Abstract
The existence of Salmonella enterica serovar Typhimurium viable-but-nonculturable (VBNC) cells is a public health concern since they could constitute unrecognized sources of infection if they retain their pathogenicity. To date, many studies have addressed the ability of S. Typhimurium VBNC cells to remain infectious, but their conclusions are conflicting. An assumption could explain these conflicting results. It has been proposed that infectivity could be retained only temporarily after entry into the VBNC state and that most VBNC cells generated under intense stress could exceed the stage where they are still infectious. Using a Radioselectan density gradient centrifugation technique makes it possible to increase the VBNC-cell/culturable-cell ratio without increasing the exposure to stress and, consequently, to work with a larger proportion of newly VBNC cells. Here, we observed that (i) in the stationary phase, the S. Typhimurium population comprised three distinct subpopulations at 10, 24, or 48 h of culture; (ii) the VBNC cells were detected at 24 and 48 h; (iii) measurement of invasion gene (hilA, invF, and orgA) expression demonstrated that cells are highly heterogeneous within a culturable population; and (iv) invasion assays of HeLa cells showed that culturable cells from the different subpopulations do not display the same invasiveness. The results also suggest that newly formed VBNC cells are either weakly able or not able to successfully initiate epithelial cell invasion. Finally, we propose that at entry into the stationary phase, invasiveness may be one way for populations of S. Typhimurium to escape stochastic alteration leading to cell death.
Like several readily culturable pathogenic bacterial species, Salmonella enterica has been shown to enter into a viable-but-nonculturable (VBNC) state in response to environmental stresses (25, 33). In this state, cells display integrity and activities but escape detection by conventional culture-based monitoring (24). The physiological significance of this phenotype is unclear: some authors have proposed that it is part of an adaptive response aimed at long-term survival under adverse conditions (22, 32); others argue that it is a consequence of stochastic cellular deterioration and that VBNC cells are on their way to death (4, 10, 12, 23). In any case, the existence of VBNC pathogens is a public health concern since they may constitute unrecognized sources of infection if they retain their pathogenicity.
To date, many studies have addressed the ability of VBNC pathogens to remain infectious, but the conclusions of some investigators are conflicting (15, 36). In vitro experiments have shown that VBNC cells of Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Oranienburg can recover their culturability (13, 27, 30, 31). This phenomenon, called resuscitation, confirms that at least some VBNC cells ultimately remain able to multiply and are therefore potentially infectious. On the other hand, most in vivo studies ruled out the ability of S. Typhimurium VBNC cells to initiate infection in mice and chicken or to resuscitate during their passage in the animal gut (6, 17, 34, 35). However, one study reported evidence of the maintenance of pathogenicity by VBNC cells of S. Oranienburg in a model of morphine-immunosuppressed mice (1). An assumption could explain these apparently opposite results. It has been proposed that infectivity could be retained only temporarily after entry into the VBNC state (8, 19, 26). Experiments intended for testing the ability of VBNC cells to retain their pathogenicity cannot be fully conclusive if the inocula still contain culturable cells. Therefore, all previously published animal experiments with S. Typhimurium were conducted on populations with VBNC-cell/culturable-cell ratios around 10,000:1. Such populations were obtained after strong exposure to stress, either under intense stressing factors for a short period (e.g., germicidal UV-C for 2 min [6]) or under mild stressing factors for a long period (e.g., starvation for a minimum of 1 week [35]). In such populations, most VBNC cells could exceed the stage where they are still infectious, and the negative outcomes of infection studies could actually reflect their inability to specifically address the fraction of recent VBNC cells.
A Radioselectan density gradient centrifugation technique was shown to fractionate stationary-phase populations of Escherichia coli into two subpopulations (10, 12, 18). Interestingly, the VBNC cells formed during a 48-h E. coli culture were specifically recovered in the high-density (HD) subpopulation (12). This technique thus gives the opportunity to increase the VBNC-cell/culturable-cell ratio without increasing exposure to stress and, consequently, to work with a larger proportion of cells having recently entered the VBNC state.
Here, this technique was used to discriminate different stationary-phase S. Typhimurium subpopulations. We further investigated the invasiveness of these cell subpopulations by using both gene expression assays of invasion genes and in vitro invasion tests. Thus, the aim of this study was to assess the invasiveness of the cell subpopulations in accordance with their cellular states.
MATERIALS AND METHODS
Bacterial strains.
The virulent strain S. Typhimurium SL1344 (14) was used in this study. The derivative strains SL1344 hilA080::Tn5lacZY, SL1344 invF12-5::Tn5lacZY, and SL1344 orgA::Tn5lacZY (2) were used to assess the transcriptional activities of three invasion gene promoters (hilA, invF, and orgA) (11). All strains were kindly provided by Karsten Tedin from the Institute of Microbiology and Animal Epidemic Diseases of Berlin.
Generation of stationary-phase populations of S. Typhimurium.
Cells were grown aerobically in trypticase soy broth (AES Laboratories) at 37°C with vigorous shaking (220 rpm). An overnight culture was subjected to two successive rounds of a 1:100 dilution in fresh trypticase soy broth, followed by growth to an optical density at 600 nm (OD600) of 0.2, and then was used to inoculate experimental batch cultures at an initial OD600 of 0.03. Batch cultures were incubated for 10, 24, or 48 h. They reached the stationary phase at 6 h. At the time requested, 10 ml of the stationary-phase cultures was centrifuged at 6,000 × g for 10 min at 4°C, and the cells were washed with cold (4°C) sterile 0.9% NaCl and resuspended in 1 or 2 ml of cold 26.5% Radioselectan for the separation procedure.
Radioselectan density gradient centrifugation.
The separation procedure was performed as previously described (12). Nevertheless, the pathogenicity of S. Typhimurium required an adaptation of the initial protocol. Polycarbonate centrifuge tubes with aerosol-proof tops (Beckman Coulter) were used, and as they must be completely full for mechanical resistance, 13 ml of 0.9% NaCl was added on top of the Radioselectan solution to complete the volume. This 13 ml of 0.9% NaCl was deposited first, and then 10 ml of 32% Radioselectan was carefully layered underneath by using a syringe. Finally, 1 ml of the 26.5% Radioselectan cell suspension was similarly layered at the interface between the 0.9% NaCl and the 32% Radioselectan solutions. Cells were centrifuged at 55,000 rpm at 4°C for 3 h with a Ti70 rotor in a Beckman Coulter ultracentrifuge. After separation, each distinguishable subpopulation was sampled (100 to 200 μl), and cells were pelleted, washed, and resuspended in the appropriate buffer for subsequent analysis. It was determined that the separation procedure had no effect on the parameters measured.
Culturability and viability assays.
Cell culturability was assessed by a spread plate procedure on trypticase soy agar (AES Laboratories). The percentage of culturable cells was expressed with respect to total cell counts, determined by DAPI (4′,6-diamidino-2-phenylindole; Sigma) staining and microscopic enumeration (29). Membrane integrity was used as a viability indicator in this culture-independent assessment. Nonviable cells do not necessarily display a damaged membrane, as observed with UV-killed cells (38). Nevertheless, loss of membrane integrity is commonly regarded as a sufficient condition for loss of viability (3). Cells were stained using a Live/Dead BacLight bacterial-viability kit (Molecular Probes) according to the manufacturer's recommendations. Samples were analyzed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) as described elsewhere (5). Viable (intact membrane) and nonviable (damaged membrane) cells were differentiated by their signatures in a plot of green (SYTO 9) fluorescence versus red (propidium iodide) fluorescence, respectively. VBNC cells were identified as the cells responsible for the discrepancy between the percentages of culturable and viable cells.
β-Galactosidase and invasion assays.
β-Galactosidase assays were performed as described by Miller (20). β-Galactosidase activity was expressed as Miller units (OD420 min−1/OD600 ml−1 × 1,000). The invasion assays were performed with HeLa cell monolayers by using 24-well plates (16).
RESULTS
Separation of culturable and nonculturable cells from stationary-phase populations of S. Typhimurium.
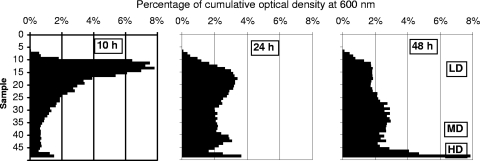
Radioselectan density gradient centrifugation had previously been shown to separate 10-h and 48-h cultures of E. coli into two subpopulations. At 48 h, the HD subpopulation was enriched with nonculturable cells (12). At 10 h, the HD subpopulation contained only culturable cells that display an increased sensitivity to various stresses (10). We first investigated if such results could be obtained with S. Typhimurium. Stationary-phase populations from 10-, 24-, and 48-h cultures of S. Typhimurium were submitted to the separation procedure. In each case, the total population was fractionated into three distinct subpopulations, but their sizes varied according to the age of the culture (Fig. 1). At 10 h, the low-density (LD) subpopulation contained 90% of the cells (Table 1). As the stationary phase continued (24 and 48 h), the LD subpopulation size decreased and a growing number of cells were present in the medium-density (MD) and HD subpopulations. We subsequently looked for the presence of nonculturable cells in the total population and the three subpopulations (Table 2). At 10 h, while nonculturable cells were below the detection limit in the total population, the separation procedure enabled recovery of them specifically in HD cells, which represent only 3.8% of the total cell population. With the extension of the stationary phase, the nonculturable-cell contents of the subpopulations increased both with time and with density in the gradient.
FIG. 1.
Distribution of the cells in a Radioselectan density gradient after ultracentrifugation of a 10-, 24-, or 48-h culture of S. Typhimurium. Fifty successive samples of 200 μl have been collected from the gradient from top to bottom of the ultracentrifuge tube. Bars represent the OD600s of the individual samples expressed as percentages of the cumulated OD600 of the 50 samples. In the graphs, cell density increases from their upper part to their lower part. LD, MD, and HD cell subpopulations are indicated.
TABLE 1.
Percentages of total S. Typhimurium cells represented by the subpopulations obtained after a Radioselectan density gradient centrifugation of 10-h, 24-h, and 48-h cultures
| Subpopulation | % of total cells
|
||
|---|---|---|---|
| 10 h | 24 h | 48 h | |
| LD | 89.6 | 60.3 | 29.9 |
| MD | 6.6 | 29.9 | 52.1 |
| HD | 3.8 | 9.8 | 18 |
TABLE 2.
Percentages of culturable cells in the total population and the subpopulations of S. Typhimurium obtained after a Radioselectan density gradient centrifugation of 10-h, 24-h, and 48-h cultures
| Cell populationa | % Culturable cellsb
|
||
|---|---|---|---|
| 10 h | 24 h | 48 h | |
| Total population* | 104 ± 11 | 82 ± 5.9 | 62 ± 0.66 |
| Total population** | 94 ± 6.6 | 77 ± 5.0 | 61 ± 9.1 |
| LD | 100 ± 5.5 | 104 ± 15 | 101 ± 11 |
| MD | 100 ± 2.1 | 55 ± 7.5 | 43 ± 14 |
| HD | 8.5 ± 1.6 | 2.4 ± 0.50 | 1.9 ± 0.61 |
*, before ultracentrifugation. **, after ultracentrifugation and rehomogenization of cells in the tube.
Means ± standard deviations for triplicate cultures.
This result was similar to the one observed with E. coli, except that three subpopulations were obtained instead of two (10, 12, 18). Nevertheless, the same E. coli strain gave in our hands three subpopulations, indicating that the differences between the two studies were attributable to our separation conditions (data not shown). Moreover, as with E. coli, we observed with S. Typhimurium that (i) at 24 and 48 h, the cellular levels of oxidized proteins, as measured by carbonyl content, increased with cell density and (ii) at 10 h, MD and HD culturable cells were more sensitive to heat shock than LD culturable cells (data not shown).
Association between cell alteration level and cell density.
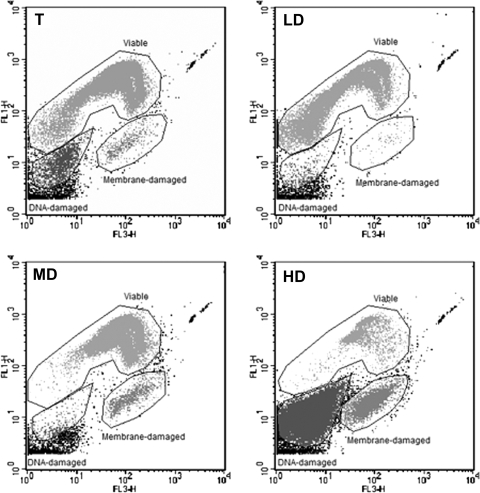
It was further investigated if, as observed with E. coli, the subpopulations enriched with nonculturable cells actually contained VBNC cells. As in the E. coli study (12), membrane integrity (Live/Dead staining) was used for viability assessment. Stained cells displayed three distinct fluorescence signatures when analyzed by flow cytometry (Fig. 2). The first signature was representative of viable cells; it was displayed by all the cells of the 100% culturable populations. The second signature was representative of nonviable cells with damaged membranes, and the third signature corresponded to nonviable cells with damaged DNA, which appeared as “ghost” cells (i.e., non-nucleoid-containing cells [39]) in epifluorescence microscopy. Therefore, four cell types were distinguishable with the combination of culturability and viability assays: culturable, VBNC, membrane-damaged, and DNA-damaged cells. As seen in Table 3, VBNC cells were detected at 24 h in the total population. After the separation, they were observed in both the MD and the HD subpopulations. At 10 h, VBNC cells were observed in the HD subpopulation, whereas they were not detected in the total population. The best VBNC enrichment was observed in the HD subpopulation at 24 h and showed a 40-fold increase in VBNC-cell/culturable-cell ratio in comparison to the level for the total population. With regard to nonviable cells, membrane-damaged cells appeared at 24 h in the total population whereas DNA-damaged cells appeared only at 48 h. Interestingly, the MD subpopulation contained only membrane-damaged cells, whereas the HD subpopulation contained both cell types, with DNA-damaged cells being a majority at 24 h. Taken together, these results suggest that cells with increasing alteration levels are formed during the stationary phase and that this alteration level is associated with apparent cell density in the Radioselectan gradient.
FIG. 2.
Cytometric analyses of the S. Typhimurium total cell population (T) and of the LD, MD, and HD cell subpopulations obtained after a Radioselectan density gradient centrifugation of a 48-h culture. Cells were stained using a Live/Dead BacLight bacterial-viability kit (Molecular Probes). The abscissa indicates the green fluorescence intensity (FL 1) of cells stained with SYTO 9. The ordinate indicates the red fluorescence intensity (FL 3) of cells stained with propidium iodide.
TABLE 3.
Percentages of each S. Typhimurium cell type distinguished by a combination of culturability and viability (cytometric analysis) assays
| Cell type | % of cellsa
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 h
|
24 h
|
48 h
|
||||||||||
| Total | LD | MD | HD | Total | LD | MD | HD | Total | LD | MD | HD | |
| Culturable | 100 | 100 | 100 | 8 | 81 | 100 | 55 | 0.6 | 59 | 100 | 43 | 0.33 |
| VBNC | 38 | 17 | 38 | 5 | 34 | 53 | 5 | |||||
| Membrane-damaged | 29 | 2 | 8 | 19 | 3 | 4 | 10 | |||||
| DNA-damaged | 24 | 76 | 4 | 85 | ||||||||
“Total” refers to the total population observed after ultracentrifugation and rehomogenization of cells in the centrifuge tube. The VBNC-cell/culturable-cell ratios were as follows: for the HD subpopulation after 10 h of culture, 4.8; for the total population after 24 h of culture, 0.2; for the MD subpopulation after 24 h of culture, 0.69; for the HD subpopulation after 24 h of culture, 8.3; for the total population after 48 h of culture, 0.58; for the MD subpopulation after 48 h of culture, 1.2; and for the HD subpopulation after 48 h of culture, 15.
Heterogeneity of invasiveness within culturable cells. (i) Expression assays of invasion genes.
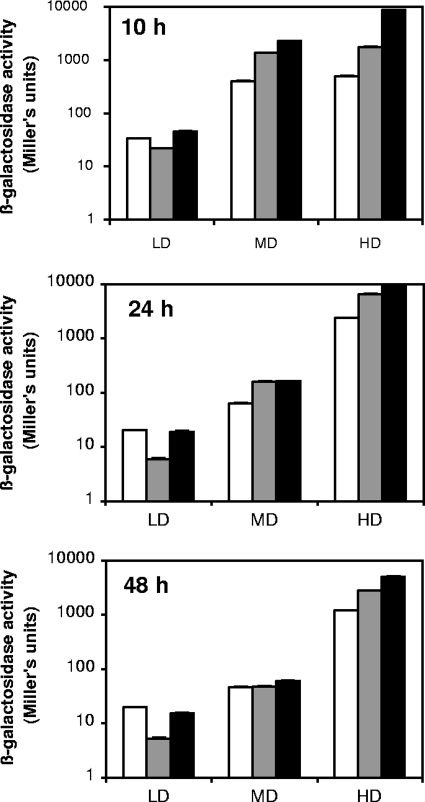
Because invasion of the intestinal epithelium is a fundamental step in Salmonella infections, we assessed the expression of three invasion genes (hilA is the central transcriptional activator of the invasion genes, invF secondarily activates the genes of secreted effector proteins, and orgA is involved in the formation of a type III secretion system responsible for the translocation of the effector proteins into the epithelial cells) by using lacZ transcriptional fusions (2). For this purpose, we analyzed the changes in the β-galactosidase levels of the three transcriptional fusions over time and in all subpopulations. As depicted in Fig. 3, the three lacZ fusions displayed very similar results. The expression levels of the three invasion genes were constant in the total population all along the stationary-phase incubation (10, 24, and 48 h). However, regardless of the age of the culture and the invasion gene-lacZ fusion used, the β-galactosidase level increased with apparent cell density, resulting in differences ranging from 10- to 500-fold between LD and HD cells. Our results demonstrate that the expression levels of the three invasion genes were therefore highly heterogeneous within the stationary-phase cells and were associated with their apparent cell densities.
FIG. 3.
β-Galactosidase activity of lacZ transcriptional fusions of three invasion genes (white bars, hilA; gray bars, invF; black bars, orgA) in S. Typhimurium subpopulations. The LD, MD, and HD cell subpopulations were obtained using Radioselectan density gradient ultracentrifugation after 10, 24, and 48 h of culture. The standard deviations for the triplicate β-galactosidase assays are so small that error bars are not visible. Results are representative of three independent experiments.
(ii) Invasion assay.
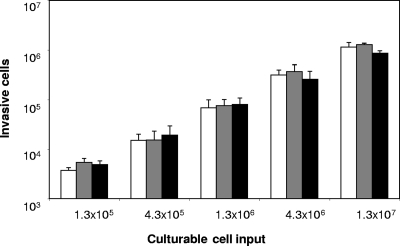
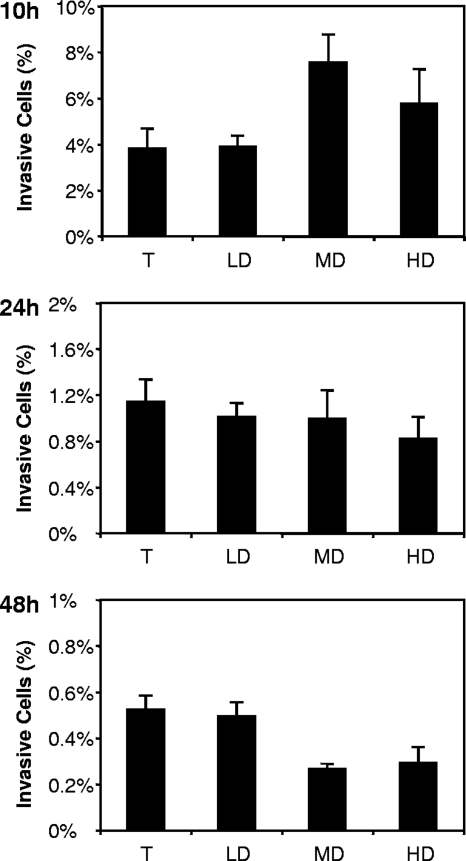
Next, we used a standard invasion assay to test if the invasiveness of the cells reflected the differences detected by the expression of invasion genes. First, it was necessary to ascertain that the nonviable cells present in various quantities in the MD and HD subpopulations did not affect the invasion efficiency of the potentially invasive cells (i.e., the culturable and VBNC cells). As depicted in Fig. 4, the addition of heat-killed cells to an inoculum of culturable cells at a 10:1 or 100:1 ratio had no effect on the number of invasive cells, suggesting that nonviable cells do not interact with the invasion process. Then, we standardized the infectious dose so that each inoculum contained 1 × 106 culturable cells, and we expressed the invasion rate with respect to this culturable-cell input. At 10 h, MD and HD cells displayed higher invasion rates than LD cells (P ≤ 0.001 and P ≤ 0.05, respectively) (Fig. 5). This result was in agreement with their higher level of expression of invasion genes. Moreover, since at 10 h, both the LD and the MD subpopulations contained only culturable cells, their difference evidenced that at least two subpopulations of culturable cells coexisted in the culture, one being more invasive than the other. Conversely, no differences were observed between the three subpopulations at 24 h. At 48 h, whereas the MD and HD subpopulations were inoculated with the same quantity of culturable cells as the LD subpopulation, they displayed a lower invasion rate (P ≤ 0.001). This result similarly demonstrated that culturable cells from different subpopulations do not show the same invasiveness. Finally, whereas fresh VBNC cells were concentrated in the MD and HD subpopulations up to 40-fold in comparison to the level for the total population, no significant increase in invasion rate that could not be explained by the culturable-cell heterogeneous invasiveness was observed. Taken together, the results suggested that (i) the difference between the subpopulation invasion rates is mostly due to the fact that culturable cells have a heterogeneous invasiveness associated with their apparent density and (ii) at 10 h, this heterogeneity could reflect differences in invasion gene expression.
FIG. 4.
Effect of the addition of heat-killed cells on the invasion rate of S. Typhimurium culturable cells on HeLa cells. Heat-killed (121°C, 15 min) S. Typhimurium cells were added to a 100% culturable cell inoculum. No heat-killed cells (white bars), 10 heat-killed cells (gray bars), or 100 heat-killed cells (black bars) were added for each culturable cell. Error bars represent standard deviations for triplicate invasion assays.
FIG. 5.
Invasion rates of S. Typhimurium on HeLa cells after 10, 24, and 48 h of culture expressed in the total (T) cell population and in the LD, MD, and HD cell subpopulations obtained using Radioselectan density gradient centrifugation. The invasion rate represents the percentage of invasive cells expressed with respect to the number of culturable cells inoculated (106 for each inoculum). Error bars represent the standard deviation of sextuplicate β-galactosidase assays. Results are representative of three independent experiments.
DISCUSSION
In this study, we show for the first time that invasiveness is heterogeneous in the culturable cells within a stationary-phase population of S. Typhimurium. In fact, whereas the LD and MD subpopulations from a 10-h culture both contain culturable cells only, we clearly demonstrate that MD cells are twice as invasive as LD cells. This result is in good agreement with the expression of invasion genes observed in these subpopulations. Besides, this study indicates that, under our conditions, specific activities coded for by selected genes tested in newly formed VBNC cells either do not initiate and carry out epithelial cell invasion or do so poorly.
The similar behavior patterns of E. coli and S. Typhimurium are evidenced by the facts that (i) at 10 h, with S. Typhimurium, as with E. coli, MD and HD culturable cells were more sensitive to stress than the LD ones and (ii) at 24 and 48 h, the level of oxidized proteins measured by carbonyl content increased with the apparent density of the subpopulation (unpublished results). This result suggests that, as with E. coli, nonculturable S. Typhimurium cells that appear during the stationary phase predominantly originate from the culturable cells in the MD and HD subpopulations (10) and result from stochastic alteration (12, 23).
In order to better characterize the nonculturable cells, besides the culturability measurement, we used a Live/Dead test combined with flow cytometry analyses. Thus, we were able to distinguish four cellular types in the total stationary-phase population, which we referred to as culturable, newly formed VBNC, membrane-damaged, and DNA-damaged cells. In fact, each type is representative of a stage in the process of cellular alteration, according to the model generally accepted that states that a starved cell begins to lose its culturability, then its membrane integrity, and finally its DNA integrity (5, 9, 21, 32, 37). At each given time of culture, the cells appear separated mostly according to their degree of alteration, since their pattern of distribution results in a gradient of increasingly altered cells, superimposed with their density gradient. Strikingly, the gradient of alteration thus obtained is in perfect agreement with the succession of alteration events acknowledged in the usual model (28). To our knowledge, this is the first time that this alteration model is corroborated in a time-independent way, since it is generally extrapolated from the chronological order of appearance of the different cellular types in a global, starved population (7).
Since at 10 h, 100% of the cells were culturable, the VBNC cells formed between 10 and 24 h could be considered newly formed VBNC cells. If we propose that newly formed VBNC bacteria may temporarily be a source or reservoir of infection, our ability to enhance the ratio between newly formed VBNC cells and culturable cells in MD or HD subpopulations gives us the possibility of testing this hypothesis. Using an invasion test on cultured epithelial cells, we observed that at 10 h, whereas the same quantities of culturable cells were loaded (106 cells) and both LD and MD subpopulations contain only culturable cells, the MD subpopulation is more invasive than the LD subpopulation. This result is corroborated by the fact that the expression levels of the three invasion genes used are more than 10-fold higher in MD than in LD subpopulations. Conversely, at 48 h, whereas inocula contain the same amount of culturable cells, we observed lower invasion rates for HD and MD subpopulations than for the LD subpopulation. This result was not corroborated by the expression of the three invasion genes (β-galactosidase levels), probably because of the high stability of the β-galactosidase enzyme. These results demonstrate that culturable cells have a heterogeneous invasiveness. Moreover, whereas at 10, 24, or 48 h, MD or HD inocula contain between 1- and 10-fold more newly formed VBNC cells than culturable cells (with the exception of the MD subpopulation at 10 h, which contains no VBNC cells), we were not able to observe a higher invasion rate than that of the corresponding LD subpopulation. The simplest explanation for the absence of invasion rate increases was that newly formed VBNC cells make a minor contribution to the invasion rate.
Finally, it is interesting to point out the fact that at 10 h of culture, culturable cells in the MD subpopulation, which will form the major part of the VBNC cells, are the ones that have the most induced invasiveness. All together, our results lead us to hypothesize that at entry into the stationary phase, invasiveness is at least one way for cells to escape stochastic alteration leading to cell death. Indeed, future nonculturable bacteria appear to be more invasive than culturable bacteria.
Acknowledgments
This work was supported by a fund from the French Environment Ministry (Programme Environnement Santé; no. EN00C11) and a doctoral fellowship (to J.P.) from CNRS and Région Languedoc Roussillon.
We also thank Rita Colwell, University of Maryland, for her critical reading of the manuscript.
Footnotes
Published ahead of print on 12 June 2009.
REFERENCES
- 1.Asakura, H., M. Watarai, T. Shirahata, and S. Makino. 2002. Viable but nonculturable Salmonella species recovery and systemic infection in morphine-treated mice. J. Infect. Dis. 186:1526-1529. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj, V., R. L. Lucas, C. Hwang, and C. A. Lee. 1996. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 22:703-714. [DOI] [PubMed] [Google Scholar]
- 3.Barer, M. R., and C. R. Harwood. 1999. Bacterial viability and culturability. Adv. Microb. Physiol. 41:93-137. [DOI] [PubMed] [Google Scholar]
- 4.Bogosian, G., and E. V. Bourneuf. 2001. A matter of bacterial life and death. EMBO Rep. 2:770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caro, A., P. Got, and B. Baleux. 1999. Physiological changes of Salmonella typhimurium cells under osmotic and starvation conditions by image analysis. FEMS Microbiol. Lett. 179:265-273. [DOI] [PubMed] [Google Scholar]
- 6.Caro, A., P. Got, J. Lesne, S. Binard, and B. Baleux. 1999. Viability and virulence of experimentally stressed nonculturable Salmonella typhimurium. Appl. Environ. Microbiol. 65:3229-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaiyanan, S., A. Huq, T. Maugel, and R. R. Colwell. 2001. Viability of the nonculturable Vibrio cholerae O1 and O139. Syst. Appl. Microbiol. 24:331-341. [DOI] [PubMed] [Google Scholar]
- 8.Colwell, R. R., P. Brayton, D. Herrington, B. Tall, A. Huq, and M. M. Levine. 1996. Viable but non culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J. Microbiol. Biotechnol. 12:28-31. [DOI] [PubMed] [Google Scholar]
- 9.Colwell, R. R., and D. J. Grimes. 2000. Nonculturable microorganisms in the environment. ASM Press, Washington, DC.
- 10.Cuny, C., L. Dukan, L. Fraysse, M. Ballesteros, and S. Dukan. 2005. Investigation of the first events leading to loss of culturability during Escherichia coli starvation: future nonculturable bacteria form a subpopulation. J. Bacteriol. 187:2244-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darwin, K. H., and V. L. Miller. 1999. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin. Microbiol. Rev. 12:405-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desnues, B., C. Cuny, G. Grégori, S. Dukan, H. Aguilaniu, and T. Nyström. 2003. Differential oxidative damage and expression of stress defence regulons in culturable and nonculturable Escherichia coli cells. EMBO Rep. 4:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupte, A. R., C. L. E. de Rezende, and S. W. Joseph. 2003. Induction and resuscitation of viable but nonculturable Salmonella enterica serovar Typhimurium DT104. Appl. Environ. Microbiol. 69:6669-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoiseth, S. K., and B. A. D. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 15.Kell, D. B., A. S. Kaprelyants, D. H. Weichart, C. R. Harwood, and M. R. Barer. 1998. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie van Leeuwenhoek 73:169-187. [DOI] [PubMed] [Google Scholar]
- 16.Lee, C. A., B. D. Jones, and S. Falkow. 1992. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc. Natl. Acad. Sci. USA 89:1847-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesne, J., S. Berthet, S. Binard, A. Rouxel, and F. Humbert. 2000. Changes in culturability and virulence of Salmonella typhimurium during long-term starvation under desiccating conditions. Int. J. Food Microbiol. 60:195-203. [DOI] [PubMed] [Google Scholar]
- 18.Maisonneuve, E., B. Ezraty, and S. Dukan. 2008. Protein aggregates: an aging factor involved in cell death. J. Bacteriol. 190:6070-6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDougald, D., S. A. Rice, D. Weichart, and S. Kjelleberg. 1998. Nonculturability: adaptation or debilitation? FEMS Microbiol. Ecol. 25:1-9. [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 21.Monfort, P., and B. Baleux. 1994. Effects of environmental factors present in the St. Lawrence Estuary (Québec, Canada) on experimental survival of Salmonella salamae as determined by flow cytometry. Can. J. Microbiol. 40:712-719. [DOI] [PubMed] [Google Scholar]
- 22.Mukamolova, G. V., A. S. Kaprelyants, D. B. Kell, and M. Young. 2003. Adoption of the transiently non-culturable state: a bacterial survival strategy? Adv. Microb. Physiol. 47:65-129. [DOI] [PubMed] [Google Scholar]
- 23.Nyström, T. 2003. Nonculturable bacteria: programmed survival forms or cells at death's door? Bioessays 25:204-211. [DOI] [PubMed] [Google Scholar]
- 24.Oliver, J. D. 1993. Formation of viable but nonculturable cells, p. 239-272. In S. Kjelleberg (ed.), Starvation in bacteria. Plenum Press, New York, NY.
- 25.Oliver, J. D. 2005. The viable but nonculturable state in bacteria. J. Microbiol. 43:93-100. [PubMed] [Google Scholar]
- 26.Oliver, J. D., and R. Bockian. 1995. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl. Environ. Microbiol. 61:2620-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panutdaporn, N., K. Kawamoto, H. Asakura, and S. I. Makino. 2006. Resuscitation of the viable but non-culturable state of Salmonella enterica serovar Oranienburg by recombinant resuscitation-promoting factor derived from Salmonella Typhimurium strain LT2. Int. J. Food Microbiol. 106:241-247. [DOI] [PubMed] [Google Scholar]
- 28.Petit, M., I. George, and P. Servais. 2000. Survival of Escherichia coli in freshwater: beta-D-glucuronidase activity measurements and characterization of cellular states. Can. J. Microbiol. 46:679-684. [PubMed] [Google Scholar]
- 29.Porter, K. G., and Y. S. Feig. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25:943-948. [Google Scholar]
- 30.Reissbrodt, R., H. Heier, H. Tschape, R. A. Kingsley, and P. H. Williams. 2000. Resuscitation by ferrioxamine E of stressed Salmonella enterica serovar Typhimurium from soil and water microcosms. Appl. Environ. Microbiol. 66:4128-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reissbrodt, R., I. Rienaecker, J. M. Romanova, P. P. E. Freestone, R. D. Haigh, M. Lyte, H. Tschape, and P. H. Williams. 2002. Resuscitation of Salmonella enterica serovar Typhimurium and enterohemorrhagic Escherichia coli from the viable but nonculturable state by heat-stable enterobacterial autoinducer. Appl. Environ. Microbiol. 68:4788-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roszak, D. B., and R. R. Colwell. 1987. Survival strategies of bacteria in the natural environment. Microbiol. Rev. 51:365-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roszak, D. B., D. J. Grimes, and R. R. Colwell. 1984. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can. J. Microbiol. 30:334-338. [DOI] [PubMed] [Google Scholar]
- 34.Smith, R. J., S. C. Kehoe, K. G. McGuigan, and M. R. Barer. 2000. Effects of simulated solar disinfection of water on infectivity of Salmonella typhimurium. Lett. Appl. Microbiol. 31:284-288. [DOI] [PubMed] [Google Scholar]
- 35.Smith, R. J., A. T. Newton, C. R. Harwood, and M. R. Barer. 2002. Active but nonculturable cells of Salmonella enterica serovar Typhimurium do not infect or colonize mice. Microbiology 148:2717-2726. [DOI] [PubMed] [Google Scholar]
- 36.Sylvester, D. M., R. Taylor, and T. R. LaHann. 2001. Viable but nonculturable bacteria: a public health threat? Infect. Dis. Rev. 3:70-82. [Google Scholar]
- 37.Troussellier, M., J. L. Bonnefont, C. Courties, A. Derrien, E. Dupray, M. Gauthier, M. Gourmelon, F. Joux, P. Lebaron, Y. Martin, and M. Pommepuy. 1998. Responses of enteric bacteria to environmental stresses in seawater. Oceanol. Acta 21:965-981. [Google Scholar]
- 38.Villarino, A., O. Bouvet, B. Regnault, S. Delautre, and P. A. D. Grimont. 2000. Cellular activities in ultra-violet killed Escherichia coli. Int. J. Food Microbiol. 55:245-247. [DOI] [PubMed] [Google Scholar]
- 39.Zweifel, U. L., and A. Hagstrom. 1995. Total counts of marine bacteria include a large fraction of non-nucleoid-containing bacteria (ghosts). Appl. Environ. Microbiol. 61:2180-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]