Abstract
Background
We previously reported that extending an overnight continuous posterior lumbar plexus nerve block to 4 days after hip arthroplasty provides clear benefits during the perineural infusion in the immediate postoperative period. However, it remains unknown if the extended infusion improves subsequent health-related quality-of-life.
Methods
Patients undergoing hip arthroplasty received a posterior lumbar plexus perineural infusion of ropivacaine 0.2% from surgery until the following morning, at which time patients were randomized to either continue perineural ropivacaine (n=24) or normal saline (n=23) in a double-masked fashion. Patients were discharged with their catheter and a portable infusion pump, and catheters were removed on postoperative day 4. Health-related quality-of-life was measured using the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index preoperatively and then at 7 days, as well as 1, 2, 3, 6, and 12 months after surgery. The WOMAC evaluates 3 dimensions of health-related quality-of-life: pain, stiffness, and physical functional disability (global score of 0–96, lower scores indicate lower levels of symptoms or physical disability). For inclusion in the primary analysis, we required a minimum of 3 of the 6 time points, including day 7 and at least 2 of months 3, 6, and 12.
Results
The 2 treatment groups had similar global WOMAC scores for the mean area under the curve calculations (point estimate for the difference in mean area under the curve for the 2 groups [extended infusion group – overnight infusion group] = 0.8, 95% confidence interval: −5.3 to +6.8 [−5.5% to +7.1%]; p=0.80) and at all individual time points (p>0.05).
Conclusions
This investigation found no evidence that extending an overnight continuous posterior lumbar plexus nerve block to 4 days improves (or worsens) subsequent health-related quality-of-life between 7 days and 12 months after hip arthroplasty.
Introduction
Although hip arthroplasty reduces chronic joint pain and improves patients’ functional status, the prostheses rarely completely abolish pain and restore functional performance to a normal level. Improved surgical outcomes, such as knee range-of-motion after knee arthroplasty, are associated with improved analgesia and physical therapy in the immediate postoperative period.1,2 Furthermore, improving postoperative analgesia may decrease the incidence of chronic pain,3 and increasing joint motion may optimize subsequent functioning by decreasing the effects of immobilization on muscles and synovial joints.4 Thus, there is indirect evidence that maximizing analgesia in the immediate postoperative period may reduce long-term pain, joint stiffness, and functional disability.
One intervention that has been shown to improve analgesia and decrease time to discharge-readiness after hip arthroplasty is a continuous posterior lumbar plexus nerve block.5,6 Unlike traditional IV opioid administration or epidural infusion, a continuous posterior lumbar plexus nerve block may be continued after discharge using a portable infusion pump, providing extended-duration treatment without requiring prolonged hospitalization.5,7 Therefore, an extended-duration continuous posterior lumbar plexus nerve block after hip arthroplasty offers the theoretical possibility of “long-term benefits from a short-term intervention.”8
The most important outcomes for patients are measures of functional status and well-being.9 These measures reflect the dimensions of health as they are conceptualized and valued by patients themselves.10 Indeed, evaluating quality-of-life is explicitly recommended for chronic pain clinical trials in the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) consensus statement.11 Although health-related quality-of-life is a subjective concept, various instruments are available that convert health status into quantifiable values.11,12 The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is an instrument specifically designed to evaluate clinically important, patient-relevant changes in health-related quality-of-life after treatment interventions in patients with osteoarthritis of the hip.13,14 The WOMAC evaluates 3 dimensions of health-related quality-of-life: pain, stiffness, and physical functional disability. Whether the improved analgesia and accelerated time to discharge-readiness that result from a continuous posterior lumbar plexus nerve block translate into increased health-related quality-of-life remain unknown.
Therefore, we completed this prospective follow-up study of a previously-reported, randomized, controlled, clinical trial.5 We hypothesized that, as measured using the WOMAC instrument, the improvement in pain, stiffness, and functional ability would be greater not only at one week, but also at 1, 2, 3, 6 and 12 months after hip arthroplasty in patients who received a 4-day continuous posterior lumbar plexus nerve block compared with an overnight continuous posterior lumbar plexus nerve block in the immediate postoperative period.
Materials and Methods
The IRB approved all study procedures and all subjects provided written, informed consent. Details of the study methods have been published previously.5 In brief, patients offered enrollment included adults (18–80 years) with osteoarthritis scheduled for primary, unilateral hip arthroplasty via a 15–25 cm curvilinear lateral skin incision over the greater trochanter (either hip resurfacing or hip replacement via the posterior approach with a posterior capsulotomy) who desired a continuous posterior lumbar plexus nerve block for postoperative analgesia.
Study intervention
Subjects received a posterior lumbar plexus nerve block and perineural catheter (Contiplex, B. Braun Medical Inc., Bethlehem, PA) followed by a perineural ropivacaine, 0.2%, infusion (8 mL/h basal; 4 mL patient-controlled bolus; 30-min lockout) from surgery until the following morning, at which time patients were randomized to either continue perineural ropivacaine (“extended infusion,” n=24) or switched to normal saline (“overnight infusion,” n=23). Randomization was performed in a triple-masked fashion (patients, investigators, statisticians) with stratification according to clinical site. Additional analgesics included one week of oral acetaminophen (975 mg every 6 h) and either oral aspirin (650 mg daily, University of Florida) or celecoxib (200 mg every 12 h, University of California at San Diego). Patients were provided oral (oxycodone 5 mg tablets) and/or IV opioids (morphine sulfate 2–4 mg) for breakthrough pain.
At 18:00 on postoperative day (POD) 2 (36 h after randomization), a portable infusion pump (Pain Pump 2 Blockaid, Stryker Instruments, Kalamazoo, MI) containing 400 mL of the same study solution (basal 5 mL/h; bolus 4 mL; lockout 60 min) replaced the previous infusion pump. Patients were discharged with their pump and perineural catheter in situ as early as 10:00 on POD 3. In the evening of POD 4, patients’ caretakers removed the posterior lumbar plexus catheters with physician instructions provided by telephone.
Outcome measurements
The current study was a pre-planned secondary analysis of prospectively collected health-related quality-of-life data, as measured with the WOMAC questionnaire. This instrument evaluates 3 dimensions: pain, stiffness, and physical functional disability with 5, 2, and 17 questions, respectively. An ordinal Likert scale from 0 to 4 is used for each question, with lower scores indicating lower levels of symptoms or physical disability.13 Each subscale is summated to a maximum score of 20, 8, and 68, respectively. The individual dimensions are always analyzed separately, and investigators have often added a “global” score, which is calculated by summating the scores for the 3 subscales.15,16 As eloquently explained by Hajiro and Nishimura,17 “Important concepts when evaluating measurements of health status are the clinically significant threshold or the minimal clinically significant difference. When health status is measured using a continuous scale, it needs to be known whether an observed difference indicates a clinically significant or trivial effect on the patients’ health status or quality of life. A statistically significant difference in health status might be of little practical importance; it is more important to know the minimal clinically significant difference.” Using the transition method, in an osteoarthritis rehabilitation intervention setting, effects larger than 12% of baseline score (6% of maximal score) can be attained and detected as the minimal clinically significant difference by the WOMAC.18
Since its inception 2 decades ago, the WOMAC has been translated into 60 languages and used in several hundred published clinical trials.19 It has been rigorously examined, demonstrating excellent construct validity, responsiveness, and test-retest reliability in patients after hip arthroplasty,13,15,20–23 and is therefore recommended in the Osteoarthritis International Research Society’s guidelines for clinical trials.19,23–26 The questionnaire may be self-administered or administered via a telephone call, and takes 5–10 minutes to complete.20,27,28 Because it is a proprietary instrument, the questionnaire itself may not be published and is therefore not included in an appendix.
Therefore, to investigate the relationship between postoperative analgesic technique and subsequent health-related quality-of-life, a baseline WOMAC was administered before surgery (POD 0), and again at 7 days as well as 1, 2, 3, 6 and 12 months after surgery. The baseline measurement was a self-administered written questionnaire, whereas subsequent measurements after hospital discharge were administered via telephone. Scores from self-administered and telephone-administered WOMAC instruments have a demonstrated error rate of only 0.9–2.6%.28
Statistical Analysis
The study was powered for the 2 previously published primary end-points (1) time to attain 3 discharge criteria (adequate analgesia, independence from IV analgesics, and ambulation of at least 30 m); and (2) ambulatory distance in 6 minutes the afternoon after surgery.26 To analyze the WOMAC scores, the WOMAC responses were joined by straight lines between timepoints from POD 7 (t=0.25 months) to t=12 months. The personal progress estimated mean area under the curve was defined as the integral of this curve from 0.25 to 12, divided by 11.75 months. The WOMAC hypotheses asked the question of whether overall personal means over a continuum for 12 months of the WOMAC scores (mean area under the curve) differ between treatment groups.
The mean area under the curve measurements were compared by a 2-sided t-test with Satterthwaite correction for unequal variance as the primary question of the null hypothesis that the 2 groups have the same WOMAC profile over time.29 To be included in this specific analysis, we required a day 7 result and at least 2 of months 3, 6, and 12. The trapezoidal rule, above, effectively imputes missing values by linear interpolation between the values on either side of the one missing, or in the case of month 12, linear extrapolation from the values of months 3 and 6. A missing-at-random assumption is made for the inference. However, under the null hypothesis that the treatments are equivalent with respect to the WOMAC, the method does provide a valid approximation to the permutational t-test and hence a valid p-value.27 Additional secondary analysis involved timepoint by timepoint comparisons for the raw values and for changes from baseline, also using the 2-sided t-test with Satterthwaite correction for unequal variance.
Results
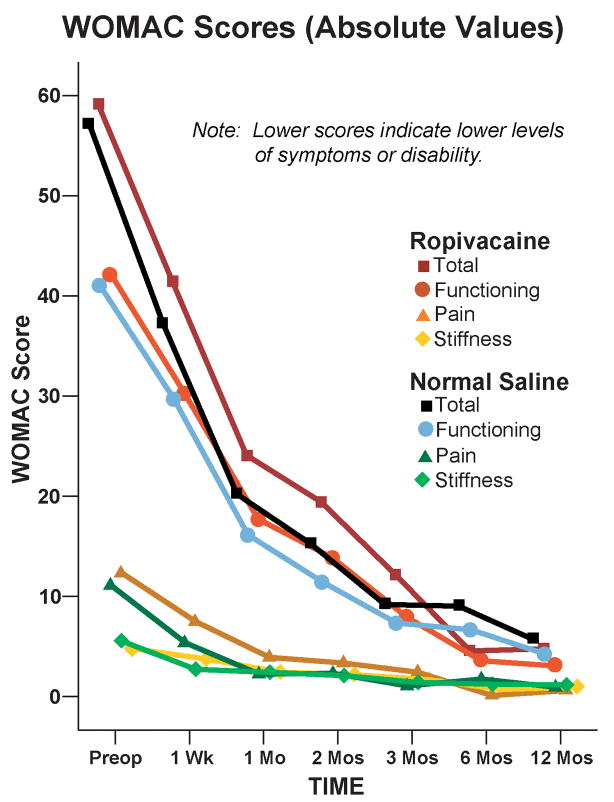
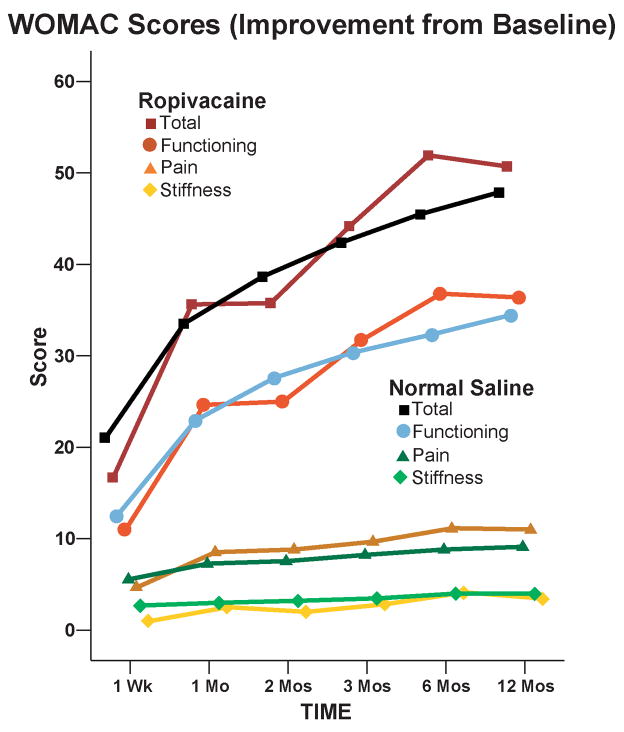
Details of the study results for the immediate postoperative period have been published previously.5 For the mean area under the curve calculations, follow-up WOMAC data meeting our stringent inclusion criteria (a minimum of 3 of the 6 timepoints, including day 7 and at least 2 of months 3, 6 and 12) were available from 17 subjects (71%) from the extended infusion and 17 (74%) subjects from the overnight infusion groups. The 2 treatment groups had similar WOMAC scores for the mean area under the curve calculations (Point estimate for the difference in mean area under the curve for the 2 groups [extended infusion group – overnight infusion group]=0.8, 95% confidence interval: −5.3 to +6.8 [−5.5% to +7.1%]; p=0.80). As expected from the statistical section, we obtained virtually the same P-value (P=0.794) by a permutational t-test of the same question. For the remaining analyses, only 3 subjects from the extended duration infusion group were completely lost to follow-up, resulting in available data for 44 subjects (94%). However, the 2 treatment groups had similar WOMAC scores at all individual time points in terms of both raw scores and changes from baseline (p>0.05; Fig. 1 and 2, and Tables 1 and 2).
Figure 1.
Effect of an extended posterior lumbar plexus perineural ropivacaine infusion on health-related quality-of-life after hip arthroplasty, as measured with the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Index. Data are expressed as means for patients randomly assigned to an extended continuous posterior lumbar plexus nerve block (perineural ropivacaine from surgery through postoperative day 4) or overnight continuous posterior lumbar plexus nerve block (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). The 2 treatment groups had similar WOMAC scores for the mean area under the curve calculations (Point estimate for the difference in mean area under the curve for the 2 groups [extended infusion group – overnight infusion group]=0.8, 95% confidence interval: −5.3 to +6.8; p=0.80) and at all individual time points (p>0.05).
Figure 2.
Effect of an extended posterior lumbar plexus perineural ropivacaine infusion on improvement from preoperative baseline of health-related quality-of-life after hip arthroplasty, as measured with the Western Ontario and McMaster Universities Osteoarthritis Index. Data are expressed as means for patients randomly assigned to an extended continuous posterior lumbar plexus nerve block (perineural ropivacaine from surgery through postoperative day 4) or overnight continuous posterior lumbar plexus nerve block (perineural ropivacaine from surgery through 06:00 postoperative day 1 followed by perineural normal saline through postoperative day 4). The 2 treatment groups had similar scores at all individual time points (p>0.05).
Table 1.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Scores: Absolute Values
| Infusion: | Extended | Overnight | P-Value |
|---|---|---|---|
| Time | Mean (SD) [N] | Mean (SD) [N] | |
| 0 (at surgery) | 59.2 (18.6) [24] | 57.1 (13.1) [23] | N/A |
| 1 Week | 41.5 (13.8) [19] | 37.1 (15.5) [22] | 0.35 |
| 1 Month | 24.1 (14.7) [13] | 20.1 (2.6) [10] | 0.36 |
| 2 Months | 19.4 (17.4) [14] | 15.1 (11.3) [15] | 0.44 |
| 3 Months | 12.2 (12.6) [11] | 9.1 (7.5) [11] | 0.49 |
| 6 Months | 4.6 (3.9) [13] | 8.9 (8.8) [14] | 0.11 |
| 12 Months | 4.8 (9.6) [17] | 5.6 (7.8) [16] | 0.79 |
| AUC | 10.8 (9.4) [17] | 11.6 (7.8) [17] | 0.80 |
N/A: Not applicable
AUC: Area Under the Curve
Table 2.
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Scores: Total Minus Baseline
| Infusion: | Extended | Overnight | P-Value |
|---|---|---|---|
| Time | Mean (SD) [N] | Mean (SD) [N] | |
| 1 Week | −16.7 (21.2) [19] | −20.0 (18.5) [22] | 0.60 |
| 1 Month | −36.2 (21.9) [13] | −31.9 (14.9) [10] | 0.58 |
| 2 Months | −35.8 (15.3) [14] | −38.9 (17.0) [15] | 0.61 |
| 3 Months | −44.2 (17.7) [11] | −42.5 (14.1) [11] | 0.80 |
| 6 Months | −51.9 (17.3) [13] | −44.7 (11.0) [14] | 0.21 |
| 12 Months | −50.7 (16.1) [17] | −47.5 (11.4) [16] | 0.51 |
Discussion
This prospective investigation found no evidence that extending an overnight continuous posterior lumbar plexus nerve block to 4 days improves subsequent health-related quality-of-life between 7 days and 12 months after hip arthroplasty. Lack of treatment effect after perineural catheter removal contrasts with the benefits provided during the infusion, as demonstrated in 2 randomized, controlled trials.5,6 Benefits during the infusion included a decrease in postoperative pain, opioid requirements, opioid-related side effects, and time to meet 3 critical discharge criteria.5,6 Absence of long-term benefit from extended-duration posterior lumbar plexus perineural infusion is disappointing as there are both theoretical reasons and clinical data suggesting that improving analgesia in the immediate postoperative period might decrease long-term pain, reduce joint stiffness, and improve functional status.1–4 However, extending the continuous posterior lumbar plexus nerve block to 4 days also resulted in no apparent outcome detriments, and therefore the previously reported continuous posterior lumbar plexus nerve block benefits in the immediate postoperative period are not negated by this WOMAC follow-up data. Of note, similar investigations involving the extension of femoral perineural infusion from 1 to 4 days after total knee arthroplasty found clear patient benefits during the infusion,30 but no subsequent improvement in health-related quality-of-life after catheter removal.31
Study Limitations
The WOMAC scores were secondary outcomes for the original study and thus do not have the statistical strength of primary outcomes. Although this study in and of itself has limited statistical power as similar studies are completed by ourselves and others, it might be possible in the future to conduct a well powered meta-analysis of the randomized intervention with respect to mid- to long-term WOMAC scores. In addition, the individual means, variances, and covariances at and between specific time points provided by this study may be used as planning parameters for future investigations. Future studies should consider the probable difficulties in contacting subjects over the course of a full year. Of 47 subjects randomized in the current study, only 34 (72%) provided a minimum of 3 of the 6 WOMACs, including day 7 and at least 2 of months 3, 6 and 12. Simple subject retention is far easier (in our study we had only 3 subjects lost to follow-up) but collecting a nearly complete sample at all timepoints proved to be more challenging.
Furthermore, the intervention protocol used in this investigation reflected our clinical practice during the study period. However, little data are available to define the optimal post-hip arthroplasty infusion protocol. Importantly, 43% of the ropivacaine group had their basal ropivacaine infusion halved the day after surgery because of quadriceps weakness versus 17% of the placebo group (Appendix).5 It is possible that an alternative infusion protocol would result in different findings than the current study. It is also noteworthy that, in the current study, pain scores from 3–12 months were exceedingly low for both treatment groups. It thus remains possible that in other patient populations with a higher risk of chronic post-hip arthroplasty pain, an extended-duration continuous lumbar plexus nerve block might yet provide a long-term benefit.
In summary, we previously reported that extending an overnight continuous posterior lumbar plexus nerve block to 4 days after hip arthroplasty provides clear benefits in the immediate postoperative period. However, the extended perineural infusion did not improve subsequent health-related quality-of-life between 7 days and 12 months.
Acknowledgments
The authors gratefully acknowledge the invaluable assistance of Eliza Ferguson, BS, and Marina Nekhendzy, Research Coordinators, Department of Anesthesiology, University of California San Diego (San Diego, California); Jennifer Woodard, BS, Research Coordinator, Department of Anesthesiology, University of Florida (Gainesville, Florida); and the entire staff of the University of Florida General Clinical Research Center (Gainesville, Florida).
Financial Suport: Funding for this project provided by the National Institutes of Health grant GM077026 from the National Institute of General Medical Sciences (Bethesda, Maryland, United States); the Foundation of Anesthesia Education and Research (Rochester, Minnesota, United States); NIH grants RR00082 and RR000827 from the National Center for Research Resources (Bethesda, Maryland, United States); the Departments of Anesthesiology, University of Florida (Gainesville, Florida, United States) and University of California San Diego (San Diego, California, United States); Stryker Instruments (Kalamazoo, Michigan, United States); and B. Braun Medical (Bethlehem, Pennsylvania, United States). Dr. Sessler is supported by the Joseph Drown Foundation (Los Angeles, California, United States). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of these entities.
Appendix
Adverse events
One subject from the Placebo group experienced a vasovagal episode on POD 3 at home, was readmitted, underwent a negative work-up for instigating conditions, and discharged home the following day without negative sequelae. Subjects from the Ropivacaine group had their catheter inadvertently dislodged (evening POD 2), occlusive dressing inadvertently removed with subsequent purposeful catheter removal (morning POD 4), and catheter purposefully removed as requested by patient (morning of POD 3). One subject from the Placebo group had her infusion pump tubing disconnected from the catheter and the catheter was subsequently purposefully removed out of concern for sterility (morning of POD 4). For purposes of analysis, each of these subjects was retained in their respective treatment group per the intention-to-treat principle. One subject from the Ropivacaine group requested study withdrawal the afternoon of POD 1 in the belief that the perineural infusion was causing her nausea.
Three subjects from the Ropivacaine group experienced a fall during the infusion period. The first ambulated 13–18 m twice on POD 1 without apparent quadriceps weakness, self-administered local anesthetic boluses every 30 min after her afternoon therapy session, and then fell immediately upon attempting to stand without assistance that evening (she described her thigh as “numb” when she fell which it had not been previously). A second subject ambulated over 30 m on 5 occasions over the course of 3 days without apparent quadriceps weakness, was discharged home on POD 3, lost her balance, then experienced what she described as a “slow, controlled fall” onto her buttocks, and was readmitted for one night. The third subject had experienced weak quadriceps on POD 1 and her basal infusion and bolus dose volumes were halved per study protocol, after which she ambulated over 30 m 5 times without difficulty. However, she experienced dizziness on POD 3 and fell that evening when attempting to walk without assistance, had her catheter removed the following day and was discharged home without further incident. The patient attributed her fall to the dizziness (presumed etiology: anemia) which did not recur. None of these 3 falls resulted in physical injury.
Footnotes
Abbreviated, preliminary results of this investigation were submitted as an abstract for the Annual Meeting of the American Society of Regional Anesthesiologists, Phoenix, Arizona, United States, April 30 May 3, 2009.
Conflict of Interest: B. Braun Medical (Bethlehem, Pennsylvania, United States) and Stryker Instruments (Kalamazoo, Michigan, United States) provided funding and donated portable infusion pumps and perineural catheters for this investigation. These 2 companies had absolutely no input into any aspect of study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation. Drs. Enneking and Mariano conduct continuous peripheral nerve block workshops for Stryker Instruments (Kalamazoo, Michigan, United States). None of the other authors has any personal financial interest in this research.
References
- 1.Ryu J, Saito S, Yamamoto K, Sano S. Factors influencing the postoperative range of motion in total knee arthroplasty. Bull Hosp Jt Dis. 1993;53:35–40. [PubMed] [Google Scholar]
- 2.Shoji H, Solomonow M, Yoshino S, D’Ambrosia R, Dabezies E. Factors affecting postoperative flexion in total knee arthroplasty. Orthopedics. 1990;13:643–9. doi: 10.3928/0147-7447-19900601-08. [DOI] [PubMed] [Google Scholar]
- 3.Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93:1123–33. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 4.Akeson WH, Amiel D, Abel MF, Garfin SR, Woo SL. Effects of immobilization on joints. Clin Orthop. 1987:28–37. [PubMed] [Google Scholar]
- 5.Ilfeld BM, Ball ST, Gearen PF, Le LT, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Meyer RS. Ambulatory continuous posterior lumbar plexus nerve blocks after hip arthroplasty: a dual-center, randomized, triple-masked, placebo-controlled trial. Anesthesiology. 2008;109:491–501. doi: 10.1097/ALN.0b013e318182a4a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddiqui ZI, Cepeda MS, Denman W, Schumann R, Carr DB. Continuous lumbar plexus block provides improved analgesia with fewer side effects compared with systemic opioids after hip arthroplasty: a randomized controlled trial. Reg Anesth Pain Med. 2007;32:393–8. doi: 10.1016/j.rapm.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Ilfeld BM, Gearen PF, Enneking FK, Berry LF, Spadoni EH, George SZ, Vandenborne K. Total hip arthroplasty as an overnight-stay procedure using an ambulatory continuous psoas compartment nerve block: a prospective feasibility study. Reg Anesth Pain Med. 2006;31:113–8. doi: 10.1016/j.rapm.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Todd MM, Brown DL. Regional anesthesia and postoperative pain management: long-term benefits from a short-term intervention. Anesthesiology. 1999;91:1–2. doi: 10.1097/00000542-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5:231–41. doi: 10.1007/BF02032362. [DOI] [PubMed] [Google Scholar]
- 10.Wu CL, Raja SN. Optimizing postoperative analgesia: the use of global outcome measures. Anesthesiology. 2002;97:533–4. doi: 10.1097/00000542-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Wu CL, Naqibuddin M, Rowlingson AJ, Lietman SA, Jermyn RM, Fleisher LA. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97:1078–85. doi: 10.1213/01.ANE.0000081722.09164.D5. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 14.Davies GM, Watson DJ, Bellamy N. Comparison of the responsiveness and relative effect size of the western Ontario and McMaster Universities Osteoarthritis Index and the short-form Medical Outcomes Study Survey in a randomized, clinical trial of osteoarthritis patients. Arthritis Care Res. 1999;12:172–9. doi: 10.1002/1529-0131(199906)12:3<172::aid-art4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. The effect of age on pain, function, and quality of life after total hip and knee arthroplasty. Arch Intern Med. 2001;161:454–60. doi: 10.1001/archinte.161.3.454. [DOI] [PubMed] [Google Scholar]
- 16.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–91. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Hajiro T, Nishimura K. Minimal clinically significant difference in health status: the thorny path of health status measures? Eur. Respir J. 2002;19:390–1. doi: 10.1183/09031936.02.00283402. [DOI] [PubMed] [Google Scholar]
- 18.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–8. [PubMed] [Google Scholar]
- 19.Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473–6. [PubMed] [Google Scholar]
- 20.Stucki G, Sangha O, Stucki S, Michel BA, Tyndall A, Dick W, Theiler R. Comparison of the WOMAC (Western Ontario and McMaster Universities) osteoarthritis index and a self-report format of the self-administered Lequesne-Algofunctional index in patients with knee and hip osteoarthritis. Osteoarthritis Cartilage. 1998;6:79–86. doi: 10.1053/joca.1997.0097. [DOI] [PubMed] [Google Scholar]
- 21.Jones CA, Voaklander DC, Johnston DW, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol. 2000;27:1745–52. [PubMed] [Google Scholar]
- 22.Fortin PR, Clarke AE, Joseph L, Liang MH, Tanzer M, Ferland D, Phillips C, Partridge AJ, Belisle P, Fossel AH, Mahomed N, Sledge CB, Katz JN. Outcomes of total hip and knee replacement: preoperative functional status predicts outcomes at six months after surgery. Arthritis Rheum. 1999;42:1722–8. doi: 10.1002/1529-0131(199908)42:8<1722::AID-ANR22>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 23.Wright JG, Young NL. A comparison of different indices of responsiveness. J Clin Epidemiol. 1997;50:239–46. doi: 10.1016/s0895-4356(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 24.Theiler R, Sangha O, Schaeren S, Michel BA, Tyndall A, Dick W, Stucki G. Superior responsiveness of the pain and function sections of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) as compared to the Lequesne-Algofunctional Index in patients with osteoarthritis of the lower extremities. Osteoarthritis Cartilage. 1999;7:515–9. doi: 10.1053/joca.1999.0262. [DOI] [PubMed] [Google Scholar]
- 25.Angst F, Aeschlimann A, Steiner W, Stucki G. Responsiveness of the WOMAC osteoarthritis index as compared with the SF-36 in patients with osteoarthritis of the legs undergoing a comprehensive rehabilitation intervention. Ann Rheum Dis. 2001;60:834–40. [PMC free article] [PubMed] [Google Scholar]
- 26.Jones JG, Leighton F. Comparison of WOMAC with SF-36 for OA of the knee or hip. Ann Rheum Dis. 2002;61:182–3. doi: 10.1136/ard.61.2.182-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45:453–61. doi: 10.1002/1529-0131(200110)45:5<453::aid-art365>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Bellamy N, Campbell J, Hill J, Band P. A comparative study of telephone versus onsite completion of the WOMAC 3.0 osteoarthritis index. J Rheumatol. 2002;29:783–6. [PubMed] [Google Scholar]
- 29.Satterthwaite FW. An approximate distribution of estimates of variance components. Biometrics Bulletin. 1947;2:110–114. [PubMed] [Google Scholar]
- 30.Ilfeld BM, Le LT, Meyer RS, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Berry LF, Spadoni EH, Gearen PF. Ambulatory continuous femoral nerve blocks decrease time to discharge readiness after tricompartment total knee arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology. 2008;108:703–13. doi: 10.1097/ALN.0b013e318167af46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilfeld BM, Meyer RS, Le LT, Mariano ER, Williams BA, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Maldonado RC, Gearen PF. Health-related quality of life after tricompartment knee arthroplasty with and without an extended-duration continuous femoral nerve block: a prospective, one-year follow-up of a randomized, triple-masked, placebo-controlled study. Anesth Analg. doi: 10.1213/ane.0b013e3181964937. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]