Abstract
Interferon-β (IFN-β has been found to have anti-tumor properties against a variety of malignancies through different mechanisms. However, clinical trials involving systemic administration of IFN-β have been hampered by secondary toxicity and the short half-life of IFN-β in the circulation. In order to circumvent these limitations we have developed an adeno-associated viral (AAV) vector gene therapy approach to deliver IFN-β to tumors. In this study, we tested the efficacy of AAV mediated local delivery of IFN-β for the treatment of retinoblastoma in preclinical models. Retinoblastoma is an ideal candidate for gene-therapy based anti-cancer treatment because target cell transduction and, therefore, IFN-β delivery can be contained within the ocular environment, thereby minimizing systemic toxicity. We report here that retinoblastoma cell lines exhibit pleiotropic responses to IFN-β consistent with previous studies on a variety of tumor cell lines. Intravitreal injection of AAV-IFN-β resulted in efficient retinal infection and sustained IFN-β production in the eye with minimal systemic exposure. Vector spread outside of the eye was not detected. Using our orthotopic xenograft model of retinoblastoma we found that intravitreal injection of AAV-IFN-β had a potent anti-tumor effect in vivo. These data suggest that AAV mediated delivery of IFN-β may provide a complementary approach to systemic chemotherapy which is the standard of care for retinoblastoma around the world.
Keywords: Retinoblastoma, Interferon-β, AAV, gene therapy
Introduction
Retinoblastoma is a cancer of the developing retina that begins in utero and is usually diagnosed in the first few years of life. After neuroblastoma and leukemia, retinoblastoma is the third most common form of cancer in infants with an incidence of 1/20,000 live births in the United States 1. Children with unilateral retinoblastoma typically undergo surgical enucleation, which results in a 95% cure rate in developed countries. However, about 40% of children with retinoblastoma are born with a germ-line mutation of the RB1 gene and develop multiple bilateral retinoblastomas at an earlier age2. The current approach to treatment of these patients includes systemic chemotherapy, to reduce tumor burden, followed by a combination of focal therapies that include laser therapy and cryotherapy. Even with this intensive treatment, approximately 40% of eyes of children with advanced bilateral retinoblastoma are still lost 3. Poor intravitreal penetration of anti-cancer drugs is one of the limitations of current therapies. To overcome this hurdle many researchers and clinicians are now focusing on novel methods to deliver anti-tumorigenic compounds to the eyes of children with retinoblastoma by subconjunctival injection 4–6 or direct injection of chemotherapeutic agents into the vitreous (Yanagisawa, T., unpublished). The advantage of these approaches is that they can lead to much higher intravitreal levels of chemotherapeutic drugs while avoiding many of the side effects associated with systemic broad-spectrum chemotherapy.
Another strategy to specifically target retinoblastoma cells in the eye is gene therapy. Using an innovative suicide gene therapy approach, Hurwitz et al, injected an adenoviral vector (AdV-TK) that expresses the herpes simplex virus thymidine kinase (HSV-TK) gene into established tumors. Cells expressing HSV-TK are sensitive to subsequent exposure to ganciclovir. Following a preclinical study using this strategy in animal models 7, the investigators tested the safety of this approach in children with refractory tumor seeding in the vitreous. Vector was injected via a transcorneal approach adjacent to vitreal seeds. Therapy was well tolerated and 7/8 patients showed a clinical response, demonstrating that this strategy is feasible and potentially effective 8.
Adeno-associated virus (AAV) is a nonpathogenic human parvovirus, which is dependent on a helper virus, usually adenovirus or herpesvirus, to propegate. AAV virions are small nonenveloped particles with a 4.7 kb linear single-stranded DNA genome and are capable of infecting both dividing and quiescent cells. AAV infection involves a multistep process starting with cell surface binding, followed by viral uptake, intracellular trafficking, nuclear localization, uncoating, and second-strand DNA synthesis 9, this later process being the rate limiting step to successful target cell transduction.
AAV has a number of desirable characteristics for gene therapy including a lack of pathogenicity, minimal immunogenicity, and the ability to transduce postmitotic cells in a stable and efficient manner 10. The retina is a sheltered environment in which AAV vectors are able to maintain high levels of transgene expression in the retinal pigmented epithelium (RPE), photoreceptors, or ganglion cells for long periods of time after a single treatment 10 with little if any viral spread to other tissues outside of the eye. Subretinal injection of AAV vectors leads to RPE and photoreceptor transduction 11–14 while intravitreal injection leads to efficient ganglion cell transduction 15–18. AAV-mediated gene delivery is currently being tested in a variety of animal models of retinal disease including recessive Leber congenital amaurosis (LCA), both dominant and recessive retinitis pigmentosa (RP), X-linked reinoschisis (RS), and retinal neovascular disease 10, 19–21.
In this study the efficacy of AAV-mediated delivery of interferon-β (IFN-β) for the treatment of retinoblastoma in preclinical models was evaluated. Interferon-β is a pleiotropic cytokine that targets a variety of intracellular signaling pathways 22, 23. First identified for their potent antiviral activities in the 1950s, type I interferons (α/β) have become recognized for their anti-tumor activities in both animal models as well as in clinical studies against solid and hematologic malignancies 24–28. Documented activity as an anticancer compound as single therapy or as a combined modality with other treatments has been shown in a variety of hematologic diseases including hairy cell leukemia, chronic myeloid leukemia (CML), myeloma, and B and T cell lymphomas 29–32. A number of solid tumors have also been found to be responsive to IFN, including melanoma, renal cell carcinoma, and midgut cardinoids 33, 34.
Despite the potent anti-neoplastic activity of IFN-β, clinical trials using IFN-β to treat human cancer patients have been disappointing 35. This is due in part to the pharmacology of systemic IFN-β administration. Side effects limit the maximum dose that can be administered and the short half-life limits the duration of IFN-β exposure in the tumor. To overcome these limitations of IFN-β treatment of cancer, a gene therapy approach has been shown to result in local target cell transduction and continuous IFN-β production at the site of the tumor 36–40.
Retinoblastoma is an ideal cancer for this type of treatment approach because an intravitreal injection of a viral vector encoding IFN-β may lead to long-term local exposure of IFN-β to the growing tumor in the retina, subretinal space, choroids and vitreous. In this study we tested the efficacy of AAV-mediated delivery of IFN-β using preclinical models of retinoblastoma. We found that a potent anti-tumor effect can be achieved using this strategy with minimal systemic exposure of IFN-β or spread of the AAV viral vector outside of the eye. AAV-mediated delivery of IFN-β may serve to complement the existing conventional approachs for treating this debilitating childhood cancer.
Materials and Methods
Cell culture
Y79 and Weri1 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained in culture in RPMI with 10% FCS 41. SEM cells were generously provided by Dr. Wing Leung (St. Jude Children’s Research Hospital, Memphis, TN). Y79 cells were engineered to stably express luciferase using a method previously described42.
Immunolabeling, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL), Caspase, and bromodeoxyuridine assays
BrdU, Caspase, and TUNEL assays were carried out as described previously43–45. Detailed protocols are available at http://www.stjude.org/dyer.
Fluorescent-activated cell sorting
DNA content was analyzed by fluorescent-activated cell sorting. Cells were washed with PBS and resuspended in a solution containing 0.05 mg/mL propidium iodide, 0.1%sodium citrate, and 0.1% Triton X-100. Samples were then treated with RNase, filtered through a 40-μm nylon mesh, and analyzed on a FACScan (Becton Dickinson, Franklin Lakes, NJ).
Viable cell calculation following drug treatment
Following drug treatment, the total number of cells (N) from hemacytometer cell counting were scored followed by Guava Viacount® and Guava Nexin™ assays (Guava technologies, Haywood, CA) to determine the proportion of cells that were metabolically active (M) and the proportion of cells that had initiated apoptosis (T). All of these data were combined to obtain the number of viable cells (Cv) in the following equation: Cv=N×M×(1-T).
The proportion of viable cells is the ratio of the number of viable cells (Cv treated) in the treated sample divided by the number of viable cells (Cv untreated) in the untreated sample. All of the cell culture experiments were carried out twice, in triplicate to generate the data shown in Fig 1.
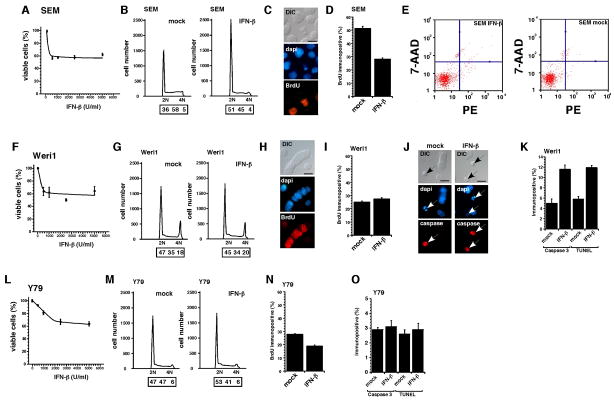
Fig 1. Retinoblastoma cell lines are sensitive to interferon-β in culture.
SEM (leukemia positive control) showed a maximum reduction in viability at 500 IU/mL of IFN-β (A). Cell cycle arrest at G0/G1 (B) and suppression of proliferation indicated by BrdU labeling (C,D) are the primary mechanisms for this response. There was no significant increase in apoptosis of SEM cells following exposure to 500 IU/ml of IFN-β. The Weri1 retinoblastoma cells showed a similar sensitivity to IFN-β with a maximum reduction in viability at 500 IU/mL (F). However, in contrast to SEM cells, the Weri1 cells exhibited no detectable change in proliferation (G–I) and a significant increase in apoptosis following exposure to IFN-β as measured by expression of activated Caspase-3 and the presence of TUNEL+ cells (J,K). Y79 retinoblastoma cells were the least sensitive cells tested in our experiments (L). Following exposure to IFN-β Y79 cells exhibited a small increase in the proportion of cells in the G1 phase (M) of the cell cycle consistent with a decrease in the proportion of BrdU labeled cells following IFN-β treatment (N). There was no significant increase in apoptosis in Y79 cells exposed to IFN-β (O). Scale bars: 10μm.
Animals/tissue
Wild-type Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA).
Microscopy
Bright-field and single-cell fluorescent images were obtained using a Zeiss Axioplan 2 fluorescent mircroscope with the Zeiss AxioCam digital camera.
Xenogen imaging of Y79-LUC cells in vivo
Orthotopic retinoblastoma xenografts were established in wild-type rats by intravitreal injection of 1×103 Y79-LUC cells using 33 gauge syringe (Hamilton, Reno, NV) at P0. AAV-IFN-β was injected using 33 gauge syringe at P7. To monitor luciferase activity, rats were anesthetized with 2% inhaled isofluorane followed by an i.p. injection of luciferin (Xenogen, INC., Alameda, CA) according to the manufacturer’s instructions. Thirty minutes later, the eyes were imaged using the Xenogen bioluminescence imaging system. Animals were monitored for health and humanely sacrificed prior to rupture of eye due to rapid growth of tumor.
rAAV vector construction, production, and purification
The cDNAs for human interferon-beta (hIFN-β) and human placental alkaline phosphatase (PLAP) were purchased from InvivoGen (San Diego, CA). AAV vector plasmids based on the ITRs of AAV serotype 2 and containing these cDNAs were then constructed. Each included the CMV-IE enhancer, β-actin promoter, a chicken β-actin/rabbit β-globin composite intron and a rabbit β-globin polyadenylation signal. Functional AAV vectors in which the AAV-2 vector genome was encapsidated with capsid proteins of AAV serotype 1, 2, 5, or 8 were then were generated by the methods described previously 46. Standard slot-blot analysis was used to determine the vector particle titer. The vector stocks were consistently free of contamination with wild type AAV and cellular and adenoviral proteins Intravitreal injections consisted of 1×109 vector particles in 1μl PBS.
Human interferon-β immunoassay
Levels of human IFN-β in mouse vitreous and plasma were determined using a commercially available immunoassay (ELISA) (Biosource International, Camarillo, CA). The standard used in the human IFN-β ELISA kit is calibrated against the NIH reference for human interferon-beta. The reported level of sensitivity for hIFN-β is 100 pg/ml in this kit.
Determination of vector biodistribution
To evaluate the biodistribution of AAV8 CAG hIFN-β vectors, 1μg of genomic DNA extracted from the retina and liver was subjected to PCR using primers which amplified a 434bp region (5′ primer: 5′ CTGTTGTGCTTCTCCACTACAG 3′ and 3′ primer: 5′ GCCTTCAGGTAATGCAGAATCC 3′), as described previously 47.
Detection of anti-AAV8 antibodies
Serum samples from rats were screened for the presence of antibodies against AAV8 by determining the ability of the serum to inhibit transduction of 293T cells by an AAV8 vector encoding GFP, as previously described47. The neutralizing antibody titer was arbitrarily calculated as reciprocal of the highest dilution, which inhibited transduction of 293T cells by 50%.
Results
Retinoblastoma cell lines are sensitive to interferon-β in culture
A variety of tumor cell types have been found to be sensitive to IFN-β. In order to test the sensitivity of retinoblastoma cells to IFN-β, we performed a series of experiments on cultured retinoblastoma cell lines. As a positive control, we used SEM leukemia cells which have been shown to be sensitive to a variety of cytokines including IFN-β 48. Cultured cells were exposed to human recombinant IFN-β (Avonex®) at concentrations ranging from 500–10000 IU/mL for 72 hours. At the end of the culture period, the cell number, proportion of viable cells, and proportion of apoptotic cells was scored in triplicate for each IFN-β concentration.
SEM cells showed a maximum reduction in viability at 500 IU/mL (Fig 1A). Cell cycle analysis demonstrated that there is a G0/G1 arrest in SEM cells exposed to IFN (Fig 1B) and this was consistent with data from BrdU labeling studies (Fig. 1C,D). There does not appear to be any increase in apoptosis in SEM cells exposed to IFN-β as measured by FACS with 7-AAD or Annexin-V staining (Fig 1E). These data are consistent with previously published data showing that SEM cells undergo cell cycle exit in response to IFN-β exposure 48.
Weri1 retinoblastoma cells 41 also showed a maximum reduction viability at 500 IU/mL IFN-β (Fig 1F). However, in contrast to the SEM cells, there is no significant cell cycle arrest in IFN-β treated Weri1 cells as measured by FACS or BrdU labeling (Fig G–I). Immunostaining for activated Caspase 3 and TUNEL showed a significant increase in apoptosis of Weri1 cells exposed to IFN-β (Fig 1J,K) suggesting that this was the predominant mechanism for the reduction in cell viability.
Maximum reduction in viability of Y79 retinoblastoma cells 49 occurred at 2500 IU/mL of IFN-β (Fig 1L). There was a small increase in cells in the G1 phase of the cell cycle following exposure to IFN-β (Fig 1M), and this is consistent with the decrease in the proportion of BrdU labeled cells following IFN-β treatment (Fig. 1N). Little if any increase in cell death was observed in Y79 cells exposed to IFN-β (Fig 10).
Quantitation of retinoblastoma growth in orthotopic xenografts
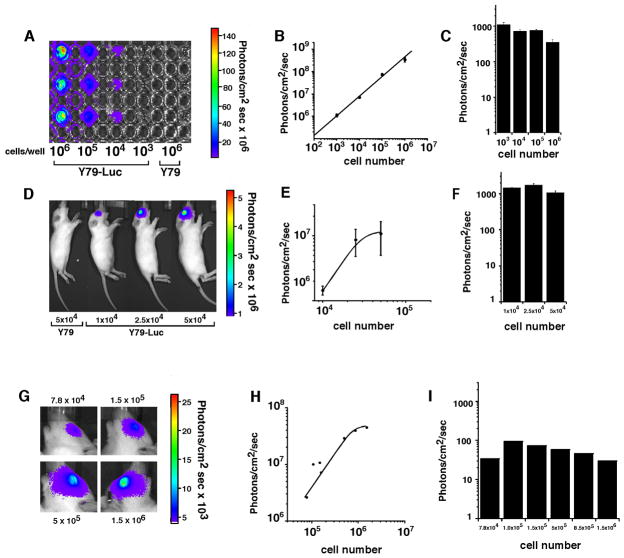
We have previously published the development of an orthotopic retinoblastoma xenograft model using Y79 cells that constitutively express the Luciferase reporter gene (Y79-Luc) 50. However, we have not directly analyzed the efficiency of luciferase quantitation per cell in this xenograft model. In order to characterize the Y79 cell line itself, we plated 103, 104, 105, and 106 cells in triplicate in a 96 well dish. We added the luciferase substrate (luciferin) and imaged using the Xenogen IVIS 200 (Fig. 2A). As expected, there was a linear relationship between cell number and photons/sec/cm2 (Fig 2B). Importantly, the photons/cm2-sec per cell was very similar over this broad range of cell density (Fig 2C).
Fig 2. Characterization of an orthotopic xenograft model of retinoblastoma.
103, 104, 105, 106 Y79-Luc cells were plated in a 96-well dish triplicate, incubated with luciferin and imaged with the Xenogen IVIS 200 (A). Photons/sec/cm2 correlate directly with cell number (B) and photons/sec/cm2 per cell were constant over all of the different cell densities tested (C). 10,000, 25,000,and 50,000 Y79-Luc cells were mixed with luciferin and injected into the vitreous of P14 rat pups and subsequently imaged (D). The photons/sec/cm2 increased as expected with increasing cell number (E) and the photons/sec/cm2 per cell was similar to that seen in culture (F). Next, Y79-Luc cells were injected to the vitreous of P14 rats and luciferin was administered by i.p. injection to measure the efficiency of luciferin transport across the blood-retinal barrier (G). There was a correlation between the number of cells and the photons/sec/cm2 (H) but the photons/sec/cm2 per cell was approximately 10-fold lower due to the transport of luciferin across the blood-retinal barrier (I).
To measure the efficiency of luciferase detection in vivo, we injected 10,000, 25,000 and 50,000 Y79-Luc cells into the vitreous of P14 rat pups (Fig. 2D). Prior to injection, the cells were mixed with luciferin to quantitate the photons/sec/cm2 per eye in vivo. The photons/sec/cm2 increased as expected with increasing cell number (Fig 2E) and the number of photons/sec/cm2 per cell (Fig. 2F) was very similar to that seen for cultured cells (compare Fig 2F to 2C). These data suggest that there is little if any reduction in the efficiency of luciferase detection when the cells are inside the eye compared to a tissue culture dish.
Next, we tested the efficiency of luciferin transport across the blood retinal barrier. Newborn rat pups received an intraocular injection of 1,000 Y79-Luc cells and 14 days later the Luciferase activity was measured using the Xenogen IVIS 200 system (Fig. 2G). We then removed the tumor from each eye, dissociated the cells and scored the proportion of viable cells. The photons/sec/cm2 increased with increasing cell number in each eye providing a strong correlation between luciferase activity and tumor burden (Fig. 2H). However, the overall efficiency of detection (photons/sec/cm2 per cell) was approximately 10-fold lower than the cultured cells or direct injection of luciferin (Fig. 2C,F) due to the restricted penetration of luciferin across the blood-retinal barrier (Fig. 2I).
AAV-mediated delivery of IFN-β to the vitreous
As a first step, we tested AAV vectors encoding luciferase with encapsidated with serotypes 1, 2, 5 and 8 to determine which was the most efficient in transducing the neonatal rat eye. Bioluminescent imaging revealed that vectors pseudotyped with AAV8 capsid generated the highest mean signal (data not shown). Therefore, all subsequent studies were performed with AAV8 vectors.
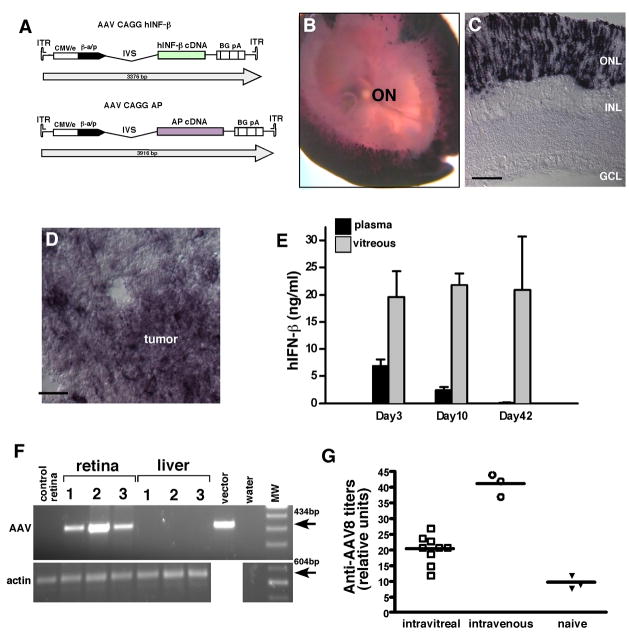
Next, we injected an AAV vector encoding alkaline phosphatase (Fig. 3A) into the vitreous of P14 rats to determine which cell types were infected in the retina. We found that the photoreceptors were the predominant cell type infected (Fig. 3B,C). When we performed a similar experiment with P14 tumor bearing rats, we observed a similar pattern of retinal infection as well as infection of the Y79 tumor cells themselves (Fig. 3D).
Fig 3. AAV infection and spread following intravitreal injection.
The human placental alkaline phosphatase (AP) gene and the human IFN-β genes were cloned into the AAV vector to generate AAV-AP and AAV- IFN-β vectors (A). Vitreal injection led to efficient infection of the photoreceptor layer across the entire retina as shown in whole mount AP stained retinae (B) and cryosections (C). In tumor bearing animals, the AAV-AP virus also infected the human Y79 retinoblastoma cells (D). The vitreal and plasma levels of IFN-β were measured following intravitreal injection of AAV- IFN-β 3, 10 and 42 days after injection (E). There was no evidence of viral spread outside of the eye as measured by by PCR analysis of liver or other tissues (F). As additional evidence for minimal AAV spread outside of the eye following an intravitreal injection, an ELISA was performed to measure anti-AAV8 antibody titer in naieve rats, those that received an intravitreal injection and rats that received an intravenous injection of AAV8 (G).
In order to determine how much IFN-β is produced in the eye following intravitreal injection of AAV- IFN-β virus we injected the virus at P14 and then monitored the levels of IFN-β in the vitreous and plasma at 3 timepoints (3, 10 and 42 days after injection). Three days following vector injection, intravitreal levels of IFN-β were 20ng/ml and remained stable for 6 weeks (Fig. 3E). The plasma levels of IFN-β were 7ng/ml three days following vector injection, 2ng/ml by day 10 and undetectable at 42 days (Fig. 3E). To determine whether the vector had spread outside of the eye, we performed PCR analysis of DNA extracted from the retina and liver (the site of greatest tropism for systemic AAV8 46 to detect the viral genome. We found no detectable vector genome in the liver (Fig. 3F). We also measured the level of systemic anti-AAV8 antibodies after intravitreal injection of AAV8 CAG hIFN-β and found the development of a low level titer (Fig. 3G), suggesting that there had been some systemic exposure to the vector upon intravitreal administration.
Response of orthotopic retinoblastoma xenografts to local AAV-IFN-β
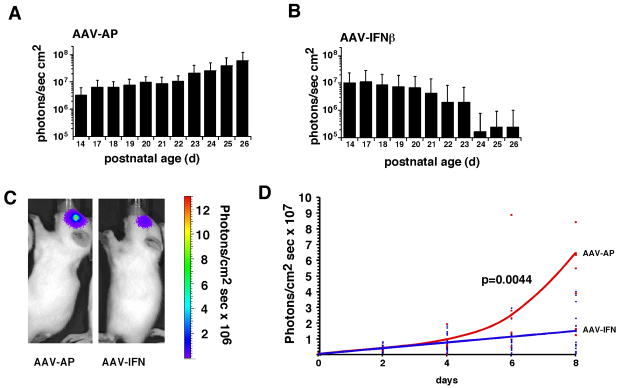
To determine if of AAV-IFN-β could mediate an antitumor effect in vivo, we mixed either AAV-IFN-β or AAV-AP with Y79-Luc retinoblastoma cells and injected 1,000 cells into the eyes of newborn rat pups. We monitored the luciferase expression from P14–P26 and found that of AAV-IFN-β led to a considerable reduction in luciferase expression during this time period (Fig. 4A,B). Next, we established tumors by injecting Y79-Luc cells into the eyes of newborn rat pups and at P14, injected AAV IFN-β or AAV AP virus into the vitreous. The dose of AAV-IFN-β (or AAV-AP) was 2×109 vector particles per eye.
Fig 4. Response of orthotopic retinoblastoma xenografts to local AAV-IFN-β.
Y79-Luc cells co-injected with AAV-IFN-β show poor tumor growth compared to Y79-Luc co-injected with AAV-AP (A,B). In established tumors, AAV-IFN-β injection reduced tumor growth as compared to AAV-AP control injected tumors (C,D). For these studies, tumors were first established and quantitated using the Xenogen IVIS-200 system and then imaged sequentially following infection with AAV- IFN-β or AAV-AP.
In the control eyes (AAV AP), there was an increase in luciferase expression over time until the animals had to be euthanized for humane reasons due to tumor burden (Fig. 4C,D). In the eyes injected with AAV IFN-β, there was little if any increase in tumor burden over this time period (Fig. 4C,D). In total, 18 treated eyes and 9 control eyes were assayed.
DISCUSSION
Previous studies have established the feasibility of gene therapy to treat retinoblastoma in preclinical models and Phase I clinical trials 8. Here we extended those studies and tested the efficacy of AAV mediated delivery of IFN-β for the treatment of retinoblastoma. We found that Weri1 retinoblastoma cells undergo apoptosis in the presence of IFN-β while Y79 retinoblastoma cells undergo cell cycle arrest in the presence of IFN-β. In vivo, AAV vectors efficiently infected the retina and retinoblastoma xenografts and continued to produce IFN-β for up to 6 weeks. There was no detectable spread of the virus outside of the eye and plasma levels dropped off to undetectable levels by 6 weeks of age. Treatment of established orthotopic retinoblastoma xenografts with local administration of AAV IFN-β resulted in a cessation of tumor expansion consistent with the results from IFN-β treatment in culture.
The difference between this study and previously published data on gene therapy for the treatment of retinoblastoma are threefold. First, we delivered a cytokine (IFN-β) that directly affects tumor survival and growth. The previous studied delivered the HSV-TK gene that sensitized the tumor cells to systemic delivery of ganciclovir. Second, in our study infection of the tumor cells, the retina or other ocular tissues is sufficient to deliver IFN-β to the vitreous and reduce tumor burden. In the previous study, only the infected tumor cells were sensitized to ganciclovir. Third, we used an AAV vector delivery method and the previous study delivered HSV-TK using adenovirus. One advantage of AAV is that it is being widely used for gene therapy based approaches to treat a variety of retinopathies. This delivery method is well-characterized and there have been no reports of retinal toxicity associated with long term viral infection. Our study is the first report of retinoblastoma sensitivity to cytokines and the first report of AAV-mediated gene therapy for retinoblastoma. Based on these studies, we propose that AAV IFN-β may complement existing approaches for the treatment of retinoblastoma. We do not anticipate that it would be used as monotherapy but may be combined with local delivery of chemotherapy by subconjunctival injection as well as focal therapies such as laser and cryotherapy.
It is also important to note that we used the cell line (Y79-Luc, see Fig. 1) that was the least sensitive to IFN-β in our xenograft cell experiments. We felt this was appropriate because gene therapy to treat retinoblastoma would provide the greatest potential benefit for patients with tumors that were refractory to other treatments and Y79 cells are the most aggressive cell line we have available.
Cytokines such as IFN-β are useful in cancer therapy because they can directly affect tumor cell viability and growth. In addition, cytokines can modulate the immune response to tumors providing a complementary anti-tumor mechanism of action. Unfortunately, systemic administration of recombinant cytokines can lead to toxicity and side effects, which include flu-like symptoms, fever, chills, muscle aches, weakness, diarrhea, and depression 51. Short half-life of cytokines such as IFN-β pose an additional challenge for their application as anti-cancer agents 37. We addressed both of these challenges by delivering IFN-β to the eye with an AAV vector. We found that AAV-mediated delivery of IFN-β led to vitreal levels of IFN-β sufficient to mediate an anti-tumor effect even using our least sensitive cell line (Y79 cells). In addition, there was not significant spread of the virus to other organs such as the liver and the plasma levels dropped to undetectable levels by 6 weeks of age even though the levels in the vitreous were maintained.
It is interesting that there was IFN-β in the plasma a few days after the injection of the virus but these levels tapered off by 6 weeks of age. It is possible that the retinal vasculature was transiently compromised as a result of the injection. It is also possible that intraocular pressure was changed transiently as a result of the injection and this led to higher IFN-β penetration into the circulation. The highest levels detected in the plasma were not sufficient to mediate the side effects typically associated with IFN-β so we believe this transient systemic exposure would not lead to significant side effects in humans. Our studies did not address this directly because we used human IFN-β against human retinoblastoma cells in a rodent host. The rodent IFN-β receptor does not bind the human IFN-β so any side effects that may result from systemic exposure to IFN-β were masked in our study. In addition, any immunomodulatory effects were also masked because our experiments only addressed direct cytotoxicity of IFN-β against retinoblastoma cells. Therefore, it is possible that the combination of direct cytotoxicity and immunomodulatory effects may lead to an even greater reduction in tumor burdern following infection with AAV-IFN-β.
This study sets the framework for a series of future studies in preclinical models prior to Phase I clinical trials. Future studies will focus on elucidating these immunomodulatory effects of IFN-β by using rodent IFN-β in a rodent host. These experiments will also address the systemic toxicity associated with the low levels of IFN-β in the plasma shortly after the viral injection. Long-term toxicity in the retina and surrounding ocular tissues must also need to be addressed prior to clinical trials. Finally, the best combination of chemotherapy to add to the AAV-IFN-β gene therapy should be analyzed along with the best method of drug delivery (systemic versus subconjunctival).
Acknowledgments
We thank Dr. John Gray and the staff of the Vector Core Facility at St Jude Children’s Research Hospital for their assistance with pseudotyped AAV. This work was supported by the Alliance for Cancer Gene Therapy (AMD), grant number T32-CA070089 from the National Cancer Institute, grants (to M.A.D.) from the National Institutes of Health (EY04867), Cancer Center Support from the National Cancer Institute (CA21766), the American Cancer Society (RSG-06-03-01), Research to Prevent Blindness, and by the American Lebanese Syrian Associated Charities. M.A.D. is a Pew Scholar.
References
- 1.Ries LAG, Smith MA, Gurney JG, et al. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Bethesda, MD: 1999. NIH Pub. No. 99–4649; [Google Scholar]
- 2.Naumova A, Sapienza C. The genetics of retinoblastoma, revisited. Am J Hum Genet. 1994 Feb;54(2):264–273. [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Galindo C, Wilson MW, Haik BG, et al. Treatment of metastatic retinoblastoma. Ophthalmology. 2003 Jun;110(6):1237–1240. doi: 10.1016/S0161-6420(03)00258-6. [DOI] [PubMed] [Google Scholar]
- 4.Hayden BH, Murray TG, Scott IU, et al. Subconjunctival carboplatin in retinoblastoma: impact of tumor burden and dose schedule. Arch Ophthalmol. 2000 Nov;118(11):1549–1554. doi: 10.1001/archopht.118.11.1549. [DOI] [PubMed] [Google Scholar]
- 5.Murray TG, Cicciarelli N, O’Brien JM, et al. Subconjunctival carboplatin therapy and cryotherapy in the treatment of transgenic murine retinoblastoma. Arch Ophthalmol. 1997 Oct;115(10):1286–1290. doi: 10.1001/archopht.1997.01100160456013. [DOI] [PubMed] [Google Scholar]
- 6.Van Quill KR, Dioguardi PK, Tong CT, et al. Subconjunctival carboplatin in fibrin sealant in the treatment of transgenic murine retinoblastoma. Ophthalmology. 2005 Jun;112(6):1151–1158. doi: 10.1016/j.ophtha.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz MY, Marcus KT, Chevez-Barrios P, Louie K, Aguilar-Cordova E, Hurwitz RL. Suicide gene therapy for treatment of retinoblastoma in a murine model. Hum Gene Ther. 1999 Feb 10;10(3):441–448. doi: 10.1089/10430349950018887. [DOI] [PubMed] [Google Scholar]
- 8.Chevez-Barrios P, Chintagumpala M, Mieler W, et al. Response of retinoblastoma with vitreous tumor seeding to adenovirus-mediated delivery of thymidine kinase followed by ganciclovir. J Clin Oncol. 2005 Nov 1;23(31):7927–7935. doi: 10.1200/JCO.2004.00.1883. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Bowles DE, van Dyke T, Samulski RJ. Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther. 2005 Dec;12(12):913–925. doi: 10.1038/sj.cgt.7700876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinculescu A, Glushakova L, Min SH, Hauswirth WW. Adeno-associated virus-vectored gene therapy for retinal disease. Hum Gene Ther. 2005 Jun;16(6):649–663. doi: 10.1089/hum.2005.16.649. [DOI] [PubMed] [Google Scholar]
- 11.Rolling F. Recombinant AAV-mediated gene transfer to the retina: gene therapy perspectives. Gene Ther. 2004 Oct;11( Suppl 1):S26–32. doi: 10.1038/sj.gt.3302366. [DOI] [PubMed] [Google Scholar]
- 12.Ali RR, Reichel MB, De Alwis M, et al. Adeno-associated virus gene transfer to mouse retina. Hum Gene Ther. 1998 Jan 1;9(1):81–86. doi: 10.1089/hum.1998.9.1-81. [DOI] [PubMed] [Google Scholar]
- 13.Ali RR, Reichel MB, Thrasher AJ, et al. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum Mol Genet. 1996 May;5(5):591–594. doi: 10.1093/hmg/5.5.591. [DOI] [PubMed] [Google Scholar]
- 14.Bennett J, Maguire AM, Cideciyan AV, et al. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc Natl Acad Sci U S A. 1999 Aug 17;96(17):9920–9925. doi: 10.1073/pnas.96.17.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudus L, Anand V, Acland GM, et al. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision Res. 1999 Jul;39(15):2545–2553. doi: 10.1016/s0042-6989(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 16.Folliot S, Briot D, Conrath H, et al. Sustained tetracycline-regulated transgene expression in vivo in rat retinal ganglion cells using a single type 2 adeno-associated viral vector. J Gene Med. 2003 Jun;5(6):493–501. doi: 10.1002/jgm.367. [DOI] [PubMed] [Google Scholar]
- 17.Guy J, Qi X, Muzyczka N, Hauswirth WW. Reporter expression persists 1 year after adeno-associated virus-mediated gene transfer to the optic nerve. Arch Ophthalmol. 1999 Jul;117(7):929–937. doi: 10.1001/archopht.117.7.929. [DOI] [PubMed] [Google Scholar]
- 18.Liang FQ, Aleman TS, Dejneka NS, et al. Long-term protection of retinal structure but not function using RAAV. CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001 Nov;4(5):461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- 19.Campochiaro PA. Gene therapy for retinal and choroidal diseases. Expert Opin Biol Ther. 2002 Jun;2(5):537–544. doi: 10.1517/14712598.2.5.537. [DOI] [PubMed] [Google Scholar]
- 20.Dejneka NS, Rex TS, Bennett J. Gene therapy and animal models for retinal disease. Dev Ophthalmol. 2003;37:188–198. doi: 10.1159/000072047. [DOI] [PubMed] [Google Scholar]
- 21.Surace EM, Auricchio A. Adeno-associated viral vectors for retinal gene transfer. Prog Retin Eye Res. 2003 Nov;22(6):705–719. doi: 10.1016/s1350-9462(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 22.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fish EN, Uddin S, Korkmaz M, Majchrzak B, Druker BJ, Platanias LC. Activation of a CrkL-stat5 signaling complex by type I interferons. J Biol Chem. 1999 Jan 8;274(2):571–573. doi: 10.1074/jbc.274.2.571. [DOI] [PubMed] [Google Scholar]
- 24.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004 Jan;4(1):11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer LM, Dinarello CA, Herberman RB, et al. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998 Jun 15;58(12):2489–2499. [PubMed] [Google Scholar]
- 26.Sondak VK. How does interferon work? Does it even matter? . Cancer. 2002 Sep 1;95(5):947–949. doi: 10.1002/cncr.10779. [DOI] [PubMed] [Google Scholar]
- 27.Einhorn S, Strander H. Interferon treatment of human malignancies--a short review. Med Oncol Tumor Pharmacother. 1993;10(1–2):25–29. doi: 10.1007/BF02987765. [DOI] [PubMed] [Google Scholar]
- 28.Kirkwood J. Cancer immunotherapy: the interferon-alpha experience. Semin Oncol. 2002 Jun;29(3 Suppl 7):18–26. doi: 10.1053/sonc.2002.33078. [DOI] [PubMed] [Google Scholar]
- 29.Gibson AD. Updated meta analysis finds that interferon-alpha improves progression-free and overall survival in low-grade non-Hodgkin’s lymphoma when administered with chemotherapy that contains anthracycline or mitoxantrone. Clin Lymphoma. 2002 Sep;3(2):82–84. [PubMed] [Google Scholar]
- 30.Ludwig H. Supportive therapies in the management of myeloma. Oncology (Williston Park) 2004 Mar;18(3):295–296. 298. [PubMed] [Google Scholar]
- 31.Rohatiner AZ, Gregory WM, Peterson B, et al. Meta-analysis to evaluate the role of interferon in follicular lymphoma. J Clin Oncol. 2005 Apr 1;23(10):2215–2223. doi: 10.1200/JCO.2005.06.146. [DOI] [PubMed] [Google Scholar]
- 32.Voutsadakis IA. Interferon-alpha and the pathogenesis of myeloproliferative disorders. Med Oncol. 2000 Nov;17(4):249–257. doi: 10.1007/BF02782189. [DOI] [PubMed] [Google Scholar]
- 33.Kirkwood JM, Tarhini AA. Adjuvant high-dose interferon-alpha therapy for high-risk melanoma. Forum (Genova) 2003;13(2):127–140. quiz 187–128. [PubMed] [Google Scholar]
- 34.Coppin C, Porzsolt F, Awa A, Kumpf J, Coldman A, Wilt T. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev. 2005;(1):CD001425. doi: 10.1002/14651858.CD001425.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Einhorn S, Grander D. Why do so many cancer patients fail to respond to interferon therapy? . J Interferon Cytokine Res. 1996 Apr;16(4):275–281. doi: 10.1089/jir.1996.16.275. [DOI] [PubMed] [Google Scholar]
- 36.Choi EA, Lei H, Maron DJ, et al. Combined 5-fluorouracil/systemic interferon-beta gene therapy results in long-term survival in mice with established colorectal liver metastases. Clin Cancer Res. 2004 Feb 15;10(4):1535–1544. doi: 10.1158/1078-0432.ccr-0040-03. [DOI] [PubMed] [Google Scholar]
- 37.Hendren SK, Prabakaran I, Buerk DG, et al. Interferon-beta gene therapy improves survival in an immunocompetent mouse model of carcinomatosis. Surgery. 2004 Apr;135(4):427–436. doi: 10.1016/j.surg.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Lu W, Fidler IJ, Dong Z. Eradication of primary murine fibrosarcomas and induction of systemic immunity by adenovirus-mediated interferon beta gene therapy. Cancer Res. 1999 Oct 15;59(20):5202–5208. [PubMed] [Google Scholar]
- 39.Qin XQ, Tao N, Dergay A, et al. Interferon-beta gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14411–14416. doi: 10.1073/pnas.95.24.14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida J, Mizuno M, Wakabayashi T. Interferon-beta gene therapy for cancer: basic research to clinical application. Cancer Sci. 2004 Nov;95(11):858–865. doi: 10.1111/j.1349-7006.2004.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McFall RC, Sery TW, Makadon M. Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res. 1977 Apr;37(4):1003–1010. [PubMed] [Google Scholar]
- 42.Dickson PV, Hamner B, Ng CYC, Hall MM, Zhou J, Hargrove PV, McCarville ME, Davidoff AM. In vivo bioluminescence imaging for early detection and monitoring of disease progression in a murine model of neuroblastoma. J Pediatr Surg. 2007 doi: 10.1016/j.jpedsurg.2007.02.027. In press. [DOI] [PubMed] [Google Scholar]
- 43.Dyer MA, Cepko CL. p57(Kip2) regulates progenitor cell proliferation and amacrine interneuron development in the mouse retina. Development. 2000 Aug;127(16):3593–3605. doi: 10.1242/dev.127.16.3593. [DOI] [PubMed] [Google Scholar]
- 44.Dyer MA, Cepko CL. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J Neurosci. 2001 Jun 15;21(12):4259–4271. doi: 10.1523/JNEUROSCI.21-12-04259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu CC, Dyer MA, Uchikawa M, Kondoh H, Lagutin OV, Oliver G. Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development. 2002 Jun;129(12):2835–2849. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]
- 46.Davidoff AM, Gray JT, Ng CY, et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol Ther. 2005 Jun;11(6):875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 47.Nathwani AC, Davidoff A, Hanawa H, Zhou JF, Vanin EF, Nienhuis AW. Factors influencing in vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001 Mar 1;97(5):1258–1265. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- 48.Greil J, Gramatzki M, Burger R, et al. The acute lymphoblastic leukaemia cell line SEM with t(4;11) chromosomal rearrangement is biphenotypic and responsive to interleukin-7. Br J Haematol. 1994 Feb;86(2):275–283. doi: 10.1111/j.1365-2141.1994.tb04726.x. [DOI] [PubMed] [Google Scholar]
- 49.Chevez-Barrios P, Hurwitz MY, Louie K, et al. Metastatic and nonmetastatic models of retinoblastoma. Am J Pathol. 2000 Oct;157(4):1405–1412. doi: 10.1016/S0002-9440(10)64653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laurie NA, Gray JK, Zhang J, et al. Topotecan combination chemotherapy in two new rodent models of retinoblastoma. Clin Cancer Res. 2005 Oct 15;11(20):7569–7578. doi: 10.1158/1078-0432.CCR-05-0849. [DOI] [PubMed] [Google Scholar]
- 51.Wadhwa PD, Zielske SP, Roth JC, Ballas CB, Bowman JE, Gerson SL. Cancer gene therapy: scientific basis. Annu Rev Med. 2002;53:437–452. doi: 10.1146/annurev.med.53.082901.104039. [DOI] [PubMed] [Google Scholar]