Abstract
We have sequenced the double-stranded DNA genomes of six lactococcal phages (SL4, CB13, CB14, CB19, CB20, and GR7) from the 936 group that were isolated over a 9-year period from whey samples obtained from a Canadian cheese factory. These six phages infected the same two industrial Lactococcus lactis strains out of 30 tested. The CB14 and GR7 genomes were found to be 100% identical even though they were isolated 14 months apart, indicating that a phage can survive in a cheese plant for more than a year. The other four genomes were related but notably different. The length of the genomes varied from 28,144 to 32,182 bp, and they coded for 51 to 55 open reading frames. All five genomes possessed a 3′ overhang cos site that was 11 nucleotides long. Several structural proteins were also identified by nano-high-performance liquid chromatography-tandem mass spectrometry, confirming bioinformatic analyses. Comparative analyses suggested that the most recently isolated phages (CB19 and CB20) were derived, in part, from older phage isolates (CB13 and CB14/GR7). The organization of the five distinct genomes was similar to the previously sequenced lactococcal phage genomes of the 936 group, and from these sequences, a core genome was determined for lactococcal phages of the 936 group.
The manufacture of cheeses requires the inoculation of carefully selected bacterial cultures, known as starter cultures, at concentrations of at least 107 live bacteria per ml of heat-treated milk. The purpose of this process is to control the fermentation and to obtain high-quality fermented products (29). Starter cultures are a combination of lactic acid bacteria (LAB), of which one of the most important species is Lactococcus lactis. L. lactis is a low-GC gram-positive bacterium used to metabolize lactose into lactic acid during the production of several cheese varieties. Because large amounts of lactococcal cells are cultivated each day in large-scale fermentation vats and because these cells are susceptible to bacteriophage infection, it is not surprising that most cheese factories have experienced problems with phage contamination (13). Even a single phage infecting a starter strain is enough to begin a chain reaction that can eventually inhibit bacterial growth and cause production delays, taste and texture variations, and even complete fermentation failures (1, 29).
Phage infections are unpredictable in food fermentations. Their presence and persistence in a dairy factory can be explained in many ways. First, raw milk can introduce new phages into an industrial plant (25). Madera et al. (22) also reported that newly isolated lactococcal phages were more resistant to pasteurization. Whey, a liquid by-product of cheese manufacturing, is another reservoir that can spread phages in a factory environment (25). Airborne phage dissemination may also be important since concentrations of up to 106 PFU/m3 have been observed close to a functional whey separation tank (32).
For decades, the dairy industry has been working to curtail the propagation of virulent phages using a variety of practical strategies, including, among others, sanitation, optimized factory design, air filtration units, rotation of bacterial strains, and the use of phage resistance systems (13). Yet new virulent phages emerge on a regular basis. Indeed, large-scale industrial milk fermentation processes can be slowed down by virulent phages of the Caudovirales order. Members of three lactococcal phage groups, namely, 936, c2, and P335, are mostly found in dairy plants. The 936-like phages are by far the most predominant worldwide (3, 18, 22, 27).
Phages of the 936 group have a double-stranded DNA genome and possess a long noncontractile tail connected to a capsid with icosahedral symmetry characteristic of the Siphoviridae family. Currently, six complete phage genomes of the lactococcal 936 group are available in public databases, including sk1 (6), bIL170 (10), jj50, 712, P008 (23), and bIBB29 (16). Their comparative analysis revealed a conserved gene organization despite being isolated from different countries. Most of the differences have been observed in the early gene module, where insertions, deletions, and point mutations likely occurred (16, 23). Moreover, it is assumed that these phages can also exchange DNA through recombination with other bacterial viruses present in the same ecosystem.
Because new members of this lactococcal phage group are regularly isolated, a better understanding of their evolution is warranted to better control them. A cheese factory is a particular man-made niche where rapidly growing bacterial strains encounter ubiquitous phages. Such active environments provide ample opportunities for phage evolution, especially to dodge phage resistance mechanisms that may be present in host cells. Nonetheless, the evolutionary dynamics that shape the diversity of lactococcal phage populations are still not well understood.
In this study, we analyzed the genome and structural proteome of six 936-group phages (SL4, CB13, CB14, CB19, CB20, and GR7) that infected the same L. lactis strains and were isolated over a 9-year period from a cheese factory.
MATERIALS AND METHODS
Isolation of virulent lactococcal phages.
Whey samples were obtained from a single cheese plant using defined starter cultures. The phages present in the samples were amplified using L. lactis subspecies cremoris SMQ-404 as an indicator strain, as described elsewhere (3, 28), and were propagated according to the method of Jarvis (17). The species of the lactococcal phage isolates was obtained using multiplex PCR (19).
Bacterial strains and phages.
L. lactis subsp. cremoris SMQ-404 and SMQ-438 were grown at 30°C in M17 (Difco) supplemented with 0.5% lactose. For the propagation of phages SL4, CB13, CB14, CB19, CB20, and GR7, host cells were incubated until the optical density at 600 nm reached 0.1. Phages and CaCl2 were added to the growing culture at final concentrations of 106 phages/ml and 10 mM, respectively. The phage-infected culture was incubated until complete bacterial lysis was obtained and then filtered through a 0.45-μm syringe filter (Fisher Scientific). To obtain highly concentrated phage preparations, lysates were mixed with polyethylene glycol (34) and purified on a discontinuous CsCl gradient, followed by a continuous one-step CsCl gradient. The first centrifugation was performed at 35,000 rpm for 3 h in a Beckman SW41 Ti rotor. The second ultracentrifugation was performed using a Beckman NVT65 rotor at 60,000 rpm for 18 h (7, 14).
DNA sequencing and sequence analysis.
The DNA of virulent lactococcal phages SL4, CB13, CB14, CB19, CB20, and GR7 was isolated from high-titer lysates using a Lambda Maxi kit (Qiagen), and the modifications for the 936 group were suggested by Deveau et al. (11). To confirm the identity of the isolated DNA, digestions with EcoRI and EcoRV endonucleases were performed. Restriction profiles were then matched with their corresponding patterns in our database. Restriction endonucleases (Roche Diagnostics) were used as recommended by the manufacturer. Primers previously designed to sequence the genome of lactococcal phage P008 (23) were used for the direct sequencing of conserved regions in the genomes of the six new phages. Primers were then designed to complete the sequencing of both strands using an ABI Prism 3100 apparatus from the genomic platform at the Centre Hospitalier de l'Université Laval. The cos site was determined as reported elsewhere (23). Sequence assembly was performed with Staden software (http://staden.sourceforge.net/), and BioEdit 7.0.5.3 software (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) was used for alignment editing. Open reading frames (ORFs) were predicted using ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). The assignment of ORFs was performed using criteria that were described previously (23). The translated ORF products were compared with known protein sequences using BLASTP (2). The estimated molecular masses and pIs were obtained using the tool Compute pI/Mw (http://ca.expasy.org).
Structural protein identification.
Approximately 8 μg of phage proteins (5) were added to a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel (1.5 mm thick). The protein samples were mixed with 4× loading buffer (0.250 M Tris-HCl [pH 6.8], 40% [wt/vol] glycerol, 8% [wt/vol] SDS, 20% [vol/vol] β-mercaptoethanol, 0.1% [wt/vol] bromophenol blue) and boiled for 5 min before loading. Proteins were detected using Coomassie blue staining. For protein identification, bands were cut from the gel, digested with trypsin, and identified by nano-high-performance liquid chromatography-tandem mass spectrometry (nano-HPLC-MS/MS) at the Génome Québec Innovation Centre at McGill University.
DNA-DNA hybridizations.
The Southern blot DNA analysis of phage and bacterial genomes was done using the method described by Deveau et al. (12). Phage genomic DNAs were used as probes. Bacterial DNA was isolated by the method described by Fortier and Moineau (15) with the following modifications: cell pellets were suspended in 1 ml saline (0.85% NaCl), transferred to an Eppendorf tube, and centrifuged for 10 min at full speed in a microcentrifuge. The pellets were then suspended in 200 μl of a 25% sucrose solution containing 30 mg/ml lysozyme and incubated at 37°C for 15 min. Finally, 400 μl of 3% SDS was added and the preparation incubated at room temperature with agitation for 7 min.
Electron microscopy.
A 1.5-ml sample from a high-titer phage lysate was centrifuged (23,500 × g) at 4°C for 1 h. The supernatant was discarded and the residual 100 μl washed twice with 1.5 ml ammonium acetate (0.1 M). A final volume of 100 μl was saved for observation. Grids were prepared by adding 7.5 μl of washed phages to a copper Formvar carbon-coated grid (200 mesh; Pelco International). Uranyl acetate (7.5 μl of a 2% solution) was immediately added and mixed by pipetting up and down. The liquid was removed after 30 to 60 s by touching the edge of the grid with blotting paper (12). Phages were observed at 80 kV with a JEOL 1230 transmission electron microscope. Dimensions given were the means of 10 specimens. Phage dimensions were measured using purified phage preparation diluted 1:10 rather than washed phage lysate.
Nucleotide accession numbers.
The genome sequences were submitted to the GenBank database under the following accession numbers: FJ848881 (phage SL4), FJ848882 (phage CB13), FJ848883 (phage CB14), FJ848884 (phage CB19), and FJ848885 (phage CB20).
RESULTS AND DISCUSSION
Isolation of the phages.
The six virulent phages analyzed in this study (SL4, CB13, CB14, CB19, CB20, and GR7) were isolated from different cheddar cheese whey samples from the same Canadian cheese factory over a 9-year period (1996 to 2004) and, at the beginning of the study, were the only ones infecting the industrial L. lactis strain SMQ-404 (Table 1). A host-range analysis indicated that all six phages infected the same two L. lactis subsp. cremoris strains (SMQ-404 and SMQ-438) out of 30 L. lactis subsp. cremoris strains tested. So far, L. lactis SMQ-404 and SMQ-438 are sensitive to only 936-like phages. The EcoRV and EcoRI restriction patterns of isolated phage DNAs were compared and found to be related (data not shown). Multiplex PCR analysis confirmed that they all belonged to the 936 species (12, 19). The genomes were sequenced to shed more light on their origins.
TABLE 1.
Characteristics of the lactococcal phages analyzed in this study
| Phagea | Year of isolation | Country | Host strain | Genome characteristics
|
Reference or source | |||
|---|---|---|---|---|---|---|---|---|
| Length (bp) | % G+C | No. of ORFs | cos site | |||||
| P008 | 1971 | Germany | IL1403 | 28,538 | 34.7 | 58 | 5′-CACAAAGGATT-3′ | 23 |
| bIL170 | 1973 | France | IL1403 | 31,754 | 34.4 | 64 | 5′-CACAAAGGACT-3′ | 10 |
| sk1 | Before 1976 | Australia | MG1363 | 28,451 | 34.5 | 55 | 5′-CACAAAGGACT-3′ | 6 |
| jj50 | 1985 | Denmark | MG1363 | 27,453 | 34.9 | 50 | 5′-CACAAAGGACT-3′ | 23 |
| 712 | Before 1988 | New Zealand | MG1363 | 30,510 | 33.9 | 55 | 5′-CACAAAGGACT-3′ | 23 |
| SL4 | 1996 | Canada | SMQ-404 | 28,144 | 35.0 | 52 | 5′-CACAAAGGACT-3′ | This study |
| CB13 | 2003 | Canada | SMQ-404 | 32,182 | 34.7 | 55 | 5′-CACAGAGGACT-3′ | This study |
| CB14 | 2003 | Canada | SMQ-404 | 29,459 | 34.8 | 52 | 5′-CACAGAGGACT-3′ | This study |
| CB19 | 2003 | Canada | SMQ-404 | 28,643 | 35.2 | 51 | 5′-CACAAAGGACT-3′ | This study |
| CB20 | 2003 | Canada | SMQ-404 | 28,625 | 35.0 | 51 | 5′-CACAAAGGACT-3′ | This study |
| GR7 | 2004 | Canada | SMQ-404 | 29,459 | 34.8 | 52 | 5′-CACAGAGGACT-3′ | This study |
| bIBB29 | Before 2007 | Poland | IL1403 | 29,305 | 34.7 | 54 | 5′-CACAAAGGACT-3′ | 16 |
Phages in bold indicate the six lactococcal phages analyzed and sequenced in this study.
Analysis of phage genomes.
Overall, the genome length of the six lactococcal phages ranged from 28,144 to 32,182 bp (Table 1). Their GC content was 34.9%, which is similar to other lactococcal phages (Table 1) and L. lactis strains (35.7%) for which the complete genomes are available (4, 24, 40). These phage genomes possessed 51 to 55 ORFs and, overall, shared 82.1% nucleic acid identity. Interestingly, comparative genomic analyses revealed that phages CB14 and GR7 contained identical genomes (100%), even though the two phages were isolated 14 months apart, indicating that a phage can be stable in a cheese plant for a long period of time. Phages CB19 and CB20 were 99.5% identical (Table 2) and were isolated from the same whey sample. The main sequence differences were found in orf1 and orf3 (Table 3), which likely encode terminase subunits. The genome of phage SL4 possessed four additional orf genes compared to the other four distinct phage genomes. Two of the orf genes (orf10 and orf24) encode proteins containing a putative HNH endonuclease motif, indicating possible roles in replication, recombination, maturation, or encapsidation of phage DNA (10). It has been suggested that the homing endonuclease is also involved in the gene diversity observed in the early expressed genomic region of the 936-like phages (23). Phage CB13 also has four distinct genes including one (orf31) that may code for an endonuclease.
TABLE 2.
Nucleic acid identity between each lactococcal phage genome of the 936 groupa
| Phage | Nucleic acid identity (%) with indicated phage
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P008 | bIL170 | sk1 | jj50 | 712 | SL4 | CB13 | CB14/GR7 | CB19 | CB20 | bIBB29 | |
| P008 | 100 | ||||||||||
| bIL170 | 77.8 | 100 | |||||||||
| sk1 | 74.2 | 69.8 | 100 | ||||||||
| jj50 | 74.9 | 69.8 | 93.9 | 100 | |||||||
| 712 | 71.7 | 64.9 | 75.1 | 73.4 | 100 | ||||||
| SL4 | 74.1 | 69.9 | 73.2 | 74.4 | 69.1 | 100 | |||||
| CB13 | 67.2 | 72.3 | 67.1 | 67.5 | 62.2 | 72.1 | 100 | ||||
| CB14/GR7 | 71.9 | 73.7 | 72.4 | 72.6 | 66.7 | 77.1 | 79.2 | 100 | |||
| CB19 | 72.6 | 75.4 | 70.7 | 71.9 | 66.2 | 80.9 | 75.3 | 90.3 | 100 | ||
| CB20 | 72.7 | 75.6 | 70.7 | 71.9 | 66.3 | 80.9 | 75.8 | 89.9 | 99.5 | 100 | |
| bIBB29 | 77.8 | 75.7 | 70.6 | 71.2 | 68.3 | 74.1 | 67.7 | 68.1 | 68.6 | 68.8 | 100 |
Phages in bold indicate the six lactococcal phages analyzed and sequenced in this study.
TABLE 3.
Coordinates of phage CB19 ORFs and representative ORFs and genes in the other 936-type phagesa
| Strand | ORF | Start | Stop | Size (aa) | MM (kDa) | pI | SD sequence AGAAAGGAGGTb | Representative ORF or gene (% aa identity) in indicated phage
|
Putative function | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SL4 | CB13 | CB14/GR7 | CB20 | P008 | bIL170 | sk1 | jj50 | 712 | bIBB29 | |||||||||
| + | 1 | 266 | 790 | 174 | 19.9 | 5.1 | AGAAAGGATAAtATG | ORF1 (98) | ORF1 (98) | ORF1 (100) | ORF1 (98) | ORF1 (96) | L1 (97) | ORF1 (97) | ORF1 (97) | ORF1 (97) | ORF1 (95) | Terminase small subunit |
| + | 2 | 787 | 1017 | 76 | 9.0 | 4.0 | GCACCAGAGGGatttgaATG | ORF2 (96) | ORF2 (97) | ORF2 (100) | ORF2 (97) | |||||||
| + | 3 | 1029 | 2651 | 540 | 63.0 | 6.0 | AGAAAGGTAATgaATG | ORF3 (98) | ORF3 (97) | ORF3 (100) | ORF3 (97) | ORF2 (97) | L2 (95) | ORF2 (97) | ORF2 (98) | ORF2 (97) | ORF2 (97) | Terminase large subunit |
| + | 4 | 2641 | 2925 | 94 | 11.2 | 9.1 | AGAAATGGCGGtgtcagATG | ORF4 (98) | ORF4 (100) | ORF4 (100) | ORF4 (100) | ORF3 (95) | L3 (97) | ORF3 (96) | ORF3 (96) | ORF3 (96) | ORF3 (98) | HNH endonuclease |
| + | 5 | 2938 | 4074 | 378 | 43.3 | 5.0 | AGAAAGGGGAAaaaTTG | ORF5 (96) | ORF5 (97) | ORF5 (95) | ORF5 (100) | ORF4 (94) | L4 (94) | ORF4 (92) | ORF4 (92) | ORF4 (92) | ORF4 (96) | Portal protein |
| + | 6 | 4055 | 4591 | 178 | 19.9 | 4.6 | AGAAAGGACGTaacaagcacagATG | ORF6 (98) | ORF6 (96) | ORF6 (99) | ORF6 (100) | ORF5 (98) | L5 (98) | ORF5 (98) | ORF5 (97) | ORF5 (97) | ORF5 (97) | Prohead protease |
| + | 7 | 4584 | 5765 | 393 | 43.7 | 5.5 | ATTGAGGATATtaaaaagaaatATG | ORF7 (93) | ORF7 (98) | ORF7 (96) | ORF7 (100) | ORF6 (95) | L6 (95) | ORF6 (95) | ORF6 (95) | ORF6 (96) | ORF6 (94) | Minor structural protein |
| + | 8 | 5786 | 6049 | 87 | 10.0 | 6.2 | AAAACGGAGGAagtaaATG | ORF8 (98) | ORF8 (96) | ORF8 (96) | ORF8 (100) | ORF8 (98) | L8 (98) | ORF7 (98) | ORF7 (96) | ORF7 (97) | ORF7 (96) | |
| + | 9 | 6049 | 6363 | 104 | 11.8 | 9.0 | ATTATGGAGGTatttaATG | ORF9 (95) | ORF9 (94) | ORF9 (92) | ORF9 (100) | ORF9 (94) | L9 (90) | ORF8 (92) | ORF8 (96) | ORF8 (93) | ORF8 (95) | |
| + | 10 | 6353 | 6691 | 112 | 12.9 | 4.2 | AGAGGGGGTCGtaagtaATG | ORF11 (94) | ORF10 (96) | ORF10 (92) | ORF10 (100) | ORF10 (91) | L10 (93) | ORF9 (96) | ORF9 (91) | ORF9 (91) | ORF9 (93) | |
| + | 11 | 6682 | 7047 | 121 | 13.7 | 10.2 | GTGCAGGTGGTcaaccATG | ORF12 (97) | ORF11 (95) | ORF11 (93) | ORF11 (100) | ORF11 (96) | L11 (94) | ORF10 (98) | ORF10 (95) | ORF10 (95) | ORF10 (95) | |
| + | 12 | 7104 | 9074 | 656 | 71.3 | 5.2 | ACAATAATGGTattttttaATG | ORF12 (33) | ORF12 (40) | ORF12 (100) | ORF12 (63) | L12 (85) | ORF12 (53), ORF16 (18) | NPS | ||||
| + | 13 | 9098 | 10003 | 301 | 32.6 | 4.9 | AAAAAGGAAAAtaaaaaATG | ORF13 (94) | ORF13 (91) | ORF13 (96) | ORF13 (100) | ORF13 (92) | L13 (93) | ORF11 (93) | ORF11 (92) | ORF11 (85) | ORF11 (85) | Major capsid protein |
| + | 14 | 10041 | 10316 | 91 | 10.6 | 4.9 | TAAAGGGATATaaaacaaaATG | ORF14 (98) | ORF14 (100) | ORF14 (97) | ORF14 (100) | ORF14 (100) | L14 (100) | ORF12 (98) | ORF12 (100) | ORF13 (100) | ORF13 (97) | |
| + | 15 | 10336 | 10848 | 170 | 19.9 | 4.8 | TGAGAGGGCTGtgaATG | ORF15 (97) | ORF15 (96) | ORF15 (97) | ORF15 (100) | ORF15 (97) | L15 (98) | ORF13 (95) | ORF13 (98) | ORF14 (95) | ORF14 (97) | |
| + | 16 | 10848 | 13838 | 996 | 105.6 | 9.1 | AGAAAGGGTATgtaATG | ORF16 (97) | ORF16 (86) | ORF16 (85) | ORF16 (100) | ORF16 (75) | L16 (76) | ORF14 (82) | ORF14 (81) | ORF15 (74) | ORF15 (77) | TMP |
| + | 17 | 13838 | 14734 | 298 | 34.4 | 5.5 | ACTAGGGAGGGcttaATG | ORF17 (92) | ORF17 (94) | ORF17 (100) | ORF17 (100) | ORF17 (92) | L17 (91) | ORF15 (92) | ORF15 (92) | ORF16 (86) | ORF16 (95) | |
| + | 18 | 14734 | 15861 | 375 | 42.8 | 5.1 | AGAAAGGCGGActtcgtttaATG | ORF18 (94) | ORF18 (94) | ORF18 (95) | ORF18 (100) | ORF18 (92) | L18 (91) | ORF16 (91) | ORF16 (91) | ORF17 (91) | ORF17 (92) | |
| + | 19 | 15851 | 16144 | 97 | 11.4 | 9.3 | AGAAAGTGGAGacaaaccaaATG | ORF19 (97) | ORF19 (97) | ORF19 (100) | ORF19 (100) | ORF19 (96) | L19 (96) | ORF17 (96) | ORF17 (94) | ORF18 (96) | ORF18 (96) | |
| + | 20 | 16134 | 16943 | 269 | 29.1 | 5.7 | TCAAGAAAGGTtaaaaATG | ORF20 (88) | ORF20 (87) | ORF20 (100) | ORF20 (100) | ORF20 (45) | L20 (46) | ORF18 (75) | ORF18 (76) | ORF19 (59) | ORF19 (32) | RBP |
| + | 21 | 16965 | 17318 | 117 | 13.5 | 6.3 | AGAAAGCAAAAtaaaATG | ORF21 (88) | ORF21 (96) | ORF21 (96) | ORF21 (100) | ORF21 (90) | L21 (87) | ORF19 (92) | ORF19 (90) | ORF20 (91) | ORF20 (89) | Holin |
| + | 22 | 17315 | 18019 | 234 | 26.0 | 5.7 | CAAACGGAGGAtaaaaaagaATG | ORF22 (98) | ORF22 (100) | ORF22 (100) | ORF22 (100) | ORF22 (76) | L22 (76) | ORF20 (67) | ORF20 (68) | ORF21 (68) | ORF21 (76) | Endolysin |
| − | 23 | 18613 | 18464 | 49 | 6.0 | 8.5 | CAACTAGAAGTatagcgTTG | ORF23 (89) | ORF23 (59) | ORF23 (100) | ORF23 (100) | ORF23 (61) | E36 (85) | p21 (91) | ORF23 (83) | ORF22 (87) | ||
| − | 24 | 18940 | 18674 | 88 | 10.2 | 4.6 | AAATTAAAGGTatgaatagATG | ORF25 (96) | ORF24 (93) | ORF24 (100) | ORF24 (100) | ORF25 (93) | E33 (93) | ORF21 (95) | ORF21 (95) | ORF24 (85) | ORF25 (90) | |
| − | 25 | 19460 | 19113 | 115 | 13.5 | 9.1 | ATAATTGAGGTtatagcataATG | ORF26 (85) | ORF25 (79) | ORF25 (100) | ORF25 (100) | ORF26 (66) | E31 (65) | ORF23 (90) | ORF22 (90) | ORF26 (73) | ORF27 (81) | |
| − | 26 | 19627 | 19460 | 55 | 6.9 | 5.8 | AGACAGGAGTAatcggataATG | ORF27 (90) | ORF26 (90) | ORF26 (100) | ORF26 (100) | ORF27 (90) | E30 (90) | ORF24 (35) | ORF23 (35) | ORF27 (35) | ORF28 (42) | |
| − | 27 | 19803 | 19627 | 58 | 6.8 | 10.3 | AGAAAGCTAGTgaataatATG | ORF28 (96) | ORF27 (91) | ORF27 (100) | ORF27 (100) | ORF28 (88) | E29 (90) | ORF29 (90) | ||||
| − | 28 | 19996 | 19805 | 63 | 7.1 | 10.2 | AGAAAGTTTTGgtgaaaaaataaATG | ORF29 (93) | ORF28 (88) | ORF28 (100) | ORF28 (100) | |||||||
| − | 29 | 20295 | 20038 | 85 | 9.8 | 5.0 | GAAAAGGAGGTtaaataGTG | ORF30 (91) | ORF29 (96) | ORF29 (100) | ORF29 (100) | ORF29 (68) | E28 (65) | ORF25 (88) | ORF24 (86) | ORF28 (75) | ORF30 (69) | |
| − | 30 | 20676 | 20368 | 102 | 12.1 | 4.6 | GGCTTGGAGGTaacatctaATG | ORF31 (94) | ORF32 (96) | ORF30 (100) | ORF30 (100) | ORF33 (82) | E24 (91) | ORF26 (80) | ORF25 (78) | ORF33 (83) | ||
| − | 31 | 20795 | 20676 | 39 | 4.1 | 8.4 | ATAAATATAGGagaacaaaATG | ORF32 (92) | ORF33 (89) | ORF31 (100) | ORF31 (100) | ORF34 (62) | E23 (89) | ORF27 (79) | ORF26 (79) | ORF34 (79) | ||
| − | 32 | 21095 | 20853 | 80 | 9.7 | 6.7 | CGAAAGGAAGTaaatagATG | ORF33 (88) | ORF35 (88) | ORF32 (100) | ORF32 (100) | E21 (85) | ORF30 (86) | ORF29 (83) | ORF29 (88) | ORF36 (85) | ||
| − | 33 | 21206 | 21096 | 36 | 3.8 | 9.4 | ATAAAGGAGCGatacaATG | ORF36 (100) | ORF33 (100) | ORF33 (100) | E19 (91) | sk1p32 (94) | ORF30 (86) | |||||
| − | 34 | 21555 | 21241 | 104 | 11.9 | 4.6 | ACTATGGTGGTatcgtctaATG | ORF34 (92) | ORF37 (90) | ORF34 (100) | ORF34 (100) | ORF35 (86) | E17 (88) | ORF31 (83) | ORF30 (86) | ORF38 (82) | ||
| − | 35 | 21746 | 21555 | 63 | 7.4 | 6.5 | GGAAAGAGGAAaaATG | ORF35 (93) | ORF38 (90) | ORF35 (100) | ORF35 (100) | |||||||
| − | 36 | 22338 | 21826 | 170 | 20.3 | 9.7 | ATATATGAGGGagtattATG | ORF36 (98) | ORF39 (98) | ORF36 (100) | ORF36 (100) | ORF37 (94) | E15 (91) | ORF32 (91) | ORF31 (91) | ORF32 (96) | ORF39 (91) | |
| − | 37 | 22547 | 22335 | 70 | 8.3 | 4.7 | GGAAAGTTGGTttcATG | ORF37 (95) | ORF40 (92) | ORF37 (100) | ORF37 (100) | ORF38 (92) | E14 (92) | ORF33 (92) | ORF32 (92) | ORF33 (91) | ORF40 (94) | |
| − | 38 | 22922 | 22563 | 119 | 13.0 | 5.0 | AGAGGGAAAATaaaaaATG | ORF38 (100) | ORF41 (90) | ORF38 (100) | ORF38 (100) | ORF39 (92) | E13 (94) | ORF34 (92) | ORF33 (92) | ORF34 (94) | ORF41 (88) | |
| − | 39 | 23549 | 22926 | 207 | 23.9 | 6.7 | CAAATGGAGAAaaaaATG | ORF39 (61) | ORF42 (63) | ORF39 (100) | ORF39 (100) | ORF40 (61) | E12 (92) | ORF35 (96) | ORF34 (95) | ORF35 (63) | ORF42 (91) | Sak protein |
| − | 40 | 24046 | 23546 | 166 | 19.2 | 9.6 | AGAAAGAGGAGaaaATG | ORF40 (100) | ORF40 (100) | E11 (94) | HNH endonuclease | |||||||
| − | 41 | 24299 | 24033 | 88 | 10.5 | 7.8 | TATTAGAAAGTtcatttATG | ORF40 (91) | ORF43 (90) | ORF41 (100) | ORF41 (100) | ORF42 (86) | E10 (94) | ORF38 (76) | ORF37 (84) | ORF38 (76) | ORF43 (88) | |
| − | 42 | 24543 | 24256 | 95 | 11.7 | 10.0 | ATAAAGGAGAAataATG | ORF41 (91) | ORF44 (95) | ORF42 (100) | ORF42 (100) | ORF43 (93) | E9 (92) | ORF39 (95) | ORF38 (97) | ORF44 (93) | ||
| − | 43 | 24853 | 24596 | 85 | 10.1 | 9.1 | AGCAAGGAAAGgtaacagaaaATG | ORF43 (94) | ORF45 (88) | ORF43 (100) | ORF43 (100) | ORF45 (87) | E7 (87) | ORF41 (89) | ORF40 (89) | ORF40 (88) | ORF46 (89) | |
| − | 44 | 25742 | 24846 | 298 | 34.2 | 5.7 | CTAAAGGAGAAagaaATG | ORF44 (45) | ORF47 (45) | ORF44 (100) | ORF44 (100) | ORF47 (96) | E5 (45) | ORF43 (43) | ORF42 (44) | ORF42 (95) | ORF47 (46) | DNA polymerase subunit |
| − | 45 | 25947 | 25786 | 53 | 6.2 | 6.5 | AGAAAAGGAGAaaatATG | ORF45 (90) | ORF45 (100) | ORF48 (92) | ORF45 (76) | |||||||
| − | 46 | 26957 | 26769 | 62 | 7.6 | 9.6 | TTATAGGAGGTaactATG | ORF47 (93) | ORF50 (91) | ORF47 (91) | ORF46 (100) | ORF52 (85) | E1 (90) | ORF50 (88) | ORF45 (85) | ORF49 (85) | ORF50 (91) | |
| + | 47 | 26988 | 27215 | 75 | 8.5 | 4.9 | ATAAAGAGTATaacataaaATG | ORF48 (97) | ORF51 (95) | ORF48 (92) | ORF47 (100) | ORF53 (97) | M1 (95) | ORF51 (95) | ORF46 (95) | ORF51 (90) | ORF51 (95) | |
| + | 48 | 27220 | 27351 | 43 | 5.2 | 4.9 | TTAAGGGAGAAtaaagaaATG | ORF49 (93) | ORF52 (90) | ORF49 (93) | ORF48 (100) | ORF54 (93) | M2 (90) | ORF52 (93) | ORF47 (93) | ORF52 (93) | ORF52 (95) | |
| + | 49 | 27348 | 27827 | 159 | 17.9 | 6.8 | TGAATTGAGTTctgatttATG | ORF50 (96) | ORF53 (95) | ORF50 (96) | ORF49 (100) | ORF55 (96) | M3 (92) | ORF53 (96) | ORF48 (94) | ORF53 (94) | ORF53 (91) | Holliday junction endonuclease |
| + | 50 | 27828 | 27995 | 55 | 6.2 | 6.5 | AGAAAGTCAGGataagtaaATG | ORF51 (96) | ORF54 (91) | ORF51 (96) | ORF50 (100) | ORF56 (98) | M4 (98) | ORF54 (100) | ORF49 (100) | ORF54 (92) | ORF54 (100) | |
| + | 51 | 28416 | 28556 | 46 | 5.3 | 11.2 | AGATAAGGGAGaagcaaATG | ORF52 (97) | ORF55 (86) | ORF52 (86) | ORF51 (100) | ORF58 (76) | ||||||
The percentages have been calculated for the smallest proteins. ORFs in bold represent those of the CB19 genome that comprise part of the core genome of the 936 group. aa, amino acids; MM, molecular mass; SD, Shine-Dalgarno.
Lowercase bases are not part of the SD sequence.
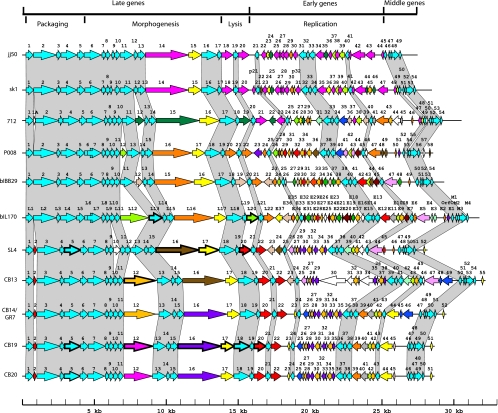
Table 1 summarizes the main characteristics of the six phages as well as their comparisons with the six other 936-like phages for which complete sequences are available in GenBank. These latter six phages were isolated outside North America, and their host strains (laboratory strains L. lactis subsp. lactis IL1403 and L. lactis subsp. cremoris MG1363) were different from the one used here (industrial strain L. lactis subsp. cremoris SMQ-404). The overall genome organization, however, was highly conserved in all 11 virulent lactococcal phage sequences of the 936 group (Fig. 1). The DNA packaging module was always found next to the morphogenesis module, followed by the lysis genes and the replication cluster. Comparative genomic analyses indicated that these 11 genomes possessed 62.2% to 99.5% identity at the nucleotide level, even though the isolates came from seven different countries (Table 2).
FIG. 1.
Schematic representation of the genomic organization of phages belonging to the 936 group. Each line represents a different phage genome and each arrow represents a putative protein. Each genome was compared only with the successive genome in this figure. ORFs of the same color represent those that share more than 80% amino acid identity. The percentages have been calculated for the smallest proteins. The white ORFs are unique. Gray shading connects genome regions conserved in all phages. Finally, arrows with thick outlines represent structural proteins observed on SDS-PAGE gels and identified by MS or by N-terminal sequencing (for the protein identification of phage bIL170, see references 9 and 33).
All 11 phages possessed cohesive genomic extremities (cos type). We identified three unique cos sites, although only one or two nucleotides separated these three genomic extremities (Table 1). Phages SL4, CB19, CB20, sk1, bIL170, 712, jj50, and bIBB29 possessed the same cos site, even though some of them were isolated 30 years apart. Phages CB13 and CB14/GR7 shared the same cos site sequence, while phage P008 had distinct genomic extremities (Table 1).
Origins of the phage genes.
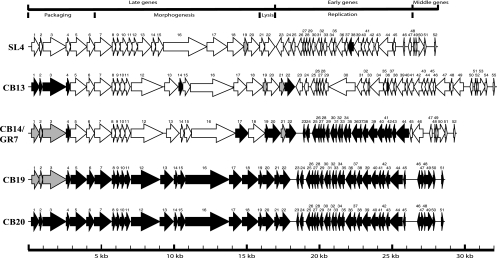
Phage SL4 was isolated in 1996, while CB13, CB14, CB19, and CB20 emerged between July 2003 and September 2003. Phage CB20 was the last distinct phage isolated from our whey samples that infected the same L. lactis strains. Thus, we hypothesized that CB20 may be derived from the other four phages. Pairwise comparisons suggested that orf4 to orf51 of phage CB20 may have derived from the genome of CB19, while orf1, orf2, and orf3 originated from the genome of phage CB13 (Fig. 2).
FIG. 2.
Schematic representation of the genomic organization of lactococcal phages SL4, CB13, CB14/GR7, CB19, and CB20. Each line represents a different phage genome, and each arrow represents a putative protein. The ORFs in black or gray represent those that share 100% amino acid identity. Arrows in white indicate proteins that share less than 100% amino acid identity.
Phage CB19 was the second-to-last phage isolated from the cheese whey samples. The orf17, orf19, orf20, and orf22 to orf44 sequences of phage CB19 may have originated from phage CB14, while orf4 and orf14 may have originated from phage CB13 (Fig. 2). However, the origins of orf5 to orf21 (except for orf14, orf17, orf19, and orf20) and orf45 to orf51 of phage CB19 remain elusive. Nonetheless, these data suggest that the most recently isolated lactococcal phages are derived, in part, from older phage isolates. The isolation of phages CB13, CB14, CB19, and CB20 in a short period of time in 2003 may explain why we could identify these shared modules. Phage SL4 was isolated 8 years previously, and it was less related to these phages.
L. lactis host strains are known to carry prophages (4, 24, 40) that participate in strain diversity and the evolution of virulent phages (20), although these prophages belong to the P335 group and are genetically distinct from the 936-like phages analyzed here (12). Nonetheless, to verify if the unknown phage DNA came from the host strain, DNA-DNA hybridization experiments were performed against total DNA from L. lactis strains SMQ-404 and SMQ-438 by using total phage DNA as the probe. No hybridization signals were observed (data not shown), indicating that these two host strains did not contribute to the genomic diversity of the 936-like phages. Taken together, these data suggest that some phage modules were swapped from other virulent lactococcal phages already in the cheese plants but not analyzed in this study.
Structural proteins.
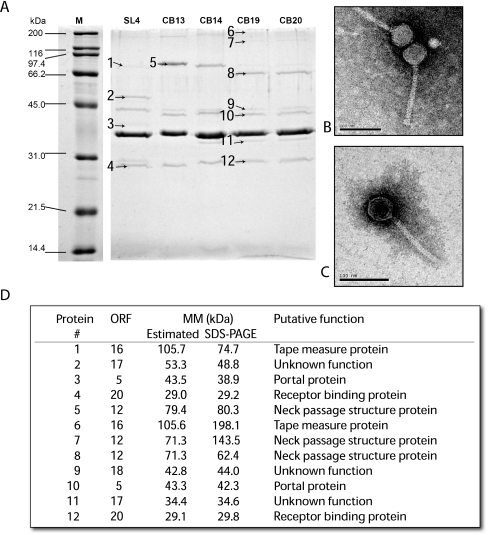
The structural protein profiles of the five lactococcal phages were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) (Fig. 3A). The five phages had similar protein profiles, including a single major structural protein, along with several minor proteins, confirming their relatedness (Fig. 3A). Phage SL4 had the most divergent profile. A total of 12 proteins were identified by nano-HPLC-MS/MS, including 7 from phage CB19, 4 from SL4, and 1 from CB13 (Fig. 3A and D). More structural proteins were selected from phage CB19 as it appeared to contain the most common proteins (based on molecular weight) among the five phages. All proteins identified by nano-HPLC-MS/MS could be linked to a phage gene. The molecular masses calculated from the SDS-PAGE were in agreement with the theoretical masses for 9 of the 12 phage proteins (Fig. 3D), including the portal protein (bands 3 and 10), the receptor binding protein (RBP; bands 4 and 12), and structural proteins of unknown function (bands 2, 9, and 11).
FIG. 3.
(A) Analysis of the structural proteins of phages SL4, CB13, CB14, CB19, and CB20 by SDS-PAGE. M is the broad-range protein marker (Bio-Rad). The numbers represent the proteins identified by MS. (B) Electron micrograph of phage CB19. (C) Electron micrograph of phage SL4. (D) Identification of the phage structural proteins by nano-HPLC coupled with MS/MS.
The putative tape measure protein (TMP; protein 6) of phage CB19 had an observed mass of 198.1 kDa, which was almost double the theoretical value estimated by bioinformatic analyses (105.6 kDa). The formation of a dimer could possibly explain this difference. Similarly, protein band 7 of phage CB19 had an estimated molecular mass of 143.5 kDa based on its migration on an SDS-PAGE gel, while its theoretical mass was calculated to be 71.3 kDa. The protein was identified as a putative neck passage structure (NPS) protein. Interestingly, the same protein was identified in bands 5 and 8 as a monomer. Structural and functional analyses have shown that some phage structural proteins are found as dimers (8, 21, 30).
As indicated above, the structural protein profiles of phage SL4 differed from the other four phages, but proteins with similar functions also differed in size. For example, the TMP (band 1) of phage SL4 was much smaller than its counterpart (band 6) in phage CB19, suggesting that it may be processed in SL4. Similarly, the portal protein was also smaller in SL4 (band 3) compared to phage CB19 (band 10). On the other hand, the RBP was a similar size in both phages. This is not surprising since the RBPs of these phages have a conserved architecture of three protomers related by a threefold axis, and each protomer comprises three domains: the N-terminal shoulders, the interlaced β-prism linker, and the C-terminal head (33, 36, 38). Moreover, both phages infect the same L. lactis strains.
NPS protein.
Bioinformatic and structural protein profile analyses suggested that phages CB13, CB14, CB19, and CB20 harbored an NPS protein. A gene (L12) coding for such a protein was previously observed in the 936-like lactococcal phage bIL41 (9). The NPS protein of phage bIL41 shared 69% identity with ORF12 of phages CB19 and CB20. It was also previously shown that the 936-like phages sk1 and jj50 do not carry a gene coding for an NPS protein (Fig. 1) (23). Similarly, we could not identify a gene coding for this protein in phage SL4. Observation of the five lactococcal phages (SL4, CB13, CB14, CB19, and CB20) by electron microscopy identified a collar structure for all phages but SL4 (Fig. 3B and C), confirming the presence of NPS in four of the five lactococcal phages analyzed.
Crutz-Le Coq et al. (9) previously showed that NPS protein does not seem to play an important role during the assembly of phages and that it is not essential for lactococcal phages of the 936 group. They hypothesized, however, that NPS protein may be involved in host recognition (9). That SL4 does not possess an NPS protein structure and has the same host range as CB13, CB14, CB19, and CB20 suggests that this protein may have another function. Others have determined that NPS protein forms a collar-whisker complex but is nonessential for phage assembly, stability, and host range of lactococcal phages of the P335 group (39).
Core genome of 936-like phages.
A core genome is defined as a set of genes invariably present and conserved in a group of isolates (37). According to Muzzi et al. (31), a gene is considered conserved when two proteins can be aligned with a minimum of 50% sequence conservation over 50% of the protein length. Using these definitions and the 11 genomes known for the lactococcal 936-like phages, a core genome was determined for this group of phages. A total of 33 ORFs were conserved (363 proteins out of 597 proteins analyzed [60.8%]) and are part of the core genome of the 936 group. ORFs of phage CB19 that are part of the core genome of the 936 group are highlighted in Table 3. Most of these ORFs are likely structural proteins. The most conserved protein was ORF14 from phages SL4, CB13, CB14, CB19, CB20, and P008. This ORF also corresponded to L14 in phage bIL170, ORF13 in phages 712 and bIBB29, and ORF12 in phages sk1, jj50, and p2. It was recently proposed that the nonstructural phage protein ORF12 of phage p2 might act as a chaperone, maintaining the phage TMP in solution during the tail assembly of lactococcal phages (35).
A comparative analysis was also performed with the 33 core-deduced proteins of the 936-like phages and deduced proteins found in other lactococcal phage groups. None of the 936 core proteins gave a significant match to other lactococcal phage proteins, except endolysin, which might also be conserved in the lactococcal Q54 and P087 phage groups.
Based on bacterial studies (26), the core genome includes all genes/proteins responsible for the basic biological characteristics of a species as well as its major phenotypic traits. Clearly, structural proteins represent major constituents of any given phage group, leading to a conserved morphotype. It remains to be seen if this core genome will be upheld as more phage genomes of the 936 group become available.
Conclusions.
To our knowledge, this is the first report on the genomic characterization of North American lactococcal phages of the predominant 936 group. The analysis of these Canadian lactococcal phages has almost doubled the number of genomes available for 936-like phages. Sequence comparisons provided valuable information about the evolutionary history of these phages. One phage was found to persist in a cheese factory for more than a year, indicating that the industrial practice of removing a starter culture for a short period of time (weeks/months) is unlikely to be effective in the long term. This study also showed that the genome architecture of lactococcal phages of the 936 group is highly conserved and probably reflects an optimal organization to rapidly multiply in a dairy environment. Such a conserved genetic structure likely facilitated the functional exchange of genes or groups of genes (modules) between virulent phage genomes in response to various host and/or environmental factors. Despite the conserved structure, our analysis identified considerable genetic flux between phage genomes, particularly in the early expressed region. Future studies aimed at understanding this natural genomic variation will likely provide clues to improved control strategies for lactococcal phage populations.
Acknowledgments
We thank Hélène Deveau and Julie Samson for helpful discussions, Denise Tremblay for assistance with the figures, Claudia Bergeron and Steve Labrie for phage isolation, and Barbara-Ann Conway for editorial assistance. We are grateful to M. Lamoureux (Agropur) for providing whey samples.
This work was funded by a strategic grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada.
Footnotes
Published ahead of print on 19 June 2009.
REFERENCES
- 1.Accolas, J. P., C. Peigney, G. K. Y. Limsowtin, P.-J. Cluzel, and L. Séchaud. 1994. Lutte contre les bactériophages dans l'industrie laitière, p. 473-492. In H. de Roissart and F. M. Luquet (ed.), Bactéries lactiques. Lorica, Uriage, France.
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissonnette, F., S. Labrie, H. Deveau, M. Lamoureux, and S. Moineau. 2000. Characterization of mesophilic mixed starter cultures used for the manufacture of aged cheddar cheese. J. Dairy Sci. 83:620-627. [DOI] [PubMed] [Google Scholar]
- 4.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Chandry, P. S., S. C. Moore, J. D. Boyce, B. E. Davidson, and A. J. Hillier. 1997. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol. 26:49-64. [DOI] [PubMed] [Google Scholar]
- 7.Chibani Azaïez, S. R., I. Fliss, R. E. Simard, and S. Moineau. 1998. Monoclonal antibodies raised against native major capsid proteins of lactococcal c2-like bacteriophages. Appl. Environ. Microbiol. 64:4255-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cingolani, G., D. Andrews, and S. Casjens. 2006. Crystallogenesis of bacteriophage P22 tail accessory factor gp26 at acidic and neutral pH. Acta Crystallogr. Sect. F 62:477-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crutz-Le Coq, A.-M., F. Cantele, S. Lanzavecchia, and S. Marco. 2006. Insights into structural proteins of 936-type virulent lactococcal bacteriophages. Arch. Virol. 151:1039-1053. [DOI] [PubMed] [Google Scholar]
- 10.Crutz-Le Coq, A. M., B. Cesselin, J. Commissaire, and J. Anba. 2002. Sequence analysis of the lactococcal bacteriophage bIL170: insights into structural proteins and HNH endonucleases in dairy phages. Microbiology 148:985-1001. [DOI] [PubMed] [Google Scholar]
- 11.Deveau, H., M. R. Van Calsteren, and S. Moineau. 2002. Effect of exopolysaccharides on phage-host interactions in Lactococcus lactis. Appl. Environ. Microbiol. 68:4364-4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deveau, H., S. J. Labrie, M.-C. Chopin, and S. Moineau. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72:4338-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emond, E., and S. Moineau. 2007. Bacteriophages and food fermentations, p. 93-124. In S. McGrath and D. van Sinderen (ed.), Bacteriophage: genetics and molecular biology. Horizon Scientific Press/Caister Academic Press, Norfolk, United Kingdom.
- 14.Fortier, L.-C., A. Bransi, and S. Moineau. 2006. Genome sequence and global gene expression of Q54, a new phage species linking the 936 and c2 phage species of Lactococcus lactis. J. Bacteriol. 188:6101-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortier, L.-C., and S. Moineau. 2007. Morphological and genetic diversity of temperate phages in Clostridium difficile. Appl. Environ. Microbiol. 73:7358-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hejnowicz, M. S., M. Golebiewski, and J. Bardowski. 2009. Analysis of the complete genome sequence of the lactococcal bacteriophage bIBB29. Int. J. Food Microbiol. 131:52-61. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis, A. W. 1978. Serological studies of a host range mutant of a lactic streptococcal bacteriophage. Appl. Environ. Microbiol. 36:785-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josephsen, J., N. Andersen, H. Behrndt, E. Brandsborg, G. Christiansen, S. Hansen, and E. W. Nielsen. 1994. An ecological study of lytic bacteriophages in Lactococcus lactis subsp. cremoris isolated in a cheese plant over a five year period. Int. Dairy J. 4:123-140. [Google Scholar]
- 19.Labrie, S., and S. Moineau. 2000. Multiplex PCR for detection and identification of lactococcal bacteriophages. Appl. Environ. Microbiol. 66:987-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Labrie, S. J., and S. Moineau. 2007. Abortive infection mechanisms and prophage sequences significantly influence the genetic make-up of emerging lytic lactococcal phages. J. Bacteriol. 189:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leiman, P. G., M. M. Shneider, V. A. Kostyuchenko, P. R. Chipman, V. V. Mesyanzhinov, and M. G. Rossmann. 2003. Structure and location of gene product 8 in the bacteriophage T4 baseplate. J. Mol. Biol. 328:821-833. [DOI] [PubMed] [Google Scholar]
- 22.Madera, C., C. Monjardin, and J. E. Suarez. 2004. Milk contamination and resistance to processing conditions determine the fate of Lactococcus lactis bacteriophages in dairies. Appl. Environ. Microbiol. 70:7365-7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahony, J., H. Deveau, S. McGrath, M. Ventura, C. Canchaya, S. Moineau, G. F. Fitzgerald, and D. van Sinderen. 2006. Sequence and comparative genomic analysis of lactococcal bacteriophages jj50, 712, and P008: evolutionary insights into the 936 phage species. FEMS Microbiol. Lett. 261:253-261. [DOI] [PubMed] [Google Scholar]
- 24.Makarova, K., A. Slesarev, Y. Wolf, A. Sorokin, B. Mirkin, E. Koonin, A. Pavlov, N. Pavlova, V. Karamychev, N. Polouchine, V. Shakhova, I. Grigoriev, Y. Lou, D. Rohksar, S. Lucas, K. Huang, D. Goodstein, T. Hawkins, V. Plengvidhya, D. Welker, J. Hughes, Y. Goh, A. Benson, K. Baldwin, J. Lee, I. Diaz-Muniz, B. Dosti, V. Smeianov, W. Wechter, R. Barabote, G. Lorca, E. Altermann, R. Barrangou, B. Ganesan, Y. Xie, H. Rawsthorne, D. Tamir, C. Parker, F. Breidt, J. Broadbent, R. Hutkins, D. O'Sullivan, J. Steele, G. Unlu, M. Saier, T. Klaenhammer, P. Richardson, S. Kozyavkin, B. Weimer, and D. Mills. 2006. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. USA 103:15611-15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntyre, K., H. A. Heap, G. P. Davey, and G. K. Y. Limsowtin. 1991. The distribution of lactococcal bacteriophage in the environment of a cheese manufacturing plant. Int. Dairy J. 1:183-197. [Google Scholar]
- 26.Medini, D., C. Donati, H. Tettelin, V. Masignani, and R. Rappuoli. 2005. The microbial pan-genome. Curr. Opin. Genet. Dev. 15:589-594. [DOI] [PubMed] [Google Scholar]
- 27.Moineau, S., M. Borkaev, B. J. Holler, S. A. Walker, J. K. Kondo, E. R. Vedamuthu, and P. A. Vandenbergh. 1996. Isolation and characterization of lactococcal phages from U.S. buttermilk plants. J. Dairy Sci. 79:2104-2111. [Google Scholar]
- 28.Moineau, S., J. Fortier, H.-W. Ackermann, and S. Pandian. 1992. Characterization of lactococcal bacteriophages from Québec cheese plants. Can. J. Microbiol. 38:875-882. [Google Scholar]
- 29.Moineau, S., D. Tremblay, and S. Labrie. 2002. Phages of lactic acid bacteria: from genomics to industrial applications. ASM News 68:388-393. [Google Scholar]
- 30.Morais, M. C., S. Kanamaru, M. O. Badasso, J. S. Koti, B. A. Owen, C. T. McMurray, D. L. Anderson, and M. G. Rossmann. 2003. Bacteriophage phi29 scaffolding protein gp7 before and after prohead assembly. Nat. Struct. Biol. 10:572-576. [DOI] [PubMed] [Google Scholar]
- 31.Muzzi, A., V. Masignani, and R. Rappuoli. 2007. The pan-genome: towards a knowledge-based discovery of novel targets for vaccines and antibacterials. Drug Discov. Today 12:429-439. [DOI] [PubMed] [Google Scholar]
- 32.Neve, H., A. Laborius, and K. J. Heller. 2003. Testing of the applicability of battery-powered portable microbial air samplers for detection and enumeration of air-borne dairy Lactococcus lactis bacteriophages. Kieler Milchwirtschaftliche Forschungsberichte 55:301-315. [Google Scholar]
- 33.Ricagno, S., V. Campanacci, S. Bangy, S. Spinelli, D. Tremblay, S. Moineau, M. Tegoni, and C. Cambillau. 2006. Crystal structure of the receptor-binding protein head domain from Lactococcus lactis phage bIL170. J. Virol. 80:9331-9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., and D. W. Russell. 2001. Molecular cloning, a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Siponen, M., G. Sciara, M. Villion, S. Spinelli, J. Lichière, C. Cambillau, S. Moineau, and V. Campanacci. 2009. Crystal structure of ORF12 from Lactococcus lactis phage p2 identifies a tape measure protein chaperone. J. Bacteriol. 191:728-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spinelli, S., A. Desmyter, C. T. Verrips, H. J. de Haard, S. Moineau, and C. Cambillau. 2006. Lactococcal bacteriophage p2 receptor-binding protein structure suggests a common ancestor gene with bacterial and mammalian viruses. Nat. Struct. Mol. Biol. 13:85-89. [DOI] [PubMed] [Google Scholar]
- 37.Tettelin, H., V. Masignani, M. J. Cieslewicz, C. Donati, D. Medini, N. L. Ward, S. V. Angiuoli, J. Crabtree, A. L. Jones, A. S. Durkin, R. T. DeBoy, T. M. Davidsen, M. Mora, M. Scarselli, I. Margarit y Ros, J. D. Peterson, C. R. Hauser, J. P. Sundaram, W. C. Nelson, R. Madupu, L. M. Brinkac, R. J. Dodson, M. J. Rosovitz, S. A. Sullivan, S. C. Daugherty, D. H. Haft, J. Selengut, M. L. Gwinn, L. Zhou, N. Zafar, H. Khouri, D. Radune, G. Dimitrov, K. Watkins, K. J. B. O'Connor, S. Smith, T. R. Utterback, O. White, C. E. Rubens, G. Grandi, L. C. Madoff, D. L. Kasper, J. L. Telford, M. R. Wessels, R. Rappuoli, and C. M. Fraser. 2005. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome.” Proc. Natl. Acad. Sci. USA 102:13950-13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tremblay, D. M., M. Tegoni, S. Spinelli, V. Campanacci, S. Blangy, C. Huyghe, A. Desmyter, S. Labrie, S. Moineau, and C. Cambillau. 2006. Receptor-binding protein of Lactococcus lactis phages: identification and characterization of the saccharide receptor-binding site. J. Bacteriol. 188:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vegge, C. S., H. Neve, L. Brøndsted, K. J. Heller, and F. K. Vogensen. 2006. Analysis of the collar-whisker structure of temperate lactococcal bacteriophage TP901-1. Appl. Environ. Microbiol. 72:6815-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wegmann, U., M. O'Connell-Motherwa, A. Zomer, G. Buist, C. Shearman, C. Canchaya, M. Ventura, A. Goesmann, M. J. Gasson, O. P. Kuipers, D. van Sinderen, and J. Kok. 2007. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 189:3256-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]