Abstract
Shewanella oneidensis MR-1, a facultatively anaerobic gammaproteobacterium, respires a variety of anaerobic terminal electron acceptors, including the inorganic sulfur compounds sulfite (SO32−), thiosulfate (S2O32−), tetrathionate (S4O62−), and elemental sulfur (S0). The molecular mechanism of anaerobic respiration of inorganic sulfur compounds by S. oneidensis, however, is poorly understood. In the present study, we identified a three-gene cluster in the S. oneidensis genome whose translated products displayed 59 to 73% amino acid similarity to the products of phsABC, a gene cluster required for S0 and S2O32− respiration by Salmonella enterica serovar Typhimurium LT2. Homologs of phsA (annotated as psrA) were identified in the genomes of Shewanella strains that reduce S0 and S2O32− yet were missing from the genomes of Shewanella strains unable to reduce these electron acceptors. A new suicide vector was constructed and used to generate a markerless, in-frame deletion of psrA, the gene encoding the putative thiosulfate reductase. The psrA deletion mutant (PSRA1) retained expression of downstream genes psrB and psrC but was unable to respire S0 or S2O32− as the terminal electron acceptor. Based on these results, we postulate that PsrA functions as the main subunit of the S. oneidensis S2O32− terminal reductase whose end products (sulfide [HS−] or SO32−) participate in an intraspecies sulfur cycle that drives S0 respiration.
Microbial reduction of inorganic sulfur compounds is central to the biogeochemical cycling of sulfur and other elements such as carbon and metals (29). The ability to reduce elemental sulfur (S0) is found in members of both prokaryotic domains (20), including mesophilic deltaproteobacteria (Desulfovibrio vulgaris, Pelobacter carbinolicus, Geobacter sulfurreducens) (6, 9, 36, 51), thermophilic deltaproteobacteria (Desulfurella acetivorans) (39), gammaproteobacteria (Shewanella putrefaciens) (41), epsilonproteobacteria (Wolinella succinogenes) (49), cyanobacteria (“Oscillatoria limnetica”) (45), and hyperthermophilic archaea (1, 53). Partially reduced inorganic sulfur compounds such as tetrathionate (S4O62−), thiosulfate (S2O32−), and sulfite (SO32−) are also important electron acceptors in the biogeochemical cycling of sulfur (29, 51). S4O62−-reducing bacteria, for example, may produce S2O32− as a metabolic end product of S4O62− reduction, while S2O32− disproportionation is a key reaction catalyzed by sulfate-reducing bacteria, resulting in the formation of sulfate (SO42−) and sulfide (S2−) (26).
Shewanella oneidensis MR-1, a facultatively anaerobic gammaproteobacterium, respires a variety of compounds as an anaerobic electron acceptor, including the inorganic sulfur compounds S0, SO32−, S2O32−, and S4O62−; transition metals [e.g., Fe(III) and Mn(IV)]; and radionuclides [e.g., U(VI) and Tc(VII)] (8, 21, 41, 44, 50, 55, 56). The majority of studies of anaerobic respiration by S. oneidensis have focused on the mechanism of electron transport to transition metals and radionuclides (11, 14, 34, 46, 58, 59), while the mechanism of electron transport to inorganic sulfur compounds has not been thoroughly examined.
Microbial S0 respiration is postulated to occur via two pathways, both of which are based on an intraspecies sulfur cycle. In the first pathway (catalyzed by members of the genus Salmonella [20]), S2O32− is reduced, yielding HS− and SO32− (24). SO32− diffuses from the cell and reacts chemically with extracellular S0 to form S2O32−, which reenters the periplasm and is rereduced, thereby sustaining an intraspecies sulfur cycle. In the second pathway (catalyzed by W. succinogenes [24]), water-soluble polysulfides (Sn2−; n > 2), formed by chemical interactions of S0 at pHs >7 (52), are reduced stepwise in the periplasm to Sn − 12− and HS−. Similarly to what occurs with the first pathway, microbially produced HS− diffuses from the cell and reacts chemically with S0 to produce additional Sn2−, which reenters the periplasm and is rereduced to sustain an analogous intraspecies sulfur cycle (24).
Genetic analyses of S2O32− reduction-deficient mutants of Salmonella enterica serovar Typhimurium have demonstrated that phsA (denoting production of hydrogen sulfide) is required for HS− production during S2O32− respiration (10, 17, 22). In addition, phsA-deficient mutants are unable to reduce S0 as an electron acceptor (24). The phsA homolog of W. succinogenes (annotated as psrA, for polysulfide reduction) is required for S0 respiration (32, 37). W. succinogenes psrA is the first gene of a three-gene cluster (including psrA, psrB, and psrC) whose products encode a polysulfide reductase, a quinol oxidase, and a membrane anchor, respectively (15). In addition, the structure of the polysulfide reductase complex (PsrABC) from Thermus thermophilus has recently been solved, and results indicate that PsrC acts as a quinol oxidase that transfers electrons stepwise via PsrB and PsrA to Sn2− during anaerobic S0 respiration (27). The main objectives of the present study were to (i) identify the S. Typhimurium phsA homolog in the S. oneidensis genome, (ii) employ a newly constructed suicide cloning vector for in-frame gene deletion mutagenesis in S. oneidensis to delete the S. Typhimurium phsA homolog of S. oneidensis, and (iii) test the S. oneidensis psrA deletion mutant for respiratory activity on a combination of two electron donors and 11 electron acceptors, including the inorganic sulfur compounds S4O62−, S2O32−, and S0.
MATERIALS AND METHODS
Growth media and cultivation conditions.
S. oneidensis MR-1 was cultured at 30°C in Luria-Bertani (LB) medium (10 g liter−1 NaCl, 5 g liter−1 yeast extract, 10 g liter−1 tryptone) for genetic manipulations. For anaerobic growth experiments, cells were cultured in a defined salts medium (44) supplemented with lactate (18 mM) or formate (30 mM) as the carbon/energy source. Anaerobic growth experiments were carried out with 13-ml Hungate tubes (Bellco Glass, Inc.) filled with 10 ml of salts medium and sealed with black butyl rubber stoppers under an N2 atmosphere. Growth experiments were performed in two parallel yet independent incubations. In a second set of anaerobic growth experiments with S0 and S2O32−, S. oneidensis cultures were incubated with continual flushing of the headspace with N2 to remove bacterially produced HS− (37). Electron acceptors were added from filter-sterilized stocks (synthesized as described previously [13]), except where indicated, at the following final concentrations: O2 (atmospheric); NO3−, 10 mM; Fe(III) citrate, 50 mM; hydrous ferric oxide and Mn(III)-pyrophosphate, 40 mM (30) and 10 mM, respectively; trimethylamine-N-oxide (TMAO), 25 mM; S2O32−, 10 mM; S4O62−, 2 mM; fumarate, 30 mM; dimethyl sulfoxide (DMSO), 25 mM; and S0, 20 mM (41). When required, antibiotics were supplemented at the following final concentrations: gentamicin (Gm), 15 μg ml−1, and chloramphenicol, 25 μg ml−1. For growth of Escherichia coli β2155 λ pir (12), diaminopimelate was supplemented at a final concentration of 100 μg ml−1.
Analytical techniques.
Cell growth was monitored by direct cell counts via epifluorescence microscopy and by measuring terminal electron acceptor depletion or end product accumulation. Acridine orange-stained cells were counted (Carl Zeiss AxioImager Z1 microscope) according to previously described procedures (35). Cell numbers at each time point were calculated as the average of 10 counts from two parallel yet independent anaerobic incubations. NO2− was measured spectrophotometrically with sulfanilic acid-N-1-naphthyl-ethylene-diamine dihydrochloride solution (40). Fe(III) reduction was monitored by measuring Fe(II) production with the ferrozine technique (54). Mn(III)-pyrophosphate concentration was measured colorimetrically as previously described (30). S2O32− and S4O62− concentrations were measured by cyanolysis as previously described (28). Growth on O2, TMAO, DMSO, and fumarate was detected by monitoring cell growth only. Control experiments consisted of incubations with cells that were heat killed at 80°C for 30 min prior to inoculation or by omission of the terminal electron acceptor.
Nucleotide and amino acid sequence analyses.
Genome sequence data for S. oneidensis MR-1 (21), S. Typhimurium LT2 (38), and W. succinogenes (5) were obtained from the comprehensive microbial resource (J. Craig Venter Institute; http://cmr.jcvi.org). S. oneidensis proteins that displayed significant similarity to S. Typhimurium PhsA (designated PsrA in the S. oneidensis genome), tetrathionate reductase (TtrA), and anaerobic sulfite reductase (AsrA) were identified via BLAST analysis (3). Functional motifs were analyzed via Pfam (http://pfam.sanger.ac.uk) and ClustalW (33). The S. oneidensis PsrA sequence was used as the query sequence for BLAST analysis of several other recently sequenced Shewanella genomes, including S. putrefaciens strain 200, S. putrefaciens CN32, S. putrefaciens W3-18-1, S. amazonensis SB2B, S. denitrificans OS217, S. baltica OS155, S. frigidimarina NCIMB400, S. pealeana ATCC 700345, S. woodyi ATCC 51908, Shewanella sp. strain ANA-3, Shewanella sp. strain MR-4, Shewanella sp. strain MR-7, S. loihica PV-4, S. halifaxensis HAW-EB4, S. piezotolerans WP3, S. sediminis HAW-EB3, and S. benthica KT99. Preliminary genome sequence data for these strains were obtained from the Department of Energy, Joint Genome Institute (http://www.jgi.doe.gov).
Construction of suicide vector pKO2.0.
The strategy for construction of the suicide vector pKO2.0 (Table 1) is outlined in Fig. S1 in the supplemental material. The R6K oriV and the RP4 origin of transfer (oriT) genes of pKNOCK-Gm (2) were PCR amplified with NheI restriction sites engineered onto the ends of the oriV-oriT fragment. The chloramphenicol acetyltransferase (cat) gene and the LacZ-containing multiple-cloning site (MCS) were PCR amplified from pBBR1MCS (31) with NheI sites also engineered onto the ends (see Table S1 in the supplemental material for corresponding primers). The resulting PCR products were ligated to form pKOCat-MCS. The open reading frames encoding SacB and Gm acetyltransferase (conferring Gm resistance [Gmr]) were PCR amplified from pJQ200 (48) as a contiguous fragment with ApaLI and MunI restriction sites engineered onto the ends. The cat gene was removed from pKOCat-MCS by inverse PCR, resulting in a fragment containing R6K oriV, RP4 oriT, and the LacZ-MCS with ApaLI and MunI sites on the ends. These two fragments were ligated to form pKO2.0 (R6K oriT LacZ-MCS Gmr sacB).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Feature(s) | Source or reference |
|---|---|---|
| Strains | ||
| Shewanella oneidensis | ||
| MR-1 | Wild-type strain | ATCC |
| PSRA1 | In-frame deletion mutant | This study |
| PSRA+ | “Knock-in” complementation mutant | This study |
| Escherichia coli | ||
| EC100D pir-116 | F−mcrA Δ(mrr-hsdRMS-mcrBC) | Epicentre |
| φ80dlacZΔM15 ΔlacX74 recA1 endA1 | ||
| araD139 Δ(ara leu)7697galUgalK λ− | ||
| β2155 λ pir | thrB1004 pro thi strA hsdS lacZΔM15 | 12 |
| (F9 lacZΔM15 lacIqtraD36 proA1 proB1) | ||
| ΔdapA::erm pir::RP4 Kmr | ||
| Plasmids | ||
| pKO2.0 | 4.5-kb γR6K, mob RP4 sacB GmrlacZ | This study |
| pKNOCK-Gm | 1.6-kb γR6K, mob RP4 Gmr | 2 |
| pBBR1MCS | CmrlacZ | 31 |
| pJQ200 | ColE1 sacB Gmr | 48 |
| pKOPSRA | pKO2.0 containing in-frame deletion of psrA | This study |
| pKOPSRA+ | pKO2.0 containing wild-type psrA | This study |
In-frame gene deletion mutagenesis.
psrA was deleted from the S. oneidensis genome by the following method. Regions corresponding to ∼750 bp upstream and downstream of the psrA open reading frame were PCR amplified using iProof ultrahigh-fidelity polymerase (Bio-Rad, Hercules, CA). Primers used for construction of the psrA deletion mutant are listed in Table 2. PCR cycling routines consisted of 98°C for 30 s, 35 cycles of 98°C for 15 s, 60°C for 30 s, and 72°C for 30 s, concluding with a final extension step at 72°C for 7 min. PCRs (50-μl total mixture volume) were performed on a Bio-Rad iCycler (Bio-Rad, Hercules, CA), and each mixture contained 20 ng of S. oneidensis genomic DNA (DNAzol; Invitrogen), 250 μM of the deoxynucleoside triphosphates, and 50 ng of each oligonucleotide. The resulting fragments were separated by agarose gel electrophoresis, isolated from the gel (QIAquick gel extraction kit; Qiagen), and subsequently joined using overlap extension PCR (25) to generate a DNA fragment containing regions homologous to the regions flanking psrA. This region was cloned into the newly constructed knockout vector pKO2.0, and the resulting plasmid (pKOPSRA) was electroporated into E. coli EC100D pir-116 (Epicentre) (Bio-Rad Xcell electroporation system; Bio-Rad, Hercules, CA). Recipients were detected on LB agar medium containing Gm and 40 μg ml−1 X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). The recombinant plasmid was confirmed by detection of an ∼1.5-kb insertion following digestion with BamHI. Following confirmation, pKOPSRA was electroporated into E. coli β2155 λ pir (12) (Bio-Rad Xcell electroporation system; Bio-Rad, Hercules, CA), and the resulting donor strain was mated biparentally with S. oneidensis on LB agar medium supplemented with diaminopimelate. Following an 18-h incubation, the mating mix was resuspended in LB liquid medium and subsequently plated onto LB agar medium containing Gm.
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| PSRAD1 | GACTGGATCCCACAGCTTATTTGGTCG |
| PSRAD2 | ATATCTTTTCGTCATTTGAGCCTCCAAGGTTACCTCCATCACACTCA |
| PSRAD3 | TGAGTGTGATGGAGGTAACCTTGGAGGCTCAAATGACGAAAAGATAT |
| PSRAD4 | GACTGTCGACACAAGCCAAGCCTAAGCTGATGG |
| PSRADTF | GCGTGCATTTTGAACGACAG |
| PSRADTR | GCTTTCTAAAGGGCATAAGCAGC |
| RT-PSRAF | GTCGCCGATAAAGCCGATGAATGGTA |
| RT-PSRAR | GGCATATCTTTTACGCCGGGCTTAC |
| RT-PSRBF | TATGGCGAAATGCCCAATCTGC |
| RT-PSRBR | AAGCGGGTGTCCTTACAGAAGT |
| RT-PSRCF | AGTCACGACAAGACGTTAGCCA |
| RT-PSRCR | GCTTTGGCCCGCATACACAATA |
| RT-RPOAF | GTTCAACACGAGCTGCTTCTA |
| RT-RPOAR | GGCTTAGCAGTGACTATCGAG |
S. oneidensis MR-1 colonies with pKOPSRA integrated into the genome were screened by PCR using primers flanking the recombination region. The resulting strain (MR-1::pKOPSRA) was grown in LB medium with NaCl omitted and subsequently transferred to LB agar medium with NaCl omitted and containing 10% (wt/vol) sucrose. Colonies were patched to LB agar medium containing Gm to confirm the loss of pKO2.0, and the deletion in psrA was confirmed in the Gm-sensitive colonies via PCR using primers flanking the recombination region. One mutant strain (PSRA1) was also confirmed by direct DNA sequencing of the targeted region (University of Nevada, Reno Genomics Center).
Confirmation of in-frame deletions.
Liquid cultures of S. oneidensis wild-type and deletion mutant strains were grown to mid-log phase (corresponding to 1.8 × 109 cells ml−1) in LB medium, and total RNA was extracted with PureZol reagent (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. RNA (∼150 ng μl−1) was further purified (RNeasy Kit; Qiagen) with on-column DNase I treatment according to the manufacturer's instructions. RNA was reversely transcribed with a high-capacity cDNA reverse transcription (RT) kit (Applied Biosystems). RT reaction mixtures (20 μl) consisted of 150 ng RNA, 50 U MultiScribe reverse transcriptase, 4 mM deoxynucleoside triphosphates, and random hexamers. Cycling routines were 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min. The resulting cDNA was PCR amplified with primers specific for internal regions of psrA, psrB, and psrC (Table 2). RNA polymerase factor rpoA served as a positive control. Negative controls consisted of identical reactions with reverse transcriptase omitted.
Knock-in complementation analysis of PSRA1.
PSRA1 was complemented via a strategy analogous to that followed in the gene deletion protocol. Wild-type psrA was PCR amplified from MR-1 genomic DNA with psrA-specific primers PSRAD1 and PSRAD4. The resulting amplicon contained the entire open reading frame and ∼750 bp of upstream and downstream DNA for subsequent recombination into PSRA1. The amplicon was cloned into pKO2.0 using identical restriction sites, and the resulting construct was subsequently transformed into E. coli strains as described above. “Knock-in” complementation was performed as described for in-frame deletion above, except that PSRA1 was used as the recipient strain. Insertion into the proper location of the genome was confirmed via PCR amplification with flanking primers PSRADTF and PSRADTR (Table 2) followed by DNA sequencing (University of Nevada, Reno Genomics Center). The resulting “knock-in” complementation strain was designated PSRA+.
RESULTS
Construction of new suicide vector pKO2.0.
Suicide vector pKO2.0 was constructed in a stepwise fashion, integrating the hallmark components of previously constructed vectors. The R6K oriV and RP4 oriT origins from pKNOCK-Gm and the chloramphenicol resistance marker (cat) and the LacZ-containing polylinker (MCS) from the broad-host-range vector pBBR1MCS were independently PCR amplified and joined to form pKOCat-MCS (See Fig. S1 in the supplemental material). The cat gene was subsequently removed via inverse PCR, and the resulting product (containing R6Kori, oriT, and MCS) was ligated with the PCR product containing the Gmr gene and sacB, encoding levansucrase (see Fig. S1 in the supplemental material).
Identification of S. oneidensis gene products that display similarity to the PhsA homolog of S. Typhimurium.
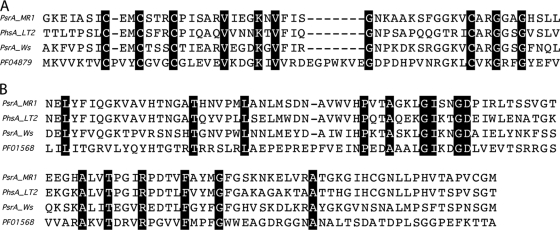
BLAST analysis revealed that the S. oneidensis MR-1 genome contained a three-gene cluster (annotated as psrABC) whose translated products displayed 59 to 73% and 65 to 80% amino acid similarity to the phsABC (psrABC) clusters of S. Typhimurium LT2 and W. succinogenes, respectively (Table 3; see also Fig. S3 in the supplemental material). The three-gene psr cluster in S. oneidensis MR-1 has the same genomic organization as the three-gene psr clusters in both S. Typhimurium and W. succinogenes (data not shown). ClustalW multiple alignments of PsrA functional domains with those of other proteobacteria indicated that S. oneidensis PsrA contains the conserved 4Fe-4S cluster and molybdopterin guanine-dinucleotide (MGD) binding motifs postulated to contribute to PsrA activity (Fig. 1). PsrA contained the predicted Pfam domains, consistent with other members of the polysulfide (thiosulfate) reductase family of proteins, including the 4Fe-4S cluster (Pfam accession no. PF04879) and MGD binding motifs (accession no. PF01568) (Fig. 1). Proteins homologous to S. Typhimurium TtrA (tetrathionate reductase) or AsrA (anaerobic sulfite reductase) were not detected in the S. oneidensis genome (data not shown).
TABLE 3.
Identities and similarities between PsrA, PsrB, and PsrC homologs
| Protein |
Shewanella spp.a
|
S. Typhimurium LT2
|
GenBankb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sim | %ID | E value | Sim | %ID | E value | Best hit | Sim | %ID | E value | |
| PsrA | 85-98 | 75-96 | 0.0 | 73 | 56 | 0.0 | W. succinogenes | 65 | 47 | 0.0 |
| PsrB | 90-99 | 79-98 | 10−87-10−107 | 71 | 54 | 10−57 | W. succinogenes | 80 | 65 | 10−63 |
| PsrC | 66-99 | 51-96 | 10−57-10−137 | 59 | 40 | 10−38 | W. succinogenes | 67 | 45 | 10−62 |
Percentages of sequence similarity (Sim), percentages of identity (%ID), and expect values (E values) between S. oneidensis Psr predicted amino acid sequences obtained from TIGR. Ranges were determined by pairwise comparison with translated sequence data from draft genome sequences of 17 recently sequenced Shewanella strains, including S. putrefaciens 200, S. putrefaciens CN32, S. putrefaciens W3-18-1, S. amazonensis SB2B, S. denitrificans OS217, S. baltica OS195, S. frigidimarina NCIMB400, S. pealeana ATCC 700345, S. woodyi ATCC 51908, Shewanella strain ANA-3, Shewanella strain MR-4, Shewanella strain MR-7, S. loihica PV-4, S. halifaxensis, S. piezotolerans, S. benthica, and S. sediminis.
Organism outside the genus Shewanella or Salmonella, with the ortholog of highest similarity (best hit) determined by BLASTP analysis of the nonredundant GenBank database.
FIG. 1.
Identification of conserved Pfam domains in the PsrA family of proteins. ClustalW multiple alignment of PsrA family proteins containing conserved Pfam domains. (A) Multiple alignment of Pfam domain PF04879 (4Fe-4S binding domain) with S. oneidensis MR-1 PsrA (PsrA_MR1), S. Typhimurium LT2 PhsA (PhsA_LT2), and W. succinogenes PsrA (PsrA_Ws). (B) Multiple alignments of Pfam domain PF01568 (bis-MGD binding domain) with PsrA and PhsA proteins. Identical residues are shaded.
S2O32−-reducing members of the genus Shewanella contain Psr homologs with high amino acid sequence similarity.
The psrABC cluster was also identified in the recently sequenced genomes of Shewanella strains that respire S0 and S2O32−, including S. putrefaciens CN32, S. putrefaciens 200, S. putrefaciens W3-18-1, S. amazonensis SB2B, S. frigidimarina NCIMB400, S. baltica OS155, S. pealeana, Shewanella sp. strain ANA-3, Shewanella loihica PV-4, Shewanella sp. strain MR-4, Shewanella sp. strain MR-7, S. benthica, S. halifaxensis, S. piezotolerans, and S. sediminis. The psr gene cluster was missing from the genomes of S. denitrificans OS217 and S. woodyi, a finding that correlated with the inability of these two strains to respire S2O32− (57). PsrA (85 to 98%), PsrB (90 to 99%), and PsrC (66 to 99%) amino acid sequence similarities were highly conserved among S2O32−-respiring Shewanella species (Table 3).
Construction and confirmation of in-frame deletion mutant strain PSRA1 and knock-in complementation strain PSRA+.
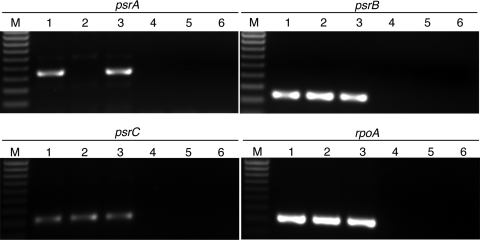
S. oneidensis psrA was deleted in frame via application of the newly developed pKO2.0-based gene deletion system. Regions flanking psrA were PCR amplified and joined to construct a region used for gene deletion. This region was cloned into pKO2.0 (creating plasmid pKOPSRA) and subsequently mobilized into S. oneidensis via conjugal transfer. Single integrants were selected on the basis of their resistance to Gm, and their sequences were confirmed via PCR using test primers flanking the recombination region. The second step involved resolution of the single integration by counterselection on medium containing sucrose. Several sucrose-resistant colonies were screened by PCR and selected for further study. A single strain (designated PSRA1) displaying a PCR product corresponding to an in-frame psrA deletion was chosen and tested for expression of downstream genes psrB and psrC via RT-PCR. Transcripts for psrB and psrC were detected in PSRA1, while transcripts for psrA were not detected (Fig. 2).
FIG. 2.
RT-PCR of genes in the psr operon. PCR products generated from cDNA from the wild type (lane 1), PSRA1 (lane 2), or PSRA+ (lane 3) were separated electrophoretically and visualized via staining with ethidium bromide. Transcripts for psr or housekeeping (rpoA) genes are indicated above the gel lanes. Negative-control lanes 4, 5, and 6 include cDNA from the wild type, PSRA1, and PSRA+, respectively, with reverse transcriptase omitted from the cDNA synthesis reaction. Lanes M, molecular size markers.
To avoid problems associated with in trans complementation (e.g., inadvertent overexpression), the newly constructed suicide vector pKO2.0 was used to generate a “knock-in” genetic complementation strain, designated PSRA+. psrA and regions upstream and downstream (identical to those used to construct PSRA1) were PCR amplified and cloned into pKO2.0 as described above. pKOPSRA+ was used to reinsert the psrA gene into PSRA1 as described for construction of the in-frame deletion, with the exception that PSRA1 was used as the recipient strain. Insertion of wild-type psrA back into PSRA1 was confirmed via DNA sequencing and RT-PCR analyses as described above (Fig. 2).
Anaerobic growth capabilities of S. oneidensis strains PSRA1 and PSRA+.
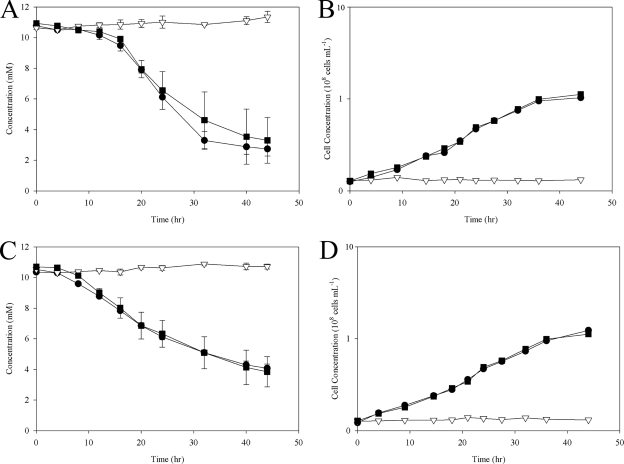
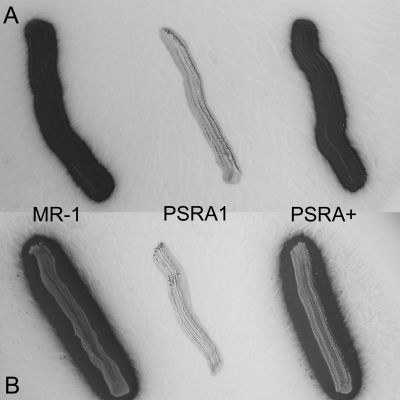
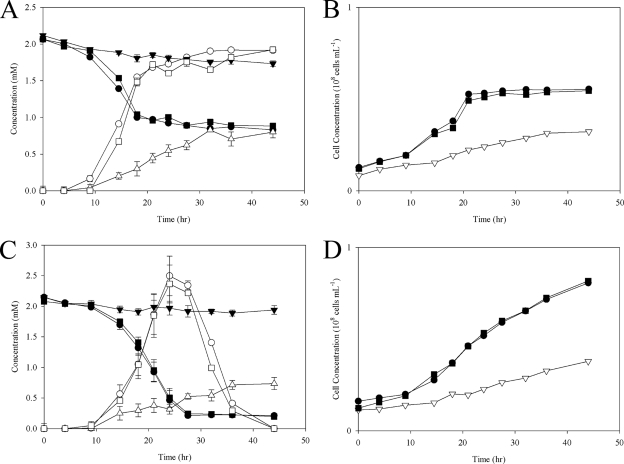
Wild-type S. oneidensis, deletion mutant PSRA1, and complemented mutant PSRA+ were tested for anaerobic respiration on a combination of two electron donors (lactate and formate) and a set of alternate electron acceptors. PSRA1 was severely impaired in its ability to respire anaerobically on S2O32− and S0 with lactate or formate (Fig. 3 and 4) yet retained the ability to grow on all other combinations of electron donors and electron acceptors, including O2, DMSO, Fe(III)-citrate, hydrous ferric oxide, NO3−, fumarate, Mn(III)-pyrophosphate, and TMAO (see Fig. S2 in the supplemental material). Anaerobic growth of wild-type S. oneidensis and PSRA+ on S0-containing solid growth medium was accompanied by the production of a clearing zone in the colony periphery. The clearing zone, indicative of S0 reduction, was more pronounced with formate than with lactate as an electron donor (Fig. 4). Neither cell mass nor a clearing zone was observed in anaerobic incubations of PSRA1 on S0-containing solid growth medium.
FIG. 3.
S2O32− reduction phenotypes of the wild-type (MR-1) (filled circles), psrA deletion mutant (PSRA1) (open triangles), and “knock-in” complementation (PSRA+) (filled squares) strains. (A) S2O32− concentration as a function of time for the wild-type, PSRA1, and PSRA+ strains with lactate (18 mM) as the electron donor. (B) Cell growth as a function of time with lactate as the electron donor. (C) S2O32− concentration as a function of time with formate (30 mM) as the electron donor. (D) Cell growth as a function of time with formate as the electron donor.
FIG. 4.
S0-respiratory phenotypes of the wild-type (MR-1), psrA deletion mutant (PSRA1), and “knock-in” complementation (PSRA+) strains. Anaerobic cell growth was measured on solid medium containing S0 (40 mM) as the electron acceptor with either lactate (18 mM) (A) or formate (30 mM) (B) as the electron donor (90% N2, 10% CO2 atmosphere). Following a 48-h anaerobic incubation, plates were visualized against a black background to observe the clearing zone associated with S0 reduction by MR-1 and PSRA+ (but not by PSRA1).
PSRA1 retained S4O62−-respiratory activity and consequently produced S2O32−, although not at wild-type rates (Fig. 5). With formate as an electron donor, the wild-type strain and PSRA+ depleted S4O62− to low levels and switched to anaerobic respiration of the produced S2O32− (noted by a depletion of S2O32− after 24 h). With lactate as an electron donor, the wild-type strain and PSRA+ reduced S4O62− to S2O32−; however, neither strain was able to respire the produced S2O32−. PSRA1, on the other hand, reduced S4O62− and produced S2O32− but was unable to respire the produced S2O32− with either lactate or formate as an electron donor. As a result, PSRA1 displayed a marked decrease in growth rate and S4O62− reduction activity (Fig. 5).
FIG. 5.
S4O62−-respiratory phenotypes of the wild-type (MR-1), psrA deletion mutant (PSRA1), and “knock-in” complementation (PSRA+) strains. (A and C) S4O62− reduction by the wild-type (filled circles), PSRA1 (filled triangles), or PSRA+ (filled squares) strain with lactate (18 mM) (A) or formate (30 mM) (C) as the electron donor. Respiration of S4O62− results in an accumulation of S2O32− by the wild-type (open circles), PSRA1 (open triangles), or PSRA+ (open squares) strain. (B and D) Cell growth as a function of time for the wild-type (filled circles), PSRA1 (open triangles), or PSRA+ (filled squares) strain with lactate (B) or formate (D) as the electron donor. In some cases, error bars are smaller than the symbol.
DISCUSSION
Metal-respiring members of the genus Shewanella provide attractive models for determining the molecular mechanism of anaerobic respiration of metals, metalloids, and radionuclides. Genetic manipulations in Shewanella can be performed aerobically prior to subsequent phenotypic tests under anaerobic conditions. With the recent release of the genomic sequences of 17 Shewanella strains (Joint Genome Institute; http://www.jgi.doe.gov), targeted, in-frame gene deletion mutagenesis may now be carried out to examine the molecular mechanism of anaerobic respiration. Earlier genetic manipulation techniques, such as transposon and chemical mutagenesis, have been problematic for a variety of reasons, including polar effects on downstream genes, inadvertent genomic rearrangements and deletions, and altered growth rates due to the energetic requirements for maintaining antibiotic resistance markers (7, 13, 43). The suicide vector constructed in the present study (pKO2.0) contains features ideal for generating in-frame, markerless gene deletions in Shewanella: pKO2.0 replicates efficiently in E. coli donor strains but not in S. oneidensis, contains an appropriate broad-host-range origin of transfer (oriT), and encodes a functional counterselection marker (SacB) (16, 18, 19). In addition, pKO2.0 is ∼5 kb smaller than previously constructed vectors (18) and contains a LacZ polylinker for more-efficient ligation of DNA constructs. Shewanella deletion mutants may therefore be constructed more efficiently due to increased transformation efficiency and elimination of problems with blunt-end cloning (19). pKO2.0 was used to construct in-frame deletions of the gene encoding the putative PsrA homolog identified in the S. oneidensis genome.
The majority of studies of anaerobic respiration by S. oneidensis have focused on the mechanism of electron transport to transition metals and radionuclides, while the mechanism of electron transport to metalloids such as inorganic sulfur compounds remains poorly understood (8, 11, 14, 46, 56). Previous studies demonstrated that several members of the Shewanella genus couple the oxidation of organic matter (or hydrogen) to the dissimilatory reduction of S0, a process that does not require direct contact between the cell and the S0 particle (41, 47). S0 respiration in S. Typhimurium and W. succinogenes involves a PhsA (PsrA)-dependent intraspecies sulfur cycle. In S. Typhimurium, PhsA reduces S2O32− to HS− and SO32−. The produced SO32− diffuses from the cell and reacts chemically with S0 to form additional S2O32−, which reenters the periplasm and is reduced by PhsA to HS− and SO32−, thereby sustaining an intraspecies sulfur cycle. In W. succinogenes, PsrA reduces Sn2− stepwise to Sn − 12− and HS−. The HS− diffuses from the cell and reacts with S0 to form additional Sn2−, which reenters the periplasm and is rereduced (24). In this manner, the terminal reduction step for S0 respiration by S. Typhimurium and W. succinogenes is driven by the abiotic interaction of S0 with either SO32− or HS− and not by direct enzymatic reduction of S0.
In the present study, all predicted PsrA homologs identified in the recently sequenced Shewanella genomes contain Pfam domains indicative of the Phs (Psr) family of thiosulfate reductases, including 4Fe-4S cluster and MGD binding motifs. psrA-like genes are not found in the genomes of S. denitrificans or S. woodyi, two Shewanella species unable to respire S0 or S2O32− (57). Interestingly, S. denitrificans and S. woodyi are also unable to respire Fe(III) oxides as the terminal electron acceptor (57), a pattern of electron acceptor utilization reflecting the close association of the S0 and Fe(III) reduction phenotypes in metal-respiring bacteria (57).
To determine if S. oneidensis psrA is required for S0 and S2O32− respiration, the S. Typhimurium phsA homolog identified in the S. oneidensis genome was deleted in frame with the new pKO2.0-based in-frame gene deletion system, and the resulting mutant was tested for anaerobic respiration on a combination of two electron donors and a set of 11 alternate electron acceptors, including S0 and S2O32−. S. oneidensis deletion mutant PSRA1 is unable to respire anaerobically on S2O32− or S0 as an electron acceptor, yet it retains respiratory activity on nine other electron acceptors tested, including S4O62−. Following depletion of S4O62−, wild-type S. oneidensis switches to anaerobic respiration of the produced S2O32−. PSRA1, on the other hand, is unable to respire the produced S2O32− and, as a result, displays decreased cell growth and S4O62− depletion rates (Fig. 5). This finding indicates that S4O62− is reduced to HS− stepwise with S2O32− as an intermediate. Cells respiring S4O62− with lactate as an electron donor were unable to reduce the produced S2O32− (Fig. 5A). The reasons for this finding are not clear, but it may be due to repression of the Psr system by residual (1 mM) S4O62− remaining in the system. A similar growth pattern was recently reported for cytochrome c maturation (ccmB) mutants of S. putrefaciens 200 that are unable to reduce NO2− but retain NO3− reduction activity (11). The S. putrefaciens CCMB1 mutant (with an in-frame deletion of ccmB) stoichiometrically converts NO3− to NO2− but is unable to respire the produced NO2−, and growth halts.
S4O62−-respiring bacteria generally contain the three-gene cluster ttrBAC, in which ttrA encodes the major subunit (TtrA) of typical S4O62− terminal reductases. TtrA contains ferredoxin and MGD binding motifs, similarly to the polysulfide (thiosulfate) reductases PhsA and PsrA (23). The ability of PSRA1 to respire S4O62− indicates that PsrA does not function as the S4O62− reductase in S. oneidensis. Interestingly, the genomes of other S4O62−-respiring members of the genus Shewanella contain the traditional ttrBAC gene cluster, while S. oneidensis does not (21). A possible candidate for S4O62− reductase in S. oneidensis is an atypical octaheme c-type cytochrome (SO4144) that displays S4O62−, NO2−, and NH2OH reduction activities in vitro (4, 42). The physiological role of SO4144 in vivo remains to be examined.
The results of the present study indicate that pKO2.0 is an effective suicide vector for generating markerless, in-frame gene deletions in S. oneidensis MR-1. The vector is also suitable for generating “knock-in” complements of the deletion mutants, thereby avoiding problems associated with genetic complementation in trans. The results also indicate that PsrA is required for S. oneidensis to respire anaerobically on either S0 or S2O32− as an electron acceptor, most likely functioning as the S2O32− terminal reductase. The large clearing zone observed in the periphery of wild-type S. oneidensis colonies incubated anaerobically on solid medium amended with S0 corroborates previous findings that direct contact is not required for S0 respiration by S. oneidensis (41). S. oneidensis may therefore respire S0 via an intraspecies sulfur cycle catalyzed by extracellular, abiotic (purely chemical) interactions between S0 and either HS− or SO32− produced during anaerobic S0 respiration. The results of the present study are unable to differentiate between the HS−- and SO32−-catalyzed pathways. One potential strategy for differentiating between the HS− and SO32− cycling pathways is to construct SO32− reductase deletion mutants. An increase in the diameters of clearing zones in the periphery of SO32− reductase deletion mutants incubated anaerobically on S0-amended solid growth medium is an indication that SO32− contributes to S0 mobilization in S. Typhimurium (24) (produced SO32− is not reduced and is therefore more readily available to interact with extracellular S0, resulting in a larger clearing zone). This experimental strategy is not currently possible for S. oneidensis, however, since a genome-wide scan indicated that genes encoding traditional dissimilatory SO32− terminal reductases (DsrA or AsrA) are missing from the S. oneidensis genome. Current work is focused on identification of the novel SO32− reductase in S. oneidensis and examination of the S0 reduction activity of the corresponding SO32− reductase deletion mutants.
Supplementary Material
Acknowledgments
Financial support for this study was provided by the National Science Foundation and the Department of Energy.
Footnotes
Published ahead of print on 19 June 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adams, M. W. 1994. Biochemical diversity among sulfur-dependent, hyperthermophilic microorganisms. FEMS Microbiol. Rev. 15:261-277. [DOI] [PubMed] [Google Scholar]
- 2.Alexeyev, M. F. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of Gram-negative bacteria. BioTechniques 26:824-825. [DOI] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson, S. J., C. G. Mowat, G. A. Reid, and S. K. Chapman. 2007. An octaheme c-type cytochrome from Shewanella oneidensis can reduce nitrite and hydroxylamine. FEBS Lett. 581:3805-3808. [DOI] [PubMed] [Google Scholar]
- 5.Baar, C., M. Eppinger, G. Raddatz, J. Simon, C. Lanz, O. Klimmek, R. Nandakumar, R. Gross, A. Rosinus, H. Keller, P. Jagtap, B. Linke, F. Meyer, H. Lederer, and S. C. Schuster. 2003. Complete genome sequence and analysis of Wolinella succinogenes. Proc. Natl. Acad. Sci. USA 100:11690-11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biebl, H., and N. Pfennig. 1977. Growth of sulfate-reducing bacteria with sulfur as electron acceptor. Arch. Microbiol. 112:115-117. [DOI] [PubMed] [Google Scholar]
- 7.Bordi, C., C. Iobbi-Nivol, V. Méjean, and J. C. Patte. 2003. Effects of ISSo2 insertions in structural and regulatory genes of the trimethylamine oxide reductase of Shewanella oneidensis. J. Bacteriol. 185:2042-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnes, B. S., M. J. Mulberry, and T. J. DiChristina. 1998. Design and application of two rapid screening techniques for isolation of Mn(IV) reduction-deficient mutants of Shewanella putrefaciens. Appl. Environ. Microbiol. 64:2716-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caccavo, F., Jr., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz, and M. J. McInerney. 1994. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark, M. A., and E. L. Barrett. 1987. The phs gene and hydrogen sulfide production by Salmonella typhimurium. J. Bacteriol. 169:2391-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale, J. R., R. Wade, Jr., and T. J. DiChristina. 2007. A conserved histidine in cytochrome c maturation permease CcmB of Shewanella putrefaciens is required for anaerobic growth below a threshold standard redox potential. J. Bacteriol. 189:1036-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehio, C., and M. Meyer. 1997. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J. Bacteriol. 179:538-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiChristina, T. J., and E. F. Delong. 1994. Isolation of anaerobic respiratory mutants of Shewanella putrefaciens and genetic analysis of mutants deficient in anaerobic growth on Fe3+. J. Bacteriol. 176:1468-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiChristina, T. J., J. K. Fredrickson, and J. M. Zachara. 2005. Enzymology of electron transport: energy generation with geochemical consequences. Rev. Mineral. Geochem. 59:27-52. [Google Scholar]
- 15.Dietrich, V., and O. Klimmek. 2002. The function of methyl-menaquinone-6 and polysulfide reductase membrane anchor (PsrC) in polysulfide respiration of Wolinella succinogenes. Eur. J. Biochem. 269:1086-1095. [DOI] [PubMed] [Google Scholar]
- 16.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong, C.-L., N. K. Heinzinger, S. Tongklan, and E. L. Barrett. 1993. Cloning of the phs genetic locus from Salmonella typhimurium and a role for a phs product in its own induction. J. Bacteriol. 175:6368-6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, W. M., Y. Q. Liu, C. S. Giometti, S. L. Tollaksen, T. Khare, L. Y. Wu, D. M. Klingeman, M. W. Fields, and J. Zhou. 2006. Knock-out of SO1377 gene, which encodes the member of a conserved hypothetical bacterial protein family COG2268, results in alteration of iron metabolism, increased spontaneous mutation and hydrogen peroxide sensitivity in Shewanella oneidensis MR-1. BMC Genomics 7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gay, P., D. Le Coq, M. Steinmetz, T. Berkelman, and C. I. Kado. 1985. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J. Bacteriol. 164:918-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedderich, R., O. Klimmek, A. Kroger, R. Dirmeier, M. Keller, and K. O. Stetter. 1998. Anaerobic respiration with elemental sulfur and with disulfides. FEMS Microbiol. Rev. 22:353-381. [Google Scholar]
- 21.Heidelberg, J. F., I. T. Paulsen, K. E. Nelson, E. J. Gaidos, W. C. Nelson, T. D. Read, J. A. Eisen, R. Seshadri, N. Ward, B. Methe, R. A. Clayton, T. Meyer, A. Tsapin, J. Scott, M. Beanan, L. Brinkac, S. Daugherty, R. T. DeBoy, R. J. Dodson, A. S. Durkin, D. H. Haft, J. F. Kolonay, R. Madupu, J. D. Peterson, L. A. Umayam, O. White, A. M. Wolf, J. Vamathevan, J. Weidman, M. Impraim, K. Lee, K. Berry, C. Lee, J. Mueller, H. Khouri, J. Gill, T. R. Utterback, L. A. McDonald, T. V. Feldblyum, H. O. Smith, J. C. Venter, K. H. Nealson, and C. M. Fraser. 2002. Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat. Biotechnol. 20:1118-1123. [DOI] [PubMed] [Google Scholar]
- 22.Heinzinger, N. K., S. Y. Fujimoto, M. A. Clark, M. S. Moreno, and E. L. Barrett. 1995. Sequence analysis of the phs operon in Salmonella typhimurium and the contribution of thiosulfate reduction to anaerobic energy metabolism. J. Bacteriol. 177:2813-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensel, M., A. P. Hinsley, T. Nikolaus, G. Sawers, and B. C. Berks. 1999. The genetic basis of tetrathionate respiration in Salmonella typhimurium. Mol. Microbiol. 32:275-287. [DOI] [PubMed] [Google Scholar]
- 24.Hinsley, A. P., and B. C. Berks. 2002. Specificity of respiratory pathways involved in the reduction of sulfur compounds by Salmonella enterica. Microbiology 148:3631-3638. [DOI] [PubMed] [Google Scholar]
- 25.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 26.Jackson, B. E., and M. J. McInerney. 2000. Thiosulfate disproportionation by Desulfotomaculum thermobenzoicum. Appl. Environ. Microbiol. 66:3650-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jormakka, M., K. Yokoyama, T. Yano, M. Tamakoshi, S. Akimoto, T. Shimamura, P. Curmi, and S. Iwata. 2008. Molecular mechanism of energy conservation in polysulfide respiration. Nat. Struct. Mol. Biol. 15:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly, D. P., and A. P. Wood. 1994. Synthesis and determination of thiosulfate and polythionates. Inorg. Microb. Sulfur Metab. 243:475-501. [Google Scholar]
- 29.Kertesz, M. A. 2000. Riding the sulfur cycle—metabolism of sulfonates and sulfate esters in Gram-negative bacteria. FEMS Microbiol. Rev. 24:135-175. [DOI] [PubMed] [Google Scholar]
- 30.Kostka, J. E., G. W. Luther, and K. H. Nealson. 1995. Chemical and biological reduction of Mn(III)-pyrophosphate complexes—potential importance of dissolved Mn(III) as an environmental oxidant. Geochim. Cosmochim. Acta 59:885-894. [Google Scholar]
- 31.Kovach, M. E., R. W. Phillips, P. H. Elzer, R. M. Roop, and K. M. Peterson. 1994. pBBR1MCS—a broad-host-range cloning vector. BioTechniques 16:800. [PubMed] [Google Scholar]
- 32.Krafft, T., R. Gross, and A. Kroger. 1995. The function of Wolinella succinogenes psr genes in electron transport with polysulphide as the terminal electron acceptor. Eur. J. Biochem. 230:601-606. [DOI] [PubMed] [Google Scholar]
- 33.Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan, H. McWilliam, F. Valentin, I. M. Wallace, A. Wilm, R. Lopez, J. D. Thompson, T. J. Gibson, and D. G. Higgins. 2007. ClustalW and ClustalX version 2. Bioinformatics 23:2947-2948. [DOI] [PubMed] [Google Scholar]
- 34.Lovley, D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219-286. [DOI] [PubMed] [Google Scholar]
- 35.Lovley, D. R., and E. J. P. Phillips. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54:1472-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lovley, D. R., E. J. P. Phillips, D. J. Lonergan, and P. K. Widman. 1995. Fe(III) and S0 reduction by Pelobacter carbinolicus. Appl. Environ. Microbiol. 61:2132-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macy, J. M., I. Schroder, R. K. Thauer, and A. Kroger. 1986. Growth of Wolinella succinogenes on H2S plus fumarate and on formate plus sulfur as energy sources. Arch. Microbiol. 144:147-150. [Google Scholar]
- 38.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Y. Du, S. F. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 39.Miroshnichenko, M. L., N. A. Kostrikina, H. Hippe, A. I. Slobodkin, and E. A. Bonch-Osmolovskaya. 1998. Biodiversity of thermophilic sulfur-reducing bacteria: new substrates and new habitats. Microbiology 67:563-568. [Google Scholar]
- 40.Montgomery, H., and J. Dymock. 1961. The determination of nitrite in water. Analyst (London) 86:414-416. [Google Scholar]
- 41.Moser, D. P., and K. H. Nealson. 1996. Growth of the facultative anaerobe Shewanella putrefaciens by elemental sulfur reduction. Appl. Environ. Microbiol. 62:2100-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mowat, C. G., E. Rothery, C. S. Miles, L. McIver, M. K. Doherty, K. Drewette, P. Taylor, M. D. Walkinshaw, S. K. Chapman, and G. A. Reid. 2004. Octaheme tetrathionate reductase is a respiratory enzyme with novel heme ligation. Nat. Struct. Mol. Biol. 11:1023-1024. [DOI] [PubMed] [Google Scholar]
- 43.Myers, C. R., and J. M. Myers. 1993. Role of menaquinone in the reduction of fumarate, nitrate, iron(III) and manganese(IV) by Shewanella putrefaciens MR-1. FEMS Microbiol. Lett. 114:215-222. [Google Scholar]
- 44.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 45.Oren, A., and M. Shilo. 1979. Anaerobic heterotrophic dark metabolism in the cyanobacterium Oscillatoria limnetica—sulfur respiration and lactate fermentation. Arch. Microbiol. 122:77-84. [Google Scholar]
- 46.Payne, A. N., and T. J. DiChristina. 2006. A rapid mutant screening technique for detection of technetium [Tc(VII)] reduction-deficient mutants of Shewanella oneidensis MR-1. FEMS Microbiol. Lett. 259:282-287. [DOI] [PubMed] [Google Scholar]
- 47.Perry, K. A., J. E. Kostka, G. W. Luther, and K. H. Nealson. 1993. Mediation of sulfur speciation by a Black Sea facultative anaerobe. Science 259:801-803. [DOI] [PubMed] [Google Scholar]
- 48.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 49.Ringel, M., R. Gross, T. Krafft, A. Kroger, and R. Schauder. 1996. Growth of Wolinella succinogenes with elemental sulfur in the absence of polysulfide. Arch. Microbiol. 165:62-64. [Google Scholar]
- 50.Saffarini, D. A., T. J. DiChristina, D. Bermudes, and K. H. Nealson. 1994. Anaerobic respiration of Shewanella putrefaciens requires both chromosomal and plasmid-borne genes. FEMS Microbiol. Lett. 119:271-277. [Google Scholar]
- 51.Schauder, R., and A. Kroger. 1993. Bacterial sulfur respiration. Arch. Microbiol. 159:491-497. [Google Scholar]
- 52.Schauder, R., and E. Muller. 1993. Polysulfide as a possible substrate for sulfur-reducing bacteria. Arch. Microbiol. 160:377-382. [Google Scholar]
- 53.Schut, G. J., S. L. Bridger, and M. W. W. Adams. 2007. Insights into the metabolism of elemental sulfur by the hyperthermophilic archaeon Pyrococcus furiosus: characterization of a coenzyme A-dependent NAD(P)H sulfur oxidoreductase. J. Bacteriol. 189:4431-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stookey, L. L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 55.Taillefert, M., J. S. Beckler, E. Carey, J. L. Burns, C. M. Fennessey, and T. J. DiChristina. 2007. Shewanella putrefaciens produces an Fe(III)-solubilizing organic ligand during anaerobic respiration on insoluble Fe(III) oxides. J. Inorg. Biochem. 101:1760-1767. [DOI] [PubMed] [Google Scholar]
- 56.Taratus, E. M., S. G. Eubanks, and T. J. DiChristina. 2000. Design and application of a rapid screening technique for isolation of selenite reduction-deficient mutants of Shewanella putrefaciens. Microbiol. Res. 155:79-85. [DOI] [PubMed] [Google Scholar]
- 57.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Evol. Microbiol. 49:705-724. [DOI] [PubMed] [Google Scholar]
- 58.Wall, J. D., and L. R. Krumholz. 2006. Uranium reduction. Annu. Rev. Microbiol. 60:149-166. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, M. N., J. R. Dale, T. J. DiChristina, and A. G. Stack. 2009. Dissolution morphology of iron (oxy)(hydr)oxides exposed to the dissimilatory iron-reducing bacterium Shewanella oneidensis MR-1. Geomicrobiol. J. 26:83-92. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.