Abstract
Sinorhizobium meliloti is a soil bacterium that fixes atmospheric nitrogen in plant roots. The high genetic diversity of its natural populations has been the subject of extensive analysis. Recent genomic studies of several isolates revealed a high content of variable genes, suggesting a correspondingly large phenotypic differentiation among strains of S. meliloti. Here, using the Phenotype MicroArray (PM) system, hundreds of different growth conditions were tested in order to compare the metabolic capabilities of the laboratory reference strain Rm1021 with those of four natural S. meliloti isolates previously analyzed by comparative genomic hybridization (CGH). The results of PM analysis showed that most phenotypic differences involved carbon source utilization and tolerance to osmolytes and pH, while fewer differences were scored for nitrogen, phosphorus, and sulfur source utilization. Only the variability of the tested strain in tolerance to sodium nitrite and ammonium sulfate of pH 8 was hypothesized to be associated with the genetic polymorphisms detected by CGH analysis. Colony and cell morphologies and the ability to nodulate Medicago truncatula plants were also compared, revealing further phenotypic diversity. Overall, our results suggest that the study of functional (phenotypic) variability of S. meliloti populations is an important and complementary step in the investigation of genetic polymorphism of rhizobia and may help to elucidate rhizobial evolutionary dynamics, including adaptation to diverse environments.
Sinorhizobium meliloti is a soil bacterium belonging to the Rhizobiales group of the Alphaproteobacteria subdivision, which includes pathogens, such as Bartonella and Brucella, and several plant-associated bacteria of relevant agricultural importance, such as Agrobacterium, Ochrobactrum, Bradyrhizobium, Mesorhizobium, and Rhizobium (21). S. meliloti forms nitrogen-fixing nodules on the roots of leguminous plants of the genera Medicago, Melilotus, and Trigonella, and it is probably the best-studied model system for the rhizobium-legume symbiosis. S. meliloti is distributed worldwide and is present in many soil types, both in association with legumes and in free-living form (40). The ubiquitous occurrence of this species suggested a wide metabolic capability allowing adaptation to very different environmental and nutritional conditions. S. meliloti has been the subject of extensive genetic, biochemical, and metabolic research (25). The sequencing of the strain Rm1021 genome (4, 11, 17, 18) provided a solid foundation for a number of molecular studies of the genetic basis of plant-bacterium interactions and of the response of S. meliloti to environmental stimuli. Moreover, the genetic diversity of natural populations of S. meliloti has been the subject of extensive analyses (1-3, 8, 12, 24, 38, 42). These investigations showed that S. meliloti populations are very diverse and that S. meliloti strains harbor a high number of different mobile genetic elements, such as insertion sequences, transposons, and bacterial mobile introns (6, 7, 22). A genome-wide investigation of the genetic polymorphism of four environmental strains in comparison with the sequenced strain Rm1021 was also carried out by comparative genomic hybridization (CGH) (19), indicating an average diversity of 5 to 6% at the open reading frame (ORF) level. Strains of S. meliloti, as for other rhizobial species (35), are known to show different nodulation capabilities and phenotypic characteristics, such as salt and stress tolerance and exopolysaccharide production (31, 43, 48). Despite the large number of genetic and molecular biology studies of the sequenced Rm1021 strain and its natural populations, little is known about the overall extent of metabolic diversity of Rm1021 and environmental strains. Consequently, clear evidence on possible functional and metabolic roles of the observed genomic polymorphism is still lacking. In particular, phenotypes that are variable and the extent of the phenotypic variation that is subjected to natural selection and could consequently have an adaptive role are still unknown for S. meliloti. In past years, more attention has been focused on that part of bacterial genetic variation which is directly related to the phenotype (44). From this perspective, comparative transcriptomic analysis has been a powerful tool for exploring the metabolic variability in both eukaryotic and prokaryotic organisms (47). Recently, direct high-throughput assessment of phenotypes (phenome) using the Phenotype MicroArray (PM) system (Biolog) (9) has stirred much attention for molecular biology, genomic, and population studies of microorganisms (14, 20, 29, 33, 34, 37, 45, 46).
Attempting to shed some light on the metabolic abilities of S. meliloti, we investigated, using 571 different conditions, the metabolic activities of strain Rm1021 and four environmental strains for which the amount of genomic polymorphism was previously assessed by CGH analysis (19). Their growth characteristics, such as colony and cell morphology, and their symbiotic abilities were also studied.
MATERIALS AND METHODS
Media and strains used.
The strains used in this work came from two world regions: two of them, BL225C and BO21CC, were trapped on Medicago sativa plants grown on soil of Lodi, Italy, in the course of previous experiments (12), while AK58 and AK83 were collected by RIAM (St. Petersburg, Russia) by trapping from soil samples of Kazakhstan, North Aral Sea region, during May 2001 using Medicago falcata. S. meliloti strains, except in the Biolog experiments, were cultured on solid or liquid TY medium (5) with 0.2 g/liter CaCO3. Strain Rm1021 (provided by F. Ausubel) was used as the reference strain.
Nodulation of M. truncatula in a sterile system.
Seedlings of M. truncatula cv. ‘Jemalong’ were germinated for 48 h in the dark at room temperature and transferred directly to sterile plastic petri dishes containing buffered Nod medium (15) supplemented with 1 μM AVG (ethylene biosynthesis inhibitor amino-ethoxy-vinyl-glycine) in 1.5% (wt/vol) noble agar (Sigma). Plantlets were grown for an additional 5 to 7 days before inoculation with S. meliloti strains. Ten plates for each strain were sealed with Parafilm, with a hole to let the plant grow outside, and transferred in a near-vertical position to a growth chamber maintained at 26°C with a 16-h photoperiod (100 microeinstein m−2 s−1). The number of nodules and weight of plant dry matter were scored after 30 days.
PM analysis.
Strains Rm1021, BL225C, B021CC, AK58, and AK83 were assayed on PM (Biolog) microplates PM1 to PM4, PM9, and PM10, testing 571 different conditions. PM technology uses the irreversible reduction of tetrazolium violet to formazan as a reporter of active metabolism (9). The reduction of the dye causes the formation of a purple color that is recorded by a charge-coupled-device camera every 15 min and provides quantitative and kinetic information about the response of the cells in PM plates. PM data were analyzed to compare the growth rates of different strains (27).
All procedures were performed as indicated by the manufacturer. Strains were grown at 30°C on BUG agar (Biolog), and then, each strain was picked with a sterile cotton swab from the agar surface and suspended in 15 ml of inoculation fluid (IF-0; Biolog) until a cell density of 85% transmittance was reached on a Biolog turbidimeter. In order to inoculate microplates PM1 to PM4, 1% tetrazolium violet (vol/vol) was added to the suspension and the mixture was inoculated (100 μl per well). Inocula for PM9 and PM10 were prepared as described by Viti et al. (46). All PM microplates were incubated at 30°C in an OmniLog reader, and changes of color in the wells were monitored automatically every 15 min. Readings were recorded for 72 h, and data were analyzed using OminoLog PM software (release OM_PM_109M), which generated a time course curve for tetrazolium color development. Each strain was analyzed at least in duplicate as previously described (9), and the results were checked for consistency (39).
Statistical analysis of PM results.
For each well, the product of the average area and the average slope of the time course data were used to define an arbitrary threshold (45, 000) to score growth in the various PM conditions. The well was scored as “metabolized by the strain” if the product exceeded the threshold value (see the green color in Table S2 in the supplemental material). Wells with a high average area value but with a slope close to zero (reduction of the tetrazolium violet by the medium) (see F7-PM10 in Table S2 and Fig. S2 in the supplemental material) were weighted as less significant than wells with a smaller area but increasing signal over time (see G1-PM9 in Table S2 and Fig. S2 in the supplemental material). Multiplication by the average slope guarantees that only wells with an increasing signal and a sufficient total area represent a “metabolically active” growth condition.
For principal component analysis (PCA), PM data were filtered using Average Height/Inflection Time as a parameter and subsequently processed with Bionumerics (Applied Math, Kortrijk, Belgium). The general metabolic diversity (see Table S4 in the supplemental material) between different plates was estimated after 72 h of growth by using average area data with the Bray-Curtis dissimilarity matrix, computed using NTSYS (pc version 2.02) (39).
The differences were calculated as the ratio between the average area of a strain for a condition and the minimal average area found among strains under that condition (see the color scale in Fig. 3). This parameter gives an indication of the spread of metabolic activities of the five strains for each PM condition that showed diversity: a well where all strains grow equally has a value close to one, while variable wells are those where at least one strain is different from the others; this parameter can be a number often larger than several units. In Fig. 3, only conditions with this parameter being larger than 2 are shown.
FIG. 3.
Metabolic differences among S. meliloti strains in PMs. Differences are calculated as the ratio between the average area and the minimal average area among strains for each condition. A color code is used (see Table S3 in the supplemental material) as follows: white (no difference), ratio of <2; light gray, ratio of between 2 and 4; dark gray, ratio of between 4 and 8; and black, ratio of >8. PM plate numbers and compounds tested are reported.
The CGH data were analyzed by PCA (see Fig. S3 in the supplemental material). The sequences' differences were first scored as a binary matrix, where “1” corresponded to identical genes and “0” corresponded to genetic differences (19). Then, a simple matching coefficient was used to infer a distance matrix among strains and used for PCA analysis by using the software NTSYSpc, version 2.02 (39).
Verification of CGH data by PCR and sequencing.
Verification of CGH data (19) was done by PCR amplification and sequencing of the genes of interest. The primer pairs for PCR amplification of gene regions included the CGH probe (19). The following primer pairs were used: for SMa0697 (5′-GCCTATCCGCTCGATATACT-3′ and 5′-TGCATTCACAAAATGGCAAG-3′), for SMa1250 (5′-ATGCCTCGATCAGGTTATGG-3′ and 5′-CGTGCACCAGGACGACTAT-3′), and for SMa1038 (5′-ACGAGTTCGACGCCAAATAC-3′ and 5′-GTTTTCGGGGATTTCCATTT-3′).
RESULTS
Preliminary analysis of phenotypes.
The four S. meliloti strains used in this work, together with reference strain Rm1021, were chosen because they were previously analyzed by CGH analysis (19). In order to start from a better phenotypical knowledge of those strains, cell and colony morphology and the ability to nodulate a model legume were analyzed.
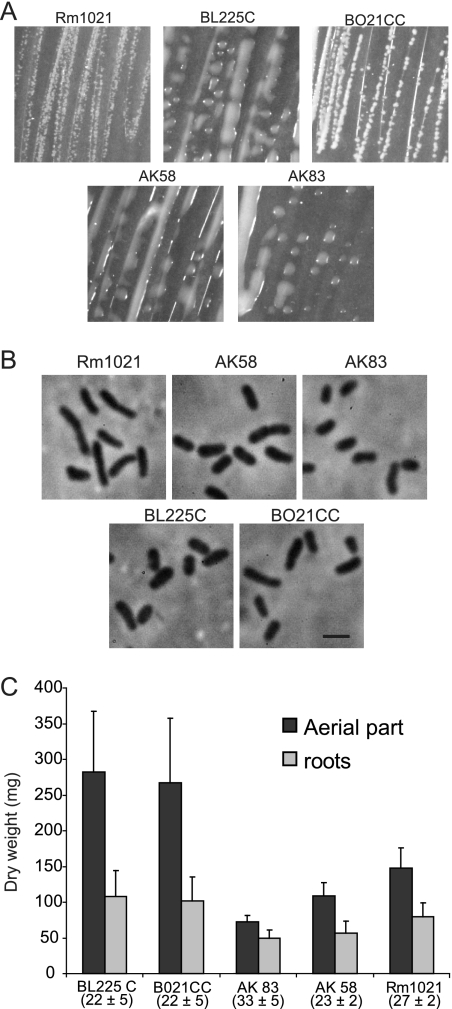
The colony morphologies of these strains varied: BL225C, AK58, and AK83 were very mucous, while BO21CC appeared more similar to Rm1021 (Fig. 1A). Also, cells, observed using phase-contrast microscopy, appeared different in shape and size among different isolates (Fig. 1B; see Table S1 in the supplemental material).
FIG. 1.
Morphology and nodulation of the investigated strains. (A) Colony morphologies of the strains. (B) Phase-contrast microscopy observations of cell shapes of the five strains (Leica microscope equipped with N Plan objective at ×100 magnification; numerical aperture, 1.25). Scale bar is 2 μm. (Cell length, diameter, and volume were estimated; see Table S1 in the supplemental material). (C) Efficiency of nodulation estimated as dry weight of nodulated root and aerial parts of M. truncatula ‘Jemalong’. Error bars in the histograms indicate standard deviations. Numbers below the strain names indicate the average number of nodules per plant, with standard deviation.
The strains were also analyzed for the ability to nodulate M. truncatula plants. The results showed a high variability of symbiosis efficiency, defined as dry weight of roots and stems of plants. BL225C and BO21CC were nearly twofold more efficient than the Aral sea isolates (Fig. 1C). Nodule numbers did not show significant differences among isolates and did not appear to be correlated with plant weight (data not shown).
These preliminary analyses revealed that natural isolates of S. meliloti are quite variable, exhibiting differences in colony/cell morphology and plant growth-promoting activity (Fig. 1A to C).
PM analysis of Sinorhizobium meliloti type strain Rm1021.
The metabolic abilities of strain Rm1021 were tested by using the PM system (Biolog). Using PM plates 1 to 4, 571 different growth conditions were tested, including 190 different carbon sources, 95 nitrogen sources, 59 phosphorus sources, 35 sulfur sources, and tolerances to different osmolytes and pH conditions (see Table S2 in the supplemental material for a complete set of results). Metabolically active conditions were scored using a threshold calculation based on growth curve data (see Materials and Methods). S. meliloti strain Rm1021 was able to metabolize 44% of tested carbon sources, 88% of nitrogen sources, 80% of sulfur sources, and 93% of phosphorus sources (see Fig S1 and Table S2 in the supplemental material). As previously reported (28), strain Rm1021 is very efficient in utilizing unusual phosphorus compounds (56/60 tested, plate PM4, wells A1-E12). The phosphorus compounds that were not utilized by S. meliloti were carbamyl phosphate, cytidine-3′,5′-cyclic monophosphate, methylene diphosphonic acid, and thymidine-5′-monophosphate.
S. meliloti was found to be able to assimilate a wide variety of S-containing compounds; only six sulfur sources, d,l-ethionine, l-methionine sulfoxide, d,l-lipoamide, p-aminobenzene-sulfonic acid, methanesulfonic acid, and tetramethylene sulfone, failed to support growth.
Using the PM3 plate, the four environmental strains were tested for their ability to grow on 95 different nitrogen sources (see Fig. S1 and Table S2 in the supplemental material). The first striking result was the apparent growth in the negative control without any nitrogen source (PM3, well A1) that was observed in all replicates and in all strains. To test whether this activity was due to real nitrogen fixation or contamination by nitrogen compounds from the preinoculum or other wells, we carried out several control experiments, including the use of a nifH mutant (23), that demonstrated that the metabolic activity observed was not due to nitrogen fixation (see Fig. S2 in the supplemental material).
Nineteen out of 95 nitrogen sources supported growth at the level of the negative control, indicating that S. meliloti cannot metabolize these compounds; these were N-amylamine, d-asparagine, β-phenylethylamine, l-threonine, putrescine, d-mannosamine, N-butylamine, d-glutamic acid, l-homoserine, tyramine, l-phenylalanine, acetamide, d-aspartic acid, d-lysine, N-acetyl-d-mannosamine, formamide, ethylamine, ɛ-amino-N-caproic acid, and l-methionine.
Twelve compounds supported growth at a level lower than the negative control; these were l-tryptophan, d-asparagine, d-serine, d-valine, N-acetyl-d,l-glutamic acid, N-phthaloyl-l-glutamic acid, hyroxylamine, ethylenediamine, d-galactosamine, d,l-α-amino-N-butyric acid, d,l-α-aminocaprylic acid, and α-amino-N-valeric acid. These compounds appear to inhibit the growth of Rm1021, although the mechanism is not yet understood.
Plates PM9 and PM10 were used to test growth under various stress conditions. Rm1021 showed active metabolism with up to 1% sodium chloride, up to 3% sodium sulfate, ethylene glycol (up to 20%), urea (up to 2%), and sodium nitrate (up to 100 mM). The pH range where S. meliloti Rm1021 exhibited active growth was between 6 and 10, with an optimal pH of around 7.0.
Metabolic differences among S. meliloti isolates.
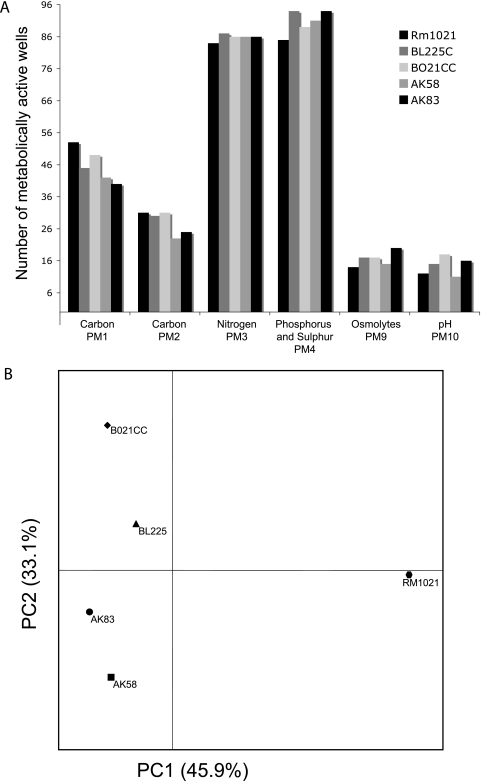
The metabolic activities of the four S. meliloti environmental isolates, BL225C, BO21CC, AK58, and AK83, were assayed with PM plates using Rm1021 as the reference strain (Fig. 2A and 3; see Table S3 in the supplemental material). We found that 18% (106 out of 571) of the conditions tested revealed a phenotypic difference among the five strains, using a criterion of at least a twofold difference in growth (Fig. 3).
FIG. 2.
Summary of phenotypic diversity of S. meliloti strains. (A) Plot of the numbers of wells per strain and per PM plate for conditions with active metabolism. (B) PCA results for PM profiles obtained from an analysis of 517 phenotypic attributes.
PCA performed on these data easily distinguished the environmental isolates from the reference one (Fig. 2B). In contrast, the same analysis carried out on CGH data did not show such striking separation among the strains (see Fig. S3 in the supplemental material).
The PM plates showed different powers of discrimination between the five S. meliloti isolates (Fig. 2A). Using data from PM1 and PM2 (carbon sources), the most metabolically versatile strains were shown to be reference strain Rm1021 and isolate BO21CC; they were able to use 104 and 102 different carbon sources, respectively. In particular, BO21CC and, partially, AK83, BL225C, and AK58 showed metabolic activity on several organic acids and glucosides (d-gluconic acid, α-keto-glutaric acid, α-ketobutyric acid, propionic acid, and α-methyl-d-glucoside), whereas Rm1021 was unable to utilize these compounds. For nitrogen, phosphorus, sulfur, osmolytes, and pH, the isolates showed higher versatilities than strain Rm1021 (Fig. 2A).
To better evaluate which PM plate was able to capture the metabolic diversity among these strains, the average Bray-Curtis dissimilarity was computed on the complete data set of 571 conditions (see Table S4 in the supplemental material). The most discriminating plates were PM1, PM2, PM9, and PM10, displaying mean similarity values of 0.155, 0.166, 0.207, and 0.206, respectively. In particular, conditions in the “osmolyte” and “pH” plates that were able to differentiate strains of S. meliloti were the following: sodium sulfate (3%; Rm1021 and AK83), sodium phosphate (20 mM; BO21CC and AK83), ammonium sulfate (10 to 20 mM; BO21CC and AK83), and sodium nitrite (40 to 60 mM, BL225C). PM3 and PM4 were less informative, displaying mean dissimilarities of 0.089 and 0.086 among the strains. In contrast, the PM5 plate had no discriminatory power (average dissimilarity, 0.067).
We then attempted to combine the results of our PM analysis with previous CGH data in order to explain the phenotypic differences observed in the metabolic capabilities of the environmental strains. Using the KEGG database (26; http://www.genome.jp/kegg/), genes predicted to be involved in metabolic pathways that showed diversity based on our PM analysis were identified. These genes were then examined for genetic differences found in the CGH analysis. With the exception of genes involved in nitrogen metabolism (see below), none of the genes were found by CGH to contain genetic differences. It appears that diversity among the environmental strains does not involve polymorphisms in metabolic enzymes.
Nitrite/ammonium resistance: comparison between phenotypes and CGH analysis.
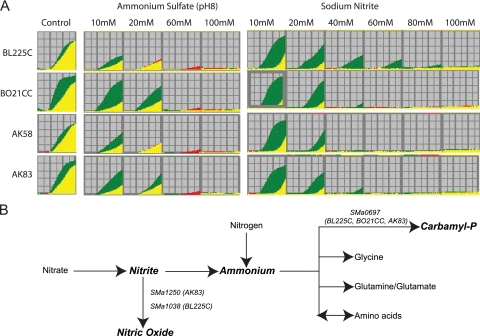
As shown in Fig. 4, strains BO21CC and AK83 displayed higher metabolic activities in 10 mM and 20 mM ammonium sulfate, pH 8, while the other strains could grow at 10 mM but with lower activities. Moreover, strain BL225C showed metabolic activity with up to 40 to 60 mM sodium nitrite, while BO21CC, AK58, and AK83 displayed active metabolism only up to 20 mM (Fig. 4A).
FIG. 4.
Phenotypic and genetic variation in ammonium and nitrite metabolism of S. meliloti strains. (A) PM kinetics from PM10 plate for wells with different concentrations of ammonium sulfate and sodium nitrite. The PM kinetic results show consensus data comparing the natural isolate (green) with strain Rm1021 (red). When the two strains reveal an equivalent activity or equivalent growth response in a well, the kinetic graphs overlap and are yellow. Red kinetic graphs indicate a stronger response by strain Rm1021, and green kinetic graphs a stronger response by the environmental isolates. (B) The metabolic pathways of ammonium and nitrite as depicted from KEGG annotation. Polymorphic genes (based on CGH data) are reported on the pathways where their products are involved. Strain names, where those genes were found to be polymorphic, are in brackets. Carbamyl-P, carbamyl phosphate.
The bioinformatic metabolic reconstruction of nitrite and ammonium metabolism in S. meliloti strains using KEGG revealed a set of genes possibly involved in determining the metabolic differences observed (Fig. 4B and Table 1). In the CGH analysis, most of those genes were found to be identical among the environmental isolates. SMa2145 was mutated (the probe region has mutations or it is absent) in all strains. Six genes were found to be polymorphic, either mutated or duplicated, in at least one strain. Within these polymorphic genes, we further analyzed three whose CGH genomic divergence from Rm1021 (see Table S5 in the supplemental material) matched the divergence of phenotypes under conditions of sodium nitrite and ammonium sulfate, pH 8; these were SMa0697, encoding a carbamate kinase; SMa1250, encoding a NirK Cu-nitrite reductase; and SMa1038, encoding a putative copper-containing oxidase. All of these genes are predicted to be involved in ammonium and nitrite metabolism and are in fact diverse among the strains analyzed.
TABLE 1.
All genes involved in nitrogen metabolism and their CGH dataa
| Reactionb | Gene; name of enzyme encoded | CGH result for indicated strainc
|
|||
|---|---|---|---|---|---|
| BL225C | BO21CC | AK58 | AK83 | ||
| Nitrate → nitrite | SMa1236; NapA periplasmic nitrate reductase | No | No | No | No |
| SMb20986; putative nitrate reductase, large subunit | No | No | No | No | |
| Nitrite → nitric oxide | SMa1250; putative NirK Cu-nitrite reductase | No | No | No | Mut |
| SMa1038; putative copper-containing oxidase | Mut | No | No | No | |
| SMc02282; putative copper-containing oxidase | No | No | No | No | |
| Nitrite → ammonium | SMb20984; NirB, putative nitrite reductase | No | No | No | No |
| SMb20985; NirD, putative nitrite reductase | No | No | No | No | |
| Ammonium → carbamyl phosphate | SMa0697; carbamate kinase | Mut | Mut | No | Mut |
| Ammonium → glycine | SMa2145; aminomethyltransferase | Mut | Mut | Mut | Mut |
| SMc04148; aminomethyltransferase | No | No | No | No | |
| SMc02047; aminomethyltransferase | No | No | No | No | |
| Ammonium → cyclic amidines | SMc00161; NAD synthetase | No | No | No | No |
| Ammonium → glutamine | SMb20745; GlnII, putative glutamine synthetase II protein | No | No | No | No |
| SMc00762; putative glutamine synthetase protein | No | No | No | No | |
| SMc00948; GlnA, glutamine synthetase I protein | No | No | No | Dup | |
| SMc01973; putative protein homologous to a glutamine synthetase protein | No | No | No | No | |
| SMc02352; putative glutamine synthetase protein | No | No | No | No | |
| SMc02613; GlnT, glutamine synthetase III protein | No | No | No | No | |
| SMc00486; GlsA, glutaminase | No | No | No | No | |
| SMa0306; putative histidine ammonia-lyase | No | No | No | No | |
| Ammonium ↔ alpha amino acids | SMb21165; HutH, histidine ammonia-lyase | No | No | No | No |
| SMc00669; HutH2, histidine ammonia-lyase | No | No | No | No | |
| SMb20267; putative d-amino acid dehydrogenase protein | No | No | No | No | |
| SMc03265; putative amino acid dehydrogenase transmembrane protein | Mut | No | No | No | |
| SMb21529; TauD, putative oxidoreductase protein | No | Dup | No | No | |
| SMb21670; taurine dehydrogenase, small subunit | No | No | No | No | |
These genes were shown by previous CGH analysis to have deletion/mutations (19) in the following strains: SMa0697 in BO21CC, BL225C, and AK83; SMa1250 in AK83; and SMa1038 in BL225C. To confirm the CGH data, we amplified a portion of those genes containing the CGH probe sequence. The results obtained showed that SMa1250, SMa1038, and SMa0697 were absent in the tested strains (data not shown).
DISCUSSION
The link between genetic and phenotypic diversity in bacterial populations is still poorly explored, especially at the systematic level. Here, for the first time, the metabolic abilities of S. meliloti Rm1021 were systematically analyzed using PMs (Biolog) and compared to those of four environmental isolates. Using PM technology, we uncovered significant metabolic diversity among these strains. We then used previous CGH data to find a correlation between genetic and phenotypic diversity. The strains used in this work showed interesting phenotypic differences even at a basic level of investigation (cell/colony morphology and nitrogen fixation efficiency). Even though colony morphology varied between mucoid and dry, the production of an extracellular matrix does not correlate with symbiotic ability, since the two most efficient strains nodulating M. truncatula have opposite colony phenotypes: BL225C is mucoid and BO21CC is nonmucoid.
Metabolic analysis by PM confirmed the large phenotypic differences previously reported among strains (19). PCA on the PM data easily captured the differences between the sequenced Rm1021 strain and environmental strains, and it was used to discriminate among the strains. In contrast, the CGH data were not able to separate the environmental isolates from strain Rm1021. These results suggest that CGH analysis, showing only differences in ORFs, does not fully address genetic variations that may directly impact phenotype and drive the adaptation of bacteria to their environment. In fact, several of the variable ORFs found in CGH analysis were classified as mobile genetic elements, like transposases, whose polymorphism is, in most cases, neutral, and consequently not directly linked to a particular phenotype or metabolic ability (41).
In comparing S. meliloti data from all PM plates with data reported on the Biolog website for other bacterial species (Escherichia coli, Burkholderia cepacia, Salmonella enterica serovar Typhimurium, Pseudomonas aeruginosa, Ralstonia solanacearum, and Ralstonia pickettii), it emerges that S. meliloti exhibits a wider range of carbon source utilization, especially for compounds present on PM2, such as galactosides. In addition, most of the nitrogen, sulfur, and phosphorus sources were also metabolized by S. meliloti. This metabolic diversity is consistent with the results of bioinformatic analysis of the genome (18) that identified a large fraction of genes (12%) as being related to transport systems. Many of these genes are found on the pSymB megaplasmid, including several genes related to metabolic pathways for carbon source utilization (17). These data confirm the general view of the lifestyle of S. meliloti, which is a bacterium that is widespread on temperate soils and has a fair ability to colonize both soils and plants (40). In order to exploit this environmental niche, the utilization of a wide range of nutrient sources has great adaptive value. We found that different PM plates have various discriminatory powers among the tested strains (Fig. 2; see Table S4 in the supplemental material). The most informative plates were PM1/PM2 (carbon sources) and PM9/PM10 (osmolytes and pH conditions). The most informative utilization patterns for carbon sources were organic acids and glucosides. These compounds are commonly found in many plant exudates (16). Also, galactoside utilization seems to play an important role in supporting the growth of S. meliloti near germinating seeds of alfalfa (10). Consequently, phenotypic differentiation for utilization of those carbon sources could have a high adaptive value in plant-microbe interaction and survival in soil and could also affect nodulation efficiency on diverse plant host species or genotypes. Moreover, this feature is consistent with the observation that the tested strains showed different growth characteristics, such as cell and colony morphology, in rich medium.
The phenotypic diversity found using plates PM9/PM10 can be rationalized by considering the seasonal variation in soil pH and osmolytes due to varying conditions of dryness and watering. Both pH and osmolarity are common stresses present in the natural habitat of S. meliloti and exert selective pressure on the population (13, 49).
Among conditions where strains of S. meliloti differed in their growth response, 65% of the test compounds could be assigned to a metabolic pathway in the KEGG database, allowing comparison between genotype (CGH data) and phenotype (PM results). In general, we did not find any CGH differences in genes related to those metabolic pathways. This lack of a correlation between genetic (CGH) and phenotypic (PM) diversity was also found in previous studies of other bacterial species (34, 36), which could be because few data on transporters are available and, also, because the KEGG database only represents the main metabolic pathways. However, in attempting to address those problems, even using a recent specific annotation (30), none of the newly annotated transporters related to the carbon sources was found among the variable genes from CGH.
Using PM10, we did find a correlation between the results for ammonium sulfate and sodium nitrite utilization and the underlying CGH data. In strains BL225C, BO21CC, and AK83, three genes, predicted to code for enzymes involved in nitrite and ammonium utilization, were mutated relative to those in strain Rm1021. In contrast, AK58, which was phenotypically most similar to the reference strain Rm1021, had no polymorphisms in those genes. Further investigations are necessary to elucidate a possible ecological role for such metabolic and genetic differences. The observation that strains resistant to high nitrate and ammonium concentrations do not possess genes that are predicted to be involved in the corresponding metabolic pathways suggests that other genetic determinants could be responsible for this phenotype.
Finally, the incongruity between the CGH and PM results revealed that phenotypic diversity is most likely due to regulatory factors or to genetic differences that are not detected by CGH analysis, such as the presence of genes carried only by natural strains or regulatory sequences that are located outside the CGH probe sequence. Although most metabolic pathways are apparently conserved among the environmental strains, differences in transcriptional regulation might explain the metabolic diversity of these strains. Our observations suggest that genome sequencing of the environmental isolates and comparative gene expression studies will be necessary to elucidate the link between the genetic and phenotypic diversity found in natural populations of S. meliloti bacteria.
Supplementary Material
Acknowledgments
We are grateful to Genexpress Laboratory (Department of Agricultural Biotechnology, University of Florence, Italy) for the OmniLog apparatus. We also thank Francesco Pini for his experimental help. Finally, we are grateful to Marina Roumiantseva for providing analyzed strains from Kazakhstan and to Delphine Capela for kindly providing the nifH::Tn5 derivative of strain Rm1021. A special acknowledgment to Jeffrey M. Skerker for his critical reading of the manuscript.
Emanuele G. Biondi is supported by a research grant from Fondazione Adriano Buzzati-Traverso.
Footnotes
Published ahead of print on 26 June 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Badri, Y., K. Zribi, M. Badri, T. Huguet, P. van Berkum, and M. E. Aouani. 2007. Comparison of rhizobia that nodulate Medicago laciniata and Medicago truncatula present in a single Tunisian arid soil. Can. J. Microbiol. 53:277-283. [DOI] [PubMed] [Google Scholar]
- 2.Bailly, X., I. Olivieri, B. Brunel, J. C. Cleyet-Marel, and G. Bena. 2007. Horizontal gene transfer and homologous recombination drive the evolution of the nitrogen-fixing symbionts of Medicago species. J. Bacteriol. 189:5223-5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailly, X., I. Olivieri, S. De Mita, J. C. Cleyet-Marel, and G. Bena. 2006. Recombination and selection shape the molecular diversity pattern of nitrogen-fixing Sinorhizobium sp. associated to Medicago. Mol. Ecol. 15:2719-2734. [DOI] [PubMed] [Google Scholar]
- 4.Barnett, M. J., R. F. Fisher, T. Jones, C. Komp, A. P. Abola, F. Barloy-Hubler, L. Bowser, D. Capela, F. Galibert, J. Gouzy, M. Gurjal, A. Hong, L. Huizar, R. W. Hyman, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, C. Palm, M. C. Peck, R. Surzycki, D. H. Wells, K. C. Yeh, R. W. Davis, N. A. Federspiel, and S. R. Long. 2001. Nucleotide sequence and predicted functions of the entire Sinorhizobium meliloti pSymA megaplasmid. Proc. Natl. Acad. Sci. USA 98:9883-9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 6.Biondi, E. G., S. Fancelli, and M. Bazzicalupo. 1999. ISRm10: a new insertion sequence of Sinorhizobium meliloti: nucleotide sequence and geographic distribution. FEMS Microbiol. Lett. 181:171-176. [DOI] [PubMed] [Google Scholar]
- 7.Biondi, E. G., A. P. Femia, F. Favilli, and M. Bazzicalupo. 2003. IS Rm31, a new insertion sequence of the IS 66 family in Sinorhizobium meliloti. Arch. Microbiol. 180:118-126. [DOI] [PubMed] [Google Scholar]
- 8.Biondi, E. G., E. Pilli, E. Giuntini, M. L. Roumiantseva, E. E. Andronov, O. P. Onichtchouk, O. N. Kurchak, B. V. Simarov, N. I. Dzyubenko, A. Mengoni, and M. Bazzicalupo. 2003. Genetic relationship of Sinorhizobium meliloti and Sinorhizobium medicae strains isolated from Caucasian region. FEMS Microbiol. Lett. 220:207-213. [DOI] [PubMed] [Google Scholar]
- 9.Bochner, B. R., P. Gadzinski, and E. Panomitros. 2001. Phenotype microarrays for high-throughput phenotypic testing and assay of gene function. Genome Res. 11:1246-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bringhurst, R., Z. G. Cardon, and D. J. Gage. 2001. Galactosides in the rhizosphere: utilization by Sinorhizobium meliloti and development of a biosensor. Proc. Natl. Acad. Sci. USA 98:4540-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capela, D., F. Barloy-Hubler, J. Gouzy, G. Bothe, F. Ampe, J. Batut, P. Boistard, A. Becker, M. Boutry, E. Cadieu, S. Dreano, S. Gloux, T. Godrie, A. Goffeau, D. Kahn, E. Kiss, V. Lelaure, D. Masuy, T. Pohl, D. Portetelle, A. Puhler, B. Purnelle, U. Ramsperger, C. Renard, P. Thebault, M. Vandenbol, S. Weidner, and F. Galibert. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc. Natl. Acad. Sci. USA 98:9877-9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carelli, M., S. Gnocchi, S. Fancelli, A. Mengoni, D. Paffetti, C. Scotti, and M. Bazzicalupo. 2000. Genetic diversity and dynamics of Sinorhizobium meliloti populations nodulating different alfalfa cultivars in Italian soils. Appl. Environ. Microbiol. 66:4785-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coba de la Peña, T., D. Verdoy, F. J. Redondo, and J. J. Pueyo. 2003. Salt tolerance in the Rhizobium-legume symbiosis: an overview, p. 187-205. In S. G. Pandalai (ed.), Recent research developments in plant molecular biology, vol. 1. Research Signpost, Trivandrum, India. [Google Scholar]
- 14.dela Cruz, T. E., B. E. Schulz, C. P. Kubicek, and I. S. Druzhinina. 2006. Carbon source utilization by the marine Dendryphiella species D. arenaria and D. salina. FEMS Microbiol. Ecol. 58:343-353. [DOI] [PubMed] [Google Scholar]
- 15.Ehrhardt, D. W., E. M. Atkinson, and S. R. Long. 1992. Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti Nod factors. Science 256:998-1000. [DOI] [PubMed] [Google Scholar]
- 16.Fan, T. W., A. N. Lane, M. Shenker, J. P. Bartley, D. Crowley, and R. M. Higashi. 2001. Comprehensive chemical profiling of gramineous plant root exudates using high-resolution NMR and MS. Phytochemistry 57:209-221. [DOI] [PubMed] [Google Scholar]
- 17.Finan, T. M., S. Weidner, K. Wong, J. Buhrmester, P. Chain, F. J. Vorholter, I. Hernandez-Lucas, A. Becker, A. Cowie, J. Gouzy, B. Golding, and A. Puhler. 2001. The complete sequence of the 1,683-kb pSymB megaplasmid from the N2-fixing endosymbiont Sinorhizobium meliloti. Proc. Natl. Acad. Sci. USA 98:9889-9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galibert, F., T. M. Finan, S. R. Long, A. Puhler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 19.Giuntini, E., A. Mengoni, C. De Filippo, D. Cavalieri, N. Aubin-Horth, C. R. Landry, A. Becker, and M. Bazzicalupo. 2005. Large-scale genetic variation of the symbiosis-required megaplasmid pSymA revealed by comparative genomic analysis of Sinorhizobium meliloti natural strains. BMC Genomics 6:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guard-Bouldin, J., C. A. Morales, J. G. Frye, R. K. Gast, and M. Musgrove. 2007. Detection of Salmonella enterica subpopulations by phenotype microarray antibiotic resistance patterns. Appl. Environ. Microbiol. 73:7753-7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerrero, G., H. Peralta, A. Aguilar, R. Diaz, M. A. Villalobos, A. Medrano-Soto, and J. Mora. 2005. Evolutionary, structural and functional relationships revealed by comparative analysis of syntenic genes in Rhizobiales. BMC Evol. Biol. 5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Lucas, I., J. A. Ramirez-Trujillo, M. A. Gaitan, X. Guo, M. Flores, E. Martinez-Romero, E. Perez-Rueda, and P. Mavingui. 2006. Isolation and characterization of functional insertion sequences of rhizobia. FEMS Microbiol. Lett. 261:25-31. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch, A. M., M. Bang, and F. M. Ausubel. 1983. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J. Bacteriol. 155:367-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jebara, M., R. Mhamdi, M. E. Aouani, R. Ghrir, and M. Mars. 2001. Genetic diversity of Sinorhizobium populations recovered from different Medicago varieties cultivated in Tunisian soils. Can. J. Microbiol. 47:139-147. [DOI] [PubMed] [Google Scholar]
- 25.Jones, K. M., H. Kobayashi, B. W. Davies, M. E. Taga, and G. C. Walker. 2007. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat. Rev. Microbiol. 5:619-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanehisa, M., M. Araki, S. Goto, M. Hattori, M. Hirakawa, M. Itoh, T. Katayama, S. Kawashima, S. Okuda, T. Tokimatsu, and Y. Yamanishi. 2008. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 36:D480-D484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koo, B. M., M. J. Yoon, C. R. Lee, T. W. Nam, Y. J. Choe, H. Jaffe, A. Peterkofsky, and Y. J. Seok. 2004. A novel fermentation/respiration switch protein regulated by enzyme IIAGlc in Escherichia coli. J. Biol. Chem. 279:31613-31621. [DOI] [PubMed] [Google Scholar]
- 28.Krol, E., and A. Becker. 2004. Global transcriptional analysis of the phosphate starvation response in Sinorhizobium meliloti strains 1021 and 2011. Mol. Genet. Genomics 272:1-17. [DOI] [PubMed] [Google Scholar]
- 29.Loh, K. D., P. Gyaneshwar, E. Markenscoff Papadimitriou, R. Fong, K. S. Kim, R. Parales, Z. Zhou, W. Inwood, and S. Kustu. 2006. A previously undescribed pathway for pyrimidine catabolism. Proc. Natl. Acad. Sci. USA 103:5114-5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mauchline, T. H., J. E. Fowler, A. K. East, A. L. Sartor, R. Zaheer, A. H. F. Hosie, P. S. Poole, and T. M. Finan. 2006. Mapping the Sinorhizobium meliloti 1021 solute binding protein-dependent transportome. Proc. Natl. Acad. Sci. USA 103:17933-17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medeot, D. B., M. A. Bueno, M. S. Dardanelli, and M. G. de Lema. 2007. Adaptational changes in lipids of Bradyrhizobium SEMIA 6144 nodulating peanut as a response to growth temperature and salinity. Curr. Microbiol. 54:31-35. [DOI] [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Mols, M., M. de Been, M. H. Zwietering, R. Moezelaar, and T. Abee. 2007. Metabolic capacity of Bacillus cereus strains ATCC 14579 and ATCC 10987 interlinked with comparative genomics. Environ. Microbiol. 9:2933-2944. [DOI] [PubMed] [Google Scholar]
- 34.Morales, C. A., S. Porwollik, J. G. Frye, H. Kinde, M. McClelland, and J. Guard-Bouldin. 2005. Correlation of phenotype with the genotype of egg-contaminating Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 71:4388-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moschetti, G., A. Peluso, A. Protopapa, M. Anastasio, O. Pepe, and R. Defez. 2005. Use of nodulation pattern, stress tolerance, nodC gene amplification, RAPD-PCR and RFLP-16S rDNA analysis to discriminate genotypes of Rhizobium leguminosarum biovar viciae. Syst. Appl. Microbiol. 28:619-631. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee, A., M. K. Mammel, J. E. LeClerc, and T. A. Cebula. 2008. Altered utilization of N-acetyl-d-galactosamine by Escherichia coli O157:H7 from the 2006 spinach outbreak. J. Bacteriol. 190:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberhardt, M. A., J. Puchałka, K. E. Fryer, V. A. P. Martins dos Santos, and J. A. Papin. 2008. Genome-scale metabolic network analysis of the opportunistic pathogen Pseudomonas aeruginosa PAO1. J. Bacteriol. 190:2790-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paffetti, D., F. Daguin, S. Fancelli, S. Gnocchi, F. Lippi, C. Scotti, and M. Bazzicalupo. 1998. Influence of plant genotype on the selection of nodulating Sinorhizobium meliloti strains by Medicago sativa. Antonie van Leeuwenhoek 73:3-8. [DOI] [PubMed] [Google Scholar]
- 39.Rohlf, F. J. 1990. NTSYS-pc, Numerical Taxonomy and Multivariate Analysis System, version 2.02. Exeter Software, Setauket, NY.
- 40.Sadowsky, M. J., and P. H. Graham. 1998. Soil biology of the Rhizobiaceae, p. 155-172. In H. P. Spaink, A. Kondorosi, and P. J. J. Hooykaas (ed.), The Rhizobiaceae: molecular biology of model plant-associated bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 41.Schneider, D., and R. E. Lenski. 2004. Dynamics of insertion sequence elements during experimental evolution of bacteria. Res. Microbiol. 155:319-327. [DOI] [PubMed] [Google Scholar]
- 42.Silva, C., F. L. Kan, and E. Martinez-Romero. 2007. Population genetic structure of Sinorhizobium meliloti and S. medicae isolated from nodules of Medicago spp. in Mexico. FEMS Microbiol. Ecol. 60:477-489. [DOI] [PubMed] [Google Scholar]
- 43.Simsek, S., T. Ojanen-Reuhs, S. B. Stephens, and B. L. Reuhs. 2007. Strain-ecotype specificity in Sinorhizobium meliloti-Medicago truncatula symbiosis is correlated to succinoglycan oligosaccharide structure. J. Bacteriol. 189:7733-7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suerbaum, S., and C. Josenhans. 2007. Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat. Rev. Microbiol. 5:441-452. [DOI] [PubMed] [Google Scholar]
- 45.Tracy, B. S., K. K. Edwards, and A. Eisenstark. 2002. Carbon and nitrogen substrate utilization by archival Salmonella typhimurium LT2 cells. BMC Evol. Biol. 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viti, C., F. Decorosi, E. Tatti, and L. Giovannetti. 2007. Characterization of chromate-resistant and -reducing bacteria by traditional means and by a high-throughput phenomic technique for bioremediation purposes. Biotechnol. Prog. 23:553-559. [DOI] [PubMed] [Google Scholar]
- 47.Whitehead, A., and D. L. Crawford. 2006. Variation within and among species in gene expression: raw material for evolution. Mol. Ecol. 15:1197-1211. [DOI] [PubMed] [Google Scholar]
- 48.Williams, A., A. Wilkinson, M. Krehenbrink, D. M. Russo, A. Zorreguieta, and J. A. Downie. 2008. Glucomannan-mediated attachment of Rhizobium leguminosarum to pea root hairs is required for competitive nodule infection. J. Bacteriol. 190:4706-4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zahran, H. 2001. Rhizobia from wild legumes: diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J. Biotechnol. 91:143-153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.