Abstract
Adverse reactions to radiation therapy represent a confounding phenomenon in Radiation Oncology. These reactions are rare, and many have been associated with individuals with DNA repair disorders such as Ataxia-telangiectasia (A-T) and Nijmegen Breakage Syndrome (NBS). There is a paucity of literature detailing such circumstances. This overview describes four exemplary situations, a comprehensive list of 32 additional cases, and some insights gleaned from this overall experience. Fanconi Anemia was associated with over half of the reports. The lowest dose given to a patient that resulted in a reaction was 3 Gy, given to an A-T patient. Most patients died within months of exposure. It is clear that the patients discussed in this paper had complicated illnesses in addition to cancer, and the radiation therapy that administered was most likely their best option. However, the underlying DNA repair defects make conventional radiation therapy doses dangerous. Our review supports prior wisdom that radiation therapy should either be avoided, or doses should be selected with great care in the case of these radiosensitive genotypes which must be recognized with their characteristic phenotypes, until more rapid, reliable and functional assays of DNA repair become available.
Keywords: Ataxia-telangiectasia, radiation sensitivity, radiation therapy, DNA repair disorders, adverse reactions
Introduction
X-rays have been a source of therapy as well as untoward biologic effects for over 100 years. Wilhelm Conrad Roentgen, a German physicist, first discovered X-rays in1895 (1). That same year Roentgen used Rudolf Albert van Kolliker, a well-known Swiss anatomy professor, as a volunteer to show the potential medical usefulness of radiation by making a radiograph of van Kolliker’s hand. Just one year later, surgeons used X-rays to locate and remove a fragment of a knife blade from the backbone of a sailor; this cured his paralysis (2). Within a year, another German surgeon, Wilhelm Alexander Freud, cured a hairy mole with radiation and presented his evidence before the Vienna Medical Society (1,3).
Shortly thereafter, radioactivity was discovered by Antoine Henri Becquerel and during the same year (1898), Pierre and Marie Curie discovered radium and polonium. Unwittingly, Becquerel became the first documented case of an adverse reaction, when he developed skin erythema and ulceration from a radium container that he accidentally left in his vest pocket. Not to be outdone, Pierre Curie intentionally used radium to create an ulcer on his forearm and noted how long it took to heal (3).
Reports of more harmful effects of radiation than skin erythema and ulcers soon followed. In 1911, Jagic reported leukemia in radiation workers (4). By the late twenties, it became apparent that radiation protection guidelines were needed and an International Committee on X-ray and Radium Protection was formed (3). By design, the new radiation protection regulations did not curtail the medical applications of X-rays as a diagnostic tool or as a means for therapy (i.e. for killing tumor cells). By 1928, Coutard had already suggested the practice of fractionated radiation treatments to reduce the adverse effects (3). Over the past century, it has become clear that while radiation is a critical component to advances in medicine and science, it needs to be used with great caution.
On the other hand, some patients are more sensitive to radiation than others. This paper reviews reports in patients with presumably inherited susceptibility. In general, these patients teach us that radiosensitivity associates closely with the homozygous inheritance of defective proteins that are necessary for the recognition and/or repair of DNA double strand breaks (5–6). It is important to appreciate the dangers associated with the administration of conventional dosage regimens of radiotherapy for such individuals.
Online Mendelian Inheritance in Man (OMIM) is an exhaustive database of human genes and phenotypes. It is updated on a daily basis and exists as a resource for medical doctors, researchers and students. Each disease and gene is assigned a six-digit number and these are provided for the disorders discussed in this overview. OMIM can be accessed online at http://www.ncbi.nlm.nih.gov/omim/.
Ataxia-telangiectasia (OMIM# 208900)
The first well-documented adverse reaction followed radiation therapy for lymphosarcoma in a 10 year-old boy with a rare autosomal recessive disorder, ataxia-telangiectasia (A-T). He had the hallmark characteristics of A-T—mask-like facial expression, slow eye movements (apraxia), and an inability to walk unassisted. The patient received 30 Gy of radiation and died of complications 8 months later (7).
This case serves as a good teaching example of the potential dangers that can confront an A-T patient when treated with conventional doses of radiation therapy. Two weeks before admission, his mother had observed a developing nasal quality to his voice and excessive saliva. The physician noted a soft palate mass that was subsequently diagnosed as lymphosarcoma by histological examination. Radiotherapy of 40 Gy to the tumor was recommended. The actual therapy delivered was fractionated into 15 total treatments over a 3-week period. The irradiated anatomy included tonsils, nasopharynx and upper neck region. Lateral portals (9×10 cm) were set up to deliver a skin dose of 1.5 Gy each for 12 treatments and 1 Gy for 3 treatments for a combined total dose of 30 Gy to the tumor volume as well as 0.8 Gy exit dose from the contralateral portal. The output factors for the machine used were 300kV, 19 mAs and a half-value layer of 4mm copper (7).
Due to an unexpected radiation reaction, therapy was interrupted after 3 weeks, with only 30 of the recommended 40 Gy being delivered to the tumor. Towards the end of the third week of treatment, the patient suffered from acute mucositis. The dose was reduced to 3 fractions of 1 Gy each to the tumor volume before ending his regimen. Twelve days later, the patient displayed a severe skin reaction to the radiation that progressed rapidly, beginning with distinct erythema, followed by seeping and crusted skin. The boy lost 8 pounds due to an inability to eat and he continued to lose weight for the next couple of months. His skin never completely healed. He was readmitted twice. On his final and third admission, he was hoarse and breathing noisily. Chest X-rays appeared normal, despite pus draining from his nose and left outer ear. He died shortly after admission, despite antibiotics and intravenous fluids.
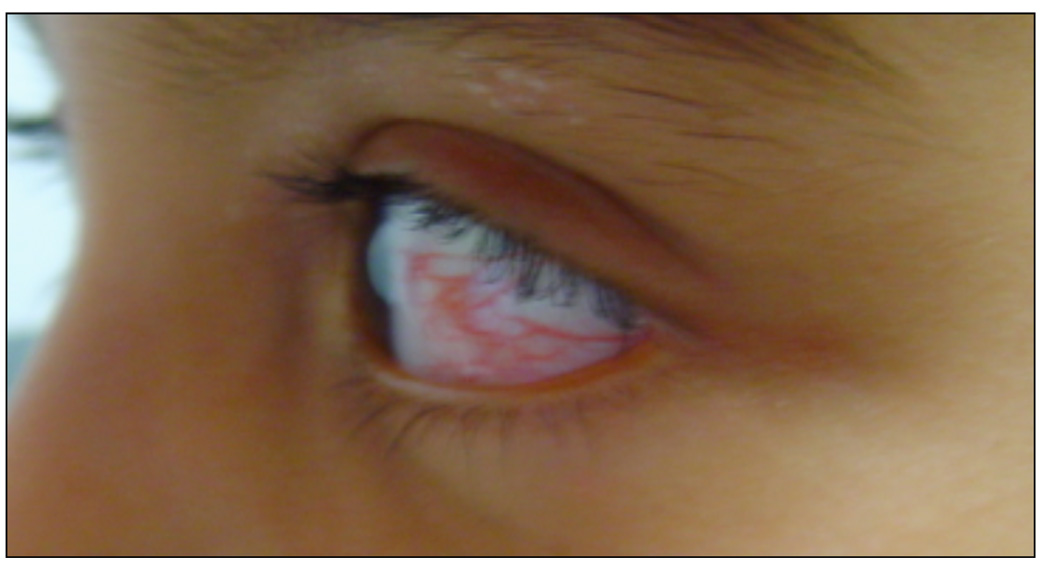
A-T was first described by Syllaba and Henner in 1926 and has a multi-faceted phenotype of immunodeficiency, cancer-predisposition, telangiectasia, ataxia, ocular apraxia, and neurodegeneration (Figure 1) (5,8–10). The incidence of A-T has been estimated to be 1 in 40,000 in the US with a carrier rate of 1 in 100 (10–11). In 1988, the responsible gene was localized by linkage analysis to chromosome 11q22–23 and later identified by positional cloning (12–14). The gene was called “Ataxia-telangiectasia mutated (ATM).” Over the past decade, ATM’s importance in understanding DNA repair and processing has expanded widely. It is a large protein, 370 kDa, with serine or threonine kinase activity, that is activated by double strand DNA breaks or chromatin perturbations (13–19). ATM phosphorylates over 900 downstream targets that coordinate cell cycle checkpoints during DNA repair, recognize and repair the broken ends of DNA, or activate apoptotic pathways in cells that have been damaged beyond repair (Figure 2–Figure 4) (14,20).
Figure 1.
Telangiectasia pattern in a patient with A-T.
Figure 2.
The two major DNA repair pathways are non-homologous end-joining (NHEJ) and homologous recombination (HR). NHEJ occurs throughout the cell cycle and is initiated by Ku70 and Ku80 binding to DNA double-stranded ends. The MRE11/RAD50/NBS (MRN) complex, DNA PK and XRCC4/DNA Ligase IV is then recruited to the site of the break. DNA PK helps to process the ends, while, DNA Ligase IV causes the ligation. In HR, ATM is recruited to the site of the double-stranded break by the MRN complex and thereafter, ATM phosphorylates other downstream targets involved in cell cycle checkpoints, DNA repair or apoptosis.
Figure 4.
Colony Survival Assay, following 1 Gy to human lymphoblastoid cell lines: 29 normals, 19 obligate heterozygotes, 104 A-T patients with varying ATM mutations, 5 NBS patients with varying mutations, 5 FA patients with varying complementation groups, and 2 LIG 4 patients (siblings). The A-T cells were more radiosensitive than the normals (P<.01).
Nijmegen Breakage Syndrome (OMIM# 251260)
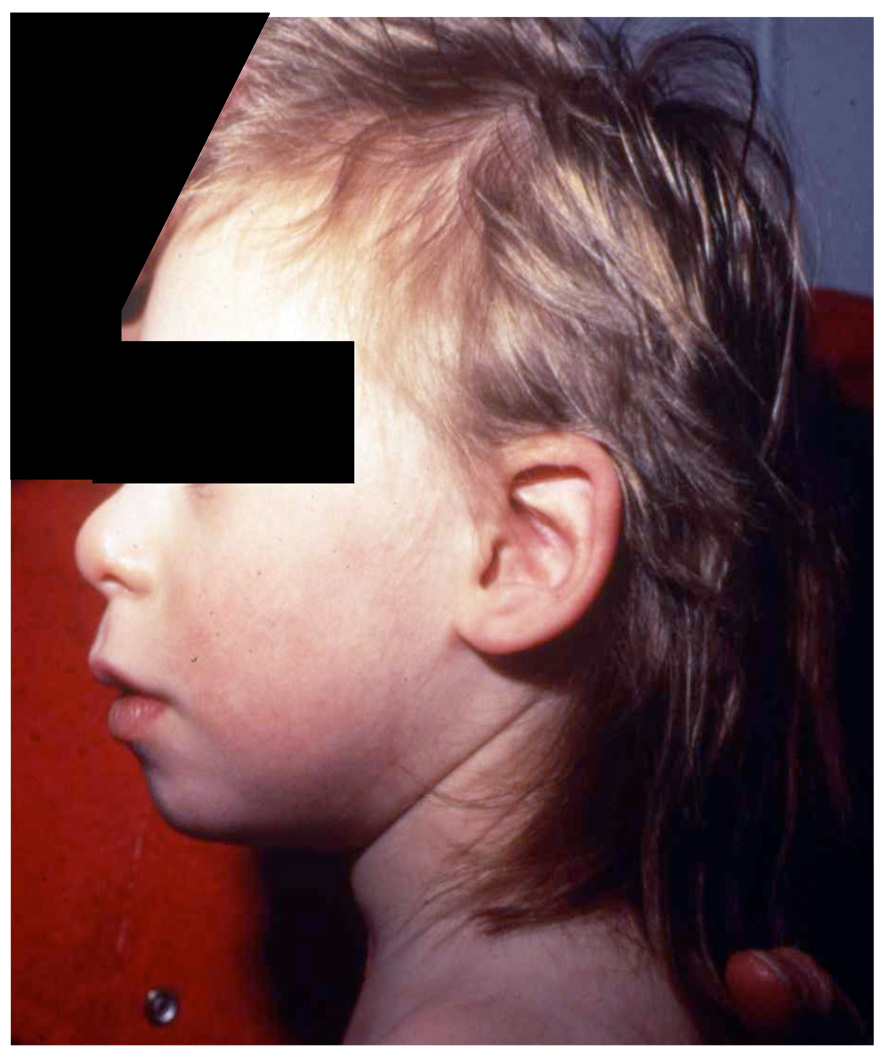
Another rare autosomal recessive disorder, Nijmegen Breakage Syndrome (NBS), has been linked to radiotherapy-induced mortality. The disease was first described by Weemaes et al in 1981 (21). NBS is characterized by growth and mental retardation, microcephaly, facial dysmorphism, immunodeficiency and cancer predisposition (Figure 5) (22). NBS patients do not manifest ataxia. Cells derived from NBS patients are also hypersensitive to ionizing radiation (23).
Figure 5.
Microcephaly and micrognathia of a patient with NBS.
NBS results from mutations in the NBS1 gene, localized to chromosome 8q21 (24–26). NBS was thought to be a cellular variant of A-T. However, NBS1 encodes a distinct protein, nibrin, which is a component of the DNA repair complex MRN (Mre11, Rad50 and Nibrin); all three proteins are activated by ATM phosphorylation at defined residues (Figure 2) (27). When considered along with A-T, this link to the DNA repair machinery helps to explain the radiosensitivity (Figure 4) (28). When activated by irradiation, the MRN complex localizes to sites of DNA damage forming protein foci at DNA breaks that are visible by fluorescence microscopy. Without nibrin, the MRN complex does not get transported into the nucleus to repair DNA (29).
The first documented case of radiation sensitivity observed in an NBS patient involved a 3-year-old microcephalic boy with medulloblastoma (23). The family history included a mother with Scottish, English and Irish roots and a Czechoslovakian father. He also had an affected older brother with microcephaly, mental retardation, porencephaly and internal hydrocephalus. The mother suffered from hypothyroidism and received thyroid supplements during pregnancy. There was a history of breast cancer in the paternal grandmother.
The child was admitted with progressive vomiting. Computed tomography of the head revealed a tumor that was histologically identified as medulloblastoma. It was removed surgically.
Weekly radiation and vincristine therapy treatments were initiated. The patient received 23.4 Gy in 13 fractions to his cranium and spine and a posterior fossa boost of up to 55.8 Gy in 18 fractions over a 7-week period. Just 2 weeks after the start of the combined chemo- and radio-therapy, the patient experienced fevers and neutropenia. He had a number of infections over the next 3 months. He developed progressive dysphagia and erosive esophagitis with esophageal basal cell hyperplasia. A month after beginning radiotherapy, a weeping dermatitis around his temples and occipital area was noted which did not respond to antibiotics and anti-fungal agents. Shortly thereafter, he expired from interstitial pneumonitis and profuse bleeding from the trachea and esophagus.
A diagnosis of NBS was made post-mortem, prompted by his marked sensitivity to radiation therapy. A western blot of the patient’s cells showed that they lacked nibrin protein and mutation analysis identified two mutations in the NBS1 gene: 657del 5 and 1142delC. Both mutations cause truncated nibrin protein, which would explain the lack of full-length nibrin on western blots.
Fanconi Anemia (OMIM# 227650)
Fanconi anemia (FA) is a rare autosomal recessive DNA repair disorder that causes chromosomal instability, cancer susceptibility and radiation sensitivity (6). The disease was first described by Fanconi when he identified two brothers with the disorder (30,31). Other typical findings include aplastic anemia and congenital abnormalities, such as microcephaly, short stature, radial ray bone abnormalities, and malformations of the kidney and heart (32–34). FA patients who live into their twenties usually develop solid tumors, such as squamous cell carcinomas of the esophagus, oropharynx, and vulva, but they tend to die more often from hematological malignancies, such as leukemia or bone marrow failure (32,33). The incidence of FA has been estimated to be 1–5 per million with a carrier frequency of 1 in 300 (35).
FA can result from mutations in one of at least 13 complementation genes (FA-A, -B, -C, -D1, -D2, -E, -F, -G, -I, -J, -L, -M and –N); FA-A alone accounts for over 66% of all patients (36). FA-C patients comprise 10–15% of FA patients; roughly half of these are of Ashkenazi Jewish background. However, all tested FA patients are radiosensitive when tested by colony survival assay (Figure 4). Only the gene FA-I has not yet been isolated. Eight of the FA proteins work together to activate FANCD2, which colocalizes with BRCA1, BRCA2, and RAD51 in order to repair DNA interstrand crosslinks, at stalked replication forks (6,35). FA cells exhibit hypersensitivity to chemotherapy and other DNA cross-linking agents such as cisplatin and mitomycin C (33,35).
One of the notable reports of extreme hypersensitivity to radiation therapy was that of a 32-year-old male admitted to Hannover Medical School in 2001 (37). This patient had been diagnosed with FA years before and had had a malignant oral tumor removed in 2000. During a 2001 hospitalization, he was discovered to have locally advanced squamous cell carcinoma of the lateral and posterior oropharyngeal wall that extended to the base of his skull. The tumor and part of his neck on the right side were resected. After the surgery, a CT scan showed that he had metastatic disease spreading from the base of his skull to his lower neck.
A 6-MV linear accelerator with 2 opposing lateral fields was used to deliver 8 Gy total to the base of his skull. The dose was given in 5 fractions of 1 Gy daily and then raised to 1.5 Gy per fraction. Radiotherapy was discontinued after a week when he developed a severe case of thrombocytopenia. He died a few days later.
DNA Ligase IV Deficiency (OMIM# 606593)
Nonhomologous end-joining (NHEJ) is one of the major pathways for DNA repair in mammalian cells (38). NHEJ occurs during G0, G1, and early S phase of the cell cycle (39). Seven components of the NHEJ pathway are presently defined: Ku70, Ku80, DNA-dependent protein kinase catalytic subunit (DNA-PKcs), Artemis, Cernunnos (XLF), XRCC4, and DNA ligase IV (LIG4) (40). During the first step of end-joining, Ku70 and Ku80 proteins bind to the broken ends, then recruit and activate DNA-PKcs. The DNA-PK and Ku complex recruits Artemis and activates its endonuclease activity; this trims the DNA ends. Finally, LIG4, XRCC4, and XLF ligate the DNA ends back together (Figure 2) (38). NHEJ is also critical for V(D)J recombination during lymphocyte maturation of T cell receptors and immunoglobulins. Disruption of this mechanism leads to immunodeficiency and has also been associated with leukemia, multiple myeloma and lymphoma (39,41). However, the bone marrow failure seen in these patients is not explained by this. In mice homozygous for null alleles of DNA LIG4, high levels of neuronal apoptosis and embryonic lethality are seen (42,43).
To date, eleven patients with DNA LIG4-deficiency have been described (39–48). The patients displayed typical characteristics such as microcephaly, growth retardation, developmental delay and various skin conditions (i.e. warts and photosensitivity) (Figure 6) (45,48). One patient exhibited a severe reaction to radiotherapy for acute lymphoblastic leukemia, another patient had a severe reaction to chemotherapy and nine were immunodeficient (43–49). Their immunodeficiencies included lymphocytopenia, absence of B lymphocytes and recurrent infections.
Figure 6.
Microcephaly and micrognathia, with low-set ears, in a patient with DNA Ligase IV deficiency.
Plowman et al. described clinical radiosensitivity with DNA LIG4-deficiency (44,45). The patient was a 14-year-old of Turkish-Cypriot background. He was the youngest of four unaffected siblings and no family history of disease. Physical examination revealed that he was anorexic, lethargic, and had multiple sites of lymphoadenopathy, as well as an enlarged liver and spleen. He was diagnosed with T cell acute lymphoblastic leukemia. The UKALL (United Kingdom Acute Lymphoblastic Leukemia) chemotherapy protocol was recommended and followed; the patient received vincristine (2 mg/week), daunorubicin (60 mg; intravenously, on Days 1 and 2), l-asparaginase (8000u; intramuscularly, alternate days) and prednisolone (50 mg/day) (44,50). Immediately after the first dose of chemotherapy, the patient showed signs of fever; intravenous antibiotics were started. The fever abated; however, the white blood cell count dropped to 0.6 × 109 /L and remained under 0.5 × 109 /L until Day 27. The patient also suffered from cardiac gallop rhythm and hypertension.
After 45 days, the white blood cell count recovered to a value above 2× 109 /L, but his platelet count remained low. A bone marrow exam showed that further chemotherapy was contra-indicated. Radiotherapy was recommended as an alternative and he received 18 Gy in 10 fractions over 12 days to his cranial meninges. Parallel-opposed lateral 6 MV X-ray portals were used to deliver the treatment. Over the course of the 12 days, the child remained leukopenic and thrombocytopenic. He showed signs of severe scalp erythema and moist desquamation behind both ears, attributable to dose “build-up” five days after the end of his radiotherapy. After 14 days, the erythema and moist desquamation ameliorated. Nineteen days later, the child became lethargic and somnolent for 3 weeks, but gradually improved.
Bilateral aural discharge was observed 2 months after the end of radiotherapy and maintenance chemotherapy was administered. On Day 115 after radiotherapy, the patient described extreme pain in the right ear and upon examination a necrotic ulcer was located above the right mastoid. He became lethargic and progressively lost weight. Seven months after his radiotherapy, he was mentally disoriented and unable to feed himself. EEG results indicated activity generally attributable to radiation-induced encephalopathy. He died eight months after the radiotherapy was discontinued.
A fibroblast cell line was established from a forearm skin biopsy of this patient (180BR), which showed evidence of radiation sensitivity similar to that of A-T. Subsequent studies on this cell line demonstrated the DNA LIG4 defect (45). Lymphoblastoid cell lines from two other LIG4 patients, who were siblings being studied in our laboratory, demonstrated similar levels of radiosensitivity and helped to establish the LIG4-deficiency syndrome (Figure 4) (45).
Discussion
Radiation therapy represents one of the most common methods used to treat cancer. Approximately one million patients in the U.S. receive radiation therapy each year (51). Ironically, there may be as many as 40 DNA repair defective disorders that are associated with radiosensitivity as well as with cancer predisposition and a sensitivity to many of the DNA damaging agents used to treat cancer (5–6,38). In our table, we summarize 36 cases of untoward outcomes to radiation therapy in such patients.
Radiosensitive individuals represent the biggest potential risk of accidental injury for a radiation oncology department. Although this includes a small number of primarily homozygotes with DNA repair disorders (A-T, NBS, FA, LIG4, MRE-11, X-linked Agammaglobulinemia SCID-ADA, SCID-Artemis, and SCID-XLF), it also includes a larger number of likely heterozygotes for of these same disorders (albeit at a higher threshold of radiosensitivity). Common characteristic phenocopies among these disorders can help physicians diagnose or suspect potentially radiosensitive individuals before radiotherapy is recommended or initiated.
Our search revealed well-documented clinical cases of radiosensitivity associated with homozygous A-T, NBS, DNA LIG4-deficiency, FA, and a single patient with Bloom’s syndrome (Table 1). We were unable to identify cases of clinical radiosensitivity involving X-linked Agammaglobulinemia, MRE11-, Artemis-, Cernunnos-deficiency (XLF) or Werner’s syndrome, most likely because of their rarity. We excluded reports in which a definitive diagnosis could not be established, such as a report of radiosensitivity associated with “chromosomal fragility” (52). We also did not attempt to include the numerous reports of adverse radiation reactions in heterozygotes for genes such as BRCA1 or BRCA2, since these are abundantly documented elsewhere and, being heterozygotes, digress from our goal (53,54). The report of clinical radiosensitivity in a patient with Bloom’s syndrome was unexpected and brought forward another issue that deserves comment (Table 1). In general, cells from homozygous patients that do not score as radiosensitive in clonogenic assays, such as the colony survival assay (CSA), would not be expected to be clinically radiosensitive (55). In a limited study, we tested lymphoblastoid cells from 5 patients with Bloom’s syndrome; they did not score in the radiosensitive range as defined for CSA using 104 A-T cell lines (55). Furthermore, cells from other helicase disorders, such as Rothmund-Thompson, have not scored as radiosensitive (unpublished). Nor have cells from Xeroderma Pigmentosum patients, which have primarily single strand repair defects and do not generally manifest adverse responses to radiation therapy (5).
Table 1.
Adverse reactions to radiation therapy
| Disorder | Years of Age/Sex |
Radiation Treatment |
Outcome | Reference |
|---|---|---|---|---|
| A-T | 10.5/M | 30 Gy | Died, 8 months | (7) |
| A-T | 9/M | 27.5 Gy (mediastinal tumor) 27.5 Gy (supraclavicular area) |
Died, 3 months | (61) |
| A-T | 3.9/M | 3 Gy | Died, < 1 month | (58) |
| A-T | 7/M | 30 Gy | Died, 3 weeks | (62) |
| A-T | 3.8/F | 30 Gy | Died, 9 months | (63) |
| A-T | 9/M | 16 Gy | Severe mucosal ulceration |
(64) |
| A-T | 4.5/M | 18 Gy | Leukoencephalopathy | (65) |
| A-T | 7/M | 24 Gy (brain) 12 Gy (spine) |
Somnolescence syndrome |
(66) |
| A-T | 9/F | 9 Gy | Died, 10 months | (67) |
| A-T | 15.2/M | 15.5 Gy | Died,1 month | (59) |
| A-T | 3.9/M | 3 Gy | Died, 3 months | (59) |
| A-T | 1.5/M | 18 Gy (brain) 3 Gy (chest) |
No excessive toxicity | (68) |
| A-T | 2.5/M | 24 Gy (brain) 6 Gy (spine) |
Leukoencephalopathy, 10 months |
(68) |
| NBS | 5/M | 36 Gy | Died, 18 months | (69) |
| NBS | 3/M | 23.4 Gy (craniospinal) 55.8 Gy (posterior fossa) |
Interstitial pneumonitis, Died, 3 months |
(23) |
| FA | 26/F | NS | Died, 4 months | (70) |
| FA | 17/F | 68 Gy | Died, 3 months | (71) |
| FA | 22/M | NS | Alive, 10 months post | (72) |
| FA | 26/M | 70 Gy | Died during RT | (73) |
| FA | 27/F | 42.5 Gy | Severe moist skin reaction, Died, 3 months |
(74) |
| FA | 23/F | NS | Died, 4 years | (75) |
| FA | 20/F | NS | Died, 2 months | (76) |
| FA | 14/M | 80 Gy | Died, 6 months | (77) |
| FA | 25,29/F | 72 Gy | Died, 6 months | (78) |
| FA | 12/M | NS | Died, 4 months | (79) |
| FA | 29/F | 32.5 Gy | Died, 12 months | (80) |
| FA | 32/F | 3.2 Gy | Died, 9 months | (81) |
| FA | 18/F | NS | Died, 3 months | (82) |
| FA | 32/F | 34 Gy | Ulcerating oropharyngeal mucositis, Alive, 3 months |
(83) |
| FA | 14/F | 21.6 Gy | Dead | (84) |
| FA | 29/F | NS | Died, 9 months | (32) |
| FA | 24/M | 67 Gy, 55.8 Gy, 25.2 Gy |
Died, 3 months | (37) |
| FA | 32/M | 8 Gy | Died, < 1 week | (37) |
| FA | 25/F | 9 Gy | Died, 1 week | (33) |
| Ligase IV |
14/M | 18 Gy | Died, 8 months | (44) |
| Bloom | 33/F | 60 Gy | severe, acute radiation toxicity |
(85) |
A-T, NBS, DNA LIG4-deficiency and FA all have radiosensitivity and cancer predisposition as common indicators of the disorder. Many are also immunodeficient. Microcephaly and growth delay are other common characteristics (6,34). The genes and missing proteins responsible for each of these disorders are known and can be identified for homozygous individuals in clinical testing labs over the course of days. Unfortunately, rapid methods for quickly screening patients prior to radiation therapy are not yet validated for clinical use; however, colony survival assays can yield definitive results in 12 weeks, thus providing some advance warning of radiosensitivity in homozygous patients (55,56). Carriers (heterozygotes) are not clearly identified by this method, although it would not be difficult to envision a rare dominant mutation causing a similar effect. Most recently, in our laboratory, a rapid method for detecting ATM heterozygotes, FC-pSMC1, appears promising for clinical use; it is based on assaying ATM kinase activity (57). This method will also reduce turnaround time for diagnosing A-T from 3 months to 3 hours, once validation is complete.
In our review, the lowest radiation dose associated with a fatal outcome was 3 Gy (58,59). However, the inherent sensitivity of radiosensitive individuals varies; this may be due to: 1) the particular genetic disorder; 2) to specific mutations within these genes; 3) to poorly documented prior radio- or chemo-therapy; 4) to particular sensitivities of the tissues exposed; 5) to our inability to accurately quantify radiosensitivity assays in the laboratory or 6) the amount of time allowed for DNA repair during radiotherapy (55). Some patients have been able to tolerate 2 Gy fractions up to a total of 30 Gy before they showed signs of radiation-induced toxicity. It is interesting that only a few cases have had non-fatal even a positive outcome. In the Hart study, an eleven-year-old male A-T patient was successfully treated for medulloblastoma by decreasing the radiation therapy dose according to his cell radiosensitivity assay results (60). Also, in some cases, it was unclear whether the patient’s underlying disease itself or the radiation therapy was causally related to the outcome. Despite this, a review of 36 cases indicates that radiation therapy should be avoided for individuals with any of the four disorders discussed above and strongly suggests that these principles can also be extrapolated to other DNA repair disorders.
The laboratory identification of heterozygosity for DNA repair disorders is only in its infancy. Thus, it has been difficult to search for correlations with clinical radiosensitivity. More sensitive, reliable assays are needed for identifying this large segment of the population, which probably comprises as much as 5% of the population (54). The use of functional assays for pathways that lead to radiosensitivity may help to resolve this clinical issue and will also provide new insights into the mechanisms of radiosensitivity.
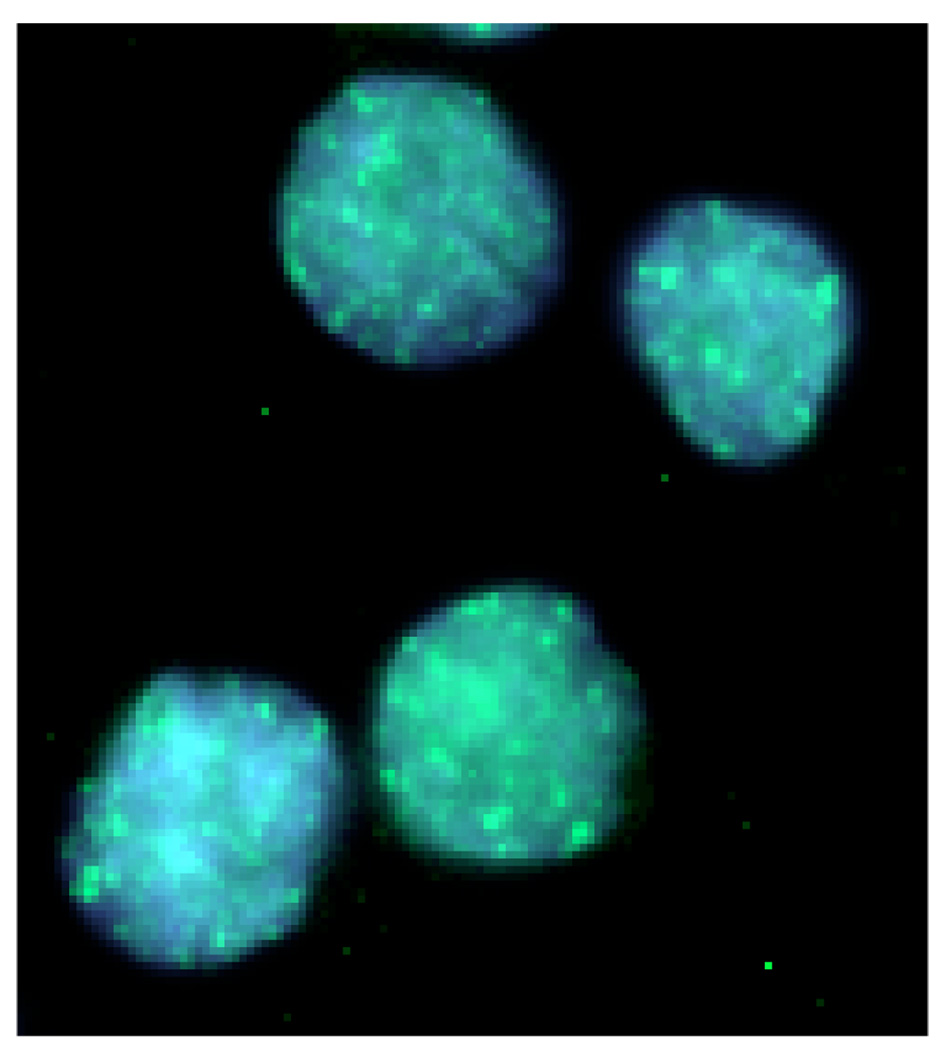
Figure 3.
ATM-S1981 immunofluorescent foci in human lymphoblastoid cells, 15 minutes after 2 Gy irradiation. Phosphorylated ATM on serine 1981 forms distinct nuclear foci at sites of DNA damage. These foci are depicted as the discrete green fluorescein isothiocyanate (FITC) stains on the blue nuclear 4, 6-diamidino-2-phenylindole (DAPI) counterstain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest.
References
- 1.Riesz PB. The life of Wilhelm Conrad Roentgen. Am J Roentgenol. 1995;165:1533–1537. doi: 10.2214/ajr.165.6.7484601. [DOI] [PubMed] [Google Scholar]
- 2.Weber AL. History of head and neck radiology: past, present, and future. Radiology. 2001;218:15–24. doi: 10.1148/radiology.218.1.r01ja2715. [DOI] [PubMed] [Google Scholar]
- 3.Hall EJ. Radiobiology for the Radiologist. 5th Edition. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 4.Clark JG, Coley WB, Gerster JCA, Jackson E, Stengel A. Progressive medicine in the medical and surgical sciences. In: Hare HA, Appleman LF, editors. Diseases of the blood. Diathetic and metabolic diseases. Diseases of the thyroid gland, nutrition and the lymphatic system. Volume 2. Philadelphia and New York: Lea and Febiger; 1912. pp. 264–265. [Google Scholar]
- 5.Gatti RA. The inherited basis of human radiosensitivity. Acta Oncologica. 2001;40(6):702–711. doi: 10.1080/02841860152619115. [DOI] [PubMed] [Google Scholar]
- 6.Gatti RA, Boder E, Good RA. Immunodeficiency, radiosensitivity, and the XCIND syndrome. Immunol Res. 2007;38:87–101. doi: 10.1007/s12026-007-0018-y. [DOI] [PubMed] [Google Scholar]
- 7.Gotoff SP, Amirmokri E, Liebner EJ. Ataxia telangiectasia. Neoplasia, untoward response to x-irradiation, and tuberous sclerosis. Am J Dis Child. 1967;114(6):617–625. doi: 10.1001/archpedi.1967.02090270073006. [DOI] [PubMed] [Google Scholar]
- 8.Syllaba L, Henner K. Contribution to the independance of the idiopathic and congenital double athetosis - Familial attack, dystophic syndrome, sign of the conjunctival vascular network, psychic integrity. Revue Neurologique. 1926;45(1):541–562. [Google Scholar]
- 9.Boder E, Sedgwick RP. Ataxia-telangiectasia: a familial syndrome of progressive cerebellar ataxia, oculocutaneous telangiectasia and frequent pulmonary infection. Pediatrics. 1958;21:526–554. [PubMed] [Google Scholar]
- 10.Chun HH, Gatti RA. Ataxia-telangiectasia, an evolving phenotype. DNA Repair. 2004;3:1187–1196. doi: 10.1016/j.dnarep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Swift M, Morrell D, Cromartie E, Chamberlin AR, Skolnick MH, et al. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet. 1986;39:573–583. [PMC free article] [PubMed] [Google Scholar]
- 12.Gatti RA, Berkel I, Boder E, Braedt G, Charmley P, et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22–23. Nature. 1988;336(6199):577–580. doi: 10.1038/336577a0. [DOI] [PubMed] [Google Scholar]
- 13.Savitsky K, Sfez S, Tagle DA, Ziv Y, Sartiel A, et al. The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum Mol Genet. 1995;4(11):2025–2032. doi: 10.1093/hmg/4.11.2025. [DOI] [PubMed] [Google Scholar]
- 14.Lange E, Borresen A, Chen X, Chessa L, Chiplunkar S, et al. Localization of an ataxia-telangiectasia gene to an ~500-kb interval on chromosome 11q23.1 linkage analysis of 176 families by an international consortium. Am J Hum Genet. 1995;57:112–119. [PMC free article] [PubMed] [Google Scholar]
- 15.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 16.Bartek J, Lukas J. DNA repair: damage alert. Nature. 2003;421(6922):486–488. doi: 10.1038/421486a. [DOI] [PubMed] [Google Scholar]
- 17.Lobrich M, Jeggo PA. The two edges of the ATM sword: co-operation between repair and checkpoint functions. Radiother Oncol. 2005;76:112–118. doi: 10.1016/j.radonc.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 18.O’Driscoll M, Jeggo PA. The role of double-strand break repair—insights from human genetics. Genetics. 2006;7:45–54. doi: 10.1038/nrg1746. [DOI] [PubMed] [Google Scholar]
- 19.Ball LG, Xiao W. Molecular basis of ataxia telangiectasia and related diseases. Acta Pharmacologica Sinica. 2005;26(8):897–907. doi: 10.1111/j.1745-7254.2005.00165.x. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, Hurov KE, et al. ATM and ATR analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316(5828):1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 21.Weemaes CM, Hustinx TW, Schores JM, van Munster PJ, Bakkeren JA, et al. A new chromosomal instability disorder: the Nijmegen breakage syndrome. Mol Immunol. 1981;37:1131–1139. doi: 10.1111/j.1651-2227.1981.tb05740.x. [DOI] [PubMed] [Google Scholar]
- 22.Hiel JA, Weemaes C, Van den Heuvel IP, van Engelen BG, Dabreets FJ, et al. International NBS Study Group. Nijmegen Breakage Syndrome. Arch Dis Child. 2000;82:400–406. doi: 10.1136/adc.82.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakhshi S, Cerosaletti KM, Concannon P, Bawle EV, Fontanesi J, Gatti RA, et al. Medulloblastoma with adverse reaction to radiation therapy in nijmegen breakage syndrome. J Pediatr Hematol Oncol. 2003;25(3):248–251. doi: 10.1097/00043426-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, et al. The hMre11/hRad50 protein complexand Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 25.Varon R, Vissinga C, Platzer M, Cerosaletti KM, Chrzanowska KH, et al. Nibrin, a novel DNA double-strand break repair protein, is mutated in nijmegen breakage syndrome. Cell. 1998;93:467–476. doi: 10.1016/s0092-8674(00)81174-5. [DOI] [PubMed] [Google Scholar]
- 26.Cerosaletti KM, Lange HM, Stringham CM, Weemaes D, Smeets B, et al. Fine localization of the Nijmegen breakage syndrome gene to 8q21: evidence for a common founder haplotype. Am J Hum Genet. 1998;63:125–134. doi: 10.1086/301927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taalman RD, Jaspers NG, Scheres JM, de Wit J, Hustinx TW. Hypersensitivity to ionizing radiation, in vitro, in a new chromosomal breakage disorder, the Nijmegen Breakage Syndrome. Mutat Res. 1983 Feb;112(1):23–32. doi: 10.1016/0167-8817(83)90021-4. [DOI] [PubMed] [Google Scholar]
- 28.Jaspers NGJ, Gatti RA, Baan C, Linssen PCML, Bootsma D. Genetic complementation analysis of ataxia telangiectasia and nijmegen breakage syndrome-survey of 50 patients. Cytogenet Cell Genet. 1988;4(49):259–263. doi: 10.1159/000132673. [DOI] [PubMed] [Google Scholar]
- 29.Desai-Mehta A, Cerosaletti KM, Concannon P. Distinct functional domains of nibrin mediate Mre11 binding, focus formation and nuclear localization. Mol Cell Biol. 2001;21:2184–2191. doi: 10.1128/MCB.21.6.2184-2191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fanconi G. Familiare infantile perniziosaartige Anamie (permizoses Blutbild Und Konstitiution) Jahrb Kinderheilkund. 1927;117:257–280. [Google Scholar]
- 31.Lobitz S, Velleuer E. Guido Fanconi (1892–1979): a jack of all trades. Nat Rev Cancer. 2006;6(11):893–898. doi: 10.1038/nrc2009. [DOI] [PubMed] [Google Scholar]
- 32.Oksuzoglu B, Yalcin S. Squamous cell carcinoma of the tongue in a patient with Fanconi’s anemia: a case report and review of the literature. Ann Hematol. 2002;81(5):294–298. doi: 10.1007/s00277-002-0455-6. [DOI] [PubMed] [Google Scholar]
- 33.Harper JL, Jenrette JM, Goddu SM, Lal A, Smith T. Vulvar cancer in a patient with Fanconi’s anemia, treated with 3D conformal radiotherapy. Am J Hematol. 2004;76:148–151. doi: 10.1002/ajh.20070. [DOI] [PubMed] [Google Scholar]
- 34.Djuzenova C, Flentje M, Plowman PN. Radiation response in vitro of fibroblasts from a Fanconi anemia patient with marked clinical radiosensitivity. Strahlenther Onkol. 2004;12:789–797. doi: 10.1007/s00066-004-1250-1. [DOI] [PubMed] [Google Scholar]
- 35.Jacquemont C, Toniguchi T. The Fanconi anemia pathway and ubiquitin. Biochem. 2007;8 Suppl 1:S1–S10. doi: 10.1186/1471-2091-8-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamary H, Bar-Yam R, Shalmon L, Rachavi G, Krostichevsky M, et al. Fanconi anemia group A mutations in Israeli non-Ashkenazi Jewish patients. Br J Haemat. 2000;111(1):338–343. doi: 10.1046/j.1365-2141.2000.02323.x. [DOI] [PubMed] [Google Scholar]
- 37.Bremer M, Schindler, Grob M, Dork T, Morlot S, Karstens JH. Fanconi’s anemia and clinical radiosensitivity. Strahlenther Onkol. 2003;11:748–753. doi: 10.1007/s00066-003-1099-8. [DOI] [PubMed] [Google Scholar]
- 38.O’Driscoll M, Gennery AR, Seidel J, Concannon P, Jeggo PA. An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA Repair. 2004;3:1227–1235. doi: 10.1016/j.dnarep.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 39.Gruhn B, Seidel J, Zintl F, Varon R, Tonnies H, et al. Successful bone marrow transplantation in a patient with DNA ligase IV deficiency and bone marrow failure. Orph J Rare Dis. 2007;2:5. doi: 10.1186/1750-1172-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche MC, et al. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with micrcephaly. Cell. 2006;124(2):260–262. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 41.Riballo E, Critchlow SE, Teo SH, Doherty AJ, Priestley A, et al. Identification of a defect in DNA ligase IV in a radiosensitive leukemia patient. Curr Biol. 1999;9(13):699–702. doi: 10.1016/s0960-9822(99)80311-x. [DOI] [PubMed] [Google Scholar]
- 42.Frank KM, Sharpless NE, Gao Y, Sekiguchi JM, Ferguson DO, et al. DNA ligase IV deficiency in mice leads to defective neurogenesis and embryonic lethality via the p53 pathway. Mol Cell. 2000;5(6):993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Omran TI, Cerosaletti KM, Concannon P, Weitzman S, Nezarati MM. A patient with mutations in DNA ligase IV: clinical features and overlap with Nijmegen breakage syndrome. Am J Med Gen. 2005;137A:283–287. doi: 10.1002/ajmg.a.30869. [DOI] [PubMed] [Google Scholar]
- 44.Plowman PN, Bridges BA, Arlett CF, Hinney A, Kingston JE. An instance of clinical radiation morbidity and cellular radiosensitivity, not associated with ataxia-telangiectasia. Br J Radiol. 1990;63:624–628. doi: 10.1259/0007-1285-63-752-624. [DOI] [PubMed] [Google Scholar]
- 45.O’Driscoll M, Cerosaletti KM, Girard PM, Dai Y, et al. DNA ligase IV mutations identified in patients exhibiting developmental delay and immunodeficiency. Mol Cell. 2001;8:1175–1185. doi: 10.1016/s1097-2765(01)00408-7. [DOI] [PubMed] [Google Scholar]
- 46.de Villartay JP, Lim A, Al-Mousa H, Dupont S, Dechanet-Merville E, et al. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest. 2005;115:3291–3299. doi: 10.1172/JCI25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Burg M, van Veelen LR, Verkaik NS, Wiegant WW, Hartwig NG, et al. A new type of radiosensitive T-B-NK+ severe combined immunodeficiency caused by a LIG4 mutation. J Clin Invest. 2006;116:137–145. doi: 10.1172/JCI26121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enders A, Fisch P, Schwarz K, Duffner U, Pannicke U, et al. A severe form of human combined immunodeficiency due to mutations in DNA ligase IV. J Immun. 2006;176:5060–5068. doi: 10.4049/jimmunol.176.8.5060. [DOI] [PubMed] [Google Scholar]
- 49.Buck D, Moshous D, de Chasseval R, Ma Y, le Deist F, et al. Severe combined immunodeficiency amd microcephaly in siblings with hypomorphic mutations in DNA ligase IV. Eur J Immun. 2006;36:224–235. doi: 10.1002/eji.200535401. [DOI] [PubMed] [Google Scholar]
- 50.Hathaway FK. The role played by radiotherapy in the UKALL III series of trials for the treatment of acute lymphoblastic leukaemia in childhood. Radiography. 1974;40(479):255–258. [PubMed] [Google Scholar]
- 51.ASTRO Fact Sheet. 2008 www.astro.org.
- 52.Alsbeih G, Story MD, Maor MH, Geara FB, Brock WA. Chromosomal fragility syndrome and family history of radiosensitivity as indicators for radiotherapy dose modification. Radiother Oncol. 2003;66(3):341–344. doi: 10.1016/s0167-8140(02)00327-4. [DOI] [PubMed] [Google Scholar]
- 53.Angele S, Hall J. The ATM gene and breast cancer: is it really a risk factor? Mutat Res. 2000;462(2–3):167–178. doi: 10.1016/s1383-5742(00)00034-x. [DOI] [PubMed] [Google Scholar]
- 54.Leong T, Chao M, Bassal S, McKay M. Radiation-hypersensitive cancer patients do not manifest protein expression abnormalities in components of the nonhomologous end-joining (NHEJ) pathway. Br J Cancer. 2003;88:1251–1255. doi: 10.1038/sj.bjc.6600897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun X, Becker-Catania SG, Chun HH, Hwang MJ, Huo Y, et al. Early diagnosis of ataxia-telangiectasia using radiosensitivity testing. J Pediatr. 2002;140:724–731. doi: 10.1067/mpd.2002.123879. [DOI] [PubMed] [Google Scholar]
- 56.Huo YK, Wang Z, Hong JH, Chessa L, McBride WH, et al. Radiosensitivity of ataxia-telangiectasia, X-linked agammaglobulinemia and related syndromes using a modified colony survival assay. Cancer Res. 1994;54:2544–2547. [PubMed] [Google Scholar]
- 57.Nahas S, Butch A, Gatti RA. Rapid flow cytometry-based SMC1 phosphorylation assay for identification of ataxia-telangiectasia homozygotes and heterozygotes. doi: 10.1373/clinchem.2008.107128. (Submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris VJ, Seeler RA. Ataxia-telangiectasia and hodgkin’s disease. Cancer. 1973;32:1415–1420. doi: 10.1002/1097-0142(197312)32:6<1415::aid-cncr2820320619>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 59.Sandoval C, Swift M. Hodgkin disease in ataxia-telangiectasia patients with poor outcomes. Med Pediatr Oncol. 2003;40:162–166. doi: 10.1002/mpo.10251. [DOI] [PubMed] [Google Scholar]
- 60.Hart RM, Kimler BF, Evans RG, Park CH. Radiotherapeutic management of medulloblastoma in a pediatric patient with ataxia telangiectasia. Int J Radiation Oncology Biol Phys. 1987;13:1237–1240. doi: 10.1016/0360-3016(87)90200-8. [DOI] [PubMed] [Google Scholar]; Pritchard J, Sandland MR, Breatnach FB, Pincott JR, Cox R, Husband P. The effects of radiation therapy for Hodgkin’s disease in a child with ataxia telangiectasia. Cancer. 1982;50:877–886. doi: 10.1002/1097-0142(19820901)50:5<877::aid-cncr2820500513>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 61.Morgan JL, Holcomb TM, Morrissey RW. Radiation reaction in ataxia telangiectasia. Amer J Dis Child. 1968;116:557–558. doi: 10.1001/archpedi.1968.02100020561022. [DOI] [PubMed] [Google Scholar]
- 62.Cunliffe PN, Mann JR, Cameron AH, Roberts KD. Radiosensitivity in ataxia-telangiectasia. Br J Radiol. 1975;48:374–376. doi: 10.1259/0007-1285-48-569-374. [DOI] [PubMed] [Google Scholar]
- 63.Pritchard J, Sandland MR, Breatnach FB, Pincott JR, Cox R, Husband P. The effects of radiation therapy for Hodgkin’s disease in a child with ataxia telangiectasia. Cancer. 1982;50:877–886. doi: 10.1002/1097-0142(19820901)50:5<877::aid-cncr2820500513>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 64.Abadir R, Hakami N. Ataxia telangiectasia with cancer. An indication for reduced radiotherapy and chemotherapy doses. Br J Radiol. 1983;56:343–345. doi: 10.1259/0007-1285-56-665-343. [DOI] [PubMed] [Google Scholar]
- 65.Eyre JA, Gardner-Medwin D, Summerfield GP. Leukoencephalopathy after prophylactic radiation for leukemia in ataxia telangiectasia. Arch Dis Childhood. 1988;63:1079–1093. doi: 10.1136/adc.63.9.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Attard-Montalto SP, Saha V, Kingston J, Plowman N, Taylor M, et al. Increased radiosensitivity in a child with T-cell non-Hodgkin’s lymphoma. Med Pediatr Oncol. 1996;27:565–570. doi: 10.1002/(sici)1096-911x(199612)27:6<565::aid-mpo11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 67.Tamminga RYJ, Dolsma WV, Leeuw JA, Kampinga HH. Chemo- and radiosensitivity testing in a patient with ataxia-telangiectasia and Hodgkin disease. Pediatr Hematol Oncol. 2002;19(3):163–171. doi: 10.1080/088800102753541314. [DOI] [PubMed] [Google Scholar]
- 68.Yanofsky R, Seshia S, Dawson A, et al. Ataxia-telangiectasia: atypical neurological features and adverse effects of cancer treatment in three Mennonite children. (Submitted). [Google Scholar]
- 69.Distel L, Neubauer S, Varon R, Holter W, Grabenbauer G. Fatal toxicity following radio- and chemotherapy of medulloblastoma in a child with unrecognized nijmegen breakage syndrome. Med Pediatr Oncol. 2003;41:44–48. doi: 10.1002/mpo.10275. [DOI] [PubMed] [Google Scholar]
- 70.Esparza A, Thompson WR. Familial hypoplastic anemia with multiple congenital anomalies (Fanconi’s syndrome)—report of three cases. R I Med J. 1966;49:103–110. [PubMed] [Google Scholar]
- 71.Vaitiekaitis AS, Grau WH. Squamous cell carcinoma of the mandible in Fanconi anemia: report of case. J Oral Surg. 1980;38:372–373. [PubMed] [Google Scholar]
- 72.Nara N, Miyamoto T, Kurisu A, et al. Two siblings with Fanconi’s anemia developing squamous cell carcinomas. Rinsho Ketsueki. 1980;21:1944–1950. [Google Scholar]
- 73.Kozarek RA, Sanowski RA. Carcinoma of the esophagus associated with Fanconi’s anemia. J Clin Gastroenterol. 1981;3:171–174. doi: 10.1097/00004836-198106000-00012. [DOI] [PubMed] [Google Scholar]
- 74.Wilkinson EJ, Morgan LS, Friedrich EG. Association of Fanconi’s anemia and squamous-cell carcinoma of the lower female genital tract with condyloma acuminatum. A report of two cases. J Reprod Med. 1984;29:447–453. [PubMed] [Google Scholar]
- 75.Helmerhorst FM, Heaton DC, Crossen PE, von Dem Borne AEGK, Engelfriet CP, Natarajan AT. Familial thrombocytopenia associated with platelet autoantibodies and chromosome breakage. Hum Genet. 1984;65:252–256. doi: 10.1007/BF00286512. [DOI] [PubMed] [Google Scholar]
- 76.Romero MG, Ortiz HC. Anemia de Fanconi. Respuesta a dosis bajass de anabolicos y asociation con carcinoma de esofago. Rev Invest Clin. 1984;36:353–356. [PubMed] [Google Scholar]
- 77.Kozhevnikov BA, Khodorenko CA. Cancer of the mucous membrane of the left side of the mouth associated with congenital hypoplastic Fanconi’s anemia in a 14-year-old boy. Vestn Khir. 1986;136:105–106. [PubMed] [Google Scholar]
- 78.Bradford CR, Hoffman HT, Wolf GT, Carey TE, Baker SR, McClatchey KD. Squamous carcinoma of the head and neck in organ transplant recipients: possible role of oncogenic viruses. Laryngoscope. 1990;100:190–194. doi: 10.1288/00005537-199002000-00016. [DOI] [PubMed] [Google Scholar]
- 79.Socie G, Henry-Amar M, Cosset JM, Devergie A, Girinsky T, Gluckman E. Increased incidence of solid malignant tumors after bone marrow transplantation for severe aplastic anemia. Blood. 1991;105:125–127. [PubMed] [Google Scholar]
- 80.Snow DG, Campbell JB, Smallman LA. Fanconi’s anemia and postcricoid carcinoma. J Laryngol Otol. 1991;105:125–127. doi: 10.1017/s0022215100115130. [DOI] [PubMed] [Google Scholar]
- 81.Lustig JP, Lugassy G, Neder A, Sigler E. Head and neck carcinoma in Fanconi’s anemia—report of a case and review of the literature. Oral Oncol Eur J Cancer. 1995;31B:68–72. doi: 10.1016/0964-1955(94)00044-5. [DOI] [PubMed] [Google Scholar]
- 82.Millen FJ, Rainey MG, Hows JM, Burton PA, Irvine GH, Swirsky D. Oral squamous cell carcinoma after allogenic bone marrow transplantation for Fanconi’s anemia. Br J Haematol. 1997;99:410–414. doi: 10.1046/j.1365-2141.1997.3683184.x. [DOI] [PubMed] [Google Scholar]
- 83.Marcou Y, Andrea AD, Jeggo PA, Plowman PN. Normal cellular radiosensitivity in an adult Fanconi anemia patient with marked clinical radiosensitivity. Radiother Onc. 2001;60:75–79. doi: 10.1016/s0167-8140(01)00370-x. [DOI] [PubMed] [Google Scholar]
- 84.Carvalho JP, Dias MLN, Carvalho FM, Diz MDPE, Petitos JW. Squamous cell vulvar carcinoma associated with Fanconi’s anemia: a case report. Int J Gynecol Cancer. 2002;12:220–222. doi: 10.1046/j.1525-1438.2002.01090.x. [DOI] [PubMed] [Google Scholar]
- 85.Leong T, Borg M, McKay M. Clinical and cellular radiosensitivity in inherited human syndromes. Clinical Oncol. 2004;16:206–209. doi: 10.1016/j.clon.2004.01.011. [DOI] [PubMed] [Google Scholar]