Abstract
The food-borne pathogen Listeria monocytogenes can grow in a wide range of temperatures, and several key virulence determinants of the organism are expressed at 37°C but are strongly repressed below 30°C. However, the impact of growth temperature on the ability of the bacteria to tolerate environmental stresses remains poorly understood. In other microorganisms, cold acclimation resulted in enhanced tolerance against freezing and thawing (cryotolerance). In this study, we investigated the impact of growth temperature (4, 25, and 37°C) on the cryotolerance of 14 strains of L. monocytogenes from outbreaks and from food processing plant environments and four strains of nonpathogenic Listeria spp. (L. welshimeri and L. innocua). After growth at different temperatures, cells were frozen at −20°C, and repeated freeze-thaw cycles were applied every 24 h. Pronounced cryotolerance was exhibited by cells grown at 37°C, with a <1-log decrease after 18 cycles of freezing and thawing. In contrast, freeze-thaw tolerance was significantly reduced (P < 0.05) when bacteria were grown at either 4 or 25°C, with log decreases after 18 freeze-thaw cycles ranging from 2 to >4, depending on the strain. These findings suggest that growth at 37°C, a temperature required for expression of virulence determinants of L. monocytogenes, is also required for protection against freeze-thaw stress. The negative impact of growth at low temperature on freeze-thaw stress was unexpected and has not been reported before with this or other psychrotrophic microorganisms.
Listeria monocytogenes remains a leading cause of deaths due to food-borne illness in the United States and other industrialized nations. Neonates, pregnant women, and immunocompromised people are at high risk for infection (15, 28, 37). Outbreaks of listeriosis tend to involve a relatively small number of closely related strains (“epidemic clones”), primarily of serotype 4b. Several major outbreaks have been attributed to epidemic clone I (ECI) and epidemic clone II (ECII), both of serotype 4b (5, 21).
Unlike most other human food-borne pathogens, L. monocytogenes grows over a wide temperature range (1 to 45°C), with optimal growth at approximately 37°C (33). It has been known for some time that expression of several key virulence genes, including hly, encoding the hemolysin listeriolysin O and actA, encoding a protein that mediates actin polymerization required for intracellular pathogenesis, is optimal at 37°C but severely repressed at temperatures below 30°C (22). In contrast, flagellin, motility, and chemotaxis genes are repressed at 37°C but optimally expressed at temperatures below 25°C (9, 10).
For an organism such as L. monocytogenes, which is commonly found in the environment but can also colonize and infect warm-blooded animals, temperature is likely to serve as a major signal differentiating environmental from vertebrate host-associated habitats (19). However, with the exception of the thermoregulated phenotypes described above (virulence factor production, motility, and chemotaxis), the impact of growth at different temperatures on specific responses and adaptations of the pathogen remains poorly understood.
L. monocytogenes may be exposed to freezing as well as thawing in the course of its existence in natural environments (e.g., soils and water in temperate or cold regions), as well as during the storage and preservation of foods. The organism has been repeatedly isolated from frozen foods (e.g., ice cream) (7). After freezing (−18°C) in laboratory media or in foods and a single thawing cycle, survival depended on strain, freezing medium, and the presence of glycerol as cryoprotectant (13, 14). The freezing and thawing of bacteria grown at 30°C, in combination with essential oil, has been explored as one means to reduce the pathogen in foods (8). The possible role of the general stress sigma factor (sigma B) in survival of bacteria grown at 30°C and exposed to repeated freezing and thawing was also investigated (43). However, there is a surprising dearth of information on the possible impact of growth temperature on tolerance of L. monocytogenes to repeated freezing and thawing (cryotolerance).
Studies with another gram-positive psychrotrophic bacterium (Exiguobacterium sibiricum and other Exiguobacterium spp.) revealed that growth in the cold (4°C), or growth on solid media regardless of temperature, resulted in increased tolerance of the bacteria against repeated freezing and thawing (40). It is not known whether low temperature or surface-associated growth may exert similar impacts on the cryotolerance of L. monocytogenes. The objective of the present study was to investigate the impact of growth temperature (4, 25, and 37°C) and of planktonic versus agar growth of L. monocytogenes on protection of the bacteria against repeated freezing and thawing.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Listeria strains used in the present study were from the Listeria strain collection of our laboratory at North Carolina State University. They included 14 strains of L. monocytogenes (10 of serotype 4b and two each of serotype 1/2a and serotype 1/2b) and two strains each of L. welshimeri and L. innocua (Table 1) . Six of the serotype 4b strains were confirmed to be ECI, including five (F2365, F2381, G3982, G4011, and G4030) implicated in outbreaks of listeriosis (37) and one isolated from a turkey processing plant environmental sample (12). Two serotype 4b strains were members of ECII, including strain H7550, implicated in the 1998 to 1999 hot dog outbreak of listeriosis (37) and strain L0226, isolated from a turkey processing plant environmental sample (12). Strain WS1 represented ECV, implicated in an outbreak of listeriosis in Winston-Salem, NC, in 2000 (5, 37), and strain 171A was isolated from a turkey processing plant and did not harbor markers specific to known epidemic clones (12). The serotype 1/2a strain J0161 represented ECIII, implicated in an outbreak of listeriosis traced to turkey deli meats in 2000 (37). Strains of L. welshimeri and L. innocua were isolated in 2004 and 2005 from a turkey processing plant and were chosen so as to represent different strain types based on pulsed-field gel electrophoresis with AscI and ApaI.
TABLE 1.
Listeria strains investigated in this study
| Strain | Source or reference |
|---|---|
| L. monocytogenes serotype 4b | |
| 171A | Turkey processing plant (12) |
| G3982 | Food, 1983-1987 Switzerland outbreak, ECI (37) |
| F2365 | Food, 1985 outbreak in California, ECI (37) |
| F2381 | Clinical, 1985 outbreak in California, ECI (37) |
| G4011 | Food, 1981 outbreak in Canada, ECI (37) |
| G4030 | Clinical, 1992 outbreak in France, ECI (37) |
| H7550 | Clinical, 1998-1999 multistate outbreak, ECII (37) |
| L0226 | Turkey processing plant, ECII (12) |
| L0228 | Turkey processing plant, ECI (12) |
| WS1 | Clinical, 2000 North Carolina outbreak (37) |
| L. monocytogenes serotype 1/2a | |
| SK2662 | Turkey processing plant, 2004 |
| JO161 | Clinical, 2000 multistate outbreak, ECIII (37) |
| L. monocytogenes serotype 1/2b | |
| 2005-625 | Clinical sporadic isolate, North Carolina, 2005 |
| G4008 | Food |
| L. welshimeri | |
| SK1523 | Turkey processing plant, 2004 |
| SK1648 | Turkey processing plant, product rinse, 2005 |
| L. innocua | |
| SK1662 | Turkey processing plant, 2005 |
| SK1512 | Turkey processing plant, 2004 |
Bacteria were grown on Trypticase soy agar with 5% sheep blood (Remel, Lenexa, KS) at 37°C for 36 h, and liquid cultures were started by transferring a single colony into 5 ml of tryptic soy broth (TSB; BBL, Cockeysville, MD) supplemented with 0.7% yeast extract (YE; Becton, Dickinson & Co., Sparks, MD) (TSBYE) and incubation at 37°C overnight. A sample (30 μl) of this culture was added to 30 ml of TSBYE and incubated at the indicated temperature (4, 25, or 37°C). Growth phase was determined by monitoring the optical density at 600 nm (OD600), using a spectrophotometer (SmartSpec 3000; Bio-Rad, Hercules, CA). Cultures at 37°C in mid-logarithmic and late-logarithmic phases had OD600s of approximately 0.6 and 1.5, respectively (approximately 11 and 14 h, respectively). Stationary phase (OD600 ∼1.8) was reached after 24 or 36 h of growth for cultures grown at either 37°C or 25°C, respectively, whereas at 4°C cells reached stationary phase after 28 days of growth. Late stationary cultures (OD600 ∼1.7) were obtained after 48 h at 37°C. To evaluate the impact of growth in liquid cultures (planktonic cells) versus solid media, strains F2365 and H7550 were grown at 4, 25, or 37°C on TSBYE containing 1.5% agar (Becton, Dickinson & Co.) (TSAYE) until the diameter of single colonies reached 2 to 4 mm (approximately 48 h at 37°C and 25°C and 30 days at 4°C). Colonies were then swabbed from the surface of the agar plate with a sterile cotton swab (Fisher Scientific, Houston, TX) and inoculated into 10 ml of TSBYE. OD600 of the cell suspension was measured as described above and adjusted so that it was comparable to the OD600 of cultures grown in broth. Cell enumerations were conducted by plating in duplicate on TSAYE after serial dilution in TSBYE and incubation at 37°C for 36 h.
Freezing and thawing treatments.
Bacteria grown at 4, 25, or 37°C (or the cell suspensions from agar-grown cultures, prepared as described above) were transferred (1.5 ml) into sterile cryovials (Nalgene, Rochester, NY) and frozen at −20°C. The freezing rate was 0.039 ml/min, and the freezing temperature for TSBYE and the cultures was ca. 0°C. Freezing rates were determined by placing a thermocouple (0.254-mm-diameter Type T thermocouples; Omega Engineering, Inc., Stamford, CT) equipped with a Digisense 12-channel scanning thermometer (Cole-Parmer Instrument Co., Vernonhills, IL) inside the cryovial and measuring the temperature every 30 s after placement of the vial at −20°C. Thawing was performed at room temperature for 10 min in a water bath. Freezing and thawing cycles were repeated every 24 h for 18 cycles. Every three cycles, cell enumerations were done in duplicate, as described above.
Assessment of injured cell prevalence following repeated freezing and thawing.
Enumerations on modified Oxford selective medium (Oxoid, Basingstoke, England) and on nonselective medium (TSAYE) were used to assess prevalence of injured cells, as described previously (20). After repeated freezing and thawing, L. monocytogenes F2365 (grown at 4 and 37°C) was serially diluted in TSBYE as described above and plated both on TSAYE and on modified Oxford selective medium. Colonies were enumerated after incubation at 37°C for 36 h.
Determination of possible impact of cryoprotectants in the culture supernatant on freezing and thawing tolerance.
Stationary-phase cultures grown at 4 and 37°C were centrifuged at room temperature for 10 min at 16,110 × g (Centrifuge 5415-D; Eppendorf, Westbury, NY), and the supernatant was filter sterilized (0.2-μm-pore-size syringe filter; Fisher Scientific). Cells grown at 4°C were resuspended in filtered supernatants from cells grown at 37°C, and the cell suspensions were frozen at −20°C as described above. Cells resuspended in filtered supernatants from the cultures in which they were grown were used as controls. Repeated freezing and thawing cycles were applied, and cell survival was determined as described above.
Statistical analysis.
All treatment combinations were replicated at least twice. Log reduction was calculated after 18 freeze-thaw cycles. The experimental design accommodated all combinations of two temperatures and 18 strains in a complete, crossed two-factor layout, but with the additional temperature level of 25°C for the two strains H7550 and F2365. Because of this incompleteness with regard to the third temperature level, we treated the 38 different temperature-strain combinations as levels of a single factor in a one-way analysis of variance with 80 observations. The lowest level of duplication of measurement was treated as subsampling, and these two measurements were averaged to obtain 80 separate means for analysis. To investigate whether the impact of growth temperature on log reduction varied across strains, the difference (between cultures grown at 37 and 4°C) in log reduction after 18 freeze-thaw cycles was computed for each strain, and these differences were compared pairwise between strains. Significance was determined at unadjusted level of alpha = 0.05. All statistical analyses were performed by using SAS v.9.1 (Cary, NC).
RESULTS
Cryotolerance was higher in stationary-phase than in logarithmic-phase cultures.
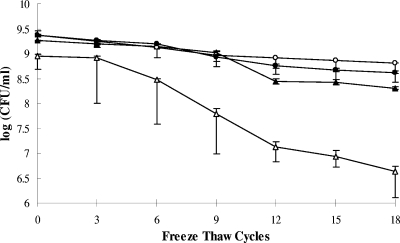
Impact of growth phase on the freeze-thaw tolerance of L. monocytogenes was investigated using L. monocytogenes F2365 (hereafter referred to as F2365) and L. monocytogenes H7550 (hereafter referred to as H7550) grown at 37°C. Cells of F2365 in the mid-logarithmic growth phase were significantly more susceptible (P < 0.05) to repeated (six or more cycles) freezing and thawing than cells from the late logarithmic or stationary phase (Fig. 1). Similar findings were obtained with H7550 (data not shown). For subsequent experiments to assess the impact of temperature on freeze-thaw tolerance, cultures in stationary phase of growth were used.
FIG. 1.
Impact of growth phase on the freeze-thaw tolerance of L. monocytogenes F2365. The data represent the means from two experiments, each performed in duplicate. Symbols: ▵, mid-logarithmic phase; ▴, late logarithmic phase; ○, stationary phase; •, late stationary phase.
L. monocytogenes F2365 and H7550 grown at 37°C in liquid were more tolerant against freeze-thaw stress than liquid cultures grown at 4 or 25°C.
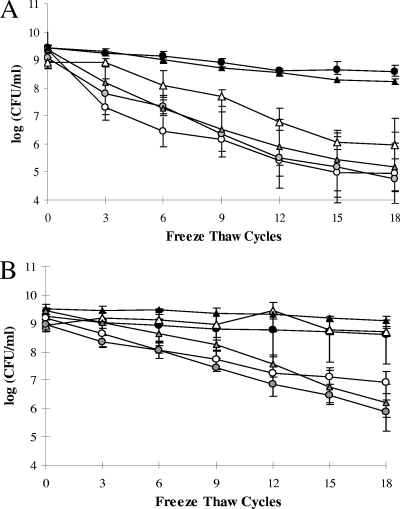
Repeated cycles of freezing and thawing appeared to have limited impact on survival of F2365 and H7550 grown at 37°C in liquid; after 18 cycles of freeze-thaw stress, the log reduction values were 0.83 ± 0.14 and 0.66 ± 0.71, respectively (Fig. 2). However, the tolerance of both strains to repeated freezing and thawing was severely impaired after growth of the bacteria at 4°C in liquid, with the decline in survival becoming increasingly pronounced with increasing numbers of freeze-thaw cycles (Fig. 2). The rate of decline in the CFU/ml of cultures grown at 4°C was significantly greater than that observed with cells at 37°C (P < 0.0001). The log decreases of cultures grown at 4°C after 18 freeze-thaw cycles were 4.39 ± 0.85 and 3.09 ± 0.46 for F2365 and H7550, respectively, suggesting significantly lower cryotolerance of F2365 (P < 0.05) (Fig. 2). Plating of cell suspensions of F2365 (grown at 37 and 4°C in liquid and subjected to repeated freezing and thawing) on TSAYE and on modified Oxford selective medium did not provide evidence for injured cells in the cell suspensions. The CFU/ml values were similar on the two types of media (data not shown).
FIG. 2.
Impact of growth temperature and agar versus liquid growth on the freeze-thaw tolerance of L. monocytogenes F2365 (A) and H7550 (B). The data represent the means from three experiments, each performed in duplicate. Symbols: •, liquid at 37°C; ○, liquid at 25°C; , liquid at 4°C; ▴, agar at 37°C; ▵, agar at 25°C; , agar at 4°C.
Rate of decline with increasing numbers of cycles was also significantly higher in cells grown at 25°C than in those grown at 37°C (P < 0.05) and did not differ significantly from that cells grown at 4°C (P > 0.05) (Fig. 2). The log decreases of F2365 and H7550 grown at 25°C after 18 cycles were 4.40 ± 1.36 and 2.26 ± 0.48, respectively, with F2365 again exhibiting a lower cryotolerance than H7550 (P < 0.05) (Fig. 2).
Impaired tolerance to repeated freezing and thawing was a general attribute of L. monocytogenes grown at low temperature and was also observed among nonpathogenic Listeria spp.
Strains F2365 and H7550 are members of two major epidemic clonal groups of L. monocytogenes serotype 4b, ECI and ECII, respectively. In order to investigate whether the impact of growth temperature on tolerance to freezing and thawing could be seen with other strains of L. monocytogenes serotype 4b, eight additional strains were characterized. This panel included strains from different outbreaks and from the food processing plant environment (Table 1).
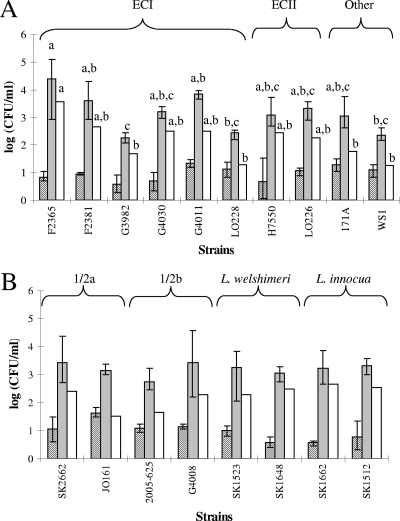
In all cases, growth of the bacteria at 37°C noticeably enhanced the tolerance of the cells to repeated freezing and thawing compared to growth at 4°C (P < 0.05) (Fig. 3A). When strains were grown at 37°C, the cryotolerance levels of the different strains were not significantly different (P > 0.05). However, we noted certain strain-specific differences in the freeze-thaw tolerance of cells grown at 4°C. Specifically, after growth at 4°C the strains G3982 and LO228 (both of ECI) and strain WS1 had significantly higher cryotolerance than F2365 (Fig. 3A). Significant differences among strains were also observed for the extent to which cryotolerance was reduced when cells were grown at 4°C compared to cells grown at 37°C. Relatively low differences (<100-fold) were observed with strains WS1, LO228, G3982, and 171A (Fig. 3A). Within ECI, the difference in cryotolerance of cells grown at 37°C versus 4°C was significantly higher for F2365 than for strains G3982 and LO228 (P < 0.05) (Fig. 3A).
FIG. 3.
Log decreases in CFU/ml for Listeria strains grown at 4 and 37°C after 18 freeze-thaw cycles. (A) L. monocytogenes serotype 4b; (B) L. monocytogenes serotypes 1/2a and 1/2b and nonpathogenic Listeria spp. (L. welshimeri and L. innocua). The data represent the means from two experiments, each performed in duplicate. Columns: ▧, cultures grown at 37°C;, cultures grown at 4°C; □, difference in CFU/ml (after 18 cycles) between cultures grown at 37 and 4°C. Different letters above columns of the same pattern indicate statistically significant differences (P < 0.05). No significant differences were noted among strains grown at 37°C (▧ columns).
To determine whether growth at 37°C also conferred protection against repeated freezing and thawing in L. monocytogenes of other serotypes, two strains each of serotype 1/2a and 1/2b were investigated. All four strains were significantly more tolerant against repeated freezing and thawing after growth at 37°C than at 4°C (P < 0.05) (Fig. 3B). No significant differences in cryotolerance after growth at either 37 or 4°C were noted among the tested strains; the cryotolerance-enhancing impact of growth at 37°C (versus growth at 4°C) also did not vary significantly among these strains (P > 0.05).
Examination of representatives of nonpathogenic Listeria spp. failed to reveal significant differences in the impact of growth temperature on cryotolerance between them and L. monocytogenes. For both tested strains of L. welshimeri and both tested strains of L. innocua, the freeze-thaw tolerance was significantly higher when bacteria were grown at 37 than at when they were grown at 4°C (P < 0.05) (Fig. 3B). Similarly to the serogroup 1/2 strains, no significant differences were noted among the tested strains of the nonpathogenic species (P > 0.05).
Enhanced cryotolerance of agar-grown versus planktonic L. monocytogenes grown at 25°C.
We determined whether agar-grown cultures differed in freeze-thaw tolerance from those grown planktonically. No significant differences were noted in cryotolerance of agar-grown versus liquid cultures when bacteria were grown at 37 or 4°C (P > 0.05). F2365 and H7550 cells grown on agar media at 37°C tolerated repeated freezing and thawing well, similarly to cultures grown at 37°C in liquid. Cells grown on agar at 4°C had markedly impaired survival, again similarly to what was observed with liquid cultures (Fig. 2).
In contrast to the lack of impact of agar versus liquid growth on cryotolerance of cells grown at 37 or 4°C, both F2365 and H7550 grown at 25°C on solid media were significantly protected against repeated freezing and thawing compared to cells grown at 25°C in liquid (P < 0.05) (Fig. 2). Agar growth at 25°C affected the cryotolerance differently for F2365 and H7550. The cryotolerance of H7550 grown on agar at 25°C was similar to that of cells grown at 37°C either in liquid or on agar (P > 0.05) (Fig. 2B), whereas F2365 grown on agar at 25°C had impaired cryotolerance in comparison to cells grown at 37°C either in liquid or on agar (P < 0.05) (Fig. 2A).
Lack of evidence for cryoprotectants in the supernatant of cells grown at 37°C.
To address the possible involvement of cryoprotectants in the enhanced freeze-thaw tolerance of cells grown at 37°C, cells (F2365 and H7550) grown at 4°C in liquid were centrifuged and resuspended in the filtered supernatants from cultures of the same strain grown at 37°C in liquid. The freeze-thaw tolerance of cells grown at 4°C resuspended in the filtered supernatant of cells grown at 37°C remained impaired, similarly to the control (i.e., cells grown at 4°C resuspended in their own filtered supernatant) (data not shown). However, cultures subjected to the centrifugation and resuspension steps had higher cryotolerance than untreated cultures of the same strain (data not shown), suggesting that centrifugation increased the freeze-thaw tolerance.
DISCUSSION
The observed impact of growth temperature on the cryotolerance of Listeria has not been reported previously and was rather unexpected. In the case of psychrotrophic strains of Exiguobacterium spp. isolated from Siberian permafrost, planktonic growth at 4°C conferred significant protection against repeated freezing and thawing than growth at higher temperatures (25°C) (40). After growth at 4°C, several other bacteria from permafrost were also found to have enhanced tolerance to long-term freezing (and subsequent thawing) (30).
Exiguobacterium spp. grown on solid media either at 4 or at 25°C had enhanced cryotolerance, similar to that of cells grown planktonically at 4°C (40). In contrast, we found that in the case of L. monocytogenes growth on solid media conferred enhanced cryotolerance in a temperature- and strain-specific fashion. H7550 and (to a lesser extent) F2365 grown on agar at 25°C had higher cryotolerance than when grown in liquid at that temperature. Such findings suggest that the impact of growth temperature and planktonic versus surface-associated growth varies among different psychrotrophic species and even among different strains of the same species.
The mechanisms that underlie the observed markedly higher cryotolerance of planktonically grown L. monocytogenes after growth at 37°C in comparison to growth at 4 or 25°C remain unknown. Extracellular cryoprotectants such as trehalose and glycerol have been found to increase cryotolerance in L. monocytogenes and other bacteria, possibly by enhancing membrane stability (11, 14, 34). However, our experiments with resuspension of cells grown at 4°C in filtered supernatants from cultures grown at 37°C failed to provide evidence for extracellular cryoprotectants in the latter. Such experiments also suggested that cryotolerance was increased through the centrifugation and resuspension steps; the impact on centrifugation and resuspension on stress responses of L. monocytogenes has been described by others as well (4).
A key finding was that cryotolerance of bacteria grown planktonically was severely impaired not only in cells grown at 4°C but also in those grown at 25°C. This suggests that impaired cryotolerance in cells grown in liquid at such temperatures was not an outcome of specific cold-induced membrane modifications or other cellular responses associated with cold stress or cold acclimation, such as those documented in numerous investigations (see, for example, references 2, 3, 23, 24, 38, and 42). The genes responsible for the enhanced cryotolerance of cells grown at 37°C may be components of a thermoregulated regulon, possibly also implicated in the virulence in L. monocytogenes. The protective impact of 37°C growth on cryotolerance was also observed with nonpathogenic Listeria spp. It is therefore tempting to speculate that the underlying thermoregulated mechanisms were present in the ancestral pathogenic Listeria lineage that is believed to have preceded the emergence of nonpathogenic species such as L. innocua and L. welshimeri (32).
Common mechanisms mediating the responses of relevance both to pathogenesis and to cryotolerance may include those that allow cells to cope with reactive oxygen species produced during infection (17, 36), as well as during freezing and thawing (25). For instance, in Campylobacter superoxide dismutase (SOD) was found to play an important role in cryotolerance (18, 35), as well as in the survival in macrophages (29) and in the colonization of chickens (31). In L. monocytogenes, SOD is required for virulence in the murine model and is controlled posttranslationally by phosphorylation (1). Even though evidence for thermoregulated transcription of sod is lacking (3, 26), earlier studies suggested that SOD production was higher in cells grown at 37°C than in cells grown at 10°C (39). The possible role of SOD in Listeria's cryotolerance remains to be characterized. Another determinant that may contribute both to cryotolerance and to virulence is the general stress sigma factor, sigma B. In Bacillus subtilis 168, sigma B was found to contribute to survival after freezing and subsequent thawing (41). In L. monocytogenes, sigma B is involved in oxidative stress responses, as well as virulence (16), but its impact on freeze-thaw survival was only evaluated in logarithmic-phase cells grown at 30°C; the observed impact was rather modest and could be detected only in cells exposed to prior additional stresses such as cold and acid shock (43). The possible role of sigma B in cryotolerance of cells grown at 37°C versus cells grown at lower temperature remains to be assessed.
Future comparative studies of the transcriptomes and proteomes of L. monocytogenes at different temperatures (e.g., 37, 4, and 25°C) will assist the identification of candidate genes responsible for temperature- and agar growth-dependent cryotolerance in this organism. Comparative studies using different strains may also elucidate the mechanisms underlying the observed strain-specific differences. It was noteworthy that following growth at 4°C certain ECI strains (e.g., F2365) had markedly lower cryotolerance than others (e.g., G3982), suggesting differences in cryotolerance of cold-grown cells, even among strains of the same clonal group. Genetic and antigenic differences among ECI strains have been described before (6, 27). It is currently not clear whether such differences, including those underlying the observed variable impact of growth temperature on cryotolerance, arose in the natural habitat of the strains, or subsequent to their isolation and in the course of their passage under laboratory conditions. It is also not clear whether the multiple frameshifts detected in the genome of F2365 (27) may contribute to the fact that this strain exhibited the greatest impairment in cryotolerance after 4°C growth (versus growth at 37°C) among the tested strains.
Temperature-dependent cryotolerance may have evolved in response to specific selection pressures in Listeria's natural habitats. For instance, bacteria amplified at body temperatures in mammalian blood or tissues as a result of listeric infection and subsequently released in the environment (e.g., in aborted fetuses or upon death of the hosts) might be better prepared to tolerate freezing and thawing that they might undergo in nature (e.g., in surface soil and water). Further studies are needed to determine whether growth at temperatures typically associated with animal infection also confers enhanced cryotolerance in other animal pathogens that have environmental lifestyles where freezing and thawing may be encountered and, if this is found to be the case, to determine whether common molecular mechanisms may be involved.
Acknowledgments
This project was partially funded by USDA grant 2006-35201-17377. S.W. was partially supported with National Science Foundation grant HRD 0102892 to St. Augustine's College.
We thank J. Simunovic and B. Farkas for assistance with determinations of freezing rate. We are grateful to R. M. Siletzky for laboratory support and to all other members of our laboratory for discussions, encouragement, and support in the course of this project.
Footnotes
Published ahead of print on 19 June 2009.
REFERENCES
- 1.Archambaud, C., M. A. Nahori, J. Pizarro-Cerda, P. Cossart, and O. Dussurget. 2006. Control of Listeria superoxide dismutase by phosphorylation. J. Biol. Chem. 281:31812-31822. [DOI] [PubMed] [Google Scholar]
- 2.Bayles, D. O., M. H. Tunick, T. A. Foglia, and A. J. Miller. 2000. Cold shock and its effect on ribosomes and thermal tolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 66:4351-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, Y. C., S. Raengpradub, K. J. Boor, and M. Wiedmann. 2007. Microarray-based characterization of the Listeria monocytogenes cold regulon in log- and stationary-phase cells. Appl. Environ. Microbiol. 73:6484-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturongakul, S., and K. Boor. 2006. σB activation under environmental and energy stress conditions in Listeria monocytogenes. Appl. Environ. Microbiol. 72:5197-5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, Y., R. M. Siletzky, and S. Kathariou. 2008. Genomic divisions/lineages, epidemic clones, and population structure, p. 337-358. In D. Liu (ed.), Handbook of Listeria monocytogenes. CRC Press, Inc., Boca Raton, FL.
- 6.Clark, E. E., I. Wesley, F. Fiedler, N. Promadej, and S. Kathariou. 2000. Absence of serotype-specific surface antigen and altered teichoic acid glycosylation among epidemic-associated strains of Listeria monocytogenes. J. Clin. Microbiol. 38:3856-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotton, L. N., and C. H. White. 1992. Listeria monocytogenes, Yersinia enterocolitica, and Salmonella in dairy plant environments. J. Dairy Sci. 75:51-57. [DOI] [PubMed] [Google Scholar]
- 8.Cressy, H. K., A. R. Jerrett, C. M. Osborne, and P. J. Bremer. 2003. A novel method for the reduction of numbers of Listeria monocytogenes cells by freezing in combination with an essential oil in bacteriological media. J. Food Prot. 66:390-395. [DOI] [PubMed] [Google Scholar]
- 9.Dons, L., O. F. Rasmussen, and J. E. Olsen. 1992. Cloning and characterization of a gene encoding flagellin of Listeria monocytogenes. Mol. Microbiol. 6:2919-2929. [DOI] [PubMed] [Google Scholar]
- 10.Dons, L., J. E. Olsen, and O. F. Rasmussen. 1994. Characterization of two putative Listeria monocytogenes genes encoding polypeptides homologous to the sensor protein CheA and the response regulator CheY of chemotaxis. DNA Seq. 4:301-311. [DOI] [PubMed] [Google Scholar]
- 11.Duong, T., R. Barrangou, W. M. Russell, and T. R. Klaenhammer. 2006. Characterization of the tre locus and analysis of trehalose cryoprotection in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 72:1218-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eifert, J. D., P. A. Curtis, M. C. Bazaco, R. J. Meinersmann, M. E. Berrang, S. Kernodle, C. Stam, L.-A. Jaykus, and S. Kathariou. 2005. Molecular characterization of Listeria monocytogenes of the serotype 4b complex (4b, 4d, 4e) from two turkey processing plants. Foodborne Pathog. Dis. 2:192-200. [DOI] [PubMed] [Google Scholar]
- 13.El-Kest, S. E., and E. H. Marth. 1991. Injury and death of frozen Listeria monocytogenes as affected by glycerol and milk components. J. Dairy Sci. 74:1201-1208. [DOI] [PubMed] [Google Scholar]
- 14.El-Kest, S. E., and E. H. Marth. 1991. Strains and suspending menstrua as factors affecting death and injury of Listeria monocytogenes during freezing and frozen storage. J. Dairy Sci. 74:1209-1213. [DOI] [PubMed] [Google Scholar]
- 15.Farber, K. R., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira, A., C. P. O'Byrne, and K. J. Boor. 2001. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl. Environ. Microbiol. 67:4454-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman, H. J., and M. Torres. 2002. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 166:S4-S8. [DOI] [PubMed] [Google Scholar]
- 18.Garénaux, A., M. Ritz, F. Jugiau, F. Rama, M. Federighi, and R. de Jonge. 2008. Role of oxidative stress in Campylobacter jejuni inactivation during freeze-thaw treatment. Curr. Microbiol. 58:134-138. [DOI] [PubMed] [Google Scholar]
- 19.Gray, M. J., N. E. Freitag, and K. J. Boor. 2006. How the bacterial pathogen Listeria monocytogenes mediates the switch from environmental Dr. Jekyll to pathogenic Mr. Hyde. Infect. Immun. 74:2505-2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayman, M. M., R. C. Anantheswaran, and S. J. Knabel. 2007. The effects of growth temperature and growth phase on the inactivation of Listeria monocytogenes in whole milk subject to high pressure processing. Int. J. Food Microbiol. 115:220-226. [DOI] [PubMed] [Google Scholar]
- 21.Kathariou, S. 2002. Listeria monocytogenes virulence and pathogenicity: a food safety perspective. J. Food Prot. 65:1811-1829. [DOI] [PubMed] [Google Scholar]
- 22.Leimeister-Wächter, M., E. Domann, and T. Chakraborty. 1992. The expression of virulence genes in Listeria monocytogenes is thermoregulated. J. Bacteriol. 174:947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, S., J. E. Graham, L. Bigelow, P. D. Morse II, and B. J. Wilkinson. 2002. Identification of Listeria monocytogenes genes expressed in response to growth at low temperature. Appl. Environ. Microbiol. 68:1697-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, S., D. O. Bayles, T. M. Mason, and B. J. Wilkinson. 2006. A cold-sensitive Listeria monocytogenes mutant has a transposon insertion in a gene encoding a putative membrane protein and shows altered (p)ppGpp levels. Appl. Environ. Microbiol. 72:3955-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazur, P. 1970. Cryobiology: the freezing of biological systems. Science 168:939-949. [DOI] [PubMed] [Google Scholar]
- 26.Milohanic, E., P. Glaser, J. Y. Coppée, L. Frangeul, Y. Vega, J. A. Vázquez-Boland, F. Kunst, P. Cossart, and C. Buchrieser. 2003. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol. Microbiol. 47:1613-1625. [DOI] [PubMed] [Google Scholar]
- 27.Nightingale, K. K., S. R. Milillo, R. A. Ivy, A. J. Ho, H. F. Oliver, and M. Wiedmann. 2007. Listeria monocytogenes F2365 carries several authentic mutations potentially leading to truncated gene products, including inlB, and demonstrates atypical phenotypic characteristics. J. Food Prot. 70:482-488. [DOI] [PubMed] [Google Scholar]
- 28.Painter, J., and L. Slutsker. 2007. Listeriosis in humans, p. 85-109. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis, and food safety, 3rd ed. CRC Press, Inc., Boca Raton, FL.
- 29.Pesci, E. C., D. L. Cottle, and C. L. Picket. 1994. Genetic, enzymatic, and pathogenic studies of the iron superoxide dismutase of Campylobacter jejuni. Infect. Immun. 62:2687-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponder. M. A., S. J. Gilmour, P. W. Bergholz, C. A. Mindock, R. Hollingsworth, M. F. Thomashow, and J. M. Tiedje. 2005. Characterization of potential stress responses in ancient Siberian permafrost psychroactive bacteria, FEMS Microbiol. Ecol. 53:103-115. [DOI] [PubMed] [Google Scholar]
- 31.Purdy, D., S. Cawthraw, J. H. Dickinson, D. G. Newell, and S. F. Park. 1999. Generation of a superoxide dismutase (SOD)-deficient mutant of Campylobacter coli: evidence for the significance of SOD in Campylobacter survival and colonization. Appl. Environ. Microbiol. 65:2540-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid, M. W., E. Y. Ng, R. Lampidis, M. Emmerth, M. Walcher, J. Kreft, W. Goebel, M. Wagner, and K. H. Schleifer. 2005. Evolutionary history of the genus Listeria and its virulence genes. Syst. Appl. Microbiol. 28:1-18. [DOI] [PubMed] [Google Scholar]
- 33.Seeliger, H. P. R., and D. Jones. 1986. Genus Listeria Pirie, 1940, p. 1235-1245. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams & Wilkins Co., Baltimore, MD. [Google Scholar]
- 34.Souzu, H., M. Sato, and T. Kojima. 1989. Changes in chemical structure and function in Escherichia coli cell membranes caused by freeze-thawing. II. Membrane lipid state and response of cells to dehydration. Biochim. Biophys. Acta 978:112-118. [DOI] [PubMed] [Google Scholar]
- 35.Stead, D., and S. F. Park. 2000. Roles of Fe superoxide dismutase and catalase in resistance of Campylobacter coli to freeze-thaw stress. Appl. Environ. Microbiol. 66:3110-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, G., Y. Wang, X. Shen, Z. Chen, and J. Yang. 2008. Mycoplasma pneumoniae infection induces reactive oxygen species and DNA damage in A459 human lung carcinoma cells. Infect. Immun. 76:4405-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swaminathan, B., and P. Gerner-Smidt. 2007. The epidemiology of human listeriosis. Microbes Infect. 9:1236-1243. [DOI] [PubMed] [Google Scholar]
- 38.Tasara, T., and R. Stephan. 2006. Cold stress tolerance of Listeria monocytogenes: a review of molecular adaptive mechanisms and food safety implications. J. Food Prot. 69:1473-1484. [DOI] [PubMed] [Google Scholar]
- 39.Vasconcelos, J. A., and H. G. Deneer. 1994. Expression of superoxide dismutase in Listeria monocytogenes. Appl. Environ. Microbiol. 60:2360-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vishnivetskaya, T. A., R. Siletzky, N. Jefferies, J. M. Tiedje, and S. Kathariou. 2007. Effect of low temperature and culture media on the growth and freeze-thawing tolerance of Exiguobacterium strains. Cryobiology 54:234-240. [DOI] [PubMed] [Google Scholar]
- 41.Volker, U., B. Maul, and M. Hecker. 1999. Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J. Bacteriol. 181:3942-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wemekamp-Kamphuis, H. H., A. K. Karatzas, J. A. Wouters, and T. Abee. 2002. Enhanced levels of cold shock proteins in Listeria monocytogenes LO28 upon exposure to low temperature and high hydrostatic pressure. Appl. Environ. Microbiol. 68:456-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wemekamp-Kamphuis, H. H., J. A. Wouters, P. P. L. de Leeuw, T. Hain, T. Chakraborty, and T. Abee. 2004. Identification of sigma factor σB-controlled genes and their impact on acid stress, high hydrostatic pressure, and freeze survival in Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 70:3457-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]