Abstract
The secreted metalloprotease ADAMTS5 is implicated in destruction of the cartilage proteoglycan aggrecan in arthritis, but its physiological functions are unknown. Its expression profile during embryogenesis and in adult tissues is therefore of considerable interest. β-galactosidase (β-gal) histochemistry, enabled by a LacZ cassette inserted in the Adamts5 locus, and validated by in situ hybridization with an Adamts5 cRNA probe and ADAMTS5 immunohistochemistry, was used to profile Adamts5 expression during mouse embryogenesis and in adult mouse tissues. Embryonic expression was scarce prior to 11.5 days of gestation (E11.5) and noted only in the floor plate of the developing brain at E9.5. After E 11.5 there was continued expression in brain, especially in the choroid plexus, peripheral nerves, dorsal root ganglia, cranial nerve ganglia, spinal and cranial nerves, and neural plexuses of the gut. In addition to nerves, developing limbs have Adamts5 expression in skeletal muscle (from E13.5), tendons (from E16.5), and inter-digital mesenchyme of the developing autopod (E13.5–15.5). In adult tissues, there is constitutive Adamts5 expression in arterial smooth muscle cells, mesothelium lining the peritoneal, pericardial and pleural cavities, smooth muscle cells in bronchii and pancreatic ducts, glomerular mesangial cells in the kidney, dorsal root ganglia, and in Schwann cells of the peripheral and autonomic nervous system. Expression of Adamts5 during neuromuscular development and in smooth muscle cells coincides with the broadly distributed proteoglycan versican, an ADAMTS5 substrate. These observations suggest the major contexts in which developmental and physiological roles could be sought for this protease.
Keywords: ADAMTS5, Adamts5, Aggrecanase, Development, Mouse, Transgenic, Arthritis, Osteoarthritis, Aggrecan, Versican, LacZ, Beta-galactosidase, In situ hybridization, Peripheral nerve, Schwann cell, Smooth muscle, Skeletal muscle, Sympathetic ganglia, Inter-digital mesenchyme, Dorsal Root Ganglia, Choroid Plexus, Cartilage, Tendon, Fibroblast
1. Results and Discussion
1.1 ADAMTS5 is a major cartilage degrading enzyme in arthritis
The ADAMTS family includes 19 secreted metalloproteases for which diverse substrates have been identified, indicative of roles in maturation of precursor proteins as well as turnover of extracellular matrix (Apte, 2004; Porter et al., 2005). Human and animal mutations have illustrated how specific members of this family are involved in maintenance of connective tissue (ADAMTS10), collagen biosynthesis (ADAMTS2), and hemostasis (ADAMTS13) (Colige et al., 1999; Dagoneau et al., 2004; Levy et al., 2001). ADAMTS5 (Abbaszade et al., 1999; Hurskainen et al., 1999) has a major role in degradation of the cartilage proteoglycan, aggrecan in arthritis (Abbaszade et al., 1999). The most compelling evidence supporting this role comes from genetic interference with ADAMTS5 in an engineered strain of mice. Specifically, mice with an in-frame deletion of a region in the catalytic domain of ADAMTS5 that rendered it proteolytically deficient, are resistant to experimentally induced arthritis (Glasson et al., 2005; Stanton et al., 2005). This observation has led to considerable interest in targeting this protease for the prevention of cartilage degradation. However, despite this critical role in disease, little is known about the physiological role of Adamts5. Adamts5 mutant mice are viable, fertile, with a normal lifespan, and neither histological analysis of organs nor blood chemistry have identified any anomalies (Glasson et al., 2005; Stanton et al., 2005). Therefore, it has been concluded that ADAMTS5 does not have a major physiological role, or that its role may be masked by redundancy with another ADAMTS protease. Since there is no information available on the normal expression profile of this protease during embryonic development and in adult tissues, we have undertaken a detailed expression analysis in the mouse. This analysis used a different strain of genetically engineered mice from those previously reported, one in which a reporter gene, LacZ, and a selectable marker, were introduced into the Adamts5 locus to inactivate the gene. The LacZ reporter, as supported by RNA in situ hybridization and immunohistochemical data provided here, enabled determination of the Adamts5 expression profile in both embryonic and adult tissues. In chronological order, there was emergent expression in developing central, peripheral and autonomic nervous system, skeletal muscle and tendons, vascular and other smooth muscle cells, and in the endocardium, pericardium, pleural and peritoneal mesothelial cells. In general, the expression of Adamts5 at many of these locations or in cell types appears to be a constitutive feature of their phenotype. Many of these sites are known to express versican, an aggregating proteoglycan that was recently identified as a substrate of ADAMTS5 (Longpre et al., 2008).
1.2. Characterization of an Adamts5 targeted allele
Adamts5 was inactivated by Deltagen Inc. (San Carlos, CA), using homologous recombination in embryonic stem cells. The targeting construct contained a 5’ homology arm of 1.2 kb and a 3’ homology arm of 2.2 kb. A cassette comprising a 5’ internal ribosome entry site (IRES) adjacent to a nuclear-targeted reporter gene (LacZ) in its 5’ half and a selectable marker (the neomycin resistance gene, driven by the Pgk promoter) in its 3’ half, was inserted into exon 2 of the Adamts5 gene. This insertion replaced 134 nucleotides of exon 2 (corresponding to nt 1962–2095 of the reference mRNA sequence NM_011782) (Fig. 1A). The deleted region includes that encoding the catalytic active site of ADAMTS5 as well as most of the putative muscle regulatory factor-binding enhancer recently identified in this exon (Barthel and Liu, 2008). Additional details of the targeting strategy, identification of targeted clones and PCR analysis of genomic DNA in targeted mice, are available at http://www.informatics.jax,org/external/ko/deltagen/1232_MolBio.html. To determine whether Adamts5 mRNA had been inactivated, as well as to confirm that IRES-lacZ was correctly integrated into the gene, we performed RT-PCR with oligonucleotide primers located upstream and downstream of the insertion site as well as across the insertion site (Fig. 1A). The results showed that PCR products could be generated with both upstream and downstream primer pairs and confirmed the interruption of exon 2 by IRES-lacZ (Fig. 1B). Because the targeting strategy interrupted the catalytic domain by inserting IRES-lacZ, ADAMTS5 protein is truncated in the catalytic domain, functional ADAMTS5 protease is not produced and this allele constitutes an Adamts5 knockout.
Figure 1. Characterization of an Adamts5 targeted allele (Adamts5).
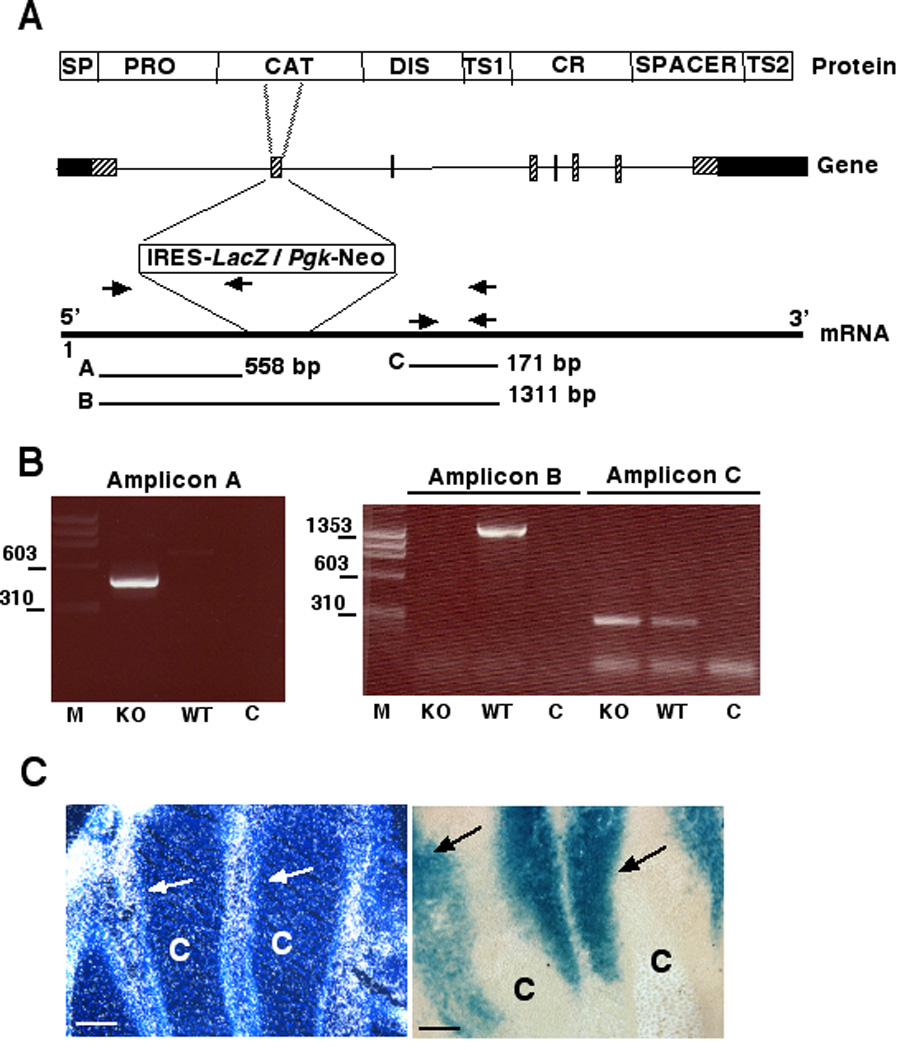
A. Scheme of the targeting strategy. The domain structure of ADAMTS5 protein (not to scale) is shown at the top, with the exon-intron structure of Adamts5 beneath it, showing the site of insertion of the IRES-lacZ-Pgk-Neomycin (Neo) resistance cassette. The primers used for RT-PCR validation of inactivation and the expected amplicons (A,B,C) are shown in relation to Adamts5 mRNA. The abbreviations used in the protein structure are: SP, Signal peptide; PRO, propeptide; CAT, catalytic domain; DIS, disintegrin-like domain; TS1 and TS2, thrombospondin type 1 repeats; CR, cysteine-rich domain. B. Ethidium bromide-stained agarose gels showing the specific amplicons associated with knockout (KO) and wild-type (WT) alleles. C indicates the control (water instead of cDNA as template). Molecular mass markers (in bp) are shown on the left of each gel. The specific amplicons demonstrated interruption of the Adamts5 mRNA (note absence of amplicon B in KO mRNA) and intactness of the targeted chimeric (with lacZ) Adamts5 mRNA (Amplicon A). Note that the presence of the targeted Adamts5-LacZ chimeric RNA in KO mice is a prerequisite for β-gal staining. C. Similar expression patterns were seen in E15.5 autopods obtained by in situ hybridization (left-hand panel) and β-gal staining (right-hand panel). C indicates digital cartilage. Arrows indicate signal in inter-digital mesenchyme. Scale bars: 50 µM.
RT-PCR showed that the disrupted RNA was stable (Fig. 1B). Thus, lacZ was expressed from the interrupted Adamts5 transcript under control of the endogenous Adamts5 promoter, and could be used to determine the expression pattern of Adamts5. Validation of the β-gal staining as a surrogate for Adamts5 mRNA was established by concordance with Adamts5 in situ hybridization done in the E15.5 hindlimb autopod, where β-gal staining was found to be the most conspicuous (Fig. 1C). Since β-gal staining was considerably stronger in Adamts5−/− mice than in Adamts5+/− mice, and because Adamts5−/− mice are phenotypically normal, we conducted studies in Adamts5−/− mice for optimal β-gal staining. To eliminate concerns that the expression pattern in Adamts5−/− mice may be different from that in Adamts5+/− mice, we compared the staining patterns in both genotypes and found it to be identical. Wild-type control littermate mice showed no β-gal staining under the conditions used for the analysis presented here. Therefore, β-gal staining represents Adamts5 expression sites.
1.3. Overview of Adamts5 expression during embryogenesis
Using whole mount β-gal staining, little focal expression of Adamts5 was noted in the conceptus during early embryogenesis (prior to E9.5). The earliest localized embryonic β-gal expression was found in the floor plate of the developing central nervous system at E9.5 (Fig. 2A, A’), with no staining detected elsewhere at this age. Whole mount β-gal staining of embryos from E11.5-E14.5 showed emerging expression in neuromuscular structures. At E11.5, expression was seen in developing muscles in the craniofacial region (Fig. 2B), accompanying the initial formation of peripheral nerves in the forelimb (Fig. 2B), and in dorsal root ganglia (Fig. 2C). Developmental processes in the hindlimb typically lag behind the forelimb by 0.5–1 day; hence, β-gal expression was noted in emerging nerves of the hindlimb after E12.5 (data not shown). From E12.5–14.5, expression continued within the arborizing nerves of all limbs, in segmental nerves growing into the trunk, and in cranial nerves (Fig. 2D). From E13.5, strong expression was noted in emerging muscle groups of the forelimb, hindlimb, and tail, as well as in facial and paraspinal muscles (Fig. 2D,E). From E13.5 to E15.5, there was striking β-gal staining in the interdigital mesenchyme and perichondrium flanking the digit skeletal elements in both forelimbs and hindlimbs. (Fig.2D–F, H,I). Cutaneous nerves at all locations in the head, trunk and limbs expressed β-gal (Fig. 2G–I).
Figure 2. Determination of Adamts5 expresison during mouse embryogenesis by whole mount β-gal staining.
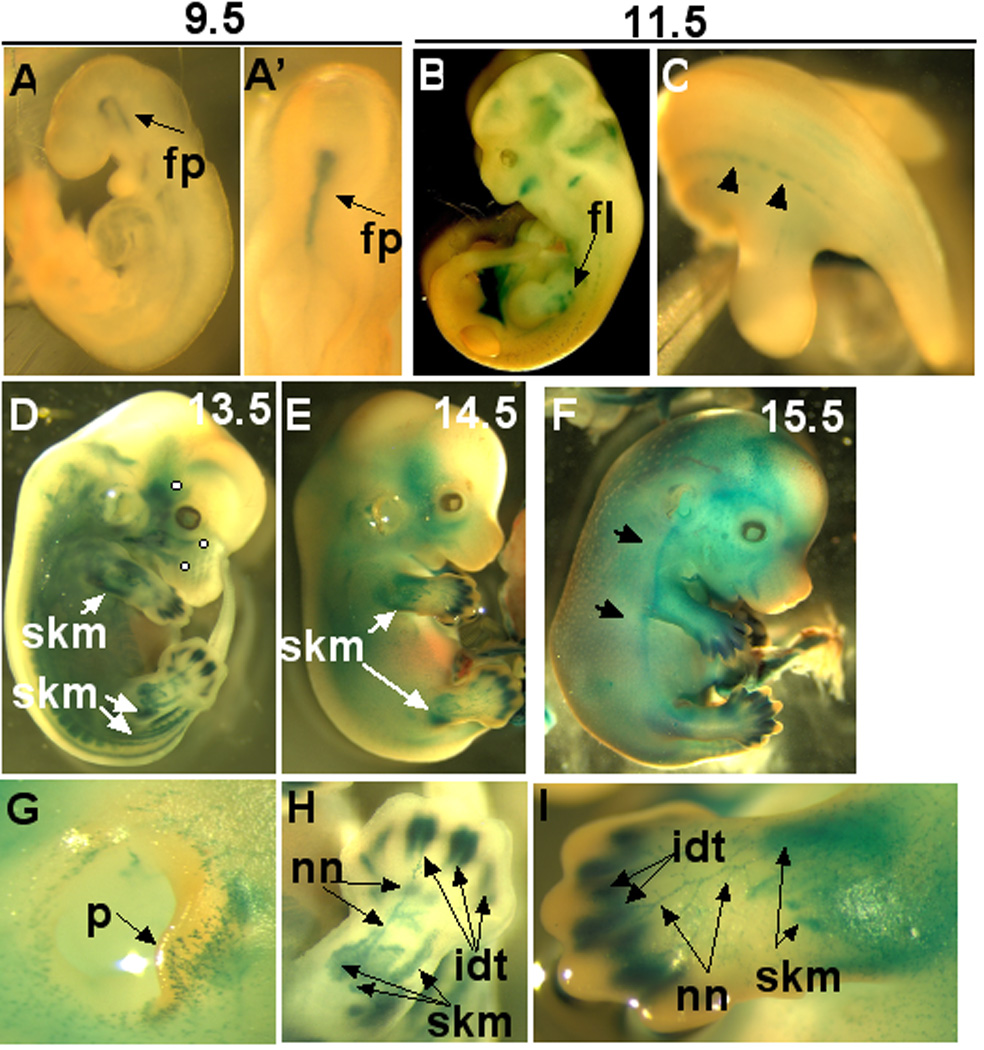
A, A’ E9.5 embryo shown from lateral (A) and cranial (A’) aspects. Note staining restricted to the neural floor plate (fp). B,C. E11.5 embryo showing β-gal staining of emerging muscle masses in the head and face as well as in forelimb. Caudal view of the E11.5 embryo in C demonstrates staining in dorsal root ganglia (arrowheads). D–F. Whole mount β-gal staining of embryos at E13.5 (D), E14.5 (E) and E15.5 (F). In D and E there is good stain penetration of internal structures and β-gal staining is seen in emerging muscle groups (skm) of the limbs and torso. There is diffuse staining of dermis at E15.5 (arrows in F). Note in D that branches of the trigeminal nerve (solid white circles) show β-gal staining. G. Close-up of the external ear shows β-gal staining in cutaneous nerves migrating to the edge of the pinna (p). H,I. Note strong staining of interdigital mesenchyme (idt) in the forelimb at E13.5 (H) and E14.5 (I), staining of nerves (nn) and skeletal muscles of the zeugopod (skm).
1.4. Expression of Adamts5 in the developing and adult head, neck, and nervous system
Although expression in the head and neck appeared at E11.5, β-gal staining was confined to the emerging skeletal muscles (Fig. 2B). After E13.5, strong staining was seen in the developing choroid plexus epithelium as well as the adjacent ependyma in all ventricles of the developing brain (Fig. 3A). In addition, specific signal was present in the developing mid-brain nuclei (Fig. 3A). Analysis of brain development at a single embryonic age (E14.5) presented at www.genepaint.org indicates additional expression at this stage in cerebellum, superior colliculus, medial and lateral ganglionic eminences, and the septum. In adult brain, we have identified continuing intense expression in the choroid plexus epithelium, and weak staining of white matter, granule layer and Purkinje cell layer of the cerebellum (data not shown). Widespread expression was seen in developing craniofacial mesenchyme from E13.5-E15.5, including around the developing calvaria, base of the skull and snout region (Fig. 3B,C), although no expression was seen within the developing cartilage skeleton. Expression was present in developing skeletal muscle of the head and in the tongue (Fig. 3D), but was lacking in the palatal shelves during palatogenesis (Fig. 3C). During tooth development (Fig. 3D) at E13.5, β-gal staining appeared in the craniofacial mesenchyme forming the dental papilla. However, at E16.5, expression was lacking in specific dental structures, and was restricted to the mesenchyme forming the tooth socket. Other sites of expression in the head and neck were the salivary glands and cranial nerve ganglia. At E13.5, expression was noted in isolated regions of the thoracic spinal cord (Fig. 3F) and isolated β-gal stained cells were also present in the adult spinal cord (Data not shown). At E13.5 β-gal staining was noted in the craniofacial mesenchyme around the developing eye and in the extraocular muscles, but not within the eye. The GenePaint website indicates retinal expression at E14.5. In adult eyes, strong expression is found in the ganglion cell layer and inner nuclear layer of the retina (Fig. 3G), the iris, and corneal endothelium (Fig. 3H). Other sites of expression in the head and neck during embryonic development were the salivary glands and cranial nerve ganglia.
Figure 3. Adamts5 expression (β-gal staining) in developing head, neck and central nervous system.
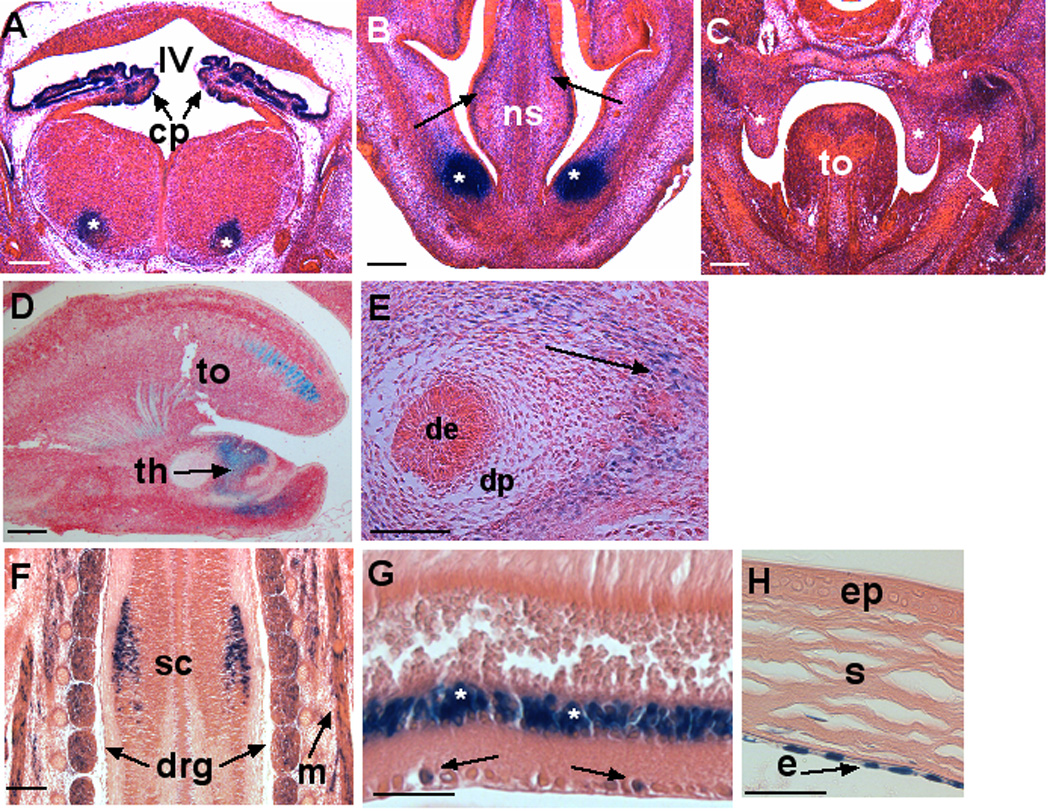
A. Coronal section at the level of the E13.5 mid-brain demonstrates strong β-gal staining (blue) in the choroid plexus (cp) epithelium of the fourth ventricle (IV). Note focal expression in mid-brain ganglia (asterisk). B. Diffuse expression (arrows) as well as strong focal expression (asterisks) in craniofacial mesenchyme is seen in this transverse section through the E13.5 snout region. Note absence of β-gal staining in the central cartilage of the nasal septum (ns), nasal epithelium, or epidermis of skin. C. Coronal section through the oral cavity of an E13.5 embryo. Note diffuse mesenchymal expression (arrows) and the lack of expression in the tongue (to) and palatal shelves (asterisks). D. Sagittal section through the E13.5 head shows expression in a subset of anterior muscle fibers in the tongue (to) and in the developing tooth (th). E. Section through an E16.5 day tooth showing β-gal staining in the mesenchyme of the tooth socket (arrow), but not in the dental epithelium (de) or dental papilla (dp). F. β-gal staining of a coronal section through an E13.5 embryo showing expression in localized regions of the spinal cord (sc), in dorsal root ganglia (drg) and paraspinal muscles (m). G. Adult retina shows strong β-gal staining in the inner nuclear layer (asterisks) and sporadic staining in the ganglion cell layer (arrows). H. β-gal staining of adult cornea showing Adamts5 expression in the endothelial cells (e), but not in the corneal stroma (s) or epithelium (ep). All sections are counterstained with eosin. Scale bars: 50 µM.
Association of β-gal stained cells with trunk segmental nerves (Fig. 4A), peripheral limb nerves (Fig. 4B,C), cranial nerves, and developing dorsal root ganglia, and cranial nerve ganglia (Fig. 4D), was confirmed using the anti-neurofilament antibody 2H3 (Dodd et al., 1988) (Fig. 4A–C). Since the cell bodies of the motor neurons reside within the spinal cord, and those of sensory neurons within dorsal root ganglia, nuclear staining of individual cells within developing peripheral nerves (e.g., Fig 4A showing spinal segmental nerves, Fig. 4B,C showing peripheral branches of the median nerve) implies expression by neural-associated Schwann cell precursors. Expression in skeletal muscle myoblasts (Fig. 4B,C) from E13.5-E16.5 was confirmed by immunohistochemistry with the muscle specific anti-myosin monoclonal antibody MF20 (Bader et al., 1982) and by α-smooth muscle actin (SMA) monoclonal antibody ( data not shown), since this smooth muscle cell marker is transiently expressed in skeletal myotubes during their differentiation (Woodcock-Mitchell et al., 1988). The expression of Adamts5 during interaction of putative motor nerves with their skeletal muscle targets was seen in both the developing limb (Fig. 4C) and axial musculature. Strong β-gal staining was present in the cranial nerve ganglia, e.g. in the trigeminal ganglion (Fig. 4D).
Figure 4. Adamts5 expression (β-gal staining) in developing nerve and skeletal muscle.
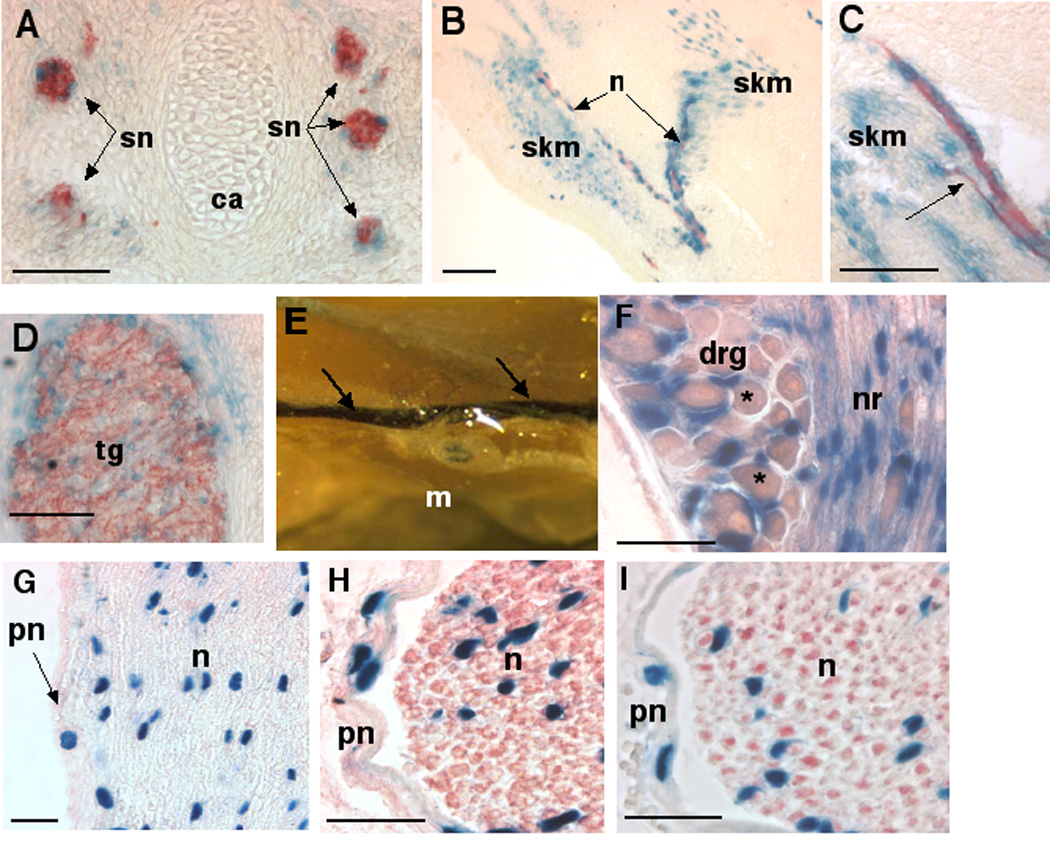
A–C. Sections were stained for β-gal (blue) followed by immunohistochemistry (red) with anti-neurofilament antibody 2H3, and illustrate Adamts5 expression in association with segmental intercostal nerves (sn, A), and motor nerves (n). In A, note the absence of staining in cartilage (ca). In B and C, the nerve runs through skeletal myoblasts (skm) that also stain positive for β-gal. The image in C shows a slender nerve branch (red) terminating in a developing muscle, indicated by an arrow. D. Combined β-gal staining and 2H3 immunohistochemistry (red) demonstrate Adamts5 expression in the trigeminal ganglion (tg). E Whole mount of adult mouse hamstring muscle with sciatic nerve (arrows) shows intense β-gal staining throughout the sciatic nerve (arrows), but no staining of muscle. F. Strong expression is seen in cells of the adult dorsal root ganglia (drg) and adjacent nerve root (nr). Asterisks indicate the ganglionic neurons, which are not positive. G. β-gal and eosin-stained longitudinal section of adult sciatic nerve demonstrates positive nuclei both within the perineurium (pn) and nerve (n). H,I. β-gal staining combined with S-100 immunohistochemistry (red, H) or 2H3 immunohistochemistry (red, I), demonstrates the distinct identity of perineural cells (pn) which are β-gal stained but do not express S-100 (a Schwann cell marker) or neurofilament (an axonal marker). β-gal staining is present in Schwann cell nuclei. Scale bars: 50 µM.
Adult peripheral nerve components such as the sciatic nerve (Fig. 4E), dorsal root ganglia and associated spinal nerve roots (Fig. 4F), stained strongly for β-gal. In the sciatic nerve, β-gal staining was present both in the perineurium and nerve proper (Fig 4G). Immunostaining of β-gal-stained sections with antibodies specific for S-100 protein, a Schwann cell marker, (Fig. 4H) or neurofilament (Fig. 4I), demonstrated that nuclear β-gal was present in mature Schwann cells.
1.5. Expression of Adamts5 in thoracic and abdominal viscera
Within the thoracic viscera, expression was present in mesothelium of the visceral pleura and parietal pleura from E13.5 (Fig. 5A shows E16.5 visceral pleura) and within the smooth muscle cells of the developing and adult bronchial tree (Fig. 5A,B). In E16.5 pancreas, stromal and ductal cells invading the acini expressed β-gal and continuing expression was seen in the smooth muscle cells of adult pancreatic ducts (not shown). Smooth muscle cells within embryonic arteries (Fig. 5C) as well as in major adult arteries such as aorta (Fig. 5D) and arterioles, constitutively expressed Adamts5. Bronchial smooth muscle and arterial smooth muscle identity was confirmed by immunostaining with anti-SMA antibody (Fig. 5B–D). Cells of the adrenal capsule had strong β-gal staining, and smooth muscle cells of the arterioles invading the adrenal cortex were also stained. At E13.5, a reticular staining was noted on the surface of all segments of the gut, from the stomach to the colon (Fig. 5E shows staining of the jejunum). Histological analysis of intestine at E13.5 as well as immunostaining with anti-SMA demonstrated specific foci of expression external to the muscularis mucosa, whereas the smooth muscle cells were not β-gal positive (Fig. 5F). At E16.5, the expression was maintained (Fig. 5G) and did not extend internal to the muscularis mucosa. Immunostaining with the neurofilament marker 2H3 confirmed that β-gal was adjacent to the myenteric plexus (of Meissner) (Fig. 5H) and the lack of β-gal stain internal to muscularis mucosa, suggests that it is not associated with the mucosal plexus of Auerbach. Expression in the myenteric plexus is seen constitutively in intestines of adult mice (Fig. 5I). β-gal staining was also present in both the sympathetic ganglia as well as the sympathetic trunk (Fig. 5J), and in ganglion cells of the prostate. Thus, in addition to the adult peripheral nervous system, Adamts5 expression is constitutively associated with components of the autonomic nervous system.
Figure 5. Expression of Adamts5 in lung, arteries and gut.
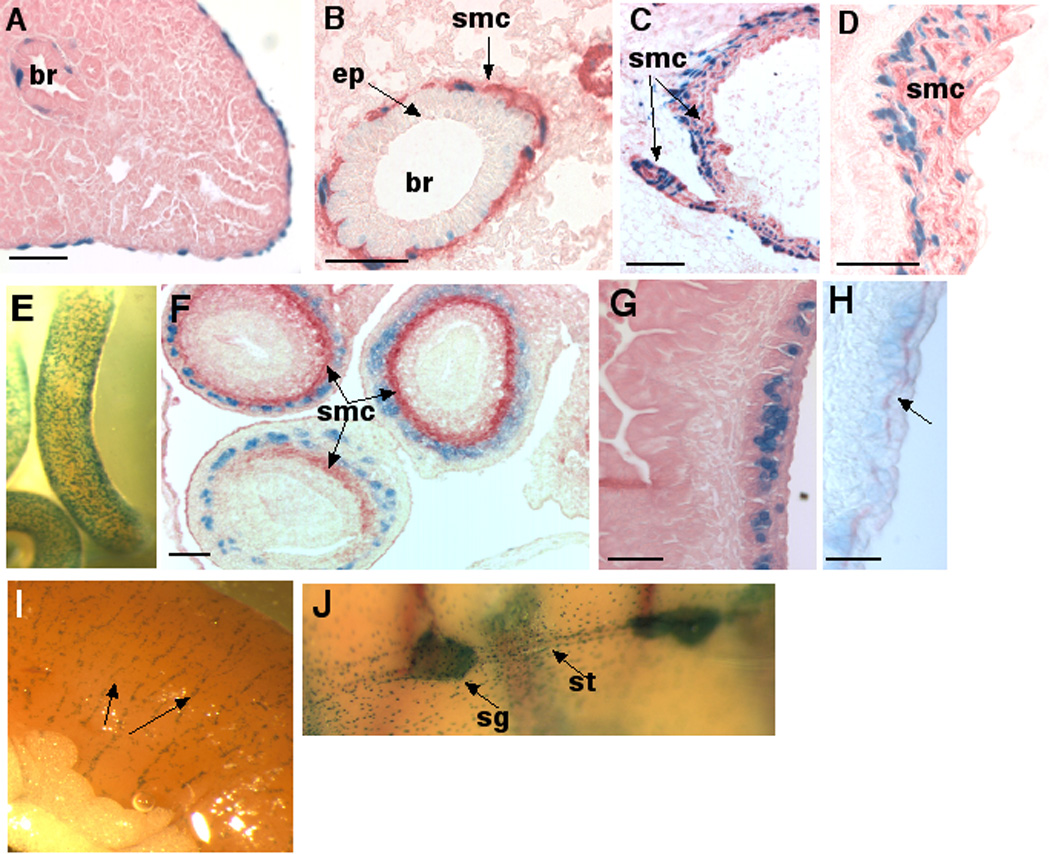
A,B Developing lung at E16.5 (A, eosin counterstain) and adult lung (B). Note β-gal positive cells lining the external surface of the lung (pleural mesothelium, A) and β-gal positive cells external to the bronchial epithelium (br). In adult lung, SMA immunohistochemistry stained smooth muscle cells (smc, red stain) have β-gal positive nuclei. Note occasional light nuclear β-gal staining in bronchial epithelium (ep). C,D Arterial expression. E16.5 (C) and adult aorta (D) were immunostained with SMA (red) to show β-gal staining in smooth muscle cells (smc). E. Whole mount stain of E13.5 intestine showing stippled surface β-gal stain. F. E13.5 intestines sequentially stained for β-gal (blue) and SMA immunostaining (red) demonstrates that Adamts5 is not expressed in intestinal smooth muscle, but external to it. G. β-gal stained section of E16.5 mouse intestine counterstained with eosin demonstrates persistant sub-adventitial β-gal staining. H. A section corresponding to G was stained with monoclonal antibody 2H3 (anti-neurofilament, red) to demonstrate the overlap of β-gal staining with the neural plexus. I. Adult small intestine showing continuing subadventitial β-gal staining (arrows indicate Meissner’s plexus). J. Whole mount staining of the interior of the adult thoracic cavity illustrates β-gal staining of the sympathetic ganglia (sg) and sympathetic trunk (st). Individual stained cells around these structures are lining the thoracic cavity and correspond to pleural mesothelium. Scale bars: 50 µM.
β-gal staining presented a dynamic pattern during development of the kidney. At E13.5, β-gal staining was present in the metanephric cap of the primitive kidney, in the collecting tubules, and in scattered cells in the mesenchyme (Fig. 6A). At E16.5, β-gal staining was present in the glomeruli and in scattered cells in the mesenchyme that are presumed to be stromal fibroblasts (Fig. 6B,B’). In the glomeruli, β-gal staining was localized to the parietal epithelial cells of Bowman’s capsule as well as to mesangial cells within the glomerular tuft (Fig. 6B’). In adult kidney, β-gal staining in the glomerulus was maintained (Fig. 6C) and in addition, there was expression in the arcuate arterioles of the kidney, where β-gal staining in the vascular smooth muscle cells was identified by SMA immunostaining (Fig. 6D). In the glomerular tuft, immunostaining with the endothelial cell marker endomucin determined that the lacZ expressing cells were neither endothelial cells nor podocytes (since they did not line the endothelial cells), and are most likely to be mesangial cells (Fig. 6E). Expression in the developing and adult reproductive tract was mostly confined to the capsular or stromal fibroblastic cells, such as in the tunica albuginea and interstitial regions of the testis, and vascular smooth muscle cells.
Figure 6. Adamts5 expression in the developing and adult kidney (A–E), and the developing heart (F–H).
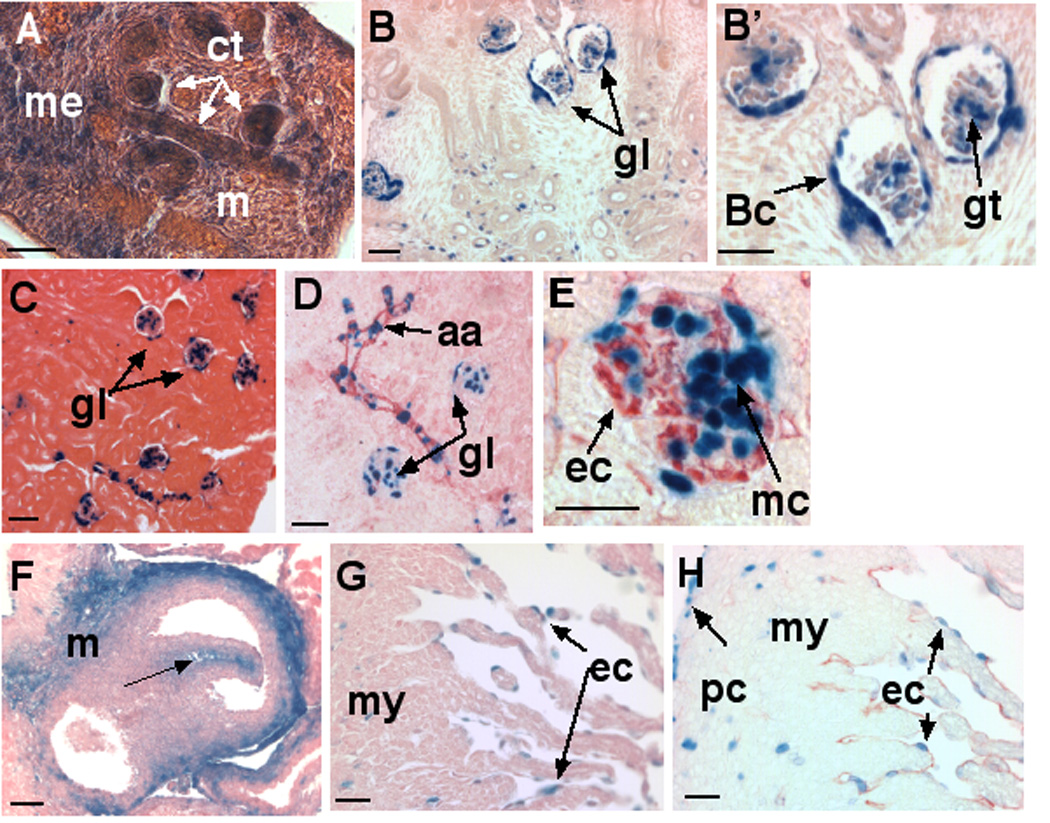
Expression is shown in E13.5 (A) and E16.5 embryonic kidney (B,B’) and adult kidney (C–). A. At E13.5, β-gal staining is present in developing undifferentiated mesonephric cap (me), collecting tubules (ct) and interstitial mesenchyme (m). B. Overview of E16.5 kidney shows expression in developing glomeruli (gl) as well as in isolated stromal fibroblasts. B’. Higher power image of glomeruli shows β-gal staining localized to parietal epithelial cells of Bowman’s capsule (Bc) and to the glomerular tuft (gt). C–E. Adult kidney shows β-gal staining in glomeruli (gl) as well as in tubular structures, which are identified by SMA immunostaining (panel D) as arcuate arterioles (aa). E. Immunostaining of glomerulus with the endothelial marker endomucin (red) demonstrates β-gal staining in mesangial cells (mc) but not endothelial cells (ec). F–H Expression in the developing heart. F. Transverse section through the E16.5 cardiac outflow tract showing strong β-gal staining in aortic mesenchyme (m) as well as valve leaflet mesenchyme (arrow). G. E16.5 heart. Scattered β-gal stained cells are seen in myocardium (my) and in the endocardium (ec). H. The identity of endocardium (ec) is confirmed by immunohistochemistry with anti-endomucin (red). Note that β-gal stained cells in the myocardium (my) and pericardium (pc) are not endomucin positive. Scale bars: 50 µM.
Sections through the E16.5 cardiac outflow tract showed intense mesenchymal expression in the aorta and pulmonic artery roots and in developing aortic valve mesenchyme (Fig. 6F). β-gal staining was present in endocardial cells lining the cardiac lumen and also within myocardium (Fig. 6G). Immunostaining of β-gal stained sections using anti-endomucin antibody confirmed that the trabecular lining cells were endothelial in origin (i.e. endocardium) (Fig. 6H). β-gal staining was noted in isolated cells scattered through the cardiac musculature, which were endomucin negative, suggesting that cardiac capillaries did not express Adamts5, and that these were myocardial or fibroblastic nuclei (Fig. 6H). Pericardial lining cells also showed β-gal staining (Fig. 6H). Consistent with expression in pleura and pericardium, β-gal staining was also found in the parietal peritoneum lining the abdominal side of the diaphragm (data not shown). Thus ADAMTS5 is a constitutive product of the mesothelial cells lining the major body cavities-pleural, peritoneal and pericardial.
During skin development, β-gal staining was present primarily in dermal mesenchyme, similar to that observed in craniofacial mesenchyme. In adult skin, β-gal staining was found in smooth muscle (arrector pili) of hair follicles in skin, but not within epithelial appendages.
1.6. Musculokeletal expression of Adamts5 and relevance to aggrecan processing
There was no association of Adamts5 mRNA or β-gal staining with developing cartilage (Fig. 1C, Fig. 2D–E, Fig. 4A), which is of interest, since the ADAMTS5 substrate aggrecan, is a major component of cartilage that is turned over rapidly during embryonic skeletogenesis. Aggrecan forms large aggregates with the glycosaminoglycan hyaluronan (HA), with the linkage being stabilized by link protein. The lack of ADAMTS5 expression in embryonic cartilage is consistent with the normal skeletal development reported in Adamts5−/− mice and Adamts4−/−; Adamts5−/− double null mice. However, aggrecan is also present in tendons, which showed β-gal staining after E16.5 as muscle staining waned, and in the adult (not shown). Tendons also contain another ADAMTS5 substrate, versican, which is abundant in the mid-tendon (Robbins and Vogel, 1994).
1.7. Distribution of ADAMTS5 protein in mouse tissues
A polyclonal antibody to a peptide from the ADAMTS5 protein that is located downstream of the gene targeting site provided specific staining in wild-type mouse tissues, but no staining was seen in knockout mice (data not shown). ADAMTS5 protein localization during mouse development precisely mirrored β-gal staining. Thus, at E15.5, protein localization was seen in dorsal root ganglia and nerves, but not in vertebral and rib cartilage (Fig. 7A–C). Immunostaining was detected in skeletal muscles such as the intercostal muscles (Fig. 7C), pectoral muscles (Fig. 7C) and the diaphragm (Fig. 7E), in the region of the myenteric plexus (Fig. 7D) and around bronchioles (Fig. 7E). As one example of the widespread distribution of the ADAMTS5 substrate versican, which we propose is relevant to the Adamts5 expression pattern, we have included a panel of versican immunostaining of E16.5 lung, showing its distribution throughout the lung mesenchyme and concentration around the basement membrane of the bronchiolar epithelium (Fig. 7F). This places versican in the immediate vicinity of ADAMTS5 expressing bronchial smooth muscle cells. ADAMTS5 localization was also seen in the aortic wall (Fig. 7G) and in myocardium (Fig. 7H).
Figure 7. Adamts5 protein localization corresponds to β-gal staining patterns.
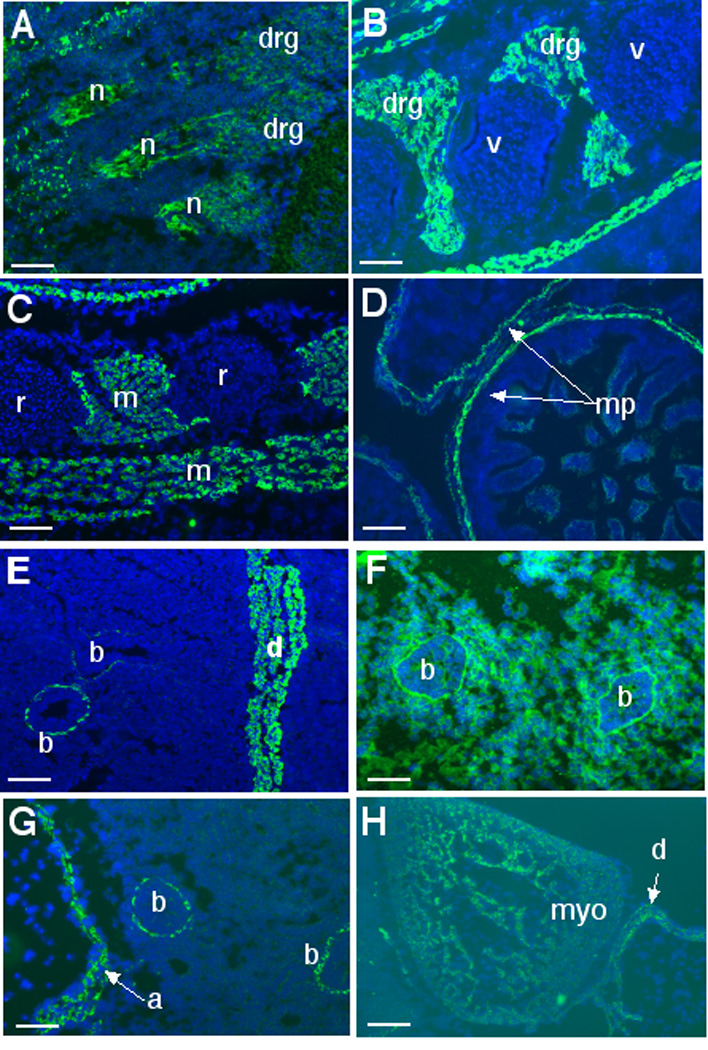
ADAMTS5 immunofluorescence is green and nuclei fluoresce blue by incorporation of DAPI. A. Immunostaining of a sagittal section of an E13.5 day mouse embryo shows expression in dorsal root ganglia (drg) and nerves (n). B. At E15.5, expression continues in the drg. Note lack of β-gal staining of the vertebral cartilage (v). C. Section through the chest wall of an E13.5 day embryo illustrates staining of muscle (m) but not of rib cartilage (r). D. In E15.5 intestine, ADAMTS5 antibody staining corresponds to the location of Meissner’s plexus (mp). E. Staining of the lung and diaphragm illustrates ADAMTS5 staining in smooth muscle cells lining the bronchial tubes (b) and diaphragm (d). F. Immunostaining of versican in E14.5 lung illustrates localization around the periphery of the bronchial tubes (b) and in the intervening lung parenchyma. G. Immunostaining of the E14.5 aorta (a) illustrates expression throughout the aortic wall. Note adjacent bronchial staining (b). H. Diffuse labeling of myocardium (myo) in the E14.5 heart is illustrated. Scale bars: 50 µM.
Of relevance to the strong β-gal staining and ADAMTS5 immunostaining of embryonic skeletal muscle observed in this study is the recent observation that exon 2 of the Adamts5 gene contains an enhancer that binds to muscle regulatory factors (Barthel and Liu, 2008). It is not yet conclusively established that this enhancer regulates Adamts5, since enhancers can act on distant genes. The ADAMTS5 antibody strongly reacts with E15.5 skeletal muscle from wild-type mice (e.g., Fig. 7C). It should be noted that β-gal staining of muscle in this study (e.g., Fig. 4 B) was obtained from mice that were null at the Adamts5 locus, i.e., they lacked exon 2, and thus the enhancer elements contained in it. If the reported enhancer indeed regulates Adamts5 expression in skeletal muscle, then it appears that even without it, the endogenous Adamts5 promoter has significant muscle-specific activity. In contrast to early myogenesis, little β-gal staining of muscle is seen in adult muscle (Fig. 4E) suggesting that expression of Adamts5 is transiently upregulated during myogenesis, but is not a feature of mature myotubes.
1.8. Relevance of Adamts5 expression and protein distribution to versican
Similar macromolecular aggregates as the aggrecan-HA complexes are present in developing non-cartilagenous mesenchyme where a related proteoglycan, versican is associated with HA, and in the brain and central nervous sytem, where brevican and versican are both present in association with HA. Versican is abundant in the central and peripheral nervous system, skin, vascular wall, developing myocardium and cardiac outflow tract (Bode-Lesniewska et al., 1996; Kern et al., 2007; Sandy et al., 2001), and is an ADAMTS5 substrate (Longpre et al., 2008). We have demonstrated here that Adamts5 expression is associated with many versican-containing structures such as the perichondrium of digit cartilage, blood vessels, brain and spinal cord, nerves, limb and trunk mesenchyme. The expression profile of Adamts5 places it in a context that suggests its ability to cleave versican may be of physiological significance. There is strong evidence that versican cleavage regulates several morphogenetic processes. Versican provides guidance cues to migrating neural-crest derived cells (Dutt et al., 2006; Perissinotto et al., 2000) and versican processing is defective in the spontaneous Adamts20 mouse mutant belted (bt), which has a defect in melanoblast survival (Silver, 2008). We have shown that Adamts5 does not have a role in melanoblast colonization of skin, since bt/bt mice lacking Adamts5 do not show an increase in the area of the of the unpigmented belt (Silver, 2008). Versican processing is critical for ovulation (Russell et al., 2003), and for vascular remodeling during the development of the cardiac outflow tract and myocardium (Kern et al., 2007; Russell et al., 2003; Stankunas et al., 2008). Indeed, a related protease, ADAMTS1 has a major function in these processes (Kern et al., 2007; Russell et al., 2003; Stankunas et al., 2008). Through its expression in both endocardium and myocardium, ADAMTS5, like ADAMTS1, may be involved in remodeling the cardiac jelly and is potentially relevant to cardiomyopathy of human myocardial non-compaction (Stankunas et al., 2008).
1.9. Relationship to the expression patterns of other proteoglycan-degrading ADAMTS proteases
The expression of Adamts5 in specific cell types, such as skeletal and smooth muscle cells, Schwann cells and connective tissue cells from cartilage and tendon, suggests an association with both neural-crest and mesoderm derivatives. However, there is not yet any evidence for functional perturbation following ADAMTS5 inactivation in these lineages. This suggests that its absence during development and in unchallenged adult null mice may be compensated by one of several related ADAMTS proteases, specifically, ADAMTS1, ADAMTS4, ADAMTS8, ADAMTS9, ADAMTS15, and ADAMTS20 all of which can process proteoglycans (Apte, 2004). Of these, ADAMTS5, ADAMTS4 and ADAMTS1 are established as efficient proteoglycan degrading enzymes in vitro (Abbaszade et al., 1999) (Sandy et al., 2001). ADAMTS9 and ADAMTS20 are relatively inefficient proteoglycanases in in vitro assays and are evolutionarily closer to each other than to ADAMTS5 (Somerville et al., 2003). Our unpublished data show that Adamts4 is extremely sparsely expressed during mouse embryonic development, and is therefore unlikely to significantly compensate for loss of Adamts5. Adamts1 is however, very strongly expressed in an overlapping pattern with ADAMTS5 at several sites, such as in vascular smooth muscle cells of arteries, endocardium, the choroid plexus, dorsal root ganglia, bone and interdigital mesenchyme (Gunther et al., 2005; Kern et al., 2007; Thai and Iruela-Arispe, 2002), suggesting it may degrade versican cooperatively with Adamts5 at these sites. Functions for Adamts1 have been established in the regulation of angiogenesis, fertility, urinary tract development and in regulatory interactions between endocardium and myocardium that are critical for cardiac development (Brown et al., 2006; Kern et al., 2007; Krampert et al., 2005; Mittaz et al., 2005; Mittaz et al., 2004; Shindo et al., 2000). Intriguingly, Adamts5 is tightly linked to Adamts1 on mouse chromosome 16 and human chromosome 21 (Koo et al., 2007). Their similar domain structure, proteolytic activity and expression profile strongly suggests they arose by duplication from a common ancestor and have conserved functions as well as conserved gene regulation, but their cooperative roles cannot be presently elucidated owing to their tight linkage. Because these two genes are only 60 kb apart, they are unlikely to segregate independently during meiosis, and thus double-null mice for these proteases cannot be generated by interbreeding Adamts5 and Adamts1 null mice. Recently, we have shown that Adamts9 and Adamts20, cooperate with each other in the colonization of skin by neural-crest derived melanoblasts (Silver, 2008). Neither protease is expressed by melanoblasts, but is expressed by mesenchyme in the dermis. The developmental expression of Adamts9 has been described in detail, showing it to be primarily a gene expressed by mesoderm-derived cells (Jungers et al., 2005), so that it may also overlap functionally with Adamts5. The expression profile of Adamts8 and Adamts15 is presently unknown. In summary, the data presented here demonstrates a strong association of Adamts5 with distinct cell lineages and indicates the specific locations at which Adamts5 functions ought to be sought, possibly in combinatorial deletion with Adamts1.
2. Experimental Procedures
2.1 Transgenic animals
Adamts5+/− mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and maintaind in the Biological Resources Unit of the Cleveland Clinic according to a protocol approved by the Institutional Animal Care And Use Committee. We have outbred them for over 8 consecutive generations into the C57Bl/6 strain. Genotyping of tail DNA was performed using primer pairs and protocols available at http://www.informatics.jax,org/external/ko/deltagen/1232_MolBio.html. Primer sequences used for RT-PCR are available on request. For determining the gestational age of embryos the day a vaginal mucus plug was observed and was designated as E0.5. Animal tissues were obtained post-mortem after euthanasia done as per recommendations of the American Veterinary Association Panel on Euthanasia.
2.2. β-galactosidase staining
Embryos and tissue fragments were fixed using freshly prepared 4% paraformaldehyde prepared in β-gal wash buffer (0.1M phosphate buffer pH 7.4, 2 mM MgCl2, 0.01% Na deoxycholate, 0.02% NP-40), rinsed in this wash buffer, and incubated overnight at 37 °C in β-gal staining solution (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mg/ml X-gal in wash buffer). After a brief rinse in wash buffer, the embryos and tissues were photographed as whole mounts or embedded in paraffin for sectioning and histology. Whole mount β-gal staining was done up to E15.5. After E16.5 organs were dissected out and stained individually. β-gal staining was substantially improved by fixing tissue in paraformaldehyde dissolved in the β-gal rinse buffer, which contains detergents, and improves penetration of the fixative and staining solutions. The presence of detergents in this buffer provides improved penetration of the staining reagents and enhances staining in deeper tissues, which are typically not stained effectively after E13.5.
2.3. RNA ISH and immunohistochemistry
Adamts5 ISH was done using [35S]-UTP labeled cRNA probes expressed from a previously described cDNA clone (Hurskainen et al., 1999). For immunohistochemistry the following antibodies were used: Anti-neurofilament M (mouse monoclonal, clone 2H3, Developmental studies Hybridoma Bank (DSHB), Iowa City, IA), anti smooth muscle α-actin (mouse monoclonal, Sigma-Aldrich, St. Lous, MO), anti-endomucin (rat monoclonal, clone V.7C7, kindly provided by Dr. Dietmar Vestweber), skeletal muscle myosin (mouse monoclonal, MF20a, DSHB), anti-versican GAGβ (rabbit polyclonal, Chemicon [Millipore], Temecula, CA). Anti-ADAMTS5 rabbit polyclonal antibody (named JSCKNG) was raised against a synthetic peptide from the cysteine-rich domain (Fig. 1A), 636KNGYQSDAKGVKTF649 (human sequence enumeration), that is identical in human and mouse ADAMTS5. The peptide was linked to ovalbumin through an N-terminal extension sequence CGG- and immunoglobulins were affinity purified on a sulfolink-peptide substituted column (Pierce Chemical/Thermo-Fisher Scientific Inc., Rockford, IL). Use of this antibody in other murine and human systems has been previously described (Plaas et al., 2007; Stewart et al., 2006).
Acknowledgements
This work was supported by NIH awards AR49930 and AR53890 and a grant from the Northeastern Ohio Chapter of the Arthritis Foundation (to S. Apte). Histological analysis was made possible by award of a NIH Core Center for Musculoskeletal Disorders (to V.C. Hascall and S.Apte). S. Bhatt was supported in part by a grant from the Howard Hughes Foundation. We thank Amanda Allamong for assistance with histology. We thank Dr. Norman Rosenblum and Dr. Wendy Macklin for useful discussion, and Dr. D. Vestweber for anti-endomucin antibody.
References
- Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, Ellis DM, Tortorella MD, Pratta MA, Hollis JM, Wynn R, Duke JL, George HJ, Hillman MC, Jr, Murphy K, Wiswall BH, Copeland RA, Decicco CP, Bruckner R, Nagase H, Itoh Y, Newton RC, Magolda RL, Trzaskos JM, Burn TC, et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274:23443–23450. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36:981–985. doi: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel KK, Liu X. A transcriptional enhancer from the coding region of ADAMTS5. PLoS ONE. 2008;3:e2184. doi: 10.1371/journal.pone.0002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode-Lesniewska B, Dours-Zimmermann MT, Odermatt BF, Briner J, Heitz PU, Zimmermann DR. Distribution of the large aggregating proteoglycan versican in adult human tissues. J Histochem Cytochem. 1996;44:303–312. doi: 10.1177/44.4.8601689. [DOI] [PubMed] [Google Scholar]
- Brown HM, Dunning KR, Robker RL, Pritchard M, Russell DL. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev Biol. 2006;300:699–709. doi: 10.1016/j.ydbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, Wilcox W, Krakow D, Cohn DH, Reardon W, Byers PH, Lapiere CM, Prockop DJ, Nusgens BV. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet. 1999;65:308–317. doi: 10.1086/302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagoneau N, Benoist-Lasselin C, Huber C, Faivre L, Megarbane A, Alswaid A, Dollfus H, Alembik Y, Munnich A, Legeai-Mallet L, Cormier-Daire V. ADAMTS10 Mutations in Autosomal Recessive Weill-Marchesani Syndrome. Am J Hum Genet. 2004;75:801–806. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Dutt S, Kleber M, Matasci M, Sommer L, Zimmermann DR. Versican V0 and V1 guide migratory neural crest cells. J Biol Chem. 2006;281:12123–12131. doi: 10.1074/jbc.M510834200. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Gunther W, Skaftnesmo KO, Arnold H, Bjerkvig R, Terzis AJ. Distribution patterns of the anti-angiogenic protein ADAMTS-1 during rat development. Acta Histochem. 2005;107:121–131. doi: 10.1016/j.acthis.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Hurskainen TL, Hirohata S, Seldin MF, Apte SS. ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. J Biol Chem. 1999;274:25555–25563. doi: 10.1074/jbc.274.36.25555. [DOI] [PubMed] [Google Scholar]
- Jungers KA, Le Goff C, Somerville RP, Apte SS. Adamts9 is widely expressed during mouse embryo development. Gene Expr Patterns. 2005;5:609–617. doi: 10.1016/j.modgep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kern CB, Norris RA, Thompson RP, Argraves WS, Fairey SE, Reyes L, Hoffman S, Markwald RR, Mjaatvedt CH. Versican proteolysis mediates myocardial regression during outflow tract development. Dev Dyn. 2007;236:671–683. doi: 10.1002/dvdy.21059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BH, Goff CL, Jungers KA, Vasanji A, O'Flaherty J, Weyman CM, Apte SS. ADAMTS-like 2 (ADAMTSL2) is a secreted glycoprotein that is widely expressed during mouse embryogenesis and is regulated during skeletal myogenesis. Matrix Biol. 2007 doi: 10.1016/j.matbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Krampert M, Kuenzle S, Thai SN, Lee N, Iruela-Arispe ML, Werner S. ADAMTS1 proteinase is up-regulated in wounded skin and regulates migration of fibroblasts and endothelial cells. J Biol Chem. 2005;280:23844–23852. doi: 10.1074/jbc.M412212200. [DOI] [PubMed] [Google Scholar]
- Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- Longpre JM, McCulloch DR, Koo BH, Alexander JP, Apte SS, Leduc R. Characterization of proADAMTS5 processing by proprotein convertases. Int J Biochem Cell Biol. 2008 doi: 10.1016/j.biocel.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Mittaz L, Ricardo S, Martinez G, Kola I, Kelly DJ, Little MH, Hertzog PJ, Pritchard MA. Neonatal calyceal dilation and renal fibrosis resulting from loss of Adamts-1 in mouse kidney is due to a developmental dysgenesis. Nephrol Dial Transplant. 2005;20:419–423. doi: 10.1093/ndt/gfh603. [DOI] [PubMed] [Google Scholar]
- Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA. Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod. 2004;70:1096–1105. doi: 10.1095/biolreprod.103.023911. [DOI] [PubMed] [Google Scholar]
- Perissinotto D, Iacopetti P, Bellina I, Doliana R, Colombatti A, Pettway Z, Bronner-Fraser M, Shinomura T, Kimata K, Morgelin M, Lofberg J, Perris R. Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development. 2000;127:2823–2842. doi: 10.1242/dev.127.13.2823. [DOI] [PubMed] [Google Scholar]
- Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, Mikecz K, Sandy JD. Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage. 2007;15:719–734. doi: 10.1016/j.joca.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JR, Vogel KG. Regional expression of mRNA for proteoglycans and collagen in tendon. Eur J Cell Biol. 1994;64:264–270. [PubMed] [Google Scholar]
- Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- Sandy JD, Westling J, Kenagy RD, Iruela-Arispe ML, Verscharen C, Rodriguez-Mazaneque JC, Zimmermann DR, Lemire JM, Fischer JW, Wight TN, Clowes AW. Versican V1 proteolysis in human aorta in vivo occurs at the Glu441- Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS- 4. J Biol Chem. 2001;276:13372–13378. doi: 10.1074/jbc.M009737200. [DOI] [PubMed] [Google Scholar]
- Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, Imai T, Wang Y, Ogata M, Nishimatsu H, Moriyama N, Oh-hashi Y, Morita H, Ishikawa T, Nagai R, Yazaki Y, Matsushima K. ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest. 2000;105:1345–1352. doi: 10.1172/JCI8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver DL, Hou L, Somerville R, Young ME, Apte SS, Pavan WJ. The secreted metalloprotease ADAMTS20 is required for melanoblast survival. PLoS Genet. 2008;4:1–15. doi: 10.1371/journal.pgen.1000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Stankunas K, Hang CT, Tsun ZY, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang CP. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Dev Cell. 2008;14:298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Stewart MC, Fosang AJ, Bai Y, Osborn B, Plaas A, Sandy JD. ADAMTS5-mediated aggrecanolysis in murine epiphyseal chondrocyte cultures. Osteoarthritis Cartilage. 2006;14:392–402. doi: 10.1016/j.joca.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Thai SN, Iruela-Arispe ML. Expression of ADAMTS1 during murine development. Mech Dev. 2002;115:181–185. doi: 10.1016/s0925-4773(02)00115-6. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J, Mitchell JJ, Low RB, Kieny M, Sengel P, Rubbia L, Skalli O, Jackson B, Gabbiani G. Alpha-smooth muscle actin is transiently expressed in embryonic rat cardiac and skeletal muscles. Differentiation. 1988;39:161–166. doi: 10.1111/j.1432-0436.1988.tb00091.x. [DOI] [PubMed] [Google Scholar]


