Abstract
Spores of Bacillus subtilis contain a number of small, acid-soluble spore proteins (SASP) which comprise up to 20% of total spore core protein. The multiple α/β-type SASP have been shown to confer resistance to UV radiation, heat, peroxides, and other sporicidal treatments. In this study, SASP-defective mutants of B. subtilis and spores deficient in dacB, a mutation leading to an increased core water content, were used to study the relative contributions of SASP and increased core water content to spore resistance to germicidal 254-nm and simulated environmental UV exposure (280 to 400 nm, 290 to 400 nm, and 320 to 400 nm). Spores of strains carrying mutations in sspA, sspB, and both sspA and sspB (lacking the major SASP-α and/or SASP-β) were significantly more sensitive to 254-nm and all polychromatic UV exposures, whereas the UV resistance of spores of the sspE strain (lacking SASP-γ) was essentially identical to that of the wild type. Spores of the dacB-defective strain were as resistant to 254-nm UV-C radiation as wild-type spores. However, spores of the dacB strain were significantly more sensitive than wild-type spores to environmental UV treatments of >280 nm. Air-dried spores of the dacB mutant strain had a significantly higher water content than air-dried wild-type spores. Our results indicate that α/β-type SASP and decreased spore core water content play an essential role in spore resistance to environmentally relevant UV wavelengths whereas SASP-γ does not.
Spores of Bacillus spp. are highly resistant to inactivation by different physical stresses, such as toxic chemicals and biocidal agents, desiccation, pressure and temperature extremes, and high fluences of UV or ionizing radiation (reviewed in references 33, 34, and 48). Under stressful environmental conditions, cells of Bacillus spp. produce endospores that can stay dormant for extended periods. The reason for the high resistance of bacterial spores to environmental extremes lies in the structure of the spore. Spores possess thick layers of highly cross-linked coat proteins, a modified peptidoglycan spore cortex, a low core water content, and abundant intracellular constituents, such as the calcium chelate of dipicolinic acid and α/β-type small, acid-soluble spore proteins (α/β-type SASP), the last two of which protect spore DNA (6, 42, 46, 48, 52). DNA damage accumulated during spore dormancy is also efficiently repaired during spore germination (33, 47, 48). UV-induced DNA photoproducts are repaired by spore photoproduct lyase and nucleotide excision repair, DNA double-strand breaks (DSB) by nonhomologous end joining, and oxidative stress-induced apurinic/apyrimidinic (AP) sites by AP endonucleases and base excision repair (15, 26-29, 34, 43, 53, 57).
Monochromatic 254-nm UV radiation has been used as an efficient and cost-effective means of disinfecting surfaces, building air, and drinking water supplies (31). Commonly used test organisms for inactivation studies are bacterial spores, usually spores of Bacillus subtilis, due to their high degree of resistance to various sporicidal treatments, reproducible inactivation response, and safety (1, 8, 19, 31, 48). Depending on the Bacillus species analyzed, spores are 10 to 50 times more resistant than growing cells to 254-nm UV radiation. In addition, most of the laboratory studies of spore inactivation and radiation biology have been performed using monochromatic 254-nm UV radiation (33, 34). Although 254-nm UV-C radiation is a convenient germicidal treatment and relevant to disinfection procedures, results obtained by using 254-nm UV-C are not truly representative of results obtained using UV wavelengths that endospores encounter in their natural environments (34, 42, 50, 51, 59). However, sunlight reaching the Earth's surface is not monochromatic 254-nm radiation but a mixture of UV, visible, and infrared radiation, with the UV portion spanning approximately 290 to 400 nm (33, 34, 36). Thus, our knowledge of spore UV resistance has been constructed largely using a wavelength of UV radiation not normally reaching the Earth's surface, even though ample evidence exists that both DNA photochemistry and microbial responses to UV are strongly wavelength dependent (2, 30, 33, 36).
Of recent interest in our laboratories has been the exploration of factors that confer on B. subtilis spores resistance to environmentally relevant extreme conditions, particularly solar UV radiation and extreme desiccation (23, 28, 30, 34 36, 48, 52). It has been reported that α/β-type SASP but not SASP-γ play a major role in spore resistance to 254-nm UV-C radiation (20, 21) and to wet heat, dry heat, and oxidizing agents (48). In contrast, increased spore water content was reported to affect B. subtilis spore resistance to moist heat and hydrogen peroxide but not to 254-nm UV-C (12, 40, 48). However, the possible roles of SASP-α, -β, and -γ and core water content in spore resistance to environmentally relevant solar UV wavelengths have not been explored. Therefore, in this study, we have used B. subtilis strains carrying mutations in the sspA, sspB, sspE, sspA and sspB, or dacB gene to investigate the contributions of SASP and increased core water content to the resistance of B. subtilis spores to 254-nm UV-C and environmentally relevant polychromatic UV radiation encountered on Earth's surface.
MATERIALS AND METHODS
Bacillus subtilis spores, sporulation, purification, and sample preparation.
Endospores of six different isogenic Bacillus subtilis 168-derived strains were used in this work: PS832, a Trp+ revertant of strain 168 (17) (wild-type strain); PS1899, lacking DacB, a d-alanyl-d-alanine carboxypeptidase (penicillin-binding protein 5*) (40); PS283, lacking SASP-α (20); PS338, lacking SASP-β (20); PS356, lacking SASP-α and -β (encoded by sspA and sspB) (17); and PS483, lacking SASP-γ (12). All strains except PS832 are resistant to chloramphenicol (5 μg/ml). Spores were obtained by cultivation at 37°C with vigorous aeration in double-strength liquid Schaeffer sporulation medium (44), under identical conditions for each strain, and the spores were purified and stored as described previously (22-27, 37). Spore preparations were free (>99%) of growing cells, germinated spores, and cell debris, as determined by phase-contrast microscopy.
Determination of spore water content.
Samples of spores in water were prepared containing known numbers of spores, determined by direct counting in a Neubauer hemocytometer. Triplicate samples of spores were placed in preweighed aluminum weighing boats and allowed to air dry at 20°C, with 33% relative humidity (RH), and weighed daily on an analytical balance to constant weight, which was reached after 3 days. Aluminum boats were transferred to a 110°C oven, dried for 24 h, cooled to room temperature in a desiccator, and weighed daily to a constant weight. Percent water was calculated as follows: [(weight of spores air-dried at 20°C − weight of spores oven dried at 110°C)/weight of spores oven dried at 110°C] × 100 (9).
Spore exposure to mono- and polychromatic UV radiation.
Wet spore samples consisted of suspensions of spores (1 × 106/ml) in water. Dry spore samples consisted of air dried (33% RH) spore monolayers (2 × 107 spores) immobilized on 7-mm-diameter quartz discs (Heraeus Quarzglas GmbH & Co. KG, Hanau, Germany) (26, 27, 41). Effects of mono- and polychromatic UV radiation on spores were determined as described previously (23, 27).
Survival assay.
To recover spores from the quartz discs after UV exposure, spore monolayers were covered by 10% aqueous polyvinyl alcohol, and after drying, the spore-polyvinyl alcohol layer was removed and dissolved in water as described previously (14, 24, 26, 27). Spore survival was determined from appropriate dilutions in distilled water as colony-forming ability after incubation overnight at 37°C on nutrient broth agar plates (Difco, Detroit, MI) as described previously (14, 22-27).
Numerical and statistical analysis.
The surviving fraction of B. subtilis spores was determined from the quotient N/N0, where N is colony formers after UV irradiation and N0 is colony formers without UV irradiation. To determine the curve parameters, the following relationship was used: ln N/N0 = −IC × F + n, where IC is the inactivation constant (m2/J), F is the applied UV fluence (J/m2), and n is the extrapolation number. The IC was determined from the slope of the fluence effect curves as described by Moeller et al. (23). All data shown are expressed as averages ± standard deviations (n = 3 or 4). The results were compared statistically using Student's t test. Values were analyzed in multigroup pairwise combinations, and differences with P values of ≤0.05 were considered statistically significant (22-27).
RESULTS
Role of SASP and spore water content in spore UV resistance in aqueous suspension.
In order to confirm previous results regarding resistance of wild-type, dacB, and sspA sspB mutant spores to 254-nm UV and to extend these results to environmentally relevant UV wavelengths, triplicate samples of spores in aqueous suspension were irradiated with UV of various wavelengths and survival kinetics determined. Spore resistance was then quantified by calculating the F10 value, the applied UV fluence leading to a spore survival of 10% for each strain, and the F10 values compared by Student's t test (Table 1). In response to 254-nm UV, the strains behaved as reported previously (20, 40); dacB spores were not significantly more sensitive than wild-type spores, and sspA sspB spores were significantly more sensitive than wild-type spores (Table 1). However, while sspA sspB spores were also significantly more sensitive to environmentally relevant UV wavelengths [280- to 400-nm and 290- to 400-nm UV-A + UV-B {UV-(A+B)} and 320- to 400-nm UV-A], dacB spores were also significantly more UV sensitive than were wild-type spores (Table 1), thus indicating a role for both α/β-type SASP and decreased spore water content in spore resistance to environmental UV when spores are in aqueous suspension.
TABLE 1.
Survival characteristics of UV-irradiated B. subtilis spores in aqueous suspensiona
| Strain description |
F10 value with treatment
|
|||
|---|---|---|---|---|
| UV-C, 254 nm (J·m−2) | UV-(A+B), 280-400 nm (kJ·m−2) | UV-(A+B), 290-400 nm (kJ·m−2) | UV-A, 320-400 nm (kJ·m−2) | |
| Wild type | 303 ± 25 | 10.1 ± 1.5 | 14.2 ± 1.6 | 498 ± 57 |
| dacB | 280 ± 23 | 7.7 ± 1.2* | 8.1 ± 1.4* | 252 ± 37* |
| sspA sspB | 78 ± 11* | 5.3 ± 0.7* | 6.3 ± 0.8* | 159 ± 26* |
Data are averages and standard deviations (n = 3). Asterisks indicate survival values that were significantly different from those for wild-type spores (P ≤ 0.05).
Role of SASP and spore water content in air-dried spore UV resistance to 254-nm UV-C radiation.
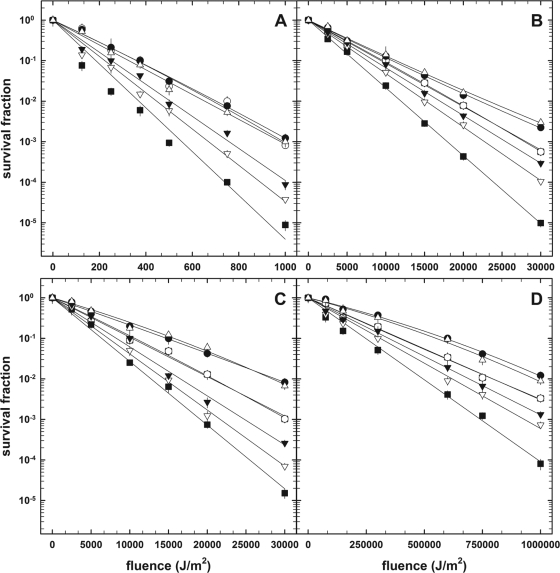
Because germicidal (254-nm) UV is often used to disinfect dry surfaces and building air (18, 31), it was of interest to understand the UV resistance of spores in the air-dried state. In order to extend results from previous studies of spore resistance to 254-nm UV in aqueous suspension, quadruplicate samples of the isogenic air-dried spores were exposed to 254-nm UV-C and spore inactivation kinetics plotted and fitted to exponential best-fit lines (Fig. 1A). Again, F10 values were calculated and compared using Student's t test (Table 2). When the data for 254-nm UV-C were analyzed, it was observed that dacB and sspE spores were not significantly more sensitive to 254-nm UV-C than were wild-type spores (Table 2) but that spores deficient in SASP-α, SASP-β, or both were significantly more sensitive to 254-nm UV-C radiation than the wild type (Table 2). Therefore, when air-dried spores of mutant strains were compared to wild-type spores, their relative sensitivities to 254-nm UV in the air-dried state were qualitatively comparable to results seen previously with spores in aqueous suspension (12, 20, 40) (Table 1).
FIG. 1.
Survival curves of air-dried spores of B. subtilis strains in response to 254-nm UV-C (A), 280- to 400-nm UV-(A+B) (B), 290- to 400-nm UV-(A+B) (C), or 320- to 400-nm UV-A (D) radiation. Strains are indicated as follows: 168 wild type, filled circles; dacB mutant, open circles; sspA mutant, open downward triangles; sspB mutant, filled downward triangles; sspE mutant, open upward triangles; and sspA sspB mutant, filled squares. Data are expressed as averages and standard deviations (n = 4). Error bars for survival data not visible were smaller than the symbol.
TABLE 2.
Survival characteristics of air-dried UV-irradiated B. subtilis sporesa
| Strain description |
F10 value with treatment
|
|||
|---|---|---|---|---|
| UV-C, 254 nm (J·m−2) | UV-(A+B), 280-400 nm (kJ·m−2) | UV-(A+B), 290-400 nm (kJ·m−2) | UV-A, 320-400 nm (kJ·m−2) | |
| Wild type | 326 ± 33 | 10.7 ± 0.9 | 14.9 ± 0.9 | 544 ± 50 |
| dacB | 295 ± 34 | 9.0 ± 0.7 | 10.2 ± 0.8* | 377 ± 31* |
| sspA | 169 ± 17* | 7.1 ± 0.6* | 7.7 ± 0.6* | 260 ± 22* |
| sspB | 216 ± 20* | 7.9 ± 0.5* | 8.4 ± 0.7* | 320 ± 23* |
| sspE | 309 ± 28 | 10.4 ± 0.8 | 14.7 ± 0.9 | 513 ± 44 |
| sspA sspB | 85 ± 13* | 5.8 ± 0.4* | 6.9 ± 0.5* | 203 ± 16* |
Data are averages and standard deviations (n = 4). Asterisks indicate survival values that were significantly different from those for wild-type spores (P ≤ 0.05).
Role of SASP and increased spore core water content in air-dried spore resistance to environmental UV radiation.
Spores are often exposed to UV in the environment in an air-dried form on surfaces or adhered to soil or dust particles (32, 59). Therefore, we examined the contribution that various SASP or DacB made to spore resistance to simulated natural UV radiation wavelengths: 280 to 400 nm (UV-B plus UV-A), 290 to 400 nm (simulating the UV-B plus UV-A radiation reaching Earth's surface), and 320 to 400 nm (UV-A).
Air-dried spores exposed to UV in the 280- to 400-nm range exhibited exponential inactivation kinetics (Fig. 1B), from which F10 values were derived and compared statistically (n = 4). The F10 values of wild-type and sspE and dacB mutant spores were not statistically different (Table 2). In contrast, air-dried spores of strains lacking SASP-α, SASP-β, or both were significantly more sensitive to 280- to 400-nm UV than wild-type spores (Table 2).
Air-dried spores exposed to 290- to 400-nm UV, which represents the range of solar UV wavelengths impinging on Earth's surface (33, 34), also exhibited exponential inactivation kinetics (Fig. 1C). The F10 values of air-dried wild-type and sspE mutant spores were not significantly different at the P = 0.05 level (Table 2), and again, spores of strains lacking SASP-α, SASP-β, or both were significantly more sensitive to 290- to 400-nm UV than wild-type spores (Table 2). Interestingly, air-dried spores of the dacB mutant were also significantly more sensitive to UV in the 290- to 400-nm range than wild-type spores (Fig. 1C and Table 2), thus indicating that spore core dehydration (see below) plays a role in spore resistance to environmentally relevant UV wavelengths.
The major part of natural solar UV consists of UV-A, comprising wavelengths of 320 to 400 nm (34, 55), so air-dried spores were exposed to UV-A wavelengths of 320 to 400 nm produced by a solar simulator and an optical filter combination. Spores exposed to 320- to 400-nm UV-A also exhibited exponential inactivation kinetics (Fig. 1D). Comparison of F10 values derived from best-fit plots showed the air-dried sspE spores lacking SASP-γ were not significantly more sensitive than wild-type spores (Table 2). However, air-dried spores of α/β-type SASP-deficient strains carrying mutations in sspA, sspB, or sspA and sspB were significantly more sensitive than wild-type spores to UV-A solar radiation, as were dacB spores (Table 2). Thus, α/β-type SASP and decreased spore core water content are also determinants of resistance of air-dried spores to environmental UV-A wavelengths.
Comparison of spore UV resistance in aqueous suspension versus the air-dried state.
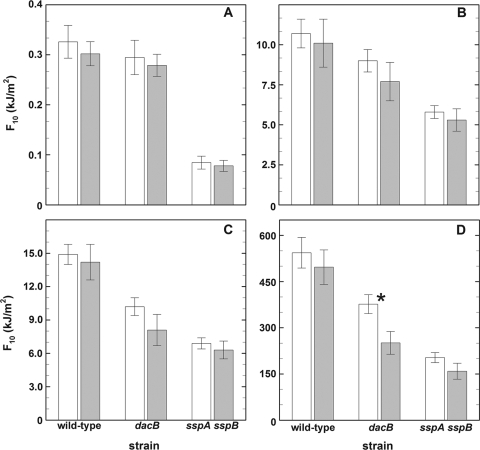
A previous study showed that spores lacking dipicolinic acid exhibited dramatically different responses to 254-nm UV depending on whether the spores were irradiated in aqueous suspension or in the air-dried state (52). The available data from the present study concerning the UV resistance of wild-type, dacB, and sspA sspB spores also allowed direct comparisons to be performed on spores exposed under identical conditions of UV irradiation but in either the aqueous (Table 1) or air-dried (Table 2) state. In response to all UV wavelengths tested, spores irradiated in aqueous suspension were slightly more UV sensitive than the same spores irradiated in the air-dried state (Fig. 2); however, in the majority of cases the differences were not significant at the P < 0.05 level. Interestingly, the sole exception was that dacB spores treated with 320- to 400-nm UV-A radiation were significantly more sensitive in the aqueous state than in the air-dried state (P = 0.011) (Fig. 2D).
FIG. 2.
Comparison of UV resistance (F10 values) of spores irradiated either in the air-dried state (white bars) or in aqueous suspension (gray bars) to 254-nm UV (A), 280- to 400-nm UV-(A+B) (B), 290- to 400-nm UV-(A+B) (C), or 320- to 400-nm UV-A (D). Data are averages and standard deviations (n = 4 for air-dried spores; n = 3 for spores in aqueous suspension). The asterisk denotes a difference significant at a P value of <0.05.
Increased water content in air-dried dacB spores of B. subtilis.
Previous studies had concluded that dacB spores exhibited decreased wet-heat and hydrogen peroxide resistance and contained higher core water content than wild-type spores (40). The observation that dacB spores were more sensitive than wild-type spores preferentially to long-wavelength UV (Fig. 1B to D), which is known to produce reactive oxygen species (ROS) especially in the presence of water, led us to hypothesize that even air-dried dacB spores may contain more water than air-dried wild-type spores. This notion was tested by determining the percentages of water in wild-type and dacB spores that had first been air-dried at 20°C with 33% relative humidity (Table 3). The data showed that wild-type spores contained 40.4% ± 2.3% water, a value in good agreement with a range of earlier measurements (32 to 55% water) conducted on spores of B. subtilis (10, 48). In contrast, air-dried dacB mutant spores contained 64.5% ± 5.5% water, a difference from wild-type spores that was highly significant by Student's t test (P = 0.0086). Therefore, air-dried dacB mutant spores contain significantly more water than do wild-type spores, and it seems highly likely that most of this difference in water content resides in the spore core (see Discussion).
TABLE 3.
Percentages of water in air-dried spores of PS832 (wild type) and PS1899 (dacB)
| Strain description | Sample | No. of spores weigheda | Mass dried at 20°C, 33% RH (g) | Mass dried at 110°C (g) | % Water | Avg ± SD |
|---|---|---|---|---|---|---|
| Wild type | 1 | 7.62 × 109 | 1.7 × 10−3 | 1.0 × 10−3 | 41.2 | 40.4 ± 2.3 |
| 2 | 6.73 × 109 | 1.9 × 10−3 | 1.1 × 10−3 | 42.1 | ||
| 3 | 9.83 × 109 | 3.7 × 10−3 | 2.3 × 10−3 | 37.8 | ||
| dacB | 1 | 3.29 × 109 | 2.3 × 10−3 | 8.0 × 10−4 | 65.2 | 64.5 ± 5.5 |
| 2 | 3.84 × 109 | 2.3 × 10−3 | 7.0 × 10−4 | 69.6 | ||
| 3 | 5.67 × 109 | 4.6 × 10−3 | 1.9 × 10−3 | 58.7 |
Obtained by direct counting.
Comparison of UV resistance of spores by inactivation constants.
A summary of spore resistance to germicidal 254-nm and environmentally relevant UV radiation for spores of the various strains is presented in Table 4. The IC data showed that killing of spores by simulated natural sunlight was much less efficient and required larger exposure fluences than killing by monochromatic 254-nm UV-C. The data also showed that spore resistance to environmentally relevant UV radiation decreased in the order of wild type (greatest resistance), followed by the sspE mutant, the sspB mutant, and then the sspA mutant, with the sspA sspB mutant showing (least resistance), similar to the trend observed after 254-nm UV-C irradiation (Table 4). From these data it can be concluded that SASP-α and -β are important determinants of spore resistance to environmentally relevant UV wavelengths, since spores of mutants lacking SASP-α, SASP-β, or both were significantly more sensitive than wild-type spores at all UV wavelengths tested (Table 4). These results confirm and extend earlier observations that the major α/β-type SASP are important determinants of spore resistance to monochromatic 254-nm UV-C (20, 21). In contrast, spores deficient in SASP-γ did not show any differences in their overall UV resistance from that of wild-type spores at any UV wavelength tested (Table 4), also confirming and extending previous results indicating that SASP-γ plays no role in spore resistance to 254-nm UV-C (12).
TABLE 4.
| Strain description | IC (m2/J) for treatment
|
|||
|---|---|---|---|---|
| UV-C, 254 nm | UV-(A+B), 280-400 nm | UV-(A+B), 290-400 nm | UV-A, 320-400 nm | |
| Wild type | (6.9 ± 0.7) × 10−3 | (1.9 ± 0.3) × 10−4 | (1.6 ± 0.2) × 10−4 | (4.5 ± 0.4) × 10−6 |
| dacB | (7.0 ± 0.9) × 10−3 | (2.5 ± 0.4) × 10−4 | (2.4 ± 0.3) × 10−4* | (6.4 ± 0.5) × 10−6* |
| sspA | (9.8 ± 0.8) × 10−3* | (3.1 ± 0.3) × 10−4* | (3.5 ± 0.2) × 10−4* | (7.6 ± 0.8) × 10−6* |
| sspB | (8.9 ± 0.6) × 10−3* | (2.8 ± 0.5) × 10−4* | (3.0 ± 0.3) × 10−4* | (6.9 ± 0.7) × 10−6* |
| sspE | (7.2 ± 0.7) × 10−3 | (2.0 ± 0.4) × 10−4 | (1.6 ± 0.1) × 10−4 | (4.8 ± 0.5) × 10−6 |
| sspA sspB | (1.1 ± 0.2) × 10−2* | (4.1 ± 0.7) × 10−4* | (4.3 ± 0.8) × 10−4* | (9.6 ± 1.3) × 10−6* |
The IC, i.e., the slope of the semilogarithmic survival curves (see Materials and Methods), was determined for each strain and each treatment.
Data are averages and standard deviations (n = 4). Asterisks indicate IC values that were significantly different from the respective wild-type IC (P ≤ 0.05).
In the same manner as that described above, a comparison was made between the IC values of wild-type and dacB spores (Table 4). As observed previously (40), dacB spores did not differ significantly from wild-type spores in their sensitivity to 254-nm UV-C in a comparison of either their IC (Table 4) or F10 values (Table 1). However, dacB spores were significantly more sensitive than wild-type spores to 280- to 400-nm UV-(A+B), 290- to 400-nm UV-(A+B), and 320- to 400-nm UV-A (Table 4).
DISCUSSION
Solar UV radiation plays an important role in regulating levels of microorganisms in the environment, and recent decreases in atmospheric ozone levels pose a serious threat to the ecological balance of bacterial populations in the environment (13, 50). In order to survive, bacterial spores must maintain the integrity of their DNA for extended periods (3, 11, 33, 38). Although spores are more resistant to UV radiation than vegetative cells, vegetative cells can constantly repair their DNA. In contrast, spores are metabolically inactive and accumulate DNA damage in their genomes during dormancy (22, 27, 30, 33, 34, 45, 48, 49). Therefore, upon germination, spores must rapidly repair the cumulative damage in their genomic DNA before transcription and replication can resume (33, 34, 38,). While spore DNA photochemistry and DNA repair have been well defined in the laboratory (33-35, 48), the present study of the roles of SASP and spore core water content in spore resistance to natural solar UV radiation provides a better understanding of the resistance of spores in the environment.
The data presented in this article augment previous data suggesting a number of conclusions about mechanisms of spore resistance to monochromatic (254-nm) and environmentally relevant polychromatic UV radiation at >290 nm. First, it appears that the major α/β-type SASP are necessary not only for spore UV-C resistance in the laboratory (20) but also for spore resistance in the solar UV environment (this report). In contrast, SASP-γ does not appear to be involved in spore resistance either to laboratory UV-C (12) or to environmental UV (this report). An unexpected result from this work was the observation that spores of strains carrying a dacB mutation with higher spore core water content than wild-type spores were not significantly more sensitive than wild-type spores to monochromatic 254-nm UV-C (40; this report) but were significantly more sensitive to environmentally relevant solar UV-B and UV-A wavelengths, particularly to UV wavelengths of >320 nm (this report).
A body of evidence has accumulated which suggests that solar UV exerts its lethal effects through direct interaction with spore DNA and indirectly via the generation of ROS, such as peroxide, superoxide, or hydroxyl radicals (34, 55). Examples for direct UV damage include pyrimidine dimers, such as the “spore photoproduct” (SP) 5-thyminyl-5,6-dihydrothymine, cyclobutane pyrimidine dimer, and pyrimidine(6-4)pyrimidone photoproducts (5, 53). These direct photoproducts are produced much more efficiently by 254-nm UV-C and the shorter-wavelength UV-B (290 to 320 nm) component of sunlight than they are by longer-wavelength UV-A (320 to 400 nm) (54). In contrast, nonspecific DNA damage, such as DSB, single-strand breaks, and AP sites, are more likely to be produced indirectly from UV-A via the production of ROS (51, 55, 59). It is well established that SP is the major UV photoproduct in spore DNA irradiated with UV-C (4, 56). While SP is also the major photoproduct in DNA of spores exposed to sunlight, less SP is detected per lethal event at solar wavelengths, suggesting that additional DNA photoproducts are also formed in spores exposed to solar radiation (36, 51, 54, 56, 59). Slieman and Nicholson (51) reported that cyclobutane pyrimidine dimers are preferentially produced in spores exposed to the UV-B component of sunlight and that DSB and single-strand breaks are formed mainly when spores are exposed to the UV-A component of sunlight (27, 51). However, AP sites were not detected in spore DNA under any of the conditions tested (51).
Regarding spore resistance to oxidative stress, it has been well established that UV-B radiation and UV-A radiation are both capable of inducing cellular oxidative stress via production of ROS and by photolysis of H2O water molecules into H· and OH· radicals (2, 13, 55). It has been observed that spores deficient in their α/β-type SASP but not SASP-γ are much more sensitive to treatments which produce ROS (e.g., ionizing radiation or oxidizing agents, such as hydrogen peroxide) than are wild-type spores (16, 24, 26, 33, 34, 48). Likewise, Popham et al. (40) reported increased hydrogen peroxide sensitivity of spores carrying the dacB mutation, suggesting that increased spore core water content results in decreased spore resistance to oxidative damage. In this study, we observed that dacB spores were also significantly more sensitive to solar wavelengths of UV-B and UV-A than wild-type spores, while dacB spores exhibited the same resistance as wild-type spores to 254-nm UV-C.
Mutation in dacB leads to higher core water content in wet spores, as determined by buoyant density centrifugation (40), but little was known about the water content of wild-type and dacB spores in the air-dried state. We determined that air-dried dacB spores also contained considerably larger amounts of water than did wild-type spores (Table 3). We reason that the extra water in dacB spores also likely resides in the spore core, since the outer spore layers (the spore coat and the cortex) are readily permeated by water and have been observed to shrink and swell dynamically with changes in relative humidity (7, 39, 58). It is thought that the cortex constrains expansion and contraction of the spore core, and the inner spore membrane also acts to greatly slow water passage, keeping the (de)hydration state of the spore core constant (7). These observations lead us to the conclusion that in addition to the α/β-type SASP, spore core dehydration is an important factor in the resistance of spores to environmentally relevant solar UV radiation, particularly the longer UV-A wavelengths, and that core dehydration specifically protects spore DNA by virtue of reducing the production and/or migration of ROS within the spore core.
Acknowledgments
We are very grateful to Justus Ackermann for his excellent, skillful technical assistance.
Footnotes
Published ahead of print on 19 June 2009.
REFERENCES
- 1.Burton, N. C., A. Adhikari, S. A. Grinshpun, R. Hornung, and T. Reponen. 2005. The effect of filter material on bioaerosol collection of Bacillus subtilis spores used as a Bacillus anthracis simulant. J. Environ. Monit. 7:475-480. [DOI] [PubMed] [Google Scholar]
- 2.Cadet, J., E. Sage, and T. Douki. 2005. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. 571:3-17. [DOI] [PubMed] [Google Scholar]
- 3.Cano, R. J., and M. K. Borucki. 1995. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science 268:1060-1064. [DOI] [PubMed] [Google Scholar]
- 4.Donnellan, J. E., Jr., and R. B. Setlow. 1965. Thymine photoproducts but not thymine dimers are found in ultraviolet irradiated bacterial spores. Science 149:308-310. [DOI] [PubMed] [Google Scholar]
- 5.Douki, T., B. Setlow, and P. Setlow. 2005. Effects of the binding of alpha/beta-type small, acid-soluble spore proteins on the photochemistry of DNA in spores of Bacillus subtilis and in vitro. Photochem. Photobiol. 81:163-169. [DOI] [PubMed] [Google Scholar]
- 6.Driks, A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 63:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driks, A. 2003. The dynamic spore. Proc. Natl. Acad. Sci. USA 100:3007-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell, S., H. B. Halsall, and W. R. Heineman. 2005. Immunoassay for B. globigii spores as a model for detecting B. anthracis spores in finished water. Analyst 130:489-497. [DOI] [PubMed] [Google Scholar]
- 9.Gerhardt, P. 1985. Diluents and biomass measurement, p. 504-507. In P. Gerhardt, R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips (ed.), Manual of methods for general bacteriology. American Society for Microbiology, Washington, DC.
- 10.Gerhardt, P., and R. E. Marquis. 1989. Spore thermoresistance mechanisms, p. 43-63. In I. Smith, R. A. Slepecky, and P. Setlow (ed.), Regulation of procaryotic development. American Society for Microbiology, Washington, DC.
- 11.Gest, H., and J. Mandelstam. 1987. Longevity of microorganisms in natural environments. Microbiol. Sci. 4:69-71. [PubMed] [Google Scholar]
- 12.Hackett, R. H., and P. Setlow. 1988. Properties of spores of Bacillus subtilis strains which lack the major small, acid-soluble protein. J. Bacteriol. 170:1403-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Häder, D. P., and R. P. Sinha. 2005. Solar ultraviolet radiation-induced DNA damage in aquatic organisms: potential environmental impact. Mutat. Res. 571:221-233. [DOI] [PubMed] [Google Scholar]
- 14.Horneck, G., P. Rettberg, G. Reitz, J. Wehner, U. Eschweiler, K. Strauch, C. Panitz, V. Starke, and C. Baumstark-Khan. 2001. Protection of bacterial spores in space, a contribution to the discussion on Panspermia. Orig. Life Evol. Biosph. 31:527-547. [DOI] [PubMed] [Google Scholar]
- 15.Ibarra, J. R., A. D. Orozco, J. A. Rojas, K. López, P. Setlow, R. E. Yasbin, and M. Pedraza-Reyes. 2008. Role of the Nfo and ExoA apurinic/apyrimidinic endonucleases in repair of DNA damage during outgrowth of Bacillus subtilis spores. J. Bacteriol. 190:2031-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, K. S., D. Bumbaca, J. Kosman, P. Setlow, and M. J. Jedrzejas. 2008. Structure of a protein-DNA complex essential for DNA protection in spores of Bacillus species. Proc. Natl. Acad. Sci. USA 105:2806-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loshon, C. A., P. C. Genest, B. Setlow, and P. Setlow. 1999. Formaldehyde kills spores of Bacillus subtilis by DNA damage and small, acid-soluble spore proteins of the alpha/beta-type protect spores against this DNA damage. J. Appl. Microbiol. 87:8-14. [DOI] [PubMed] [Google Scholar]
- 18.Luna, V. A., A. C. Cannons, P. T. Amuso, and J. Cattani. 2008. The inactivation and removal of airborne Bacillus atrophaeus endospores from air circulation systems using UVC and HEPA filters. J. Appl. Microbiol. 104:489-498. [DOI] [PubMed] [Google Scholar]
- 19.Majcher, M. R., K. A. Bernard, and S. A. Sattar. 2008. Identification by quantitative carrier test of surrogate spore-forming bacteria to assess sporicidal chemicals for use against Bacillus anthracis. Appl. Environ. Microbiol. 74:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason, J. M., and P. Setlow. 1986. Essential role of small, acid-soluble spore proteins in resistance of Bacillus subtilis spores to UV light. J. Bacteriol. 167:174-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason, J. M., and P. Setlow. 1987. Different small, acid-soluble proteins of the alpha/beta type have interchangeable roles in the heat and UV radiation resistance of Bacillus subtilis spores. J. Bacteriol. 169:3633-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moeller, R., T. Douki, J. Cadet, E. Stackebrandt, W. L. Nicholson, P. Rettberg, G. Reitz, and G. Horneck. 2007. UV radiation induced formation of DNA bipyrimidine photoproducts in Bacillus subtilis endospores and their repair during germination. Int. Microbiol. 10:39-46. [PubMed] [Google Scholar]
- 23.Moeller, R., G. Horneck, R. Facius, and E. Stackebrandt. 2005. Role of pigmentation in protecting Bacillus sp. endospores against environmental UV radiation. FEMS Microbiol. Ecol. 51:231-236. [DOI] [PubMed] [Google Scholar]
- 24.Moeller, R., G. Horneck, E. Rabbow, G. Reitz, C. Meyer, U. Hornemann, and D. Stöffler. 2008. Role of DNA protection and repair in resistance of Bacillus subtilis spores to ultrahigh shock pressures simulating hypervelocity impacts. Appl. Environ. Microbiol. 74:6682-6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moeller, R., G. Horneck, P. Rettberg, H.-J. Mollenkopf, E. Stackebrandt, and W. L. Nicholson. 2006. A method for extracting RNA from dormant and germinating Bacillus subtilis strain 168 endospores. Curr. Microbiol. 53:227-231. [DOI] [PubMed] [Google Scholar]
- 26.Moeller, R., P. Setlow, G. Horneck, T. Berger, G. Reitz, P. Rettberg, A. J. Doherty, R. Okayasu, and W. L. Nicholson. 2008. Roles of the major, small, acid-soluble spore proteins and spore-specific and universal DNA repair mechanisms in resistance of Bacillus subtilis spores to ionizing radiation from X rays and high-energy charged-particle bombardment. J. Bacteriol. 190:1134-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moeller, R., E. Stackebrandt, G. Reitz, T. Berger, P. Rettberg, A. J. Doherty, G. Horneck, and W. L. Nicholson. 2007. Role of DNA repair by non-homologous end joining in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV, and ionizing radiation. J. Bacteriol. 189:3306-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munakata, N., and C. S. Rupert. 1972. Genetically controlled removal of “spore photoproduct” from deoxyribonucleic acid of ultraviolet-irradiated Bacillus subtilis spores. J. Bacteriol. 111:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munakata, N., and C. S. Rupert. 1974. Dark repair of DNA containing “spore photoproduct” in Bacillus subtilis. Mol. Gen. Genet. 130:239-250. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson, W. L., P. Fajardo-Cavazos, R. Rebeil, T. A. Slieman, P. J. Riesenman, J. F. Law, and Y. Xue. 2002. Bacterial endospores and their significance in stress resistance. Antonie van Leeuwenhoek 81:27-32. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson, W. L., and B. Galeano. 2003. UV resistance of Bacillus anthracis spores revisited: validation of Bacillus subtilis spores as UV surrogates for spores of B. anthracis Sterne. Appl. Environ. Microbiol. 69:1327-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholson, W. L., and J. F. Law. 1999. Method for purification of bacterial endospores from soils: UV resistance of natural Sonoran desert soil populations of Bacillus spp. with reference to B. subtilis strain 168. J. Microbiol. Methods 35:13-21. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of bacterial endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson, W. L., A. C. Schuerger, and P. Setlow. 2005. The solar UV environment and bacterial spore UV resistance: considerations for Earth-to-Mars transport by natural processes and human spaceflight. Mutat. Res. 571:249-264. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson, W. L., B. Setlow, and P. Setlow. 1990. Binding of DNA in vitro by a small, acid-soluble spore protein from Bacillus subtilis and the effect of this binding on DNA topology. J. Bacteriol. 172:6900-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholson, W. L., B. Setlow, and P. Setlow. 2002. UV photochemistry of DNA in vitro and in Bacillus subtilis spores at earth-ambient and low atmospheric pressure: implications for spore survival on other planets or moons in the solar system. Astrobiology 2:417-425. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination, and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Sussex, England.
- 38.Nicholson, W. L. 2002. Roles of Bacillus endospores in the environment. Cell. Mol. Life Sci. 59:410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plomp, M., T. J. Leighton, K. E. Wheeler, and A. J. Malkin. 2005. The high-resolution architecture and structural dynamics of Bacillus spores. Biophys. J. 88:603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popham, D. L., S. Sengupta, and P. Setlow. 1995. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl. Environ. Microbiol. 61:3633-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puskeppeleit, M., L. E. Quintern, S. el Naggar, J. U. Schott, U. Eschweiler, G. Horneck, and H. Buecker. 1992. Long-term dosimetry of solar UV radiation in Antarctica with spores of Bacillus subtilis. Appl. Environ. Microbiol. 58:2355-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riesenman, P. J., and W. L. Nicholson. 2000. Role of the spore coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl. Environ. Microbiol. 66:620-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salas-Pacheco, J. M., B. Setlow, P. Setlow, and M. Pedraza-Reyes. 2005. Role of the Nfo (YqfS) and ExoA apurinic/apyrimidinic endonucleases in protecting Bacillus subtilis spores from DNA damage. J. Bacteriol. 187:7374-7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schaeffer, P., J. Millet, and J.-P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 45:704-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Setlow, P. 1995. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu. Rev. Microbiol. 49:29-54. [DOI] [PubMed] [Google Scholar]
- 47.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 48.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514-525. [DOI] [PubMed] [Google Scholar]
- 49.Setlow, P. 2008. Dormant spores receive an unexpected wake-up call. Cell 135:410-412. [DOI] [PubMed] [Google Scholar]
- 50.Sinha, R. P., and D. P. Häder. 2002. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 1:225-236. [DOI] [PubMed] [Google Scholar]
- 51.Slieman, T. A., and W. L. Nicholson. 2000. Artificial and solar UV radiation induces strand breaks and cyclobutane pyrimidine dimers in Bacillus subtilis spore DNA. Appl. Environ. Microbiol. 66:199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slieman, T. A., and W. L. Nicholson. 2001. Role of dipicolinic acid in survival of Bacillus subtilis spores exposed to artificial and solar UV radiation. Appl. Environ. Microbiol. 67:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slieman, T. A., R. Rebeil, and W. L. Nicholson. 2000. Spore photoproduct (SP) lyase from Bacillus subtilis specifically binds to and cleaves SP (5-thyminyl-5,6-dihydrothymine) but not cyclobutane pyrimidine dimers in UV-irradiated DNA. J. Bacteriol. 182:6412-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tyrrell, R. M. 1978. Solar dosimetry with repair deficient bacterial spores: action spectra, photoproduct measurements, and comparison with other biological systems. Photochem. Photobiol. 27:571-579. [DOI] [PubMed] [Google Scholar]
- 55.Tyrrell, R. M. 1992. Inducible responses to UV-A exposure, p. 59-64. In F. Urbach (ed.), Biological responses to ultraviolet-A radiation. Valdenmar Publishing, Overland Park, KS.
- 56.Varghese, A. J. 1970. 5-Thyminyl-5,6-dihydrothymine from DNA irradiated with ultraviolet light. Biochem. Biophys. Res. Commun. 38:484-490. [DOI] [PubMed] [Google Scholar]
- 57.Wang, S. T., B. Setlow, E. M. Conlon, J. L. Lyon, D. Imamura, T. Sato, P. Setlow, R. Losick, and P. Eichenberger. 2006. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 358:16-37. [DOI] [PubMed] [Google Scholar]
- 58.Westphal, A. J., P. B. Price, T. J. Leighton, and K. E. Wheeler. 2003. Kinetics of size changes of individual Bacillus thuringiensis spores in response to changes in relative humidity. Proc. Natl. Acad. Sci. USA 100:3461-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue, Y., and W. L. Nicholson. 1996. The two major spore DNA repair pathways, nucleotide excision repair and spore photoproduct lyase, are sufficient for the resistance of Bacillus subtilis spores to artificial UV-C and UV-B but not to solar radiation. Appl. Environ. Microbiol. 62:2221-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]