Abstract
Pseudomonas aeruginosa possesses three quorum-sensing (QS) systems which are key in the expression of a large number of genes, including many virulence factors. Most studies of QS in P. aeruginosa have been performed in clinical isolates and have therefore focused on its role in pathogenicity. P. aeruginosa, however, is regarded as a ubiquitous organism capable of colonizing many different environments and also of establishing beneficial associations with plants. In this study we examined the role of the two N-acyl homoserine lactone systems known as RhlI/R and LasI/R in the environmental rice rhizosphere isolate P. aeruginosa PUPa3. Both the Rhl and Las systems are involved in the regulation of plant growth-promoting traits. The environmental P. aeruginosa PUPa3 is pathogenic in two nonmammalian infection models, and only the double las rhl mutants are attenuated for virulence. In fact it was established that the two QS systems are not hierarchically organized and that they are both important for the colonization of the rice rhizosphere. This is an in-depth genetic and molecular study of QS in an environmental P. aeruginosa strain and highlights several differences with QS regulation in the clinical isolate PAO1.
Pseudomonas aeruginosa has been intensively studied by the scientific community because it is an opportunistic pathogen able to chronically colonize and infect cystic fibrosis patients (30). An important aspect of this bacterium is its capability to adapt to the host environment through the extensive and complex transcriptional regulation of an arsenal of virulence genes. A key player in this response is the quorum-sensing (QS) cell-cell communication system, which coordinates the behavior of P. aeruginosa communities. In fact, the transcriptional regulation of many virulence genes is controlled by two N-acyl homoserine lactone (AHL)-dependent QS systems called LasI/R and RhlI/R (16, 48).
In the LasI/R system, lasI directs the synthesis of N-(3-oxo-dodecanoyl)-homoserine lactone (3-oxo-C12-HSL), which binds and activates the cognate response regulator LasR, resulting in the regulation of target gene expression. In the RhlI/R system, on the other hand, rhlI directs the synthesis of N-(butanoyl)-homoserine lactone (C4-HSL), which then interacts with the cognate RhlR, influencing transcription of target genes. These two QS systems are probably among the most studied in bacteria, and their regulons are fundamental to the pathogenicity of P. aeruginosa (48). Importantly, the two systems are intimately connected, being hierarchically organized with the LasI/R system regulating the transcription of rhlI-rhlR (29). The two QS regulons overlap, and together they constitute approximately 10% of the genes in P. aeruginosa, including factors like elastase, alkaline protease, exotoxin A, rhamnolipids, and pyocyanin, as well as being important for the regulation of biofilm formation (19, 46, 58). The two systems are themselves controlled by different regulators, allowing QS to respond and be modulated also by an array of environmental stimuli (56).
The importance of QS in the pathogenicity of P. aeruginosa has been demonstrated using a number of models, including insect, animal, and worm. The sputum of cystic fibrosis patients has been found to contain AHL molecules, virulence factors, and lasI-lasR transcripts, indicating that QS is active in vivo (14). The large majority of studies on AHL-dependent QS systems in P. aeruginosa have used the model strain PAO1, which was isolated from an infected wound over 50 years ago (54). In spite of the fact that P. aeruginosa is considered to be a ubiquitously distributed bacterium and is able to competitively colonize several environments, including soil, marshes, marine habitats, and plant roots, environmental strains have been hardly investigated (7). In this study we analyzed the LasI/R and RhlI/R QS systems of P. aeruginosa strain PUPa3 (28), a plant growth-promoting rice rhizosphere isolate which exhibits a wide range of antifungal activities and other beneficial traits related to the plant-bacteria interaction. It is shown that this strain harbors two QS systems that are highly homologous to the LasI/R and RhlI/R systems of PAO1. However, in contrast to PAO1, the two systems of PUPa3 are not hierarchically arranged. The roles of these two systems in root colonization, virulence toward Caenorhabditis elegans and the wax moth Galeria melonella, and for expression of several other phenotypic traits have been investigated.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and recombinant DNA techniques.
P. aeruginosa (strain PUPa3), which has strong antifungal activity and several plant growth-promoting traits, has been previously isolated from the rice rhizosphere in India (28). This strain was grown at 28°C in either Luria-Bertani (LB) agar, M9 minimal medium (44), or King medium (24). Strains and plasmids used in this study are listed in Table 1. AHL bacterial biosensors used for AHL detection were Chromobacterium violaceum strain CVO26, Escherichia coli JM109(pSB1075), and Pseudomonas putida F117(pKRC12) (for all, see reference 53). For DNA transformations DH5α (18) was used while for triparental matings we used E. coli DH5α(pRK2013) (15). Antibiotics were added as required at the following final concentrations: ampicillin, 100 μg ml−1; tetracycline, 15 μg ml−1 (E. coli) or 100 μg ml−1 (P. aeruginosa); gentamicin, 10 μg ml−1 (E. coli) or 100 μg ml−1 (P. aeruginosa); kanamycin, 50 μg ml−1 (E. coli and C. violaceum) or 300 μg ml−1 (P. aeruginosa).
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant characteristics or sequence | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH5α | F′ endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 (lacZYA-argF)U169 deoR [80dlac(lacZ)M15recA1] | 44 |
| JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB) mcrA [F′ traD36 proAB lacIqlacZΔM15 | 63 |
| C. violaceum CV026 | Double mini-Tn5 mutant from C. violaceum ATCC 31532, AHL biosensor | 36 |
| P. putida F117 | AHL-negative derivative of P. putida IsoF; PpuI− | 50 |
| P. aeruginosa strains | ||
| PAO1 | Wild type | 21 |
| PAO1lasI::Gm | PAO1 with Gm cartridge inserted into unique EcoRI site of lasI | 3 |
| PAO1rhlI::Tc | PAO1 with Tc cartridge inserted into unique EcoRI site of rhlI | 3 |
| PAO1rsaL | rsaL::ISlacZ-hah | 42 |
| PUPa3 | Wild type, rice rhizosphere isolate | 28 |
| LASI | lasI::Km of P. aeruginosa PUPa3; Kmr | This study |
| LASR | lasR::Km of P. aeruginosa PUPa3; Kmr | This study |
| RHLI | rhlI::Gm of P. aeruginosa PUPa3; Gmr | This study |
| DMI | lasI::Km rhlI::Gm of P. aeruginosa PUPa3; Kmr Gmr | This study |
| DMR | lasR::Km rhlR::Tet of P. aeruginosa PUPa3; Kmr Tetr | This study |
| RSAL | rsaL::Tc of P. aeruginosa PUPa3; Tetr | This study |
| Plasmids | ||
| pKRC12 | pBBR1MCS-5 carrying PlasB-gfp (ASV) Plac-lasR; Gmr | 43 |
| pSB1075 | lasR-PlasI luxCDABE; Ampr | 61 |
| pRK2013 | Tra+ Mob+ ColE1 replicon; Kmr | 15 |
| pMOSBlue | Cloning vector; Ampr | Amersham-Pharmacia |
| pBluescript KS | Cloning vector; Ampr | Stratagene |
| pLAFR3 | Broad-host-range cloning vector, IncP1; Tetr | 49 |
| pIB101 | pLAFR3 containing P. aeruginosa PUPa3 DNA | This study |
| pIB103 | pLAFR3 containing P. aeruginosa PUPa3 DNA | This study |
| pMULTIAHLPROM | Broad-host-range plasmid containing eight luxI-type promoters fused to a promoterless lacZ gene; Tetr | 52 |
| pKNOCK-Km | Conjugative suicide vector; Kmr | 1 |
| pKNOCK-Gm | Conjugative suicide vector; Gmr | 1 |
| pKNOCK-Tet | Conjugative suicide vector; Tetr | 1 |
| pKNOCK-lasI | Internal PCR fragment of P. aeruginosa PUPa3 lasI cloned in pKNOCK-Km | This study |
| pKNOCK-rhlI | Internal PCR fragment of P. aeruginosa PUPa3 rhlI cloned in pKNOCK-Gm | This study |
| pKNOCK-rhlR | Internal PCR fragment of P. aeruginosa PUPa3 rhlR cloned in pKNOCK-Tet | This study |
| pKNOCK-rsaL | Internal PCR fragment of P. aeruginosa PUPa3 rsaL cloned in pKNOCK-Tet | This study |
| Primers | ||
| lasI-for | GAAATCGATGGTTATGACGC | This study |
| lasI-rev | CGGCACGGATCATCATCTTC | This study |
| rhlI-for | TCAGGTCTTCATCGAGAAGC | This study |
| rhlI-rev | CGTTGCGAACGAAATAGCG | This study |
| rhlR-for | TGGATCCGGCGATCCTCAAC | This study |
| rhlR-rev | GCTCTAGAGCTTCTGGGTCAGCAACT | This study |
| rsaL-for | TTGGATCCACCCGCACCGCCCGAC | This study |
| rsaL-rev | GCTCTAGATATATAGGGAAGGGCAGG | This study |
Recombinant DNA techniques, including digestion with restriction enzymes, agarose gel electrophoresis, purification of DNA fragments, ligation with T4 ligase, end filling with Klenow enzyme, hybridization, radioactive labeling by random priming, and transformation of E. coli were performed as described previously (44). Southern hybridizations were performed using N+ Hybond membranes (Amersham, Biosciences); plasmids were purified using Jet Star columns (Genomed GmbH, Löhne, Germany) or the alkaline lysis method (6); total DNA from Pseudomonas was isolated by Sarkosyl-pronase lysis as described previously (5).
Extraction and visualization of AHL signal molecules.
For the detection of the AHL molecules, P. aeruginosa strain PUPa3 was grown overnight in 20 ml of M9 minimal medium supplemented with glucose and Casamino Acids, and the supernatant of the culture was extracted with an equal volume of ethyl acetate acidified with 0.1% acetic acid. The preparation was centrifuged (5,000 rpm for 5 min) and the ethyl acetate phase collected. The extract was then dried and resuspended in a small volume of ethyl acetate and was run on C18 reverse-phase chromatography plates, with synthetic AHLs used as standards (which were purchased either from Fluka-Sigma-Aldrich or from P. Williams, University of Nottingham, United Kingdom), using 60% (vol/vol) methanol in water as the mobile phase. The plates were overlaid with a thin layer of LB agar seeded with either C. violaceum CVO26 or E. coli(pSB1075).
Cloning and inactivation of QS genes in P. aeruginosa PUPa3.
A cosmid library was constructed for P. aeruginosa PUPa3 using the cosmid pLAFR3 (49) as vector. Insert DNA was prepared by partial EcoRI digestion of the chromosomal DNA of strain PUPa3 and then ligated in the corresponding site of pLAFR3. The ligated DNA was then packaged into λ phage heads using Gigapack III Gold packaging extract (Stratagene), and the phage particles were transduced to E. coli HB101. In order to identify the cosmid containing the QS genes, the E. coli HB101 harboring the cosmid library was used as donor in a triparental conjugation with, as acceptors, the AHL biosensor C. violaceum CV026 or P. putida F117(pKRC12). Transconjugants in which the biosensor was activated (i.e., restoration of purple pigment or green fluorescent protein expression) were considered positive and were extracted. Cosmid pIB103 harbored the lasI-lasR genes of P. aeruginosa PUPa3; part of a ClaI-SacI fragment (approximately 5.5 kb) was sequenced and found to include a partial QS locus. To obtain the remaining part of the lasI gene, a primer walking procedure on the original cosmid was performed. Cosmid pIB101 harbored the rhlI-rhlR genes of P. aeruginosa PUPa3; two adjacent ClaI-SacI fragments (approximately 4.5 and 0.6 kb) were cloned into pBSIIKS and their sequences were found to include the rhlI-rhlR genes. The QS genomic null mutants were created via insertional mutagenesis utilizing the conjugative suicide vectors pKNOCK-Km, pKNOCK-Gm, and pKNOCK-Tc (1). For the lasI (3-oxo-C12-HSL synthase gene) mutant, part of this gene (343 bp) was amplified using oligonucleotides lasI-for and lasI-rev and cloned as an XbaI-BamHI fragment in pKNOCK-Km to yield pKNOCK-lasI. This latter plasmid was then used as a suicide delivery system in order to create a lasI knockout mutant through homologous recombination, generating LASI. Similarly, the rhlI (C4-HSL synthase gene) mutant was created by amplifying part of this gene (377 bp) using oligonucleotides rhlI-for and rhlI-rev and cloning it as a XbaI-BamHI fragment into pKNOCK-Gm to yield pKNOCK-rhlI, which was used to generate RHLI. The double mutant lasI rhlI was obtained by conjugating pKNOCK-rhlI into lasI mutant LASI, generating DMI (double mutant I). To generate the lasR (transcriptional regulator) mutant (LASR), first cosmid pIB103 was mutagenized using transposon Tn5 (as described in reference 32) with E. coli HB101::Tn5 as source of the transposon. Thereafter, the cosmids containing Tn5 were selected by conjugating them in strain P. putida WCS358, extracting, and transforming them in E. coli DH5α in order to conjugate them into the biosensor P. putida(pKRC12). Colonies that did not express the reporter gene were selected, and the position of Tn5 in the lasR gene was confirmed through arbitrary PCR as previously described (40) and sequencing. The cosmid harboring the Tn5 (pIB103::Tn5) carrying a Tn5 insertion in the lasR gene was homogenized with the corresponding target region of the genome of P. aeruginosa PUPa3 by a marker exchange procedure (8, 57). pPH1JI was used as the incoming IncP1-incompatible plasmid. The lasR rhlR (transcriptional regulators) double mutant (DMR) was created by amplifying part of the rhlR gene (266 bp) using oligonucleotides rhlR-for and rhlR-rev and cloning it as a BamHI- XbaI fragment into pKNOCK-Tc to yield pKNOCK-rhlR, and the latter was then conjugated into LASR to generate DMR (lasR rhlR double mutant). Finally, the rsaL (negative transcriptional regulator) mutant was created by amplifying part of the rsaL gene (126 bp) using oligonucleotides rsaL-for and rsaL-rev and cloning it as a BamHI- XbaI fragment into pKNOCK-Tc to yield pKNOCK-rsaL, and the latter was then conjugated into the P. aeruginosa wild-type strain to obtain the mutant RSAL. The fidelities of all marker exchange events were confirmed by Southern analysis (data not shown). All mutants were tested for their growth rate in LB and M9 minimal media and showed a behavior similar to the wild-type strain (data not shown).
In vivo models of infection. (i) Root colonization assay.
Root colonization assays were performed with the following strains: the wild-type P. aeruginosa PUPa3 (PUPA), the lasI derivative mutant (LASI), the lasR derivative mutant (LASR), the rhlI derivative mutant (RHLI), the double derivative mutant lasI rhlI (DMI), the double derivative mutant lasR rhlR (DMR), and the rsaL mutant derivative (RSAL).
Rice seeds (cultivar BALDO; kindly provided by the Ente Risi, Pavia, Italy) were first sterilized by soaking them in 5% sodium hypochlorite for 30 min and then washed six times (3 min for each wash) in sterile water. Then, under sterile conditions, each seed was placed in a 50-ml test tube containing 7.5 ml of half-diluted Hoagland solution (20) and placed at 30°C for germination. Six days later the germinated seedlings were treated with bacterial suspensions (108 CFU ml−1) for 10 min. The seedlings were subsequently transferred to new 50-ml test tubes containing sterile perlite and then filled with half-diluted Hoagland solution containing 108 CFU of P. aeruginosa PUPa3 inoculant for every gram of perlite present. After 1 day in a 30°C growth chamber the plants were transferred to a full-containment greenhouse for an additional 10 days. In the greenhouse natural daylight was supplemented with 500-W lamps with one lamp for 2 square meters and a 16-h/8-h light-dark cycle. The temperature was kept at 27°C and humidity at 70%. One day after the transfer to the greenhouse, a hole was made on the bottom of each test tube to allow drainage. From that moment onwards, 15 ml of sterile half-diluted Hoagland solution was added to each plant every other day. Eleven days after bacterial treatments, roots were harvested, separated from perlite, weighed, and finally ground in 5 ml physiological solution (0.85% NaCl). Serial dilutions were plated (on LB ampicillin), and CFU were counted on the following day after overnight incubation at 30°C. Values of root colonization are given as CFU g−1 of root. Four separate root colonization assays were performed. In the first experiment the strains tested were as follows: (i) PUPa3 wild-type strain, (ii) LASI (lasI mutant), and (iii) LASR (lasR mutant). In the second independent experiment the strains tested were (i) PUPa3, (ii) LASI (lasI mutant), and (iii) DMI (lasI rhlI double mutant). In the third experiment we tested (i) PUPa3, (ii) DMR (lasR rhlR double mutant), and (iii) RSAL (rsaL mutant). Finally in the fourth experiment we tested (i) PUPa3, (ii) LASI (lasI mutant), (iii) RHLI (rhlI mutant), (iv) DMI (lasI rhlI double mutant), (v) DMR (lasR rhlR double mutant), and (vi) RSAL (rsaL mutant). In the first two experiments we used 8 plant replicates per treatment, in the third experiment we used 8 plant replicates for the wild-type strain and 24 plants for each of the mutants, and in the fourth experiment we used 10 plant replicates for each bacterial treatment. The statistical significance of the differences between the wild type and mutant strains in root colonization ability was tested with an analysis of variance (ANOVA; with wild-type PUPa3, LASI [lasI], RHLI [rhlI], DMI [lasI rhlI], DMR [lasR rhlR], and RSAL [rsaL] as independent variables) and then the specific differences were tested using planned comparisons.
(ii) Caenorhabditis elegans and Galeria melonella killing assays.
Infection of G. mellonella larvae was performed as described previously (23, 47) with some modifications. Caterpillars in the final larval stage (Brumann or Hebeisen, Zürich, Switzerland) were stored in wooden shavings at 15°C and were used within 2 to 3 weeks. Bacterial overnight cultures grown in LB broth at 37°C were diluted 1:100 in 30 ml LB and grown to an optical density at 600 nm (OD600) of 0.3 to 0.9. Cultures were centrifuged, and pellets were resuspended in 10 mM MgSO4 (E. Merck, Dietikon, Switzerland), and the OD600 was adjusted to 1.0. A 10-μl aliquot of a 10−5 dilution of this suspension, corresponding to approximately 30 CFU/ml, was used to infect the larvae using a 1 ml syringe (BD Plastipak, Madrid, Spain) with a 27-gauge, 7/8 in. needle (Rose GmbH, Trier, Germany) via the hindmost proleg. Bacterial suspensions were supplemented with antibiotics when appropriate to prevent contamination. The injection area was disinfected with a cotton swab soaked in ethanol. Ten healthy, randomly chosen larvae were injected per strain and the infected larvae were stored in petri dishes at 30°C in the dark. To monitor killing of animals due to physical injury or infection, larvae were injected with 10 μl MgSO4 containing appropriate antibiotics. The number of dead larvae was scored 24 h postinfection. Dead larvae turned black as a result of melanization and did not respond to touch. Experiments with more than one dead larva in the control group were not considered and repeated. Data are mean values of at least three independent experiments.
C. elegans killing assays were performed essentially as described by Köthe et al. (26). Briefly, 100-μl overnight cultures of P. aeruginosa test strains were plated on nematode growth medium (NGM II) agar plates. After 24 h of incubation, approximately 20 to 40 hypochlorite-synchronized stage L4 larvae were transferred onto each test plate. The number of worms at the time of transfer was determined with a Stemi SV6 microscope (Zeiss, Oberkochen, Germany) at a magnification of 50×. Plates were then incubated at 20°C and scored for live worms at 72 h. E. coli OP50 was used as a negative control. Data are mean values of at least three independent experiments. Data were analyzed using ANOVA and planned comparisons.
Assays of motility and lipolytic, proteolytic, and antifungal activities.
The different phenotypes were tested on the wild-type P. aeruginosa PUPa3 strain and its various QS mutants. Swimming assays were performed on 0.3% LB agar plates while swarming assays were performed using M8 medium plates (M9 salts without NH4Cl [25] supplemented with 0.2% glucose and 0.05% glutamate and containing 0.5% agar) (39). The inoculation was performed with a sterile toothpick dipped in a bacterial suspension with an OD600 of 2.7. The swimming zone was measured after 24 h of incubation at 30°C, while swarming plates were incubated at 30°C overnight and then at room temperature for an additional 48 h.
Proteolytic and lipolytic activities were determined in the appropriate indicator plates as previously reported (22).
Screening for antifungal activity was performed on potato dextrose agar (PDA; Difco Laboratories, Detroit, MI) medium. Pathogens used were Fusarium graminearum, Fusarium verticillioides, Bipolaris oryzae, and Pyricularia grisea (obtained from F. Favaron, Department of Agriculture, University of Padova, Padua, Italy). A water suspension of the fungal conidia was inoculated in the melted PDA. The bacterial strains were grown to an OD600 of 1.5, and then 0.3 μl was spotted on the PDA plate containing the fungi. The plates were then grown at 25°C for approximately 1 week, during which the fungal mycelia completely covered the agar surface unless mycelial growth was inhibited by the bacteria, in which case we observed an inhibition halo surrounding the bacterial spot.
Nucleotide sequence accession numbers.
All DNA sequencing was performed either at the CRIBI center (University of Padova, Italy) or at Macrogen, and the nucleotide sequences were deposited in GenBank/EMBL/DDBJ. The Las-QS locus of P. aeruginosa PUPa3 is given as a 1,907-bp fragment of pIB103 under the accession number AM778435, and the Rhl-QS locus of P. aeruginosa PUPa3 is given as a 2,209-bp XhoI-EcoRV fragment of pIB101 under the accession number AM778436.
RESULTS
Identification of the lasI-lasR and rhlI-rhlR QS genes of P. aeruginosa PUPa3 and AHL production.
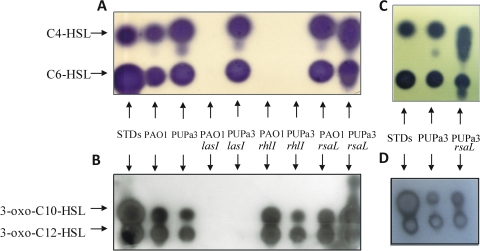
P. aeruginosa PUPa3, just like P. aeruginosa PAO1, was found to have two different AHL-dependent QS systems, namely, the LasI/R and the RhlI/R systems. P. aeruginosa PUPa3 produces 3-oxo-C12-HSL and its shorter acyl chain derivatives in addition to C4-HSL (Fig. 1A and B). As expected the lasI mutant of P. aeruginosa PUPa3 no longer produced 3-oxo-C12-HSL but retained the capability of producing the RhlI-synthesized C4-HSL. Similarly, the rhlI mutant no longer produced C4-HSL but was still able to synthesize 3-oxo-C12-HSL. These results were the first indication that in P. aeruginosa PUPa3 the two QS systems do not cross-regulate each other and thus act independently and not hierarchically, as is the case with P. aeruginosa PAO1. The P. aeruginosa PUPa3 lasI rhlI double mutant no longer produced any AHLs (data not shown). Similarly to the lasI-lasR system of P. aeruginosa PAO1, PUPa3 also contains a rsaL homologue in the intergenic region of these two genes, which is involved in negative regulation of the LasI/R system. The rsaL gene was found to affect AHL production of the LasI/R system, since the rsaL mutant produced approximately 100 times more LasI-synthesized AHLs (i.e., 3-oxo-C12-HSL and shorter-chain derivatives) while the production of AHLs by the Rhl system remained unchanged (Fig. 1C and D). The presence of two additional spots in the rsaL mutant in Fig. 1C is linked to the fact that 3-oxo-C6-HSL and 3-oxo-C8-HSL, which are produced by the Las system in high concentrations in the rsaL mutant, are also detected by the C. violaceum biosensor. In order to compare AHL thin-layer chromatography (TLC) profiles with that of the well-studied P. aeruginosa PAO1 strain, we performed a similar analysis using the PAO1 strain and AHL QS mutant derivatives. As depicted in Fig. 1A, unlike what was observed with strain PUPa3, in the lasI mutant of PAO1 no RhlI/R AHLs (i.e., C4- and C6-HSLs) were detected, indicating that under the growth conditions that we used, LasI/R is controlling the RhlI/R system (Fig. 1A and B). Another important difference between PAO1 and PUPa3 was the effect of the RsaL repressor on the production of the LasI/R AHLs. The rsaL mutant of PUPa3 resulted in considerable production of 3-oxo-C12-HSL and 3-oxo-C10-HSL, much more than what was observed with the rsaL mutant of PAO1 (Fig. 1B). This indicated that RsaL in PUPa3 was much more effective at repressing lasI gene expression than the RsaL in PAO1.
FIG. 1.
TLC analysis of AHLs produced by wild type and QS mutants. (A and B) Ethyl acetate extracts of P. aeruginosa strain PUPa3 wild type, PAO1 wild type, lasI mutants, rhlI mutants, and rsaL mutants of both PUPa3 and PAO1 strains. Standards (STDs) were synthetic C6-HSL (0.15 nmol) and C4-HSL (0.2 nmol) (for panels A and C) or 3-oxo-C12-HSL (4 nmol) and 3-oxo-C10-HSL (4 nmol) (for panels B and D). (C and D) Ethyl acetate extracts of P. aeruginosa strain PUPa3 WT and the rsaL mutant. For panels A and C TLCs were overlaid with the bacterial biosensor C. violaceum CV026; for panels B and D, TLCs were overlaid with the bacterial biosensor E. coli(pSB1075). In all the samples the equivalent of an extraction of 109 cells was run on the TLC, while for the rsaL mutant (only in panel D), the equivalent of 107 cells was run, since the rsaL mutant produces approximately 100 times more 3-oxo-C12-HSL and 3-oxo-C10-HSL.
Rice rhizosphere colonization by P. aeruginosa PUPa3 and QS mutant derivatives.
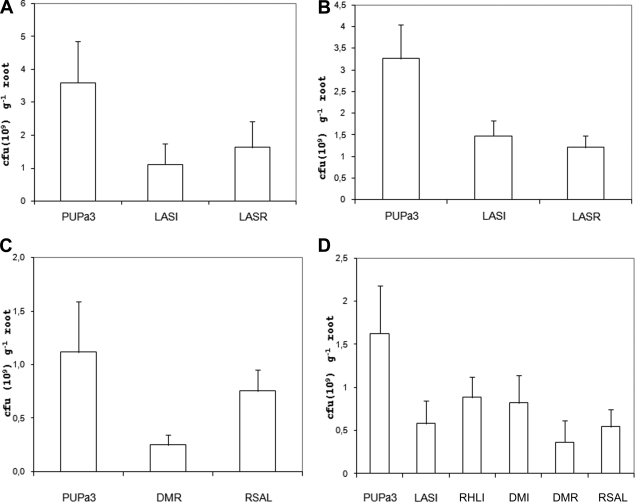
As PUPa3 was first isolated from the rhizosphere of rice roots, it was of interest to determine whether the ability to colonize and persist in the rhizosphere of roots was altered in the QS mutants compared to the parent strain. Therefore, we thoroughly and exhaustively investigated root colonization in four independent experiments with rice as host plant. From our first and second root colonization experiments it was evident that the lasI mutant, the lasR mutant, and the lasI rhlI double mutant (DMI) of P. aeruginosa PUPa3 were impaired in their root colonization abilities in comparison to their parent strain (Fig. 2A and B). These observations were supported by two separate statistical analyses performed on each experiment. The first ANOVA, including as independent variables the wild-type PUPa3, the lasI mutant LASI, and the lasR mutant LASR, yielded a significant main effect [F(2,21) = 15.56; P < 0.0001]. The root colonization ability was significantly higher in the wild-type strain relative to the two QS mutants [F(1,21) = 29.88; P < 0.0001]. The second ANOVA, including as independent variables the wild-type PUPa3, and the mutants LASI (lasI mutant) and DMI (lasI rhlI double mutant), yielded a significant main effect [F(2,21) = 36.25; P < 0.00001]. The root colonization ability was significantly higher in the wild-type strain relative to both the lasI mutant and DMI (lasI rhlI double mutant) [F(1,21) = 71.45; P < 0.00001]. A third experiment compared wild-type PUPa3 root colonization with that of the lasR rhlR double mutant (DMR) and RSAL (rsaL mutant). An ANOVA with wild-type PUPa3, DMR (lasR rhlR double mutant), and RSAL (rsaL mutant) as independent variables yielded a significant main effect [F(2,21) = 16.32; P < 0.0001]. The wild type colonized the root to a significantly higher extent [F(1,21) = 21.70; P < 0.0001] than the two mutants (Fig. 2C). The fourth experiment was done to further validate the results of the previous experiments and compared root colonization of the wild-type PUPa3 and mutants LASI (lasI mutant), RHLI (rhlI mutant), DMI (lasI rhlI double mutant), DMR (lasR rhlR double mutant), and RSAL (rsaL mutant). These experiments confirmed that the QS mutant strains all colonize rice roots to a lesser extent than the wild-type P. aeruginosa PUPa3 (Fig. 2D). The ANOVA yielded a significant main effect [F(5,54) = 18.47; P < 0.0001]. The colonization of the wild type was significantly higher than that of all mutant strains [F(1,54) = 75.72; P < 0.00001].
FIG. 2.
Root colonization assays of rice rhizosphere P. aeruginosa strain PUPa3 and QS mutant derivatives in four independent experiments (A to D). PUPa3, P. aeruginosa PUPa3 wild-type strain; LASI, lasI mutant; LASR, lasR mutant; RHLI, rhlI mutant; DMI, lasI rhlI double mutant; DMR, lasR rhlR double mutant; RSAL, rsaL mutant. The colonization of the wild-type strain was significantly higher than that of all QS mutant strains.
Pathogenicity of P. aeruginosa PUPa3 and QS mutant derivatives in C. elegans and G. melonella infection models.
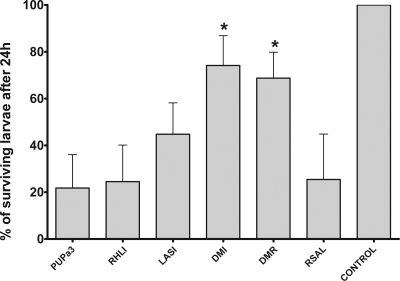
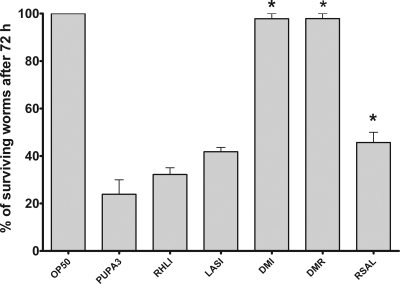
P. aeruginosa can infect several animals, including the nematode C. elegans (34) and larvae of the greater wax moth G. mellonella (37). It was of interest to determine whether this P. aeruginosa rhizosphere isolate is virulent for these two animals and if so whether QS plays a role in pathogenesis, as has been previously demonstrated for clinical isolates (for a review, see reference 33). P. aeruginosa PUPa3 was found to be very pathogenic for G. mellonella, with approximately 30 bacteria being sufficient to kill most larvae within 24 h. The ANOVA yielded a significant main effect [F(5,15) = 4.90; P < 0.01]. Virulence of the rhlI mutant RHLI was virtually indistinguishable from that of the wild type [F(1,3) = 0.35; P = 0.59], and the lasI mutant LASI virulence was found to be only slightly reduced, with a difference that was not significant [F(1,3) = 4.51; P = 0.12]. However, inactivation of both QS systems, as was the case with the two double mutants, greatly reduced pathogenicity [F(1,3) = 38.80; P < 0.01] (Fig. 3). When P. aeruginosa PUPa3 was used as a food source for C. elegans on NGM approximately 80% of the nematodes died within 72 h. The ANOVA yielded a significant main effect [F(5,10) = 37.72; P < 0.0001]. Inactivation of rhlI did not significantly reduce pathogenicity [F(1,2) = 1.22; P > 0.38], and inactivation of lasI only slightly reduced pathogenicity [just missed the significance level; F(1,2) = 17.38; P = 0.052], whereas both the DMI (lasI rhlI) and DMR (lasR rhlR) were avirulent [F(1,2) = 80.01; P < 0.05] (Fig. 4). These results show that for full virulence in the two infection models both QS systems have to be intact, and thus the results further demonstrate that the two systems are not hierarchically arranged.
FIG. 3.
Killing of G. mellonella larvae by P. aeruginosa PUPa3, the rhlI mutant RHLI, the lasI mutant LASI, the DMI (lasI rhlI) and DMR (lasR rhlR) double mutants, and the rsaL mutant RSAL. Larvae were infected with approximately 30 cells of the various P. aeruginosa strains and incubated in the dark at 30°C. Dead larvae were determined after 24 h. Data represent means and standard errors of three independent trials. The treatments that showed significant differences (using ANOVA; see text for details) from the wild-type P. aeruginosa PUPa3 strain were the two double mutants DMI and DMR and are indicated in the figure by an asterisk.
FIG. 4.
Killing of C. elegans by P. aeruginosa PUPa3, the rhlI mutant RHLI, the lasI mutant LASI, the DMI and DMR double mutants, and the rsaL mutant RSAL. P. aeruginosa strains were grown on NGM overnight, and 20 to 40 nematodes were then placed onto the plates. Surviving worms were counted after 72 h of incubation at 20°C. Data represent means and standard errors of three independent trials. The treatments that showed significant differences (using ANOVA; see text for details) from the wild-type P. aeruginosa PUPa3 strain were the two double mutants DMI and DMR and the rsaL mutant and are indicated in the figure by an asterisk.
Interestingly, the RsaL repressor of the LasI/R system did not play a role in virulence in the wax moth model [F(1,3) = 0.02; P = 0.88], whereas the P. aeruginosa rsaL mutant was slightly attenuated in pathogenicity against C. elegans [F(1,2) = 72.84; P < 0.05].
Role of QS regulation of motility and lipase, protease, and antifungal activities.
It was of interest to determine whether other important colonization-related phenotypes were regulated by QS in P. aeruginosa PUPa3. Motility assays were performed, and these showed that swimming motility of all the mutants tested (LASI, RHLI, DMI, DMR, and RSAL) was impaired relative to the wild type, implying that both the Las and the Rhl QS systems control this type of motility (Table 2). The wild-type phenotype was restored in the lasI mutant, the rhlI mutant, and lasI rhlI double mutant by addition of 3-oxo-C12-HSL, C4-HSL, and both these AHL molecules, respectively, to the medium. Swarming was found to be also positively QS regulated in P. aeruginosa PUPa3. The wild-type strain formed a typical star shape with several tendrils, the LASI (lasI) mutant swarmed but to a lesser extent than the wild type, the RHLI (rhlI) mutant swarmed even less than the LASI (lasI), and the double mutant DMI (lasI rhlI) did not swarm at all (data not shown). It was therefore evident that swarming in P. aeruginosa PUPa3 requires both the LasI/R and RhlI/R systems.
TABLE 2.
Phenotypes controlled by QS in P. aeruginosa wild type (PUPa3), mutants LASI, RHLI, DMI, DMR, and RSAL, and mutants complemented with AHLsa
| Strain | Activityb for strain
|
||
|---|---|---|---|
| SW (cm) | LI (mm) | PR (mm) | |
| PUPa3 | 7.67 ± 1.15 | 1.07 ± 0.15 | 3.33 ± 0.58 |
| LASI | 3.27 ± 0.90 | 0.50 ± 0.00 | 0.67 ± 0.29 |
| LASI + OC12 | 7.83 ± 1.26 | 0.97 ± 0.15 | 3.17 ± 0.29 |
| RHLI | 3.70 ± 1.85 | 0.47 ± 0.12 | 3.67 ± 0.58 |
| RHLI + C4 | 7.33 ± 2.89 | 0.80 ± 0.20 | 4.00 ± 1.00 |
| DMI | 2.37 ± 1.03 | 0 | 0.33 ± 0.58 |
| DMI + OC12 + C4 | 6.77 ± 3.04 | 0.73 ± 0.06 | 3.00 ± 0.00 |
| DMR | 2.43 ± 0.25 | 0 | 0 |
| RSAL | 3.07 ± 1.36 | 0.8 ± 0.10 | 3.00 ± 0.00 |
Abbreviations for AHLs: C4, C4-HSL; OC12, 3-oxo-C12-HSL.
SW, swimming activity; LI, lipase activity; PR, protease activity. Values are means ± standard deviations.
Secreted proteolytic and lipolytic activities were tested, and it was observed that P. aeruginosa PUPa3 displayed strong proteolytic activity which was reduced approximately sevenfold in both the lasI mutant and the lasI rhlI double mutant, while in the rhlI mutant the activity did not differ from that of the wild-type parent strain (Table 2). Hence, proteolytic activity appears to be only regulated by the LasI/R system and not by the RhlI/R system in P. aeruginosa PUPa3. Lipolytic activity, on the other hand, was reduced to approximately one-half in both the lasI and the rhlI mutants, and this activity was completely abolished in the lasI rhlI double mutant (Table 2). It was therefore concluded that lipolytic activity in P. aeruginosa PUPa3 is regulated cooperatively by the two QS systems.
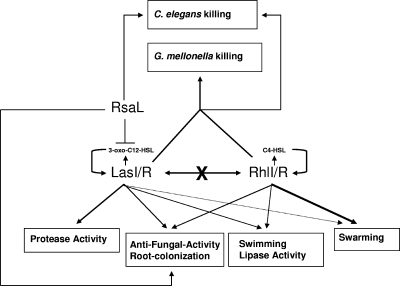
Antifungal activities were tested in wild-type P. aeruginosa PUPa3 and its QS mutant derivatives against four different fungi: Bipolaris oryzae, Fusarium graminearum, F. verticillioides, and Pyricularia grisea. The strongest antifungal activities were observed against B. oryzae and P. grisea, and in all cases the activities were found to be regulated by QS (Table 3). Figure 5 summarizes our results on QS-regulated phenotypes in P. aeruginosa PUPa3.
TABLE 3.
Antifungal activities of P. aeruginosa PUPa3 wild type and QS mutant derivatives on B. oryzae, F. graminearum, F. verticillioides, and P. grisea
| P. aeruginosa strain | Antifungal activity againsta:
|
|||
|---|---|---|---|---|
| B. oryzae | F. graminearum | F. verticillioides | P. grisea | |
| PUPA3 | +++ | ++ | ++ | +++ |
| LASI | + | + | + | +++ |
| RHLI | + | ++ | +/− | ++ |
| DMI | − | − | − | + |
| DMR | − | − | +/− | − |
| RSAL | − | + | + | + |
−, no inhibition halo; +/−, 2-mm halo; +, 4-mm halo; ++, 6-mm halo; +++, 8-mm halo.
FIG. 5.
Working model for the role of QS in P. aeruginosa PUPa3. Both QS systems positively regulate swimming, swarming (with the Rhl system being more important than the Las system), lipase, root colonization, and antifungal activity; the LasI/R system positively regulates protease activity, while RhlI/R does not regulate this activity. The two systems acting independently of each other and are not hierarchically organized. The LasI/R system produces 3-oxo-C12-HSL and undergoes positive autoregulation. The RhlI/R system produces C4-HSL and C6-HSL (C4 is the cognate AHL), and it also undergoes positive autoregulation. Both systems together are necessary for the infection of C. elegans and G. mellonella, whereas both independently are important for rhizosphere colonization. RsaL is a negative regulator of the LasI/R system and it is important for nematocidal killing, for antifungal activity, and for root colonization.
DISCUSSION
The most-studied P. aeruginosa strains are clinical isolates, mostly isolated from patients with cystic fibrosis or other immunodeficiency diseases. Considerably less information on the organism's genetics, molecular biology, and lifestyle is available from the environmental strains of P. aeruginosa. Pirnay et al. (41) studied the presence and types of P. aeruginosa in a river and found that the river community was almost as diverse as the global P. aeruginosa population; these data imply that rivers may be sources of distribution of potentially pathogenic P. aeruginosa strains. A recent study demonstrated the presence of P. aeruginosa strains also in the ocean and concluded from multilocus sequence typing analysis that these strains have diverged from other terrestrial isolates and form a distinct cluster. However, a different study that utilized multilocus sequence analysis on a large number of P. aeruginosa strains showed that the environmental isolates clustered with clinical isolates (9). This close relationship between clinical and environmental strains implies that the environment can be a reservoir for opportunistic human pathogens, especially the rhizosphere, where the characteristics that make a bacterial strain an efficient plant growth promoter (e.g., antagonistic properties, colonization ability) could also make it a threatening human opportunistic pathogen (4). P. aeruginosa has been isolated from roots of different plants, such as oilseed rape, potato, and rice (17, 27, 38, 55). However, few studies have specifically tested the abilities of these strains to act as root colonizers and plant growth promoters (13). De Vleesschauwer et al. (11) studied the mechanisms of induced systemic resistance in rice as a consequence of root colonization by P. aeruginosa strain 7NSK2. In contrast to the little information available on the beneficial interactions between environmental strains and plants, most published work thus far has focused on the ability of clinical isolates to cause disease in plants. For example, Walker et al. showed how P. aeruginosa PAO1 and PA14 colonized the roots of Arabidopsis and sweet basil, forming a biofilm and eventually killing the plants (59).
Our study analyzed for the first time the QS circuitry of an environmental P. aeruginosa strain, PUPa3, and in contrast to previous studies which demonstrated the importance of QS only for pathogenicity in various models, we showed also the involvement of QS in the regulation of beneficial traits, particularly for the interaction with the roots of rice plants. The two AHL QS systems of clinical isolate P. aeruginosa strain PAO1 (45) are organized in a hierarchical manner such that the LasI/R exerts transcriptional control over the RhlI/R system (29, 56). The Las and Rhl systems have, however, also been shown to be regulated by several environmental conditions, bringing into question the concept of an organized hierarchy between the two systems (12). Importantly, in contrast to what occurs in PAO1, in P. aeruginosa PUPa3 under the growth conditions we used, the LasI/R and RhlI/R QS systems are not hierarchically arranged but act independently and often autonomously regulate the same functions.
As P. aeruginosa PUPa3 was isolated from rice roots and had previously been described as a plant growth-promoting rhizobacteria (PGPR), we were interested in determining whether PGPR traits were under QS regulation. We therefore thoroughly tested root colonization of the different strains and showed that this trait is under positive QS regulation in P. aeruginosa PUPa3. This is similar to the results found for P. fluorescens 2P24, in which the QS mutant also showed a reduced capability for root colonization (60). Another report showing positive QS regulation of root colonization was for PGPR Pseudomonas chlororaphis strain 30-84; for this species it was suggested that production of phenazines, which are antibiotics produced by this strain, is under QS regulation. More recently it has been shown that phenazines may also have additional functions, such as being involved in attachment or for themselves serving as additional signals triggering factors important for biofilm formation on the root surface (31). Root colonization can be also negatively controlled by QS, as is the case for the rice rhizosphere Pseudomonas putida strain RD8MR3 (51).
In this study the rsaL repressor mutant of P. aeruginosa was tested for the first time in in vivo models. While rsaL is a negative regulator for the production of AHL signaling molecules, the effect of a mutation in this gene was similar to that of mutants in the AHL synthase genes, i.e., all mutants were impaired in their ability to colonize the roots. Therefore, it appears not only that signaling molecules have to be present for efficient root colonization but also that they have to be present in the correct concentration, and rsaL provides homeostasis by limiting AHL production to a physiological level, as had been shown previously in in vitro studies (42). RsaL may also interact directly with the promoters of genes linked to root colonization, as it affects gene expression also by acting as a transcriptional regulator independently of AHL production (42).
An important trait for root colonization in rhizosphere bacteria is motility, which allows the colonization of a larger area on the root surface. It was reported that enhanced motility of P. fluorescens F113 is an advantageous trait since hypermotile variants were selected during rhizosphere colonization (35). QS mutants of P. aeruginosa PUPa3 were partially impaired in swimming motility, hence the QS regulation of swimming motility was found to correlate with that of root colonization, possibly indicating that this type of movement may be important for efficient root colonization. Swarming is a different type of bacterial translocation, by which bacteria can spread as a biofilm over a surface. Similar to PAO1, swarming motility of P. aeruginosa PUPa3 was also found to be positively regulated by both QS systems. Another important characteristic of biocontrol bacteria is the ability to protect crops from fungal diseases through the production of antifungal compounds. P. aeruginosa PUPa3 possesses antifungal activity and QS was shown to be involved in its regulation. It was, however, observed that the QS systems involved in this regulation depended on the fungal species to which strain PUPa3 was exposed. This result suggests that the two QS systems may independently regulate the production of various factors having a different effect on diverse fungal species. Secreted proteases and lipases also contribute to the antifungal activity of bacterial rhizosphere biocontrol strains. P. aeruginosa PUPa3 exhibited lipolytic activity which was dependent on both QS systems. Proteolytic activity of this strain, on the other hand, was found to be regulated only by the LasI/R system. Other reports have demonstrated that lipases and proteases are also regulated by QS in Pseudomonas and Burkholderia species. However, it is not yet known whether this occurs directly or indirectly (2, 10, 62).
Kumar et al. (28) proposed P. aeruginosa PUPa3 as an isolate for potential use as a biofertilizer and antagonist against phytopathogenic fungi, as it had PGPR traits and was isolated from the rhizosphere. In this study we tested whether a P. aeruginosa PUPa3 isolate beneficial to plants was pathogenic in other models and therefore could be a threat as an opportunistic human pathogen. Importantly, it was established that P. aeruginosa PUPa3 was pathogenic in both the C. elegans and the G. mellonella wax moth model, indicating that it may behave as an opportunistic human pathogen. In addition, we established that QS was important for virulence in these two models. In both models significant attenuation in pathogenicity was only observed when both QS systems were inactivated, implying that both are independently involved in the regulation of virulence factors. This is interesting, as it could indicate that the virulence factors are independently regulated by the two systems and that only with both QS systems being nonfunctional would their levels be low enough to result in a significant decrease in pathogenicity. This is what occurs with lipase activity, since it is reduced to approximately one-half when either the Rhl or Las systems are inactivated and disappears when both systems are knocked out. The RsaL repressor mutant on the other hand did not affect pathogenicity of strain PUPa3 in the G. mellonella model, whereas it was somewhat less infectious in C. elegans. RsaL is therefore involved in the regulation of virulence factors for C. elegans infection; importantly, it has been shown that RsaL can regulate expression of certain genes independently of QS (42). In summary, the QS systems of the beneficial rhizosphere P. aeruginosa strain PUPa3 were shown to act independently, and they are important for rhizosphere colonization and act in concert for animal virulence (Fig. 5 summarizes the role of LasI/R and RhlI/R in strain PUPa3). This study also highlights that the role and mode of action of QS systems in P. aeruginosa may vary in different strains, since several aspects of QS observed in strain PUPa3 are different from what is known for P. aeruginosa PAO1.
Acknowledgments
We gratefully thank N. Sakthivel for providing the rice rhizosphere Pseudomonas aeruginosa PUPa3 strain. We thank Zulma R. Suarez-Moreno for assistance in preparing the figures.
L.S. was supported by an ICGEB fellowship. I.B. was supported by a fellowship from the Italian Cystic Fibrosis Research Foundation (project FFC no. 9/2007) with the contribution of the Delegazione FFC di Belluno. L.D.S. was supported by a fellowship from the Accademia Nazionale dei Lincei (Rome, Italy). V.V.'s laboratory in the ICGEB Biosafety Outstation in Ca'Tron is also supported by the Fondazione Cassamarca, Treviso, Italy.
Footnotes
Published ahead of print on 12 June 2009.
REFERENCES
- 1.Alexeyev, M. F. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. BioTechniques 26:824-826, 828. [DOI] [PubMed] [Google Scholar]
- 2.Arevalo-Ferro, C., M. Hentzer, G. Reil, A. Gorg, S. Kjelleberg, M. Givskov, K. Riedel, and L. Eberl. 2003. Identification of quorum-sensing regulated proteins in the opportunistic pathogen Pseudomonas aeruginosa by proteomics. Environ. Microbiol. 5:1350-1369. [DOI] [PubMed] [Google Scholar]
- 3.Beatson, S. A., C. B. Whitchurch, A. B. Semmler, and J. S. Mattick. 2002. Quorum sensing is not required for twitching motility in Pseudomonas aeruginosa. J. Bacteriol. 184:3598-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, G., L. Eberl, and A. Hartmann. 2005. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 7:1673-1685. [DOI] [PubMed] [Google Scholar]
- 5.Better, M., B. Lewis, D. Corbin, G. Ditta, and D. R. Helinski. 1983. Structural relationships among Rhizobium meliloti symbiotic promoters. Cell 35:479-485. [DOI] [PubMed] [Google Scholar]
- 6.Birnboim, H. C. 1983. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 100:243-255. [DOI] [PubMed] [Google Scholar]
- 7.Cabrol, S., A. Olliver, G. B. Pier, A. Andremont, and R. Ruimy. 2003. Transcription of quorum-sensing system genes in clinical and environmental isolates of Pseudomonas aeruginosa. J. Bacteriol. 185:7222-7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbin, D., G. Ditta, and D. R. Helinski. 1982. Clustering of nitrogen fixation (nif) genes in Rhizobium meliloti. J. Bacteriol. 149:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran, B., D. Jonas, H. Grundmann, T. Pitt, and C. G. Dowson. 2004. Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J. Clin. Microbiol. 42:5644-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devescovi, G., J. Bigirimana, G. Degrassi, L. Cabrio, J. J. Lipuma, J. Kim, I. Hwang, and V. Venturi. 2007. Involvement of a quorum-sensing-regulated secreted lipase A clinical isolate of Burkholderia glumae in severe disease symptoms in rice. Appl. Environ. Microbiol. 73:4950-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vleesschauwer, D., P. Cornelis, and M. Hofte. 2006. Redox-active pyocyanin secreted by Pseudomonas aeruginosa 7NSK2 triggers systemic resistance to Magnaporthe grisea but enhances Rhizoctonia solani susceptibility in rice. Mol. Plant-Microbe Interact. 19:1406-1419. [DOI] [PubMed] [Google Scholar]
- 12.Duan, K., and M. G. Surette. 2007. Environmental regulation of Pseudomonas aeruginosa PAO1 Las and Rhl quorum-sensing systems. J. Bacteriol. 189:4827-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egamberdieva, D., F. Kamilova, S. Validov, L. Gafurova, Z. Kucharova, and B. Lugtenberg. 2008. High incidence of plant growth-stimulating bacteria associated with the rhizosphere of wheat grown on salinated soil in Uzbekistan. Environ. Microbiol. 10:1-9. [DOI] [PubMed] [Google Scholar]
- 14.Erickson, D. L., R. Endersby, A. Kirkham, K. Stuber, D. D. Vollman, H. R. Rabin, I. Mitchell, and D. G. Storey. 2002. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 70:1783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuqua, C., and E. P. Greenberg. 2002. Listening in on bacteria: acyl-homoserine lactone signalling. Nat. Rev. Mol. Cell Biol. 3:685-695. [DOI] [PubMed] [Google Scholar]
- 17.Graner, G., P. Persson, J. Meijer, and S. Alstrom. 2003. A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol. Lett. 224:269-276. [DOI] [PubMed] [Google Scholar]
- 18.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 19.Hentzer, M., L. Eberl, J. Nielsen, and M. Givskov. 2003. Quorum sensing: a novel target for the treatment of biofilm infections. BioDrugs 17:241-250. [DOI] [PubMed] [Google Scholar]
- 20.Hoagland, D. R., and D. I. Arnon. 1950. The water-culture method for growing plants without soil. Calif. Agric. Exp. Station Circular 347:1-39. [Google Scholar]
- 21.Holloway, B. W. 1955. Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 13:572-581. [DOI] [PubMed] [Google Scholar]
- 22.Huber, B., K. Riedel, M. Hentzer, A. Heydorn, A. Gotschlich, M. Givskov, S. Molin, and L. Eberl. 2001. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology 147:2517-2528. [DOI] [PubMed] [Google Scholar]
- 23.Jander, G., L. G. Rahme, and F. M. Ausubel. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 182:3843-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 25.Kohler, T., L. K. Curty, F. Barja, C. van Delden, and J. C. Pechere. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kothe, M., M. Antl, B. Huber, K. Stoecker, D. Ebrecht, I. Steinmetz, and L. Eberl. 2003. Killing of Caenorhabditis elegans by Burkholderia cepacia is controlled by the cep quorum-sensing system. Cell. Microbiol. 5:343-351. [DOI] [PubMed] [Google Scholar]
- 27.Krechel, A., A. Faupel, J. Hallmann, A. Ulrich, and G. Berg. 2002. Potato-associated bacteria and their antagonistic potential towards plant-pathogenic fungi and the plant-parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood. Can. J. Microbiol. 48:772-786. [DOI] [PubMed] [Google Scholar]
- 28.Kumar, R. S., N. Ayyadurai, P. Pandiaraja, A. V. Reddy, Y. Venkateswarlu, O. Prakash, and N. Sakthivel. 2005. Characterization of antifungal metabolite produced by a new strain Pseudomonas aeruginosa PUPa3 that exhibits broad-spectrum antifungal activity and biofertilizing traits. J. Appl. Microbiol. 98:145-154. [DOI] [PubMed] [Google Scholar]
- 29.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 30.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051-1060. [DOI] [PubMed] [Google Scholar]
- 31.Maddula, V. S., Z. Zhang, E. A. Pierson, and L. S. Pierson III. 2006. Quorum sensing and phenazines are involved in biofilm formation by Pseudomonas chlororaphis (aureofaciens) strain 30-84. Microb. Ecol. 52:289-301. [DOI] [PubMed] [Google Scholar]
- 32.Magazin, M. D., J. C. Moores, and J. Leong. 1986. Cloning of the gene coding for the outer membrane receptor protein for ferric pseudobactin, a siderophore from a plant growth-promoting Pseudomonas strain. J. Biol. Chem. 261:795-799. [PubMed] [Google Scholar]
- 33.Mahajan-Miklos, S., L. G. Rahme, and F. M. Ausubel. 2000. Elucidating the molecular mechanisms of bacterial virulence using non-mammalian hosts. Mol. Microbiol. 37:981-988. [DOI] [PubMed] [Google Scholar]
- 34.Mahajan-Miklos, S., M. W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47-56. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Granero, F., R. Rivilla, and M. Martin. 2006. Rhizosphere selection of highly motile phenotypic variants of Pseudomonas fluorescens with enhanced competitive colonization ability. Appl. Environ. Microbiol. 72:3429-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClean, K. H., M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 37.Miyata, S., M. Casey, D. W. Frank, F. M. Ausubel, and E. Drenkard. 2003. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 71:2404-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales, A., J. L. Garland, and D. V. Lim. 1996. Survival of potentially pathogenic human-associated bacteria in the rhizosphere of hydroponically grown wheat. FEMS Microbiol. Ecol. 20:155-162. [DOI] [PubMed] [Google Scholar]
- 39.Murray, T. S., and B. I. Kazmierczak. 2006. FlhF is required for swimming and swarming in Pseudomonas aeruginosa. J. Bacteriol. 188:6995-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 41.Pirnay, J. P., S. Matthijs, H. Colak, P. Chablain, F. Bilocq, J. Van Eldere, D. De Vos, M. Zizi, L. Triest, and P. Cornelis. 2005. Global Pseudomonas aeruginosa biodiversity as reflected in a Belgian river. Environ. Microbiol. 7:969-980. [DOI] [PubMed] [Google Scholar]
- 42.Rampioni, G., M. Schuster, E. P. Greenberg, I. Bertani, M. Grasso, V. Venturi, E. Zennaro, and L. Leoni. 2007. RsaL provides quorum sensing homeostasis and functions as a global regulator of gene expression in Pseudomonas aeruginosa. Mol. Microbiol. 66:1557-1565. [DOI] [PubMed] [Google Scholar]
- 43.Riedel, K., M. Hentzer, O. Geisenberger, B. Huber, A. Steidle, H. Wu, N. Hoiby, M. Givskov, S. Molin, and L. Eberl. 2001. N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 147:3249-3262. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 45.Schuster, M., and E. P. Greenberg. 2006. A network of networks: quorum-sensing gene regulation in Pseudomonas aeruginosa. Int. J. Med. Microbiol. 296:73-81. [DOI] [PubMed] [Google Scholar]
- 46.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seed, K. D., and J. J. Dennis. 2008. Development of Galleria mellonella as an alternative infection model for the Burkholderia cepacia complex. Infect. Immun. 76:1267-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith, R. S., and B. H. Iglewski. 2003. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 49.Staskawicz, B., D. Dahlbeck, N. Keen, and C. Napoli. 1987. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J. Bacteriol. 169:5789-5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steidle, A., K. Sigl, R. Schuhegger, A. Ihring, M. Schmid, S. Gantner, M. Stoffels, K. Riedel, M. Givskov, A. Hartmann, C. Langebartels, and L. Eberl. 2001. Visualization of N-acylhomoserine lactone-mediated cell-cell communication between bacteria colonizing the tomato rhizosphere. Appl. Environ. Microbiol. 67:5761-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steindler, L., I. Bertani, L. De Sordi, J. Bigirimana, and V. Venturi. 2008. The presence, type and role of N-acyl homoserine lactone quorum sensing in fluorescent Pseudomonas originally isolated from rice rhizospheres are unpredictable. FEMS Microbiol. Lett. 288:102-111. [DOI] [PubMed] [Google Scholar]
- 52.Steindler, L., G. Devescovi, S. Subramoni, and V. Venturi. 2008. A versatile plasmid biosensor useful to identify quorum sensing LuxR-family orphans in bacterial strains. J. Microbiol. Methods 73:273-275. [DOI] [PubMed] [Google Scholar]
- 53.Steindler, L., and V. Venturi. 2007. Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiol. Lett. 266:1-9. [DOI] [PubMed] [Google Scholar]
- 54.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 55.Tripathi, A. K., S. C. Verma, and E. Z. Ron. 2002. Molecular characterization of a salt-tolerant bacterial community in the rice rhizosphere. Res. Microbiol. 153:579-584. [DOI] [PubMed] [Google Scholar]
- 56.Venturi, V. 2006. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol. Rev. 30:274-291. [DOI] [PubMed] [Google Scholar]
- 57.Venturi, V., F. Zennaro, G. Degrassi, B. C. Okeke, and C. V. Bruschi. 1998. Genetics of ferulic acid bioconversion to protocatechuic acid in plant-growth-promoting Pseudomonas putida WCS358. Microbiology 144:965-973. [DOI] [PubMed] [Google Scholar]
- 58.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker, T. S., H. P. Bais, E. Deziel, H. P. Schweizer, L. G. Rahme, R. Fall, and J. M. Vivanco. 2004. Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation, and root exudation. Plant Physiol. 134:320-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei, H. L., and L. Q. Zhang. 2006. Quorum-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie van Leeuwenhoek 89:267-280. [DOI] [PubMed] [Google Scholar]
- 61.Winson, M. K., S. Swift, L. Fish, J. P. Throup, F. Jorgensen, S. R. Chhabra, B. W. Bycroft, P. Williams, and G. S. Stewart. 1998. Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol. Lett. 163:185-192. [DOI] [PubMed] [Google Scholar]
- 62.Wopperer, J., S. T. Cardona, B. Huber, C. A. Jacobi, M. A. Valvano, and L. Eberl. 2006. A quorum-quenching approach to investigate the conservation of quorum-sensing-regulated functions within the Burkholderia cepacia complex. Appl. Environ. Microbiol. 72:1579-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanisch-Peron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-109. [DOI] [PubMed] [Google Scholar]