Abstract
We have developed a tool for controlled expression of heterologous or ectopic genes in the chestnut pathogen Cryphonectria parasitica using the promoter region from a putative copper-regulated transporter gene. In addition, we have found that expression control via this system is not affected by the virulence-attenuating hypovirus CHV1-EP713.
Cryphonectria parasitica, an ascomycete plant pathogen, is the causative agent of chestnut blight. First observed in the United States in the early part of the 20th century (21), the fungus rapidly spread throughout the natural range of Castanea dentata, the American chestnut, resulting in the near eradication of this species. The discovery of healing European chestnut (Castanea sativa) trees (A. Biraghi, presented at the 11th Rep. Congr. Int. Union Forest Res. Org., Rome, Italy, 1953) led to the subsequent isolation of hypovirulent strains of C. parasitica (14) that contained a cytoplasmically transmissible factor (15), later identified as a double-stranded RNA species (2, 10). The single most striking phenotype that these viruses impose upon the host is a reduction in pathogenesis, but hypovirus infection also retards colony growth and development (reviewed in references 9 and 22) and affects colony morphology (9, 13, 22) at least in part through major impacts on central metabolism (A. L. Dawe, W. A. Van Voorhies, and T. A. Lau, unpublished). With the availability of infectious cDNA clones of three members of the Hypoviridae (4, 5, 20), together with advances in molecular tools characteristic of other ascomycetes, including transformation (6), gene deletion (11), spotted cDNA microarrays (1), and a forthcoming genome sequence, C. parasitica has developed into an powerful model system for investigating the molecular biology of fungal-plant pathogenesis and virus-host interactions.
However, one tool lacking from the armory has been the availability of a method for controlled gene expression. Expression constructs for C. parasitica have been derived from pCPXHY1 (7), which constitutively drives gene expression with the gpd promoter. More recently, the promoter of the hydrophobin cryparin has also been characterized (18). However, neither example provides for controlled expression. In other fungi, such tools have been developed using nutritional control, such as by use of the GAL system in Saccharomyces cerevisiae (3) and the alcA mechanism in Aspergillus nidulans (28). One potential problem with nutritional control is that colony morphology and development may be impaired. This is of particular importance in the C. parasitica experimental system, since any controlled expression mechanism should ideally also function in the presence of hypovirus that itself affects colony morphology and development as noted above.
Therefore, we have explored the utility of a copper-controlled promoter. This approach has been successfully applied to a controlled expression in S. cerevisiae (16, 26) that employs the CUP1 metallothionein promoter to enhance expression in the presence of copper. More recently, studies have characterized the control regions from the copper transporters CTR4 in Cryptococcus neoformans (23) and CRP1 in Histoplasma capsulatum (12).
In order to determine whether the C. parasitica genome contained a CTR4-like sequence, we searched the draft genome sequence using an online database (http://www.jgi.doe.gov/) and identified a putative protein with approximately 30% identity to the CTR4 proteins from both C. neoformans (GenBank accession number XP_775793 [23]) and Schizosaccharomyces pombe (AAD51064 [19]). The predicted C. parasitica protein also contained features of the conserved Ctr superfamily of proteins (8, 25). We then tested whether the conditions under which the CTR4 promoter was used in C. neoformans affected the colony phenotype of C. parasitica. Twenty-five micromolar CuSO4 (for excess copper resulting in repression) or 200 μM bathocuproinedisulfonic acid (BCS; for copper depletion leading to induction) were incorporated into 3.9% (wt/vol) Difco potato dextrose agar (Becton Dickinson) and the colonies grown at room temperature (22 to 25°C) with a 12-h light/dark cycle at 1,300 to 1,600 lx for 8 days. Wild-type C. parasitica strain EP155 (ATCC 38755) was found to grow and develop with only minor differences noted: a slight decrease in aerial mycelium production in the presence of excess Cu2+ and a slight increase in sporulation when copper was depleted (data not shown). Similarly, there was no major impact of the copper concentration on the growth of the hypovirus-infected isogenic strain EP155/CHV1-EP713 (ATCC 52571) (data not shown). In all cases, colony size was not affected.
Closer examination of the sequence immediately upstream of the putative ctr4-like gene revealed a potential TATA box sequence (TATAA) at bp −126 and six sequences that match the core consensus for a metal-sensing element established for S. pombe (GCTG [17]) at positions −201, −240, −313, −522, −936, and −1237. Furthermore, searching the C. parasitica genome revealed, as was noted for C. neoformans (23), a predicted protein with expressed sequence tag support that contained 51% identity to the 60 N-terminal amino acids of S. pombe Cuf1p, the transcription factor that activates ctr4 expression (19).
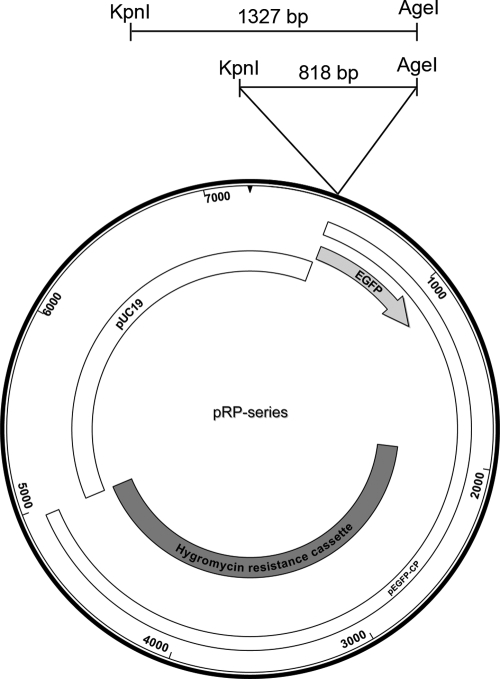
To test the potential for a copper-sensitive control mechanism in C. parasitica, we amplified this upstream region using genomic DNA isolated from strain EP155 and primer KLW1 (5′-TAGGCCCATTTGTTTCGTTTTTAT-3′) or KLW3 (5′-CGTCGTGCCCGTGATAGA-3′) in conjunction with reverse primer KLW2 (5′-CTTGGCGGCTGTTGATGATTAT-3′). The two products produced were 818 bp and 1,327 bp long and terminated immediately prior to the ATG of exon 1 of the predicted ctr4 gene. These amplicons were cloned into pSC-A using the Strataclone system (Stratagene) according to the manufacturer's instructions and verified by DNA sequencing. We built a new vector, pRP1, that included pUC19 features for maintenance in Escherichia coli and, from pEGFP-CP (27), the hygromycin resistance cassette, enhanced green fluorescent protein (EGFP) with the gpd terminator, and unique KpnI and AgeI restriction sites at the 5′ end of the reporter gene. Each of the two genomic DNA fragments was then cloned into pRP1, creating pRP1-1 (1,327-bp fragment) and pRP1-2 (818-bp fragment) by use of the KpnI and AgeI sites (Fig. 1). These two plasmids were used for transformation of C. parasitica spheroplasts as described by Churchill et al. (6). Hygromycin-resistant colonies were selected and allowed to produce asexual spores and colonies derived from single spores to ensure that nuclear homogeneity were used in all subsequent studies. No phenotypic consequences of transformation with this construct were observed under any of the growth conditions tested.
FIG. 1.
Construction of the pRP series vectors. pRP1 (7,220 bp) was created by ligating KpnI-XbaI-digested pUC19 and pEGFP-CP (27), digested with the same restriction enzymes. This vector was then augmented by cloning each of the putative ctr4 control regions into the indicated KpnI and AgeI sites to create pRP1-1 (8,557 bp) or pRP1-2 (8,038 bp). Open boxes denote regions defined by pUC19 or pEGFP-CP, as indicated. Dark-gray box, hygromycin resistance cassette; light-gray box, EGFP coding region.
To test for EGFP expression, 7- to 8-day-old liquid stationary cultures grown in potato dextrose broth (PDB) were harvested by filtration. Total protein extracts were prepared as described previously (24) and then separated by electrophoresis on a 10% denaturing polyacrylamide gel in Tris-glycine running buffer (25 mM Tris, pH 8.3; 192 mM glycine; 0.1% sodium dodecyl sulfate). The proteins were transferred to an Immobilon-P membrane (Millipore), blocked in 5% blotting-grade nonfat dry milk (Bio-Rad) in 1× TBST (20 mM Tris, 150 mM NaCl, 0.05% Tween 20, pH 7.5), and incubated in primary antibody (1:2,000 dilution of Living Colors A.v. monoclonal antibody JL-8; Clontech). Following the addition of secondary antibody (1:1,000 dilution of Immun-Star goat anti-mouse-horseradish peroxidase conjugate; Bio-Rad) the signal was detected using Immun-Star horseradish peroxidase chemiluminescent reagents (Bio-Rad) and imaged on a Chemi-Doc imaging system (Bio-Rad) for between 30 and 60 s. Inducing (copper depletion with 200 μM BCS) or repressing (25 μM CuSO4) conditions were incorporated into the growth medium as indicated for Fig. 2. As a control, we included the vector pEGFP-CP, which contains the same EGFP sequence under the control of the constitutively active gpd promoter (27).
FIG. 2.
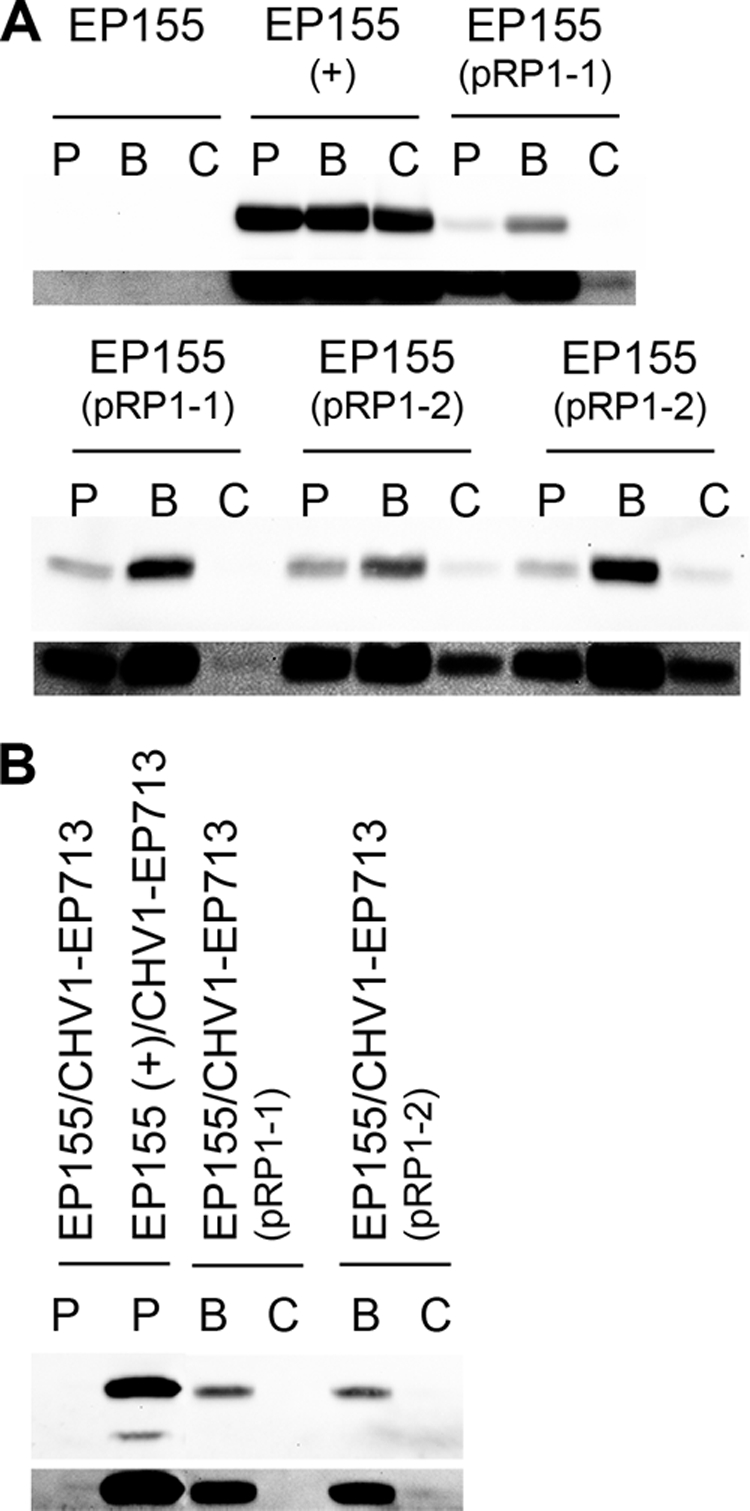
Western analysis of total proteins (20 μg) from two independent transformants reveals controlled EGFP expression under conditions of copper depletion and excess. Lanes: P, unmodified PDB medium; B, PDB medium plus 200 μM BCS; C, PDB medium plus 25 μM CuSO4. Notations in parentheses indicate the transforming construct, and (+) designates the positive control expressing EGFP under the control of the gpd promoter. Darker panels beneath each blot show the enhanced images necessary for visualizing the extremely low quantities of EGFP under repressing conditions. (A) Copper-sensitive expression of EGFP driven by either of the C. parasitica genomic fragments. EGFP is visible only under repressing conditions when the image is maximally enhanced for the black level. (B) The characteristics of induction and repression are maintained in the presence of the hypovirus CHV1-EP713.
Figure 2A shows data from two independent transformants with each construct. In all cases, the depletion of Cu2+ by addition of BCS elevated EGFP accumulation substantially above that observed for PDB medium alone, but in the presence of 25 μM CuSO4, EGFP was absent or barely detectable. No EGFP was initially detectable under repressing conditions from the pRP1-1 construct at all. However, it was possible to observe very faint bands when the image was enhanced by maximizing the black level in Adobe Photoshop following a 5-min exposure, as seen in the darker panels beneath each blot (Fig. 2). Faint bands were observed for pRP1-2 even without image enhancement or overexposure, which may suggest that the two core consensus metal-sensing elements in this region are important. These extremely low levels of basal expression are consistent with the observations of C. neoformans by Ory et al. (23). Also of note were the reduced, but consistent, levels of EGFP observed in unmodified PDB medium, indicating that the levels of Cu2+ in this environment are not sufficient to repress the expression completely. No difference in EGFP accumulation was observed in pEGFP-CP, where expression was driven by the constitutive gpd promoter.
As described earlier, an essential feature of the C. parasitica experimental system is the ability to attenuate virulence by infection with mycoviruses of the family Hypoviridae. We have also verified that infection of the transformants noted above with the prototypic hypovirus CHV1-EP713 by anastomosis does not result in an altered regulation pattern. The overall levels of EGFP accumulation are somewhat lower (Fig. 2B), but the essential feature of controlled expression was maintained. The faint shadow observed on the darker panel in the rightmost lane may suggest that, as noted for the noninfected mycelium, GFP expression under repressing conditions may be marginally less well controlled with construct pRP1-2.
In conclusion, we have established that the copper-regulated system in C. parasitica shares components previously identified in ascomycetous and basidiomycetous fungi. Exploiting the features of this system, we have identified a region upstream of the putative copper transporter gene ctr4 that contains predicted metal-regulatory elements and used it successfully to drive the heterologous expression of EGFP. This system has desirable characteristics of strong induction when Cu2+ is depleted and strong repression when Cu2+ is in excess. In addition, we have shown that these conditions have minimal impact on the growth and phenotype of C. parasitica colonies, and crucially, the essential feature of expression control is maintained following infection with a virulence-attenuating hypovirus. This tool greatly enhances the potential of the C. parasitica experimental system as a model for plant pathogenesis and virus-host interactions. Given the conserved nature of the processes for copper-mediated transcriptional control in fungi as diverse as S. pombe, C. neoformans, and C. parasitica, it is likely that this principle may be broadly applicable for the development of similar tools for other fungi of interest, possibly even by direct use of the promoter fragments from C. parasitica.
Nucleotide sequence accession number.
The 1,500-bp sequence immediately upstream of the putative ctr4-like open reading frame has been deposited in GenBank with the accession number FJ627178.
Acknowledgments
We thank Joanna Salamon for technical assistance.
This work was supported by a Howard Hughes Medical Institute Undergraduate Science Education grant (award 52005881, for support of A.M.K.) and the National Science Foundation (award MCB-0718735 to A.L.D.).
This study would not have been possible without the efforts of the Cryphonectria Genome Sequencing Consortium and the Joint Genome Institute of the U.S. Department of Energy.
Footnotes
Published ahead of print on 19 June 2009.
REFERENCES
- 1.Allen, T. D., A. L. Dawe, and D. L. Nuss. 2003. Use of cDNA microarrays to monitor transcriptional responses of the chestnut blight fungus Cryphonectria parasitica to infection by virulence-attenuating hypoviruses. Eukaryot. Cell 2:1253-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anagnostakis, S. L., and P. R. Day. 1979. Hypovirulence conversion in Endothia parasitica. Phytopathology 69:1226-1229. [Google Scholar]
- 3.Broach, J. R., Y.-Y. Li, L.-C. Chen Wu, and M. Jayaram. 1983. Vectors for high-level inducible expression of cloned genes in yeast, p. 83-117. In M. Inouye (ed.), Experimental manipulation of gene expression. Academic Press, New York, NY.
- 4.Chen, B., and D. L. Nuss. 1999. Infectious cDNA clone of hypovirus CHV1-Euro7: a comparative virology approach to investigate virus-mediated hypovirulence of the chestnut blight fungus Cryphonectria parasitica. J. Virol. 73:985-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi, G. H., and D. L. Nuss. 1992. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science 257:800-803. [DOI] [PubMed] [Google Scholar]
- 6.Churchill, A. C. L., L. M. Ciufetti, D. R. Hansen, H. D. Van Etten, and N. K. Van Alfen. 1990. Transformation of the fungal pathogen Cryphonectria parasitica with a variety of heterologous plasmids. Curr. Genet. 17:25-31. [Google Scholar]
- 7.Craven, M. G., D. M. Pawlyk, G. H. Choi, and D. L. Nuss. 1993. Papain-like protease p29 as a symptom determinant encoded by a hypovirulence-associated virus of the chestnut blight fungus. J. Virol. 67:6513-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dancis, A., D. Haile, D. S. Yuan, and R. D. Klausner. 1994. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J. Biol. Chem. 269:25660-25667. [PubMed] [Google Scholar]
- 9.Dawe, A. L., and D. L. Nuss. 2001. Hypoviruses and chestnut blight: exploiting viruses to understand and modulate fungal pathogenesis. Annu. Rev. Genet. 35:1-29. [DOI] [PubMed] [Google Scholar]
- 10.Day, P. R., J. A. Dodds, J. E. Elliston, R. A. Jaynes, and S. L. Anagnostakis. 1977. Double-stranded RNA in Endothia parasitica. Phytopathology 67:1393-1396. [Google Scholar]
- 11.Gao, S., G. H. Choi, L. Shain, and D. L. Nuss. 1996. Cloning and targeted disruption of enpg-1, encoding the major in vitro extracellular endopolygalacturonase of the chestnut blight fungus, Cryphonectria parasitica. Appl. Environ. Microbiol. 62:1984-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebhart, D., A. K. Bahrami, and A. Sil. 2006. Identification of a copper-inducible promoter for use in ectopic expression in the fungal pathogen Histoplasma capsulatum. Eukaryot. Cell 5:935-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golinski, M. R., W. J. Boecklen, and A. L. Dawe. 2008. Two-dimensional fractal growth properties of the filamentous fungus Cryphonectria parasitica: the effects of hypovirus infection. J. Basic Microbiol. 48:426-429. [DOI] [PubMed] [Google Scholar]
- 14.Grente, J. 1965. Les formes hypovirulentes d'Endothia parasitica et les espoires de lutte contre le chancre du chataigner. C. R. Seances Acad. Agric. Fr. 51:1033-1037. [Google Scholar]
- 15.Grente, J., and S. Sauret. 1969. L'hypovirulence exclusive est-elle controllee par des determinants cytoplasmiques? C. R. Acad. Sci. Ser. D 268:3173-3176. [Google Scholar]
- 16.Hottiger, T., J. Kuhla, G. Pohlig, P. Furst, A. Spielmann, M. Garn, S. Haemmerli, and J. Heim. 1995. 2-Micron vectors containing the Saccharomyces cerevisiae metallothionein gene as a selectable marker: excellent stability in complex media, and high-level expression of a recombinant protein from a CUP1-promoter-controlled expression cassette in cis. Yeast 11:1-14. [DOI] [PubMed] [Google Scholar]
- 17.Koch, K. A., and D. J. Thiele. 1996. Autoactivation by a Candida glabrata copper metalloregulatory transcription factor requires critical minor groove interactions. Mol. Cell. Biol. 16:724-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon, B. R., M. J. Kim, J. A. Park, H. J. Chung, J. M. Kim, S. M. Park, S. H. Yun, M. S. Yang, and D. H. Kim. 2009. Assessment of the core cryparin promoter from Cryphonectria parasitica for heterologous expression in filamentous fungi. Appl. Microbiol. Biotechnol. 83:339-348. [DOI] [PubMed] [Google Scholar]
- 19.Labbe, S., M. M. Pena, A. R. Fernandes, and D. J. Thiele. 1999. A copper-sensing transcription factor regulates iron uptake genes in Schizosaccharomyces pombe. J. Biol. Chem. 274:36252-36260. [DOI] [PubMed] [Google Scholar]
- 20.Lin, H., X. Lan, H. Liao, T. B. Parsley, D. L. Nuss, and B. Chen. 2007. Genome sequence, full-length infectious cDNA clone, and mapping of viral double-stranded RNA accumulation determinant of hypovirus CHV1-EP721. J. Virol. 81:1813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merkel, H. W. 1906. A deadly fungus on the American chestnut. N. Y. Zool. Soc. Annu. Rep. 10:97-103. [Google Scholar]
- 22.Nuss, D. L. 2005. Hypovirulence: mycoviruses at the fungal-plant interface. Nat. Rev. Microbiol. 3:632-642. [DOI] [PubMed] [Google Scholar]
- 23.Ory, J. J., C. L. Griffith, and T. L. Doering. 2004. An efficiently regulated promoter system for Cryptococcus neoformans utilizing the CTR4 promoter. Yeast 21:919-926. [DOI] [PubMed] [Google Scholar]
- 24.Parsley, T. B., G. C. Segers, D. L. Nuss, and A. L. Dawe. 2003. Analysis of altered G-protein subunit accumulation in Cryphonectria parasitica reveals a third Gα homologue. Curr. Genet. 43:24-33. [DOI] [PubMed] [Google Scholar]
- 25.Puig, S., J. Lee, M. Lau, and D. J. Thiele. 2002. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J. Biol. Chem. 277:26021-26030. [DOI] [PubMed] [Google Scholar]
- 26.Sone, T., D. P. McDonnell, B. W. O'Malley, and J. W. Pike. 1990. Expression of human vitamin D receptor in Saccharomyces cerevisiae. Purification, properties, and generation of polyclonal antibodies. J. Biol. Chem. 265:21997-22003. [PubMed] [Google Scholar]
- 27.Suzuki, N., L. M. Geletka, and D. L. Nuss. 2000. Essential and dispensable virus-encoded replication elements revealed by efforts to develop hypoviruses as gene expression vectors. J. Virol. 74:7568-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waring, R. B., G. S. May, and N. R. Morris. 1989. Characterization of an inducible expression system in Aspergillus nidulans using alcA and tubulin-coding genes. Gene 79:119-130. [DOI] [PubMed] [Google Scholar]