Taylor HA, Miles JS, Chambless L. Trends in prehospital delay time and use of emergency medical services for acute myocardial infarction: experience in 4 US communities from 1987–2000. Am.Heart J. 2005;150:392–400. doi: 10.1016/j.ahj.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 40.Rosamond WD, Chambless LE, Sorlie PD, Bell EM, Weitzman S, Smith JC, Folsom AR. Trends in the sensitivity, positive predictive value, false-positive rate, and comparability ratio of hospital discharge diagnosis codes for acute myocardial infarction in four US communities, 1987–2000. Am.J Epidemiol. 2004;160:1137–1146. doi: 10.1093/aje/kwh341. [DOI] [PubMed] [Google Scholar]