Abstract
By employing an intramolecular Pd(0)-mediated ring opening of an acylnitroso-derived cycloadduct, new hydroxamic acid containing benzodiazepines have been synthesized and have demonstrated biological activity in MCF-7 and PC-3 tumor cell lines. Subsequent N–O bond reduction of the hydroxamate has provided access to amide analogs for SAR studies. During the course of our syntheses, an intermediate oxazoline-N-oxide was isolated and gave insight into the mechanism of the key Pd(0)-mediated reaction.
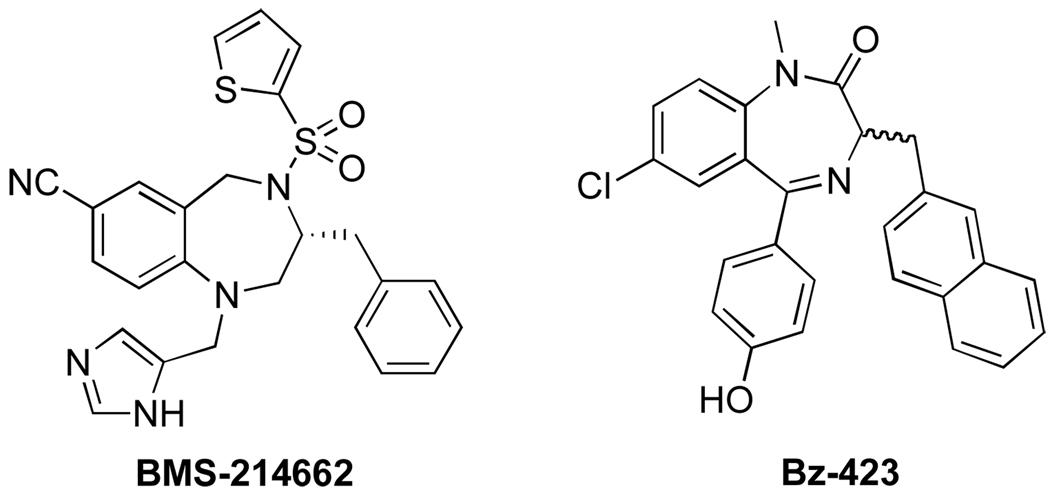
1,4-Benzodiazepines are important biomolecules with a wide array of biological activities and therapeutic functions. Benzodiazepines are primarily known for their actions in the central nervous system. In addition to their established anxiolytic activites, 1,4-benzodiazepines also demonstrate activities as antibiotics,1 anti-malarial,2 and anti-HIV3 agents. Additionally, there have been several reports of benzodiazepines as anti-cancer agents,4 including BMS-214662,4a a known farnesyltransferase (FTase) inhibitor, and Bz-423,4b which has antiproliferative effects through the regulation of the c-myc protein (Figure 1).
Figure 1.
Benzodiazepines with anti-cancer activity.
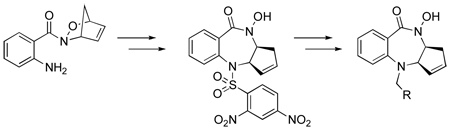
Herein we report the synthesis of 1,4-benzodiazepines via a Pd(0)-mediated ring opening of cycloadduct 3a and subsequent elaboration and evaluation of their anti-cancer activity in MCF-7 and PC-3 tumor cell lines. In contrast to the benzodiazepines disclosed in the literature, the benzodiazepines synthesized from cycloadduct 3a contain a hydroxamate embedded within the core structure. This presents the possibility of direct metal binding with the benzodiazepine ring system and makes these compounds potential inhibitors of metalloenzymes such as the aforementioned farnesyltransferase protein.5 Whereas BMS-214662 contains an imidazole group that coordinates to zinc (II) in the FTase active site,6 our benzodiazepine derivatives may potentially bind to the active site of FTase through a monoanionic bidentate chelation of the hydroxamate to the zinc (II) ion.
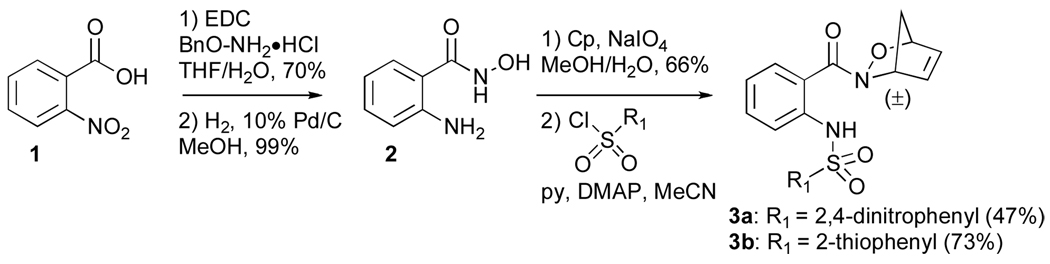
Acylnitroso-derived hetero-Diels–Alder adducts, generated from the reaction of transient acylnitroso species with cyclopentadiene, are synthetically important precursors to a variety of bioactive molecules.7 Pd(0)-mediated ring openings of acylnitroso-derived cycloadducts have been employed to provide syn-1,4-disubstituted cyclopentene derivatives.8 Construction of the appropriately functionalized cycloadducts 3a and 3b began with commercially available 2-nitrobenzoic acid 1 (Scheme 1), which was coupled with O-benzyl hydroxylamine and subsequently deprotected and reduced under hydrogenolysis conditions to yield hydroxamic acid 2. In situ oxidation of hydroxamic acid 2 in the presence of cyclopentadiene afforded the anthranilic acid based cycloadduct, which was reacted with the appropriate sulfonyl chloride to give cycloadducts 3a and 3b in 47% and 73% yield, respectively.
Scheme 1.
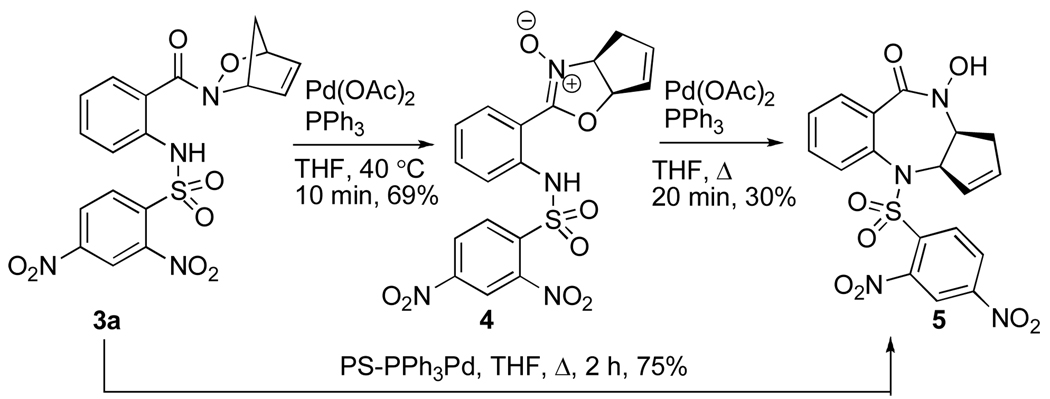
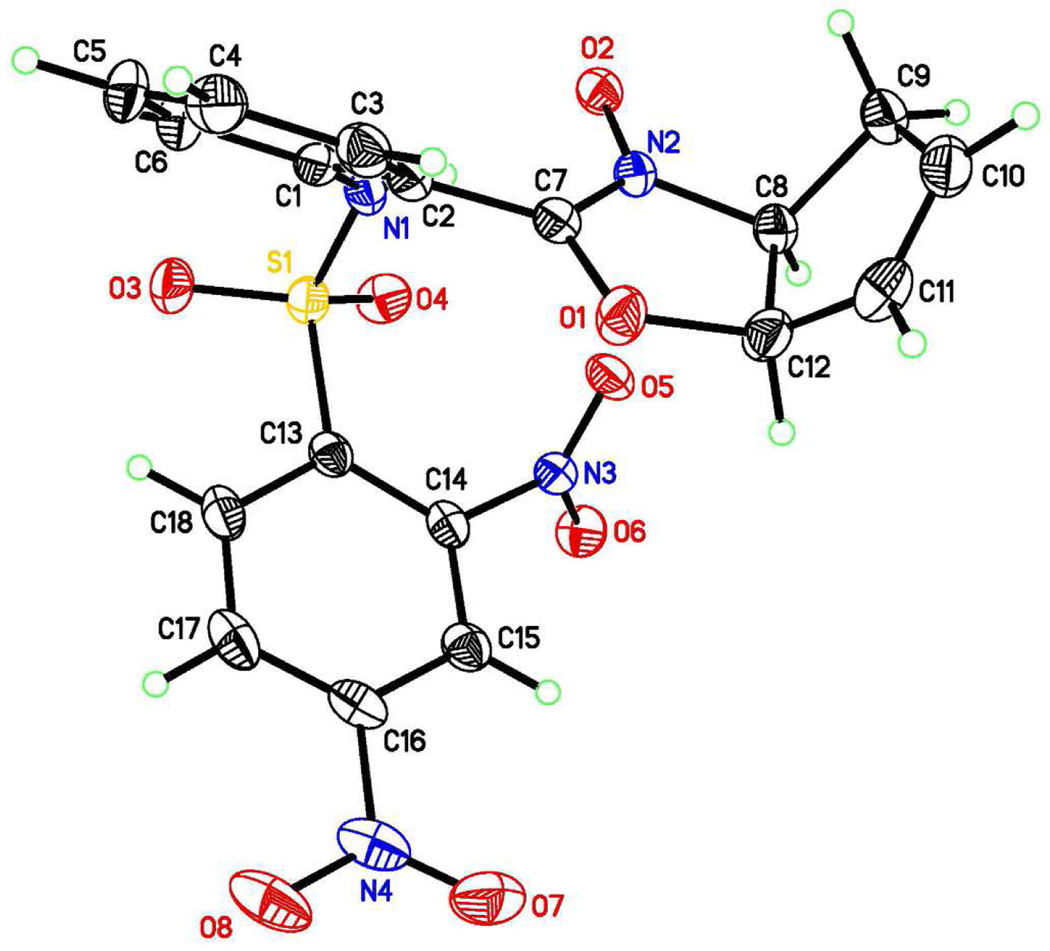
Treatment of cycloadduct 3a with Pd(OAc)2 and PPh3 at 40 °C for 10 min generated nitrone 4 (Scheme 2). The structure was confirmed by X-ray crystallographic analysis (Figure 2). To our knowledge, this is the first reported example of the formation of an oxazoline N-oxide from an acylnitroso-derived hetero-Diels–Alder adduct. Oxazoline N-oxides are versatile synthons and have been used in [3+2] cycloadditions.9 After isolation and purification, resubjection of nitrone 4 to Pd(OAc)2 and PPh3 in refluxing THF gave benzodiazepine 5 in 30% yield.10
Scheme 2.
Figure 2.
X-ray structure of 4 with thermal ellipsoids drawn at 50% probability.
Treatment of cycloadduct 3a with 3.5 mol% polymer-bound triphenylphosphine-Pd(0) in refluxing THF for 2 h facilitated direct conversion to benzodiazepine 5 in an optimized 75% yield.11 Several palladium reagents were explored and the polymer-bound triphenylphosphine-Pd(0) emerged as the most practical due to the ease of product isolation12 and increased yield. Direct transformation of adduct 3a to benzodiazepine 5 was ideal for further elaboration to biologically relevant molecules.
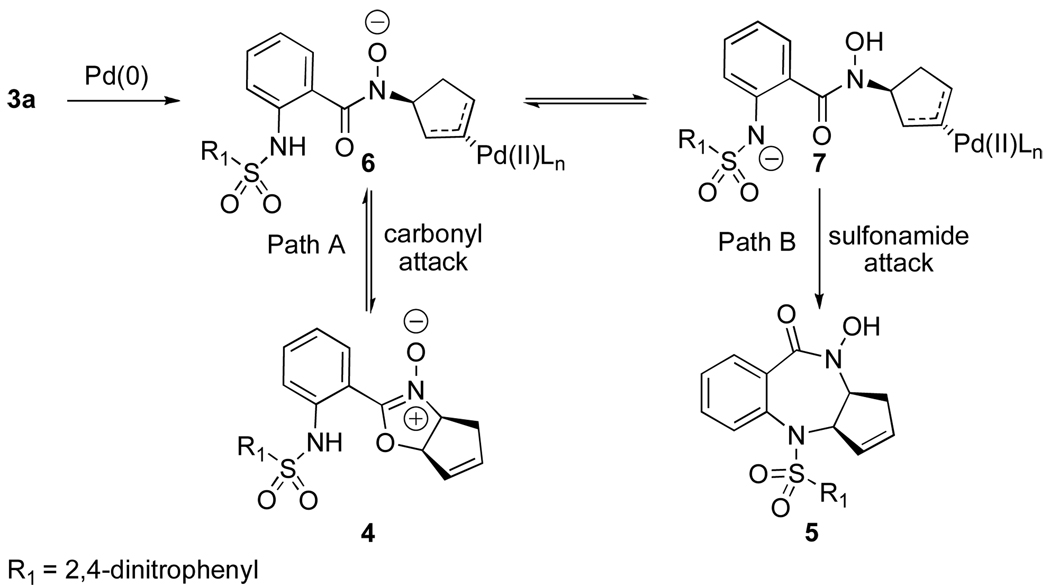
A plausible mechanism for the formation of benzodiazepine 5 is proposed in Scheme 3. The hydroxamate carbonyl oxygen may act as a nucleophile and allow for reversible attack on π-allyl complex 6 to form nitrone 4. Cyclization to the [5, 5] system (Path A) is faster than formation of the [7, 5] system. Prolonged reaction times and/or heat would allow Pd(0) complexation with 4, which would reintroduce the intermediate π-allyl complex 6. Intramolecular proton transfer to complex 7 followed by irreversible nucleophilic attack (Path B) of the sulfonamide nitrogen provides the thermodynamically preferred benzodiazepine 5. Further evidence that this pathway could be operative was found when the dinitrobenzenesulfonamide group was replaced with a 2-thiophene sulfonamide.
Scheme 3.
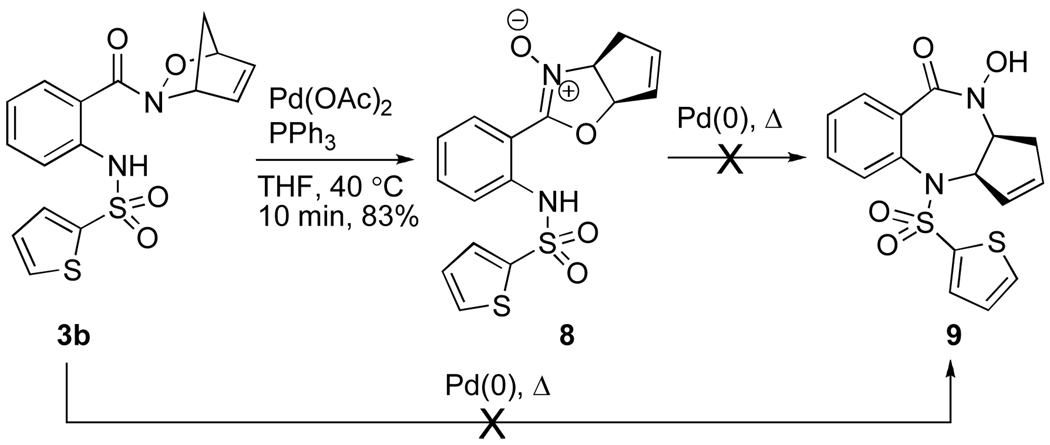
Treatment of cycloadduct 3b with Pd(OAc)2 and PPh3 at 40 °C for 10 min resulted in formation of nitrone 8 in 83% yield (Scheme 4). Prolonged heating of 3b in the presence of various palladium reagents (Pd(OAc)2, Pd(dba)2, Pd2(dba)3•CHCl3, PS-PPh3Pd), phosphine ligands, solvents and bases resulted in either no reaction or decomposition of the reaction mixture. Isolation and purification of nitrone 8 followed by reexposure to Pd(0) was also unsuccessful. Since the pKa of the sulfonamide NH is significantly higher in cycloadduct 3b (compared to 3a), the essential proton transfer was expected to be less facile and the benzodiazepine would be unable to form. Still, formation of 9 was of particular interest considering its structural similarity to the known anti-cancer compound BMS-214662.4a
Scheme 4.
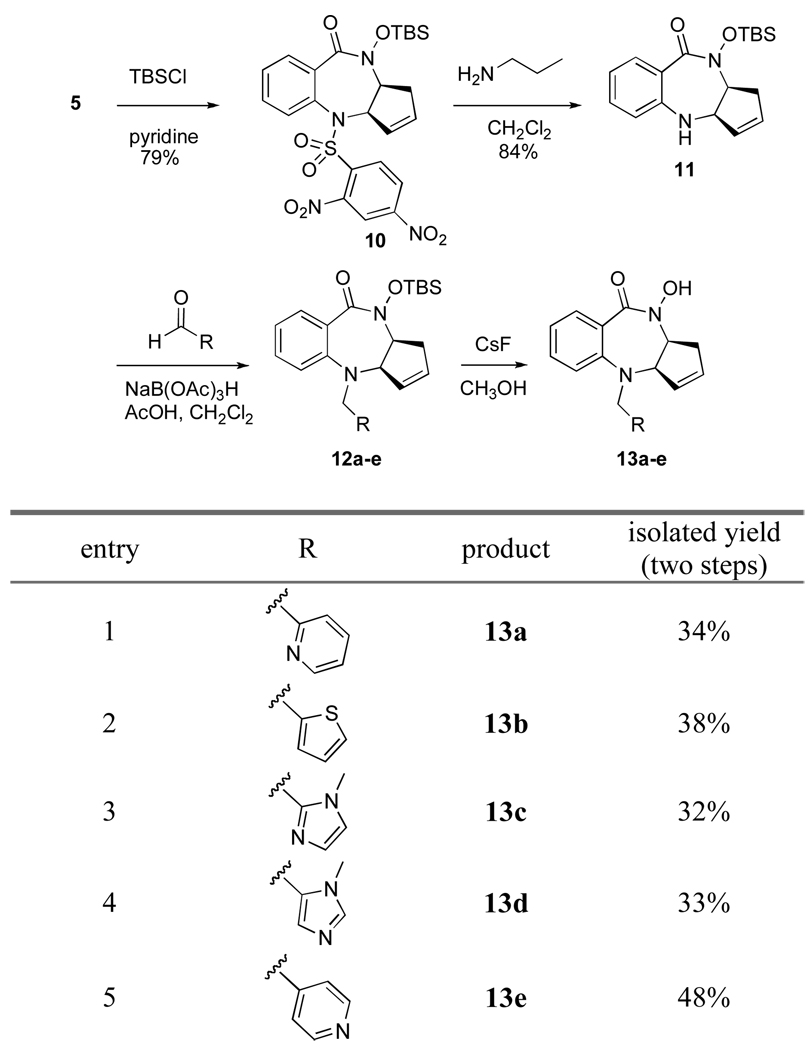
Unable to convert cycloadduct 3b directly to the benzodiazepine core, we turned our attention to an alternative multi-step route towards functionalized benzodiazepines. Thus, benzodiazepine 5 was treated with TBSCl in pyridine to give protected hydroxamate 10 in good yield (Scheme 5). Upon protection of the hydroxamate, attention was turned to functionalization of the 4-amino position. Treatment of benzodiazepine 10 with excess n-propylamine at rt allowed for the mild deprotection of the dinitrobenzenesulfonamide to afford core 11.13 The resulting deep yellow dinitro-N-propylaniline byproduct was easily separated from 11 by column chromatography. Alternatively, mercaptoacetic acid was evaluated for the deprotection of the dinitrobenzenesulfonamide, but gave inconsistent results.14
Scheme 5.
To test the reactivity of the relatively hindered and electron deficient 4-amino position, benzodiazepine 11 was first treated with several different sulfonyl chlorides. Reaction with 2-thiophenesulfonyl chloride was first explored in an effort to prepare compound 9. Unfortunately, compound 11 was completely unreactive with several sulfonyl chlorides, including 2-thiophenesulfonyl chloride, dinitrobenzene sulfonyl chloride and tosyl chloride.15 In order to derivatize 11, we focused on reductive animations, as aldehydes are unhindered and strongly electrophilic.
Benzodiazepine 11 was treated with various aldehydes in the presence of acetic acid and molecular sieves to form the corresponding iminium species, which were subsequently reduced with sodium triacetoxyborohydride to give protected benzodiazepines 12a–e. Removal of the TBS group with CsF in MeOH and purification using iron-free silica gel16 provided hydroxamate containing benzodiazepines 13a–e in 32–48% yield over two steps (Scheme 5).
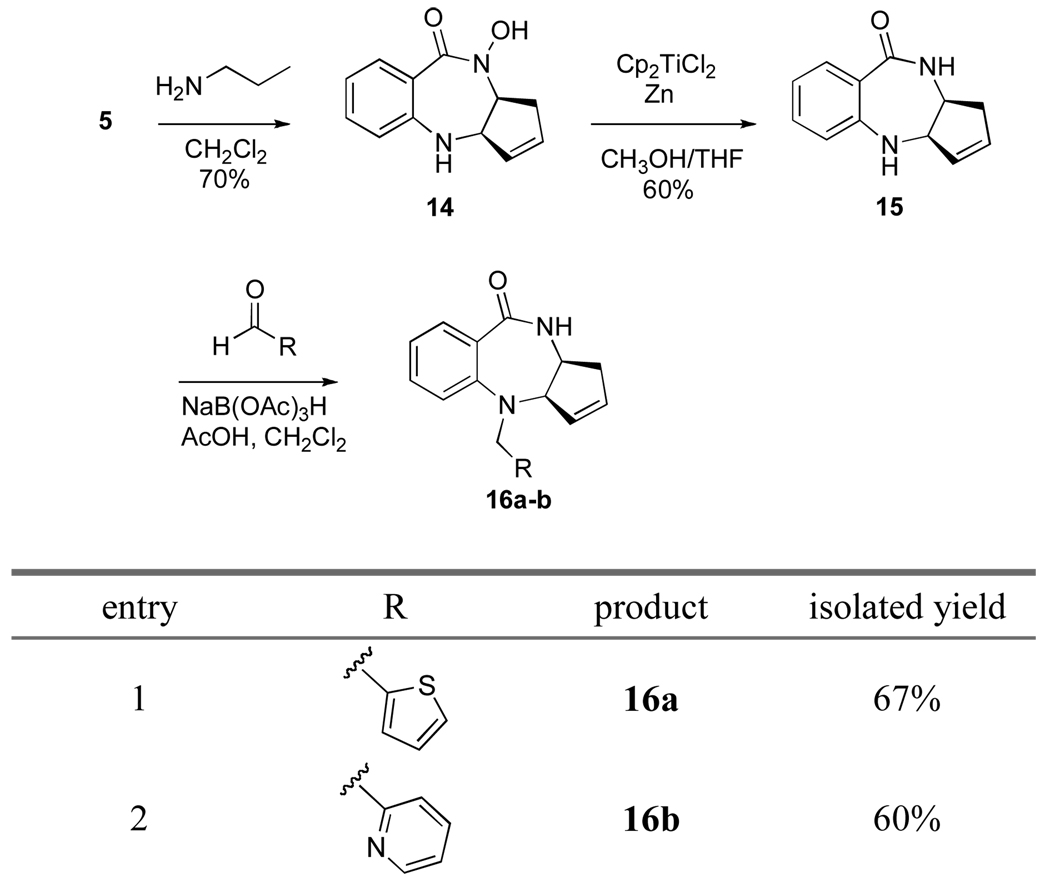
An important functionality present in benzodiazepines 13a–e is the hydroxamic acid. This group was anticipated to play a role in the biological activity of these compounds. Therefore, it was important to synthesize several analogs that do not contain the hydroxamate. Benzodiazepine 5 was deprotected with n-propylamine in 70% yield (Scheme 6). Benzodiazepine 14 was then subjected to stoichiometric titanocene chloride reduction conditions,17 which produced amide 15 in 60% yield. Benzodiazepine 15 proved more amenable to the same reductive amination conditions (NaBH(OAc)3/AcOH) and analogs 16a–b were synthesized in improved yield.
Scheme 6.
Several benzodiazepines demonstrated growth-inhibitory activity in MCF-7 (breast cancer) and PC-3 (prostate cancer) tumor cell assays (Table 1). Compounds 5, 13a, and 13b reached the low micromolar range of inhibition against MCF-7 cells. In general, all compounds showed significantly improved inhibitory activity against the MCF-7 cell line. The substituent at the aniline nitrogen of the benzodiazepine seems to plays a rather important role in determining activity. Based on the results of compounds 16a–b, the hydroxamate has also been demonstrated to be a necessary functionality for activity in this class of benzodiazepines.
Table 1.
Results of Anti-Cancer Screenings.a
| % inhibition at 20 µM | IC50 (µM) | |||
|---|---|---|---|---|
| compound | PC-3 | MCF-7 | PC-3 | MCF-7 |
| 5 | 100 | 96 | 4 | 3 |
| 13a | 32 | 92 | – | 8 |
| 13b | 31 | 90 | – | 1.8 |
| 13c | <10 | 15 | – | – |
| 13d | 16 | 22 | – | – |
| 13e | <10 | 39 | – | – |
| 16a | 11 | 29 | – | – |
| 16b | <10 | 13 | – | – |
Trichostatin A was used as the positive control (MCF-7 IC50 = 16 nM, PC-3 IC50 = 160 nM)
In summary, we have reported a new class of benzodiazepines that have shown encouraging biological activity in MCF-7 cell lines. Benzodiazepine 13b has shown low micromolar activity and is a suitable lead for further SAR studies. The exact mechanism of action for this new class of 1,4-benzodiazepines is currently unknown. Enzyme assays and other means to determine mechanism of action are under investigation.
Supplementary Material
General methods, experimental details, and 1H and 13C NMR spectra for 3b, 4, 8, 10, 11, 13a-e, 14, 15, and 16a-b. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We gratefully acknowledge Mrs. Patty Miller (University of Notre Dame) for performing MCF-7 and PC-3 cellular assays and Dr. Bruce Noll (Univeristy of Notre Dame) and Dr. Allen Oliver (Univeristy of Notre Dame) for X-ray crystal determination and data manipulation. We also thank Nonka Sevova (Univeristy of Notre Dame) for mass spectroscopic analyses, Dr. Jaroslav Zajicek (Univeristy of Notre Dame) for NMR assistance and Dr. Jed Fisher (Univeristy of Notre Dame) for helpful discussions. We acknowledge the University of Notre Dame and NIH (GM068012 and GM075855) for support of this work.
References
- 1.Thuston DE, Bose DS. Chem. Rev. 1994;94:433. [Google Scholar]
- 2.Nallan L, Bauer KD, Bendale P, Rivas K, Yokoyama K, Hornéy CP, Pendyala PR, Floyd D, Lombardo LJ, Williams DK, Hamilton A, Sebti S, Windsor WT, Weber PC, Buckner FS, Chakrabarti D, Gelb MH, Van Voorhis WC. J. Med. Chem. 2005;48:3704. doi: 10.1021/jm0491039. [DOI] [PubMed] [Google Scholar]
- 3.Kukla MJ, Breslin HJ, Diamond CJ, Grous PP, Ho CY, Miranda M, Rodgers JD, Sherrill RG, De Clercq E, Pauwels R, Andries K, Moens LJ, Janssen MAC, Janssen PAJ. J. Med. Chem. 1991;34:3187. doi: 10.1021/jm00115a007. [DOI] [PubMed] [Google Scholar]
- 4.(a) Hunt JT, Ding CZ, Batorsky R, Bednarz M, Bhide R, Cho Y, Chong S, Chao S, Gullo-Brown J, Guo P, Kim SH, Lee FYF, Leftheris K, Miller A, Mitt T, Patel M, Penhallow BA, Ricca C, Rose WC, Schmidt R, Slusarchyk WA, Vite G, Manne V. J. Med. Chem. 2000;43:3587. doi: 10.1021/jm000248z. [DOI] [PubMed] [Google Scholar]; (b) Sundberg TB, Ney GM, Subramanian C, Opipari AW, Glick GD. Cancer Res. 2006;66:1775. doi: 10.1158/0008-5472.CAN-05-3476. [DOI] [PubMed] [Google Scholar]; (c) Dourlat J, Liu W, Greash N, Garbay C. Bioorg. Med. Chem. Lett. 2007;17:2527. doi: 10.1016/j.bmcl.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Bell IM. J. Med. Chem. 2004;47:1869. doi: 10.1021/jm0305467. [DOI] [PubMed] [Google Scholar]
- 6.Reid TS, Beese LS. Biochemistry. 2004;43:6877. doi: 10.1021/bi049723b. [DOI] [PubMed] [Google Scholar]
- 7.(a) Jiang MXW, Warshakoon NC, Miller MJ. J. Org Chem. 2005;70:2824. doi: 10.1021/jo0484070. [DOI] [PubMed] [Google Scholar]; (b) Li F, Brogan JB, Gage JL, Zhang D, Miller MJ. J. Org. Chem. 2004;69:4538. doi: 10.1021/jo0496796. [DOI] [PubMed] [Google Scholar]
- 8.Mulvihill MJ, Surman MD, Miller MJ. J. Org. Chem. 1998;63:4874. doi: 10.1021/jo016275u. [DOI] [PubMed] [Google Scholar]
- 9.Dirat O, Kouklovsky C, Langlois Y. Org. Lett. 1999;1:753. doi: 10.1021/ol990734k. [DOI] [PubMed] [Google Scholar]
- 10.Low yield due to difficulty with purification. Yield would be expected to be higher using polymer bound-Pd(0).
- 11.Unoptimized yield with Pd(OAc)2/PPh3/THF/Δ was 56%. See Surman MD, Mulvihill MJ, Miller MJ. Org. Lett. 2002;4:139. doi: 10.1021/ol017036w.
- 12.Simply filtering the resin and concentrating the filtrate afforded a yellow solid, which upon trituration with CH2Cl2 gave product 5 as a pure white powder.
- 13.Fukuyama T, Cheung M, Jow C, Hidai U, Kant T. Tetrahedron Lett. 1997;38:5831. [Google Scholar]
- 14.Decomposition of mercaptoacetic acid due to oxidation and occasional deprotection of TBS group proved problematic.
- 15.Several bases, solvents and temperatures were exhaustively explored.
- 16.Procedure for the preparation of iron free silica gel may be found in the Supporting Information.
- 17.Cesario C, Tardibono L, Miller MJ. J. Org. Chem. 2009;74:448. doi: 10.1021/jo802184y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General methods, experimental details, and 1H and 13C NMR spectra for 3b, 4, 8, 10, 11, 13a-e, 14, 15, and 16a-b. This material is available free of charge via the Internet at http://pubs.acs.org.