Abstract
Bradyrhizobium japonicum, a symbiotic nitrogen-fixing soil bacterium, has multiple gene copies for aromatic degradation on the genome and is able to use low concentrations of vanillate, a methoxylated lignin monomer, as an energy source. A transcriptome analysis indicated that one set of vanA1B, pcaG1H1, and genes for C1 compound catabolism was upregulated in B. japonicum USDA110 cells grown in vanillate (N. Ito, M. Itakura, S. Eda, K. Saeki, H. Oomori, T. Yokoyama, T. Kaneko, S. Tabata, T. Ohwada, S. Tajima, T. Uchiumi, E. Masai, M. Tsuda, H. Mitsui, and K. Minamisawa, Microbes Environ. 21:240-250, 2006). To examine the functions of these genes in vanillate degradation, we tested cell growth and substrate consumption in vanA1B, pcaG1H1, and mxaF mutants of USDA110. The vanA1B and pcaG1H1 mutants were unable to grow in minimal media containing 1 mM vanillate and protocatechuate, respectively, although wild-type USDA110 was able to grow in both media, indicating that the upregulated copies of vanA1B and pcaG1H1 are exclusively responsible for vanillate degradation. Mutating mxaF eliminated expression of gfa and flhA, which contribute to glutathione-dependent C1 metabolism. The mxaF mutant had markedly lower cell growth in medium containing vanillate than the wild-type strain. In the presence of protocatechuate, there was no difference in cell growth between the mxaF mutant and the wild-type strain. These results suggest that the C1 pathway genes are required for efficient vanillate catabolism. In addition, wild-type USDA110 oxidized methanol, whereas the mxaF mutant did not, suggesting that the metabolic capability of the C1 pathway in B. japonicum extends to methanol oxidation. The mxaF mutant showed normal nodulation and N2 fixation phenotypes with soybeans, which was not similar to symbiotic phenotypes of methylotrophic rhizobia.
Naturally occurring aromatics are important sources of energy and carbon for soil-dwelling microorganisms. Lignin represents an abundant carbon constituent of the vascular plant cell wall. Soluble phenolic lignin monomers, such as vanillate, are released by complex oxidative cleavage by some fungi (16). Indeed, vanillate and other lignin monomers have been found to be crucial components of dissolved organic matter in terrestrial (21) and marine (20) environments.
Bradyrhizobium japonicum, a symbiotic nitrogen-fixing soil bacterium, is able to aerobically catabolize low concentrations of aromatic compounds, such as vanillate and protocatechuate (3, 12, 24). Although many redundant copies of genes encoding proteins involved in aromatic degradation are scattered over nine loci of the B. japonicum genome, a previous transcriptome analysis (12) showed that vanillate and protocatechuate markedly upregulated the expression of only one set of oxygenase genes, pcaG1H1 and vanA1B (Fig. 1).
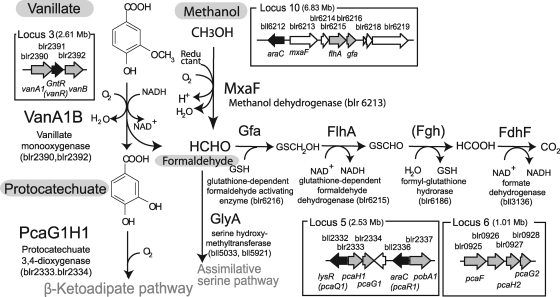
FIG. 1.
Organization of genes and pathways in the genome of B. japonicum strain USDA110 for the degradation of vanillate (adapted from reference 12). vanA1B and pcaG1H1 were exclusively expressed in vanillate-fed cells of B. japonicum USDA110 (12). The glutathione-dependent pathway from formaldehyde to CO2 was derived from previous transcriptome data that included locus 10 (see the text). mxaF (blr6213) encodes a putative methanol dehydrogenase at locus 10 (12). The numbers in parentheses in the loci show genomic positions on B. japonicum USDA110 (14). GSH, glutathione.
This previous transcriptome analysis also indicated that genes for glutathione-dependent formaldehyde oxidation were highly expressed in B. japonicum cells grown in vanillate (12) (Fig. 1). The bacterial pathway for aerobic degradation of aromatic compounds has been investigated extensively (8). Although vanillate is demethylated to yield protocatechuate and formaldehyde (32) (Fig. 1), little is known about the fate of the methoxy group during degradation of methoxylated aromatics (10, 19, 32).
In Burkholderia cepacia, a bacterium that grows on several lignin monomers, the formaldehyde-fixing enzymes play important roles in the scavenging and assimilatory fixation of formaldehyde during vanillate degradation (18). A growth phase-specific activation of C1 compound metabolism has also been observed in Burkholderia xenovorans LB400 during the degradation of polychlorinated biphenyl, although the involvement of C1 metabolism in that degradation process remains unclear (4).
In this study, we examined the functions of the vanA1B, pcaG1H1, and mxaF genes during vanillate degradation by disrupting the B. japonicum USDA110 genes that were upregulated in the transcriptome analysis (12). The goal was to address whether the upregulated genes vanA1B and pcaG1H1 are responsible for the degradation of vanillate and protocatechuate, a vanillate degradation product, in the bacterium. We also examined whether C1 compound catabolism contributes to vanillate degradation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used are listed in Table 1. Bradyrhizobium cells were grown at 30°C in HM salt medium (2, 12) supplemented with 0.1% (wt/vol) arabinose and 0.025% (wt/vol) yeast extract (Difco, Detroit, MI) for preculture. For feeding of aromatic compounds or succinate, the cells were grown in a defined mineral medium that was a minimal medium for bradyrhizobia (12, 24). Cells in liquid media were cultured with reciprocal shaking at 300 rpm. Growth was monitored by measuring absorbance at a wavelength of 660 nm with a spectrophotometer (UV-1200; Shimazu, Kyoto, Japan). Escherichia coli cells were grown at 37°C in Luria-Bertani medium (28). Antibiotics were added to the media at the following concentrations: for B. japonicum, 100 μg/ml of tetracycline, 100 μg/ml of spectinomycin, 100 μg/ml of streptomycin, 100 μg/ml of kanamycin, and 50 μg/ml of polymyxin B; for E. coli, 15 μg/ml of tetracycline, 50 μg/ml of spectinomycin, 50 μg/ml of streptomycin, 50 μg/ml of kanamycin, and 100 μg/ml of ampicillin.
TABLE 1.
Bacterial strains and plasmid used in this study
| Strain or plasmid | Characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| B. japonicum | ||
| USDA110 | Wild-type strain | 14 |
| vanA1B mutant | USDA110 vanA1B::del/ins Ω cassette; Smr Spr | This study |
| pcaG1H1 mutant | USDA110 pcaG1H1::del/ins Ω cassette; Smr Spr | This study |
| mxaF mutant | USDA110 mxaF::del/ins Ω cassette; Smr Spr | This study |
| E. coli | ||
| JM109 | recA cloning strain | Toyobo Inc.b |
| Plasmids | ||
| pRK2013 | ColE1 replicon carrying RK2 transfer genes; Kmr | 6 |
| pHP45Ω | Plasmid carrying 2.1-kb Ω cassette; Spr Smr Apr | 25 |
| pK18mob | Cloning vector; pMB1 oriT; Kmr | 31 |
| pK19mob | Cloning vector; pMB1 oriT; Kmr | 31 |
| brp14282 | pUC18 carrying vanA1B gene cluster; Apr | 14 |
| brp01186 | pUC18 carrying pcaG1H1 gene cluster; Apr | 14 |
| brp12520 | pUC18 carrying mxaF gene cluster; Apr | 14 |
| pBI01 | pK19mob carrying 4.5-kb vanA1B fragment; Kmr | This study |
| pBI07 | pK19mob carrying 3.9-kb vanA1B::del/ins Ω cassette; Smr Spr Kmr | This study |
| pJN001 | pK18mob carrying 3.7-kb pcaG1H1 fragment; Kmr | This study |
| pJN002 | pK18mob carrying 5.8-kb pcaG1H1::del/ins Ω cassette; Smr Spr Kmr | This study |
| pJN003 | pK18mob carrying 4-kb mxaF fragment; Kmr | This study |
| pJN004 | pK18mob carrying 6-kb mxaF::del/ins Ω cassette; Smr Spr Kmr | This study |
Apr, ampicillin resistant; Kmr, kanamycin resistant; Smr, streptomycin resistant; Spr spectinomycin resistant.
Toyobo Inc., Tokyo, Japan.
DNA manipulations.
Isolation of plasmids, DNA ligation, and transformation of E. coli were performed as described by Sambrook et al. (28). Genomic DNA was extracted from B. japonicum by using an AquaPure Genomic DNA kit (Bio-Rad Laboratories, Hercules, CA). Southern hybridization was carried out as described previously (30).
Construction of B. japonicum USDA110 mutants.
An NruI/HindIII fragment (4.5 kb) of plasmid brp14282 containing vanA1B was inserted into pK19mob to generate pB101 (Table 1). The Ω cassette (2.1 kb) from pHP45 was inserted into the XhoI/BstX1 sites of pB101, resulting in pB107. An XhoI fragment (3.7-kb) of brp001186 containing the pcaG1H1gene and a MunI/BglII fragment (4 kb) of brp12520 containing the mxaF gene were inserted into the SalI and EcoRI/BamHI sites of pK18mob to generate pJN001 and pJN003, respectively. The Ω cassette was inserted into the Eco0109I site of pJN001 and the NotI/SfiI sites of pJN003, resulting in pJN002 and pJN004, respectively. Triparental mating was conducted on HM agar plates using E. coli pRK2013 as a helper (29). The double-crossover events were verified by Southern hybridization.
Quantification of gene expression.
Primers for real-time reverse transcription (RT)-PCR of blr6215 (flhA) and blr6216 (gfa) were designed using Beacon Designer software (Premier Biosoft International, Palo Alto, CA). The primer sequences were as follows: blr6215L, 5′-GCCAGAAGACCAATCTCTGC; blr6215R, 5′-AAGTTCGAGAAGGTCGAGCA; blr6216L, 5′-GACTATTGCACTCCACCCATC; and blr6216R, 5′-GACCTGATGGTCCTTGCATT. The sigA gene (bll7349) was used as a control for quantification. PCR primers bll7349L (5′-GAGAACCAGATGTCGCTTGC) and bll7349R (5′-TGGATGTCCTGCTCCTGAAG) were used for RT-PCR of sigA. Total RNA was prepared as described previously (9). RT-PCR was carried out with the i-Cycler optical system (Bio-Rad Laboratories, Inc., Tokyo, Japan) as described previously (12, 29).
Gas chromatography.
The methanol concentration in culture was determined with a gas chromatograph (GC7A; Shimadzu, Kyoto, Japan) equipped with a Porapak Q column (80/100 mesh; diameter, 0.3 mm; length, 1 m) and a flame ionization detector (26). The temperature of injection was 150°C, and the temperature of the column was 185°C. The flow rate of the carrier gas (N2) was 60 ml/min. After the culture was centrifuged at 18,000 × g for 5 min at 4°C, an aliquot (3 μl) of the sample was directly injected into the gas chromatograph.
HPLC analysis.
Vanillate and protocatechuate in cultures were measured with a high-performance liquid chromatography (HPLC) apparatus (LC-10AD; Shimadzu, Kyoto, Japan) equipped with a reverse-phase column (ODS-80T; GL Sciences Inc., Tokyo, Japan) and UV detector (254 nm). The isotonic mobile phase consisted of methanol, water, and acetic acid (30:70:1 [vol/vol]). The flow rate was 0.9 ml min−1. After the culture was centrifuged at 18,000 × g for 5 min at 25°C, an aliquot (5 μl) of sample was injected directly into the HPLC system.
Resting-cell experiment.
Cells were grown in liquid minimum medium supplemented with 1 mM vanillate. The cells were harvested by centrifugation at 3,500 × g for 15 min at 4°C and washed twice with the medium. The washed cells were suspended in the medium at 1010 cells/ml and incubated at 30°C in the presence of 1 mM methanol. An aliquot of the cell suspension was analyzed with a gas chromatograph.
Plant test.
Surface-sterilized soybean seeds (Glycine max cv. Enrei) were germinated and transplanted into Leonard jars that contained sterile vermiculite and nitrogen-free nutrient solution (23). B. japonicum inoculation and plant cultivation were carried out as described previously (23). Plant phenotypes were observed 4 weeks after inoculation.
RESULTS
Growth and metabolism of vanA1B and pcaG1H1 mutants.
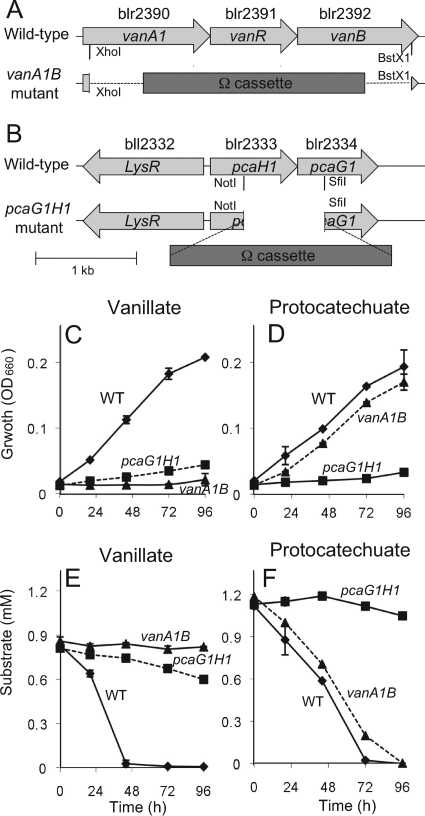
To examine whether the pcaG1H1, vanA1B, and mxaF genes in B. japonicum are responsible for vanillate degradation, we constructed pcaG1H1, vanA1B, and mxaF deletion mutants of B. japonicum USDA110 (Table 1). vanA1B and pcaG1H1 mutants (Fig. 2A and B) and wild-type USDA110 were grown in minimum medium supplemented with 1 mM of vanillate or protocatechuate, the concentration found to be optimal in this study (see Fig. S1 in the supplemental material). The vanA1B and pcaG1H1 mutants did not grow in minimum medium containing vanillate and protocatechuate, respectively (Fig. 2C and D), although the wild-type strain of USDA110 was able to grow in these media (Fig. 2C and D). The mutants showed normal growth similar to that of the wild-type strain in a minimum medium supplemented with 1 mM succinate (data not shown).
FIG. 2.
Growth and substrate consumption in vanA1B and pcaG1H1 mutants of B. japonicum USDA110. (A and B) Physical maps of vanA1B (A) and pcaG1H1 (B) deletion mutants. Cell growth was monitored by measuring turbidity (optical density at 660 nm [OD660]). (C and F) The B. japonicum cells were grown in minimal medium supplemented with vanillate (C and E) or with protocatechuate (D and F). The concentrations of vanillate (E) and protocatechuate (F) in culture were determined by HPLC. The error bars indicate standard deviations of triplicate determinations. WT, wild type. Dashed lines indicate cross-combinations of mutated genes and substrate (pcaG1H1 mutant in vanillate-amended medium [E], vanA1B mutant in protocatechuate-amended medium [F]).
The vanA1B and pcaG1H1 mutants did not consume vanillate and protocatechuate, respectively (Fig. 2E and F), although wild-type USDA110 cells consumed these aromatic substrates during cell growth (Fig. 2E and F). This result clearly indicates that the vanA1B and pcaG1H1 genes upregulated in the previous transcriptome analysis (12) are exclusively responsible for vanillate degradation in B. japonicum. In the presence of protocatechuate, the vanA1B mutant showed normal growth profiles (Fig. 2D and F) that were similar to wild-type USDA110, supporting the proposed pathway of vanillate degradation in B. japonicum USDA110 (Fig. 1).
Expression of flhA and gfa genes in the mxaF mutant.
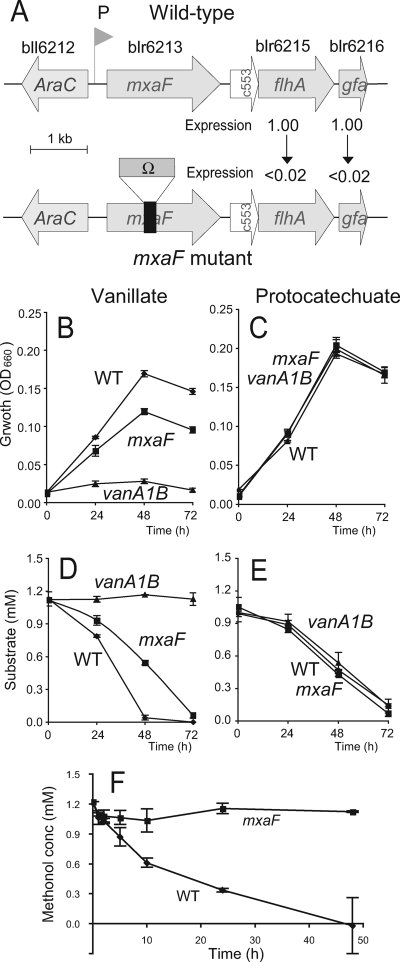
The flhA and gfa genes, which likely function in the glutathione-dependent C1 pathway (Fig. 1), were also upregulated in the cells grown in vanillate (12). Although we tried to construct flhA and gfa mutants, the double-crossover steps needed for the mutant construction failed several times. However, on the basis of their DNA sequences, it is likely that mxaF, flhA, and gfa form a transcriptional unit from a single putative promoter (Fig. 3A). We therefore constructed an mxaF mutant with the omega cassette, an interposon with a strong polar effect (25), and expected that RNA and protein synthesis would be terminated beyond the site of insertion (Fig. 3). RT-PCR analysis indicated that the expression of flhA and gfa genes was drastically downregulated in the maxF mutant compared with the wild-type USDA110 (Fig. 3A). Thus, the maxF mutant can also be used instead of the flhA and gfa mutants.
FIG. 3.
Growth and methanol oxidation of the mxaF deletion mutant of B. japonicum USDA110. (A) mxaF gene cluster in wild-type strain USDA110 (top) and its mxaF deletion mutant (bottom). Ω indicates the omega cassette from pHP45Ω (Table 1). Expression, the relative expression levels, determined with RT-PCR, of the flhA and gfa genes in the maxF mutant compared with wild-type USDA110 based on sigA expression (see the text). c553 (blr6214) is a homolog of cytochrome c-553 of Paracoccus denitrificans and Methylococcus capsulatus. Other genes are shown in locus 10 in Fig. 1. (B to E) The wild-type strain (WT), mxaF mutant, and vanA1B1 mutant of B. japonicum USDA110 cells were precultured in HM medium and then simultaneously transferred to minimal medium with vanillate (B and D) or with protocatechuate (C and E). Cell growth was monitored by turbidity (optical density at 660 nm [OD660]) (B and C). The concentrations of vanillate (D) and protocatechuate (E) in culture were determined by HPLC. (F) Methanol-oxidizing activities of the wild-type strain and the mxaF mutant of B. japonicum USDA110. Resting cells of wild-type USDA110 and mxaF mutant cells prepared from vanillate-grown culture were incubated in a buffer containing 1 mM methanol. The error bars indicate standard deviations of triplicate determinations.
Vanillate catabolism of the mxaF mutant.
The mxaF mutant was subjected to growth experiments in vanillate-supplemented medium. The wild-type strain and the vanA1B mutant of B. japonicum USDA110 were used as positive and negative controls, respectively. The mxaF mutant had markedly lower growth in minimum medium containing vanillate than the wild-type strain (Fig. 3B), whereas the mxaF mutant was able to grow at the same rate as the wild-type strain in medium containing protocatechuate (Fig. 3C). The rate of vanillate catabolism by the mxaF mutant was also lower than catabolism by the wild-type strain (Fig. 3D), whereas the mxaF mutant and wild-type strains showed similar rates of protocatechuate catabolism (Fig. 3E). These results suggest that the flhA and gfa genes are required for efficient vanillate catabolism through the C1 pathway (Fig. 3), probably because they alleviate the toxicity of the formaldehyde produced by vanillate monooxgenase, VanA1B (Fig. 1).
Methanol oxidation by the mxaF mutant.
The mxaF gene encodes methanol dehydrogenase, which oxidizes methanol to formaldehyde in methylotrophs (17, 36). The mxaF gene is commonly used as a functional marker gene for environmental DNA analysis (27) and metagenomics (20) of the global C1 cycle in the environment (1, 15). We therefore tested methanol oxidation in mxaF mutants and wild-type cells of B. japonicum USDA110. Since USDA110 showed very weak growth in minimum medium with methanol (data not shown), methanol oxidation was assayed using the condensed resting cells of B. japonicum.
USDA110 and mxaF mutant cells were grown in minimal medium with vanillate for 5 days. Then, the resting cells (1010 cells/ml) were incubated in the presence of 1 mM methanol. Methanol oxidation was observed in the wild-type strain, but not in the mxaF mutant (Fig. 3F). The rate of methanol oxidation from 2 h to 10 h was 18.8 ± 3.2 nmol methanol consumed h−1 (109 CFU)−1 for the wild-type and 1.5 ± 2.2 for the mxaF mutant cells (P < 0.05; t test), indicating that the metabolic capability of the C1 pathway in B. japonicum extends to methanol oxidation, as schematized in Fig. 1.
Symbiotic phenotype of the mxaF mutant.
The mxaF gene has been shown to play an important role in symbiosis (13) and in competitive fitness for plant colonization (33). However, soybean plants inoculated with the wild-type strain and the mxaF mutant showed no apparent difference in shoot growth, nodule numbers, or nodule weights (see Fig. S2 in the supplemental material). This result suggests that the methanol oxidation and C1 compound metabolism of B. japonicum are not directly involved in soybean nodulation and symbiotic nitrogen fixation.
Vanillate catabolism genes in Bradyrhizobiaceae.
To examine whether vanA1B, pcaG1H1, and mxaF clusters are present on the genomes in members of the Bradyrhizobiaceae (19 strains, including B. japonicum, Agromonas oligotrophica, Rhodopseudomonas palustris, and Bradyrhizobim sp.), we extracted the previous array results (11) and summarized them in Table S1 in the supplemental material. Consequently, loci 3, 5, and 10, including vanA1B, pcaG1H1, mxaF, flhA, and gfa, were well conserved on the genomes of the 19 strains. Indeed, Bradyrhizobium sp. strains BTAi1 and ORS278 (7) carried van and mxaF gene clusters that were similar to those of USDA110 (see Fig. S3 in the supplemental material).
DISCUSSION
Recent efforts to find novel functional genes through global expression analyses have often failed in complicated biological systems; for example, the disruption mutants of genes upregulated in rhizobia during symbiosis generally show no symbiotic phenotype (9, 34). In the current study, the vanA1B and pcaG1H1 genes that were upregulated in B. japonicum cells grown in vanillate (12) were shown to be exclusively responsible for the catabolism of vanillate and protocatechuate, respectively (Fig. 2). Because vanA1B (blr2390 and blr2392) at locus 3 represents the full set of vanAB gene homologs (vanillate monooxygenase) (Fig. 1), it is reasonable that the vanA1B mutant would completely lose the capability to catabolize vanillate (Fig. 2C and E). However, there are two full sets of pcaGH homologs (protocatechuate 3,4-dioxygenase): pcaG1H1 (blr2334 and blr2333) at locus 5 and pcaG2H2 (blr0928 and blr0927) at locus 6 (Fig. 1). The identities of the amino acids between pcaG1H1 and pcaG2H2 range from 48% to 54%. Thus, the results of the previous global expression study coincide with the functional information from postgenomic work for protocatechuate catabolism.
The growth and protocatechuate consumption of the vanA1B mutant were similar to those of the wild-type strain in protocatechuate-amended medium (Fig. 2D and F and 3C and E), but vanillate catabolism was drastically lower in the pcaG1H1 mutant than in the wild-type strain (Fig. 2C and E). These facts are consistent with the pathway of vanillate catabolism (vanillate → protocatechuate → β-ketoadipate pathway) and the metabolic significance of C1 compounds derived from the methoxy moiety of vanillate (Fig. 1). Interestingly, comparative genomic hybridization (11) suggested that these metabolic capabilities are conserved in members of the Bradyrhizobiaceae (see Table S1 in the supplemental material).
Bacterial cells that use vanillate as a carbon source ought to produce formaldehyde, which has a toxic effect on all organisms (5). In B. japonicum, the formaldehyde is likely to be converted into CO2 in the C1 pathway (Fig. 1), because the gfa, flhA, fdhF, and fdhD genes were coexpressed by vanillate addition (12). In this work, the mxaF mutant caused the downregulation of flhA and gfa in the glutathione-dependent C1 pathway (Fig. 3A). The decreased growth (Fig. 3B) and vanillate consumption (Fig. 3D) of mxaF mutants supported the conversion of formaldehyde into CO2 by the C1 pathway. However, it is likely that an assimilatory serine pathway (Fig. 1) also functions for formaldehyde detoxification (1, 10, 18, 35), because the mxaF mutant retained its growth ability in the vanillate-amended medium (Fig. 3B and D).
In addition to our interest in the role of mxaF in the detoxification of formaldehyde via the C1 pathway, we are interested in its ecological function in encoding methanol dehydrogenase (17, 36). The inability of the mxaF mutant to oxidize methanol in condensed resting cells (Fig. 3F) clearly shows that mxaF is responsible for methanol oxidation in B. japonicum.
It has been reported that the mxaF gene is required for symbiosis between Methylobacterium nodulans and Crotalaria pedocarpa (13) and for competitive colonization by Methylobacterium extorquens on plant surfaces (33). On the other hand, the methanol oxidation of B. japonicum USDA110 was not directly involved in nodulation and nitrogen fixation (see Fig. S2 in the supplemental material).
A sequence comparison of the mxaF gene in RhizoBase (http://genome.kazusa.or.jp/rhizobase/) found that only the stem-nodulating bradyrhizobia BTAi1 and ORS278 also carried a gene organization (araC mxaF flhA gfa) similar to that of B. japonicum USDA110 (see Fig. S3 in the supplemental material). Thus, rhizobial methanol oxidation by mxaF is likely to be restricted to Bradyrhizobium species. Because plants often produce methanol and formaldehyde (22), the ability to cope with these compounds might enhance the environmental fitness of plant-associated bradyrhizobia under competitive conditions.
Supplementary Material
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas Comparative Genomics, by a grant to K.M. (no. 17380046), by Special Coordinate Funds for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by PROBRAIN. We are grateful to UNESCO and ICB Biotech (Osaka University) for their financial support of the research of N.S.
We thank T. Kaneko and S. Tabata (Kazusa DNA Research Institute) for providing the cosmids carrying target genes.
Footnotes
Published ahead of print on 5 June 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Christoserdova, L., M. G. Kalyuzhnaya, and M. E. Lindstrom. 2005. C1-transfer modules: from genomics to ecology. ASM News 71:521-528. [Google Scholar]
- 2.Cole, M. A., and G. H. Elkan. 1973. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob. Agents Chemother. 4:248-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dao, T. V., M. Nomura, R. Hamaguchi, K. Kato, M. Itakura, K. Minamisawa, S. Sinsuwongwat, H. T. Le, T. Kaneko, S. Tabata, and S. Tajima. 2008. NAD-malic enzyme affects nitrogen fixing activity of Bradyrhizobium japonicum USDA 110 bacteroids in soybean nodules. Microbes Environ. 23:215-220. [DOI] [PubMed] [Google Scholar]
- 4.Denef, V. D., M. A. Patrauchan, C. Florizone, J. Park, T. V. Tsoi, W. Verstraete, J. M. Tiedje, and L. D. Eltis. 2005. Growth substrate- and phase-specific expression of biphenyl, benzoate, and C1 metabolic pathways in Burkholderia xenovorans LB400. J. Bacteriol. 187:7996-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferdman, M. Y. 1973. Reaction of nucleic acids and nucleoproteins with formaldehyde. Prog. Nucleic Acid Res. Mol. Biol. 13:1-49. [DOI] [PubMed] [Google Scholar]
- 6.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giraud, E., L. Moulin, D. Vallenet, V. Barbe, E. Cytryn, J. C. Avarre, M. Jaubert, D. Simon, F. Cartieaux, Y. Prin, G. Bena, L. Hannibal, J. Fardoux, M. Kojadinovic, L. Vuillet, A. Lajus, S. Cruveiller, Z. Rouy, S. Mangenot, B. Segurens, C. Dossat, W. L. Franck, W. S. Chang, E. Saunders, D. Bruce, P. Richardson, P. Normand, B. Dreyfus, D. Pignol, G. Stacey, D. Emerich, A. Verméglio, C. Médigue, and M. Sadowsky. 2007. Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316:1307-1312. [DOI] [PubMed] [Google Scholar]
- 8.Harwood, C. S., and R. E. Parales. 1996. The β-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 50:553-590. [DOI] [PubMed] [Google Scholar]
- 9.Hauser, F., G. Pessi, M. Friberg, C. Weber, N. Rusca, A. Lindermann, H. Fischer, and H. Hennecke. 2007. Dissection of the Bradyrhizobium japonicum NifA+ σ54 regulon, and identification of a ferredoxin gene (fdxN) for symbiotic nitrogen fixation. Mol. Genet. Genomics 278:255-271. [DOI] [PubMed] [Google Scholar]
- 10.Hibi, M., T. Sonoki, and H. Mori. 2005. Functional coupling between vanillate-O-demethylase and formaldehyde detoxification pathway. FEMS Microbiol. Lett. 253:237-242. [DOI] [PubMed] [Google Scholar]
- 11.Itakura, M., K. Saeki, H. Omori, T. Yokoyama, T. Kaneko, S. Tabata, T. Ohwada, S. Tajima, T. Uchiumi, K. Honnma, K. Fujita, H. Iwata, Y. Saeki, Y. Hara, S. Ikeda, S. Eda, H. Mitsui, and K. Minamisawa. 2009. Genomic comparison of Bradyrhizobium japonicum strains with different symbiotic nitrogen-fixing capabilities and other Bradyrhizobiaceae members. ISME J. 3:326-339. [DOI] [PubMed] [Google Scholar]
- 12.Ito, N., M. Itakura, S. Eda, K. Saeki, H. Oomori, T. Yokoyama, T. Kaneko, S. Tabata, T. Ohwada, S. Tajima, T. Uchiumi, E. Masai, M. Tsuda, H. Mitsui, and K. Minamisawa. 2006. Global gene expression in Bradyrhizobium japonicum cultured with vanillin, vanillate, 4-hydroxybenzoate and protocatechuate. Microbes Environ. 21:240-250. [Google Scholar]
- 13.Jourand, P., A. Renier, S. Rapior, S. M. de Faria, Y. Prin, A. Galiana, E. Giraud, and B. Dreyfus. 2005. Role of methylotrophy during symbiosis between Methylobacterium nodulans and Crotalaria pedocarpa. Mol. Plant-Microbe Interact. 18:1061-1068. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, K. Kawashima, M. Kohara, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:225-256. [DOI] [PubMed] [Google Scholar]
- 15.Levipan, H. A., R. A. Quiñones, H. E. Johansson, and H. Urrutia. 2007. Methylotrophic methanogens in the water column of an upwelling zone with a strong oxygen gradient off central Chile. Microbes Environ. 22:268-278. [Google Scholar]
- 16.Martinez, A. T., M. Speranza, F. J., Ruiz-Duenas, P. Ferreria, S. Camarero, F. Guillen, M. J. Martinez, A. Gutierrez, and J. C. del Rio. 2005. Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 8:195-204. [PubMed] [Google Scholar]
- 17.McDonald, I. R., and J. C. Murrell. 1997. The methanol dehydrogenase structural gene mxaF and its use as a functional gene probe for methanotrophs and methylotrophs. Appl. Environ. Microbiol. 63:3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitsui, R., Y. Kusano, H. Yurimoto, Y. Sakai, N. Kato, and M. Tanaka. 2003. Formaldehyde fixation contributes to detoxification for growth of a nonmethylotroph, Burkholderia cepacia TM1, on vanillic acid. Appl. Environ. Microbiol. 69:6128-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morawski, B., A. Segura, and L. N. Ornston. 2000. Substrate range and genetic analysis of Acinetobacter vanillate demethylase. J. Bacteriol. 182:1383-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mou, X., S. Sun, R. A. Edwards, R. E. Hodson, and M. A. Moran. 2008. Bacterial carbon processing by generalist species in the coastal ocean. Nature 451:708-711. [DOI] [PubMed] [Google Scholar]
- 21.Nardi, S., D. Pizzeghello, L. Bragazza, and R. Gerdol. 2003. Low-molecular-weight organic acids and hormone-like activity of dissolved organic matter in two forest soils in N Italy. J. Chem. Ecol. 29:1549-1563. [DOI] [PubMed] [Google Scholar]
- 22.Nemecek-Marshall, M., R. C. MacDonald, J. J. Franzen, C. L. Wojciechowski, and R. Fall. 1995. Methanol emission from leaves. Plant Physiol. 108:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okazaki, S., M. Sugawara, K. Yuhashi, and K. Minamisawa. 2007. Rhizobitoxine-induced chlorosis occurs in coincidence with methionine deficiency in soybeans. Ann. Bot. 100:55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parke, D., and L. N. Ornston. 1984. Nutritional diversity of Rhizobiaceae revealed by auxanography. J. Gen. Microbiol. 130:1743-1750. [Google Scholar]
- 25.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 26.Sadowsky, M. J., and B. B. Bohool. 1986. Growth of fast- and slow-growing rhizobia on ethanol. Appl. Environ. Microbiol. 52:951-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito, A., S. Ikeda, H. Ezura, and K. Minamisawa. 2007. Microbial community analysis of the phytosphere using culture-independent methodologies. Microbes Environ. 22:93-105. [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Sameshima-Saito, R., K. Chiba, J. Hirayama, M. Itakura, H. Mitsui, S. Eda, and K. Minamisawa. 2006. Symbiotic Bradyrhizobium japonicum reduces N2O surrounding the soybean root system via nitrous oxide reductase. Appl. Environ. Microbiol. 72:2526-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sameshima-Saito, R., K. Chiba, and K. Minamisawa. 2006. Correlation of denitrifying capability with the existence of nap, nir, nor and nos genes in diverse strains of soybean bradyrhizobia. Microbes Environ. 21:174-184. [Google Scholar]
- 31.Schäfer, A., A. Tauch, W. Jäger, J. Kalinowski, G. Thierbach, and A. Pühler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 32.Segura, A., P. V. Bünz, D. A. D'Argenio, and L. N. Ornston. 1999. Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J. Bacteriol. 181:3494-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sy, A., A. C. J. Timmers, C. Knief, and J. A. Vorholt. 2005. Methylotropic metabolism is advantageous for Methylobacterium extorquens during colonization of Medicago truncatula under competitive conditions. Appl. Environ. Microbiol. 71:7245-7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchiumi, T., T. Ohwada, M. Itakura, H. Mitsui, N. Nukui, P. Dawadi, T. Kaneko, S. Tabata, Y. Yokoyama, K. Tejima, K. Saeki, H. Omori, M. Hayashi, T. Maekawa, R. Sriprang, Y. Murooka, S. Tajima, K. Simomura, M. Nomura, A. Suzuki, Y. Shimoda, K. Sioya, M. Abe, and K. Minamisawa. 2004. Expression islands clustered on the symbiosis island of the Mesorhizobium loti genome. J. Bacteriol. 186:2439-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vorholt, J. A. 2002. Cofactor-dependent pathway of formaldehyde oxidation in methylotrophic bacteria. Arch. Microbiol. 178:239-249. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, M., and M. E. Lidstrom. 2003. Promoters and transcripts for genes involved in methanol oxidation in Methylobacterium extorquens AM1. Microbiology 149:1033-1040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.