Abstract
It was recently proposed that gut bacteria are required for the insecticidal activity of the Bacillus thuringiensis-based insecticide, DiPel, toward the lepidopterans Manduca sexta, Pieris rapae, Vanessa cardui, and Lymantria dispar. Using a similar methodology, it was found that gut bacteria were not required for the toxicity of DiPel or Cry1Ac or for the synergism of an otherwise sublethal concentration of Cry1Ac toward M. sexta. The toxicities of DiPel and of B. thuringiensis HD73 Cry− spore/Cry1Ac synergism were attenuated by continuously exposing larvae to antibiotics before bioassays. Attenuation could be eliminated by exposing larvae to antibiotics only during the first instar without altering larval sterility. Prior antibiotic exposure did not attenuate Cry1Ac toxicity. The presence of enterococci in larval guts slowed mortality resulting from DiPel exposure and halved Cry1Ac toxicity but had little effect on B. thuringiensis HD73 Cry− spore/Cry1Ac synergism. B. thuringiensis Cry− cells killed larvae after intrahemocoelic inoculation of M. sexta, Galleria mellonella, and Spodoptera litura and grew rapidly in plasma from M. sexta, S. litura, and Tenebrio molitor. These findings suggest that gut bacteria are not required for B. thuringiensis insecticidal activity toward M. sexta but that B. thuringiensis lethality is reduced in larvae that are continuously exposed to antibiotics before bioassay.
Bacillus thuringiensis has long been regarded as a bona fide entomopathogen that can produce an array of virulence factors including insecticidal parasporal crystal (Cry) toxins, vegetative insecticidal proteins, phospholipases, immune inhibitors, and antibiotics (31). B. thuringiensis establishes lethal infections in many insect species after intrahemocoelic inoculation (9, 10, 14, 26, 31), and the insecticidal activity of Cry toxins, which lyse the intestinal epithelium, can be synergized by the presence of viable B. thuringiensis spores (31). In each instance, synergism has been attributed to hemocoelic infection by B. thuringiensis.
A novel hypothesis (6, 7) proposed that B. thuringiensis is incapable of killing Lymantria dispar, Manduca sexta, Pieris rapae, or Vanessa cardui in the absence of gut bacteria. Prior exposure of L. dispar larvae to a combination of four antibiotics severely reduced the subsequent toxicity of the B. thuringiensis-based (spores and Cry toxins) bioinsecticide, DiPel (Valent BioSciences) (6). Both larval susceptibility to B. thuringiensis and the number of culturable gut bacteria were found to be negatively correlated with the concentration of antibiotics to which larvae were previously exposed. Furthermore, a total reduction in larval susceptibility was coincident with the elimination of any detectable gut bacteria. Experimental reinfection with Enterobacter sp. strain NAB3, found in the guts of some populations of L. dispar larvae, was found to rescue the toxicity of B. thuringiensis, whereas reinfection with Enterococcus casseliflavus and Staphylococcus xylosus did not. It was also shown that while Escherichia coli, Enterobacter sp. strain NAB3, and B. thuringiensis could all grow in tryptic soy broth, B. thuringiensis alone could not grow in filter-sterilized plasma from L. dispar larvae. Finally, it was shown that the toxicity of Cry1Aa-expressing E. coli JM103 to L. dispar larvae was reduced by the prior exposure of larvae to antibiotics and could be eliminated when E. coli was also heat killed before use. It was concluded that B. thuringiensis-induced mortality results from a mixed infection of the hemocoel that must include bacteria capable of growth within the L. dispar larval hemolymph (6).
Using the same methods, it was subsequently reported that prior exposure of Vanessa cardui, M. sexta, Pieris rapae, and Heliothis virescens larvae to antibiotics eliminated culturable bacteria and rendered larvae resistant to DiPel (7). Experimental reinfection of larvae with Enterobacter sp. strain NAB3 rescued DiPel toxicity in V. cardui, M. sexta, and P. rapae but not in H. virescens larvae. Using a continuous-exposure bioassay, the susceptibility of Pectinophora gossypiella to the Cry1Ac-based bioinsecticide MVPII was found to be increased by prior exposure to antibiotics. Toxicity from a 48-h exposure of L. dispar larvae to MVPII was reduced, but not eliminated, by prior antibiotic exposure and could be rescued by reinfection with Enterobacter sp. strain NAB3. It was concluded that “enteric bacteria have important roles in B. thuringiensis-induced killing of Lepidoptera across a range of taxonomy, feeding breadth, and relative susceptibility to B. thuringiensis” (7).
The present work shows that gut bacteria are not required for the insecticidal activity of B. thuringiensis or Cry1Ac toxin toward M. sexta but that prior antibiotic exposure reduces larval susceptibility to B. thuringiensis.
MATERIALS AND METHODS
Bacterial strains and formulations.
DiPel DF with an insecticidal activity of 32 kIU mg−1 (Valent BioSciences) was provided by Ben Raymond (University of Oxford, United Kingdom). DiPel solutions were prepared in sterile distilled water. A Cry− B. thuringiensis HD73 strain was produced for use in synergism bioassays so as not to inadvertently increase the Cry1Ac concentration. B. thuringiensis HD73 was cured of its cry-carrying plasmid by culturing in Luria-Bertani (LB) broth at 42°C. B. thuringiensis HD73 Cry− spores were prepared from colonies which had sporulated on Luria agar (LA). Spores were resuspended in sterile distilled water, washed, pasteurized (45 min at 70°C), and quantified by dilution plating.
Insect populations.
A field population of Spodoptera litura (Noctuidae) was collected from Tamil, India, in March 2007 and reared in the laboratory at 25°C on Hoffman's tobacco hornworm diet (15). Larvae of the greater wax moth, Galleria mellonella (Pyralidae), and of the darkling beetles Tenebrio molitor (Tenebrionidae) and Zophobas morio (Tenebrionidae) were obtained from HPC (West Sussex, United Kingdom). G. mellonella larvae were reared in the dark at 25°C on a grain-honey diet described elsewhere previously (11). Tenebrionids were reared at 25°C and fed wheat bran. M. sexta (Sphingidae) eggs derived from parents reared on Hoffman's tobacco hornworm diet containing 100 μg ml−1 chlortetracycline (pure veterinary-grade Aureomycin; Lederle) were supplied by Stuart Reynolds (University of Bath, United Kingdom). M. sexta eggs from a single batch were separated into the three rearing regimens described below and reared at 25°C.
Nonsterile rearing regimen.
Nonsterile M. sexta larvae were reared individually on a diet that had not been autoclaved and that did not contain antibiotics. The diet components (excluding water) were premixed and frozen at −20°C. Each batch of nonsterile diet was derived from this stock and prepared with sterile distilled water.
Aseptic rearing regimens.
Sterile diet was produced by mixing heat-stable components with distilled water followed by autoclaving at 120°C and 20 lb/in2 for 25 min. Heat-labile components were dissolved in sterile distilled water, sterilized using a 0.22-μm filter, and mixed with the diet base after cooling to 60°C. Molten diet was poured into sterile beakers and allowed to set in a laminar-flow cabinet. M. sexta eggs were given three alternating 30-min washes of 3.8% formaldehyde and sterile distilled water and hatched on a sterile diet containing 100 μg ml−1 of rifampin and streptomycin. Upon molting to the second instar, larvae were further separated into two regimens. Half of the larvae were transferred to a sterile diet without antibiotics, whereas the remaining larvae continued to be reared on a sterile diet containing 100 μg ml−1 of rifampin and streptomycin before being starved 12 h before bioassay. Thus, two groups of aseptic larvae were produced: one group was exposed to antibiotics only during the first instar, whereas the other was continuously exposed to antibiotics prior to bioassay.
M. sexta bioassays.
Three different types of feeding bioassays were conducted as described below. Third-instar larvae were used for all bioassays, and larvae were held individually in sterile universal bottles. Mortality was scored daily for 6 days, and larvae were considered dead if they were unable to move when prodded with a blunt sterile probe. All bioassays were repeated twice at least 1 month apart.
DiPel bioassays.
DiPel bioassays were conducted as described previously (7). A total of 25 IU of DiPel were applied in 1 μl of sterile distilled water to the surface of a sterile diet disc (r = 1.5 mm; h = 1 mm) without antibiotics. Larvae were starved for 12 h before being fed DiPel-contaminated diet discs for 48 h, after which larvae were fed an uncontaminated sterile artificial diet without antibiotics. Larvae were starved for 12 h only, to avoid a reingestion of antibiotic-containing frass in larvae that had been continuously exposed to antibiotics. Bioassays used at least 30 larvae from all three rearing regimens described above.
Cry1Ac bioassays.
Cry1Ac protoxins were produced as inclusion bodies in E. coli cultures and purified as previously described (30). Cry1Ac crystals were diluted in sterile distilled water and incorporated into sterile diet at five concentrations as described previously by Gilliland et al. (12). Thirty larvae were assayed against each toxin concentration. Bioassays used nonsterile larvae from the nonsterile rearing regimen and aseptic larvae that had been continuously reared on a sterile diet containing 100 μg ml−1 of rifampin and streptomycin.
Synergism bioassays.
Cry1Ac protoxins and B. thuringiensis HD73 Cry− spores were diluted in sterile distilled water and incorporated into sterile diet to concentrations of 50 ng ml−1 and 105 CFU ml−1, respectively. Cry1Ac bioassays described above had previously shown 50 ng ml−1 Cry1Ac to be a sublethal concentration that slowed larval growth. Bioassays used at least 30 larvae from all three rearing regimens described above.
Monitoring of larval sterility.
Thirty larvae from bioassay control groups representing each rearing regimen were dissected, and their guts were removed. Guts from each rearing regimen were divided into two equal groups. One group was used for dilution plating onto LA followed by incubation at 28°C. The second group of guts was used for cetyltrimethylammonium bromide DNA extraction as described previously by Ausubel et al. (3). Each DNA extract was then used as a template for PCR amplification of 16S rRNA genes using universal primers 27f (5′-GTGCTGCAGAGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-CACGGATCCTACGGGTACCTTGTTACGACTT-3′) (22). PCR contents for a 50-μl volume were 0.5 μl of 100 pmol μl−1 forward (27F) and reverse (1492R) primers, 1 μl of 10 mM deoxynucleoside triphosphates (Promega), 5 μl of 10× Taq polymerase buffer (Promega), and 0.5 μl of Taq DNA polymerase (2.5 U; Promega). A hot-start protocol was used, with an initial denaturation step at 94°C for 3 min followed by 35 cycles of denaturation at 94°C for 1 min, annealing at 52°C for 1.5 min, and elongation at 72°C for 2 min, followed by a final extension step at 72°C for 10 min. Larvae were considered to be sterile if bacteria could not be detected by culturing or by 16S rRNA gene amplification.
Intrahemocoelic inoculation.
B. thuringiensis HD73 Cry− cells were grown to an optical density at 600 nm of 1.0 (approximately 108 cells ml−1), washed with sterile water, and diluted to the required densities. Nonsterile larvae (third-instar M. sexta and S. litura or final-instar G. mellonella, T. molitor, and Z. morio larvae) were chilled on ice, and injection sites (first right proleg for Lepidoptera and third right leg for Coleoptera) were swabbed with 95% ethyl alcohol. A sterile 5-μl Hamilton syringe with a removable 26-gauge needle was used to inject 5 μl of a cell suspension directly into the hemocoel of each larva. Control groups received injections of sterile distilled water only. Larvae were placed into sterile plastic boxes with diet, and mortality was scored each day for 72 h. Larvae were considered dead if they were unable to move when prodded with a blunt sterile probe. Bioassays were repeated twice. Actual doses of cells used were calculated by dilution plating. Bacterial cell densities in dead larvae were calculated by homogenizing cadavers in sterile distilled water followed by dilution plating onto LA. For immunization, larvae were injected with approximately 104 E. coli JM109 cells or sterile distilled water 24 h before injection of B. thuringiensis.
Plasma growth assays.
For third-instar S. litura and M. sexta larvae, the dorsal surface of the antepenultimate body segment was removed with scissors, allowing hemolymph to be collected and flash-frozen on dry ice. Hemolymph from final-instar T. molitor larvae was collected from cuts made by slicing across the legs. Hemolymph was collected from >100 larvae of each species, thawed on ice, and prepared as previously described (6). Briefly, four 1-ml plasma pools were constructed for each insect species such that any single larva was represented in only one pool. Pooled hemolymph was centrifuged at 13,000 × g for 20 min at 4°C. Glutathione was added to a concentration of 5 mM to inhibit melanization, and each solution was sterilized using a 0.22-μm filter. B. thuringiensis HD73 Cry− or E. coli JM109 cells were grown overnight in LB broth and washed, and each culture was added to two separate 1-ml pools of plasma to densities of approximately 5 × 105 CFU ml−1. Actual densities were calculated by dilution plating. The growth of each strain at 28°C was monitored by dilution plating of samples every 1.5 h for 18.5 h.
Hemolymph phenoloxidase (PO) activity assays.
Hemolymph from 10 larvae was pooled, diluted with 3 volumes of ice-cold phosphate-buffered saline (PBS), and centrifuged at 13,000 × g for 10 min at 4°C. Aliquots (5 μl) of the resulting supernatant were added to 995 μl of 10 mM l-Dopa in PBS, and the absorbance (490 nm) was measured every minute for 60 min. Absorbance was corrected for spontaneous hydrolysis by using a negative control containing only 10 mM l-Dopa and PBS. Experiments were repeated three times.
Statistical analysis.
Fifty percent lethal concentration (LC50) or 50% lethal dose (LD50) estimates and their 95% fiducial limits (FLs) were calculated by logit regression using GLIM 3.77 (1985; Numerial Algorithms Group) as previously described (30). Estimates were considered significantly different if their 95% FL values did not overlap.
RESULTS
Aseptic rearing.
M. sexta eggs were derived from antibiotic-treated parents and harbored spore-forming bacilli at low densities (fewer than 10 CFU per egg). No culturable bacteria were detected after eggs were repeatedly washed in 3.8% formaldehyde. Egg washing delayed hatching by approximately 24 h and decreased total hatching by approximately 10%. Growth rates were severely reduced when M. sexta larvae were reared on a combination of antibiotics (500 μg each of gentamicin, penicillin, rifampin, and streptomycin ml−1 diet) used in other studies to rear aseptic lepidopteran larvae (6, 7). The use of streptomycin and rifampin (100 μg each ml−1 diet) from hatching to the end of the first larval instar was sufficient to maintain sterility without causing deleterious side effects. Bacterial colonization of larval intestines was not detected by culturing or by 16S rRNA gene amplification in either of the aseptic rearing regimens, whereas larvae from the nonsterile rearing regimen harbored a single morphotype of gram-positive cocci (phenotypically identical to an Enterococcus faecium group strain isolated from other lepidopterans reared in our laboratory) at an average density of 6.2 × 106 CFU per larva.
Gut bacteria and DiPel toxicity in M. sexta.
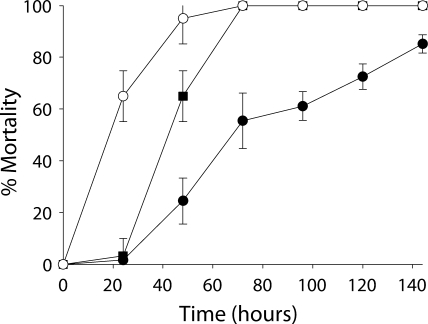
Aseptic larvae that were exposed to antibiotics only during their first instar died more rapidly than did larvae from other rearing regimens, although the final mortality rate was equivalent to that of nonsterile larvae (Fig. 1). Aseptic larvae that were continuously exposed to antibiotics prior to bioassay died more slowly than did larvae from other rearing regimens. Furthermore, the final mortality rate was approximately 20% lower than that of larvae from other rearing regimens. Dead larvae from all rearing regimens were visually indistinguishable and were characterized by progressive melanization extending from the central body segments around the first proleg (see Fig. S1 in the supplemental material). Approximately 24 h after larval death, B. thuringiensis dominated cadavers from all rearing regimens and had reached densities of >108 CFU per cadaver. Enterococcal densities were lower (8 × 103 to 4 × 104 CFU per cadaver) in nonsterile B. thuringiensis-killed larvae than in nonsterile control larvae (3 × 106 CFU per larva).
FIG. 1.
Effects of different rearing regimens on rates of mortality of third-instar M. sexta larvae exposed to DiPel. ▪, nonsterile without antibiotic exposure; ○, aseptic with antibiotic exposure during the first instar only; •, aseptic with continuous antibiotic exposure prior to bioassay. Error bars indicate 95% confidence intervals of the means of data from two repetitions.
Gut bacteria and Cry1Ac toxicity in M. sexta.
Cry1Ac was approximately twice as toxic to aseptic larvae that were reared continuously on antibiotics prior to bioassay (LC50 = 1.392 μg ml−1; 95% FL = 0.9639 to 1.959) relative to larvae from the nonsterile rearing regimen (LC50 = 3.384 μg ml−1; 95% FL = 2.219 to 4.793). Clear differences were observed between larval cadavers of aseptic and nonsterile larvae (see Fig. S2 in the supplemental material). At 24 h after death, aseptic larval cadavers showed no melanization or any other symptoms of septicemia, and cadavers retained structural integrity. In contrast, the cadavers of nonsterile larvae rapidly melanized and were internally liquefied. These cadavers were extremely fragile and oozed fluid from the cuticle. Enterococcal densities were increased (7 × 107 to 2 × 108 CFU per cadaver) in cadavers of nonsterile larvae relative to those of living control larvae (4 × 106 CFU per larva).
Cry1Ac synergism by B. thuringiensis spores.
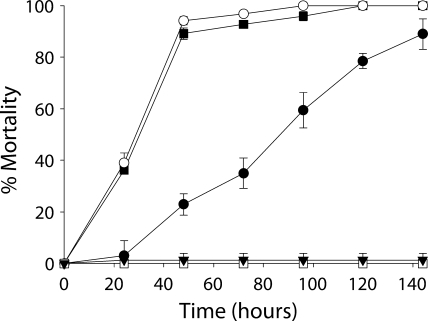
The inclusion of B. thuringiensis HD73 Cry− spores within the diet strongly synergized an otherwise sublethal concentration of Cry1Ac (Fig. 2). Spores alone caused no mortality. Mortality was slowed slightly in the presence of gut bacteria and was reduced in aseptically reared larvae that were continuously exposed to antibiotics prior to bioassay. Similar to DiPel bioassays, at approximately 24 h after larval death, B. thuringiensis densities reached 8 × 107 to 2 × 108 CFU per cadaver, whereas enterococcal densities decreased to 9 × 102 to 9 × 103 CFU per cadaver.
FIG. 2.
Effects of different rearing regimens on rates of mortality of third-instar M. sexta larvae exposed to B. thuringiensis HD73 Cry− spores and an otherwise sublethal Cry1Ac concentration. ▪, nonsterile without antibiotic exposure; ○, aseptic with antibiotic exposure during the first instar only; •, aseptic with continuous antibiotic exposure prior to bioassay. Error bars indicate 95% confidence intervals of the means of data from two repetitions. Results for Cry1Ac alone (□) and for B. thuringiensis HD73 Cry− spores alone (▾) were combined, as there were no significant differences in mortality rates between larvae from all three rearing regimens.
Intrahemocoelic toxicity of B. thuringiensis toward lepidopteran and coleopteran larvae.
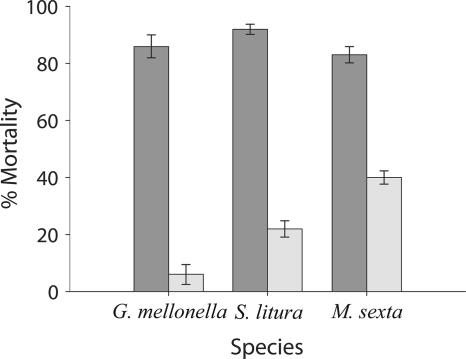
B. thuringiensis HD73 Cry− cells rapidly killed larvae when injected directly into the hemocoel of S. litura, M. sexta, or G. mellonella larvae, whereas larvae of the tenebrionid beetles T. molitor and Z. morio were less susceptible to infection, and LD50 values could not be calculated (Table 1). Prior immunization with E. coli increased immunity to subsequent B. thuringiensis infection at ∼LD85 in S. litura, M. sexta, and G. mellonella larvae (Fig. 3). In M. sexta larvae, prior immunization with E. coli elicited an increase in the amount of immunologically detectable hemolymph PO (data not shown) that coincided with increased hemolymph PO activity (slope at Vmax of 9.45; 95% confidence level of 8.96 to 9.94) relative to that of naïve larvae (slope at Vmax of 1.65; 95% CL of 0.96 to 2.34). E. coli did not grow or cause mortality after injection into any of the species tested.
TABLE 1.
Intrahemocoelic toxicity of B. thuringiensis HD73 Cry− cells to lepidopteran and coleopteran larvae
| Species | LD50 (95% FL) (CFU per larva) |
B. thuringiensis density (CFU per larva after LD50 injection)
|
|
|---|---|---|---|
| Cadaversa | Survivorsb | ||
| Spodoptera litura | 2.5 × 103 (7.8 × 102 - 6.3 × 103) | 1 × 109 | ND |
| Galleria mellonella | 6.6 × 103 (2.6 × 103 - 1.3 × 104) | 4 × 109 | ND |
| Manduca sexta | 1.6 × 103 (1.1 × 103 - 3.5 × 103) | 1 × 108 | ND |
| Tenebrio molitor | >105 | ND | |
| Zophobas morio | >106 | ND | |
Cadavers were homogenized and plated approximately 24 h after death.
ND, not detectable.
FIG. 3.
Effect of immunization on toxicity of intrahemocoelically injected B. thuringiensis HD73 Cry− cells toward larvae of three species of lepidoptera. Dark gray, naïve; light gray, immunized. Error bars indicate 95% confidence intervals of the means of data from two repetitions.
Growth of B. thuringiensis and E. coli in plasma.
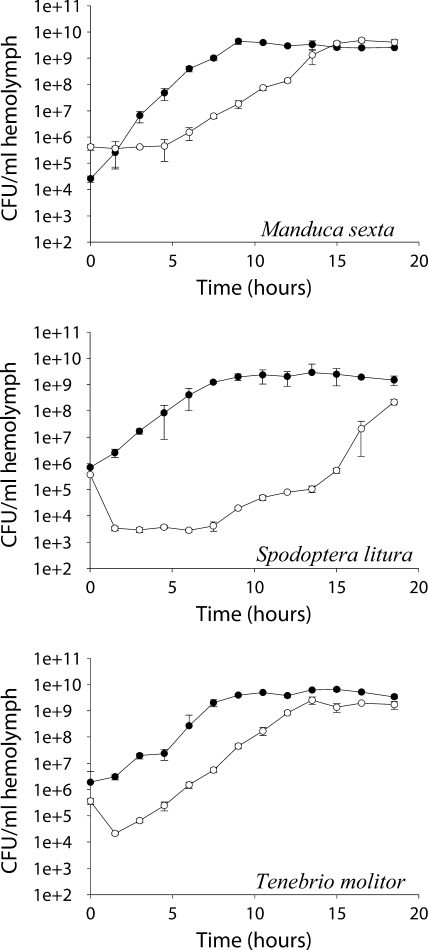
B. thuringiensis HD73 Cry− cells grew rapidly in plasma from S. litura, T. molitor, and M. sexta, whereas E. coli grew more slowly, with initial decreases in cell density apparent in plasma from S. litura and T. molitor (Fig. 4).
FIG. 4.
Growth of B. thuringiensis (•) and E. coli (○) in plasma from lepidopteran and coleopteran larvae. Error bars indicate 95% confidence intervals of the means of data from two repetitions.
DISCUSSION
The results presented here show that the toxicity of B. thuringiensis or Cry1Ac toward M. sexta does not depend upon the presence of gut bacteria. However, continuous exposure to antibiotics before bioassay reduced mortality when B. thuringiensis was subsequently fed to larvae. It is noteworthy that the antibiotic attenuation of B. thuringiensis was observed after using two antibiotics at lower concentrations than those used in similar studies (6, 7), which used gentamicin, penicillin, rifampin, and streptomycin each at 500 μg ml−1 of diet. It is known that dietary antibiotics can attenuate the toxicity of B. thuringiensis spore-crystal mixtures to many lepidopterans (5, 16, 17, 19, 21, 25) and that such attenuation can eliminate spore synergism, leaving only the effect of Cry toxins (25, 32). Therefore, it is proposed that a direct effect of residual antibiotics or an indirect effect such as an induction of a host xenobiotic detoxification response could reduce or eliminate any synergism associated with the germination of B. thuringiensis spores, leaving only the effect of Cry toxins. Previous studies (6, 7) exposed larvae to B. thuringiensis for 48 h before reverting to uncontaminated sterile diet. This method of bioassay is known to significantly increase the LC50 of Cry toxins relative to those reported previously for continuous-exposure bioassays (8). The finding that prior antibiotic exposure, and not an elimination of gut bacteria, attenuates B. thuringiensis in lepidopteran hosts is consistent with previously reported results (7), which showed that prior exposure to antibiotics completely attenuates B. thuringiensis toxicity toward H. virescens larvae. This result cannot be attributed to the removal of gut bacteria, as no bacteria were detected in H. virescens larvae before antibiotic treatment (7). Recent work has also implicated antibiotic attenuation in Plutella xylostella larvae, where prior rifampin exposure reduced the toxicity of rifampin-sensitive strains of B. thuringiensis but not of rifampin-resistant mutants (27).
The observation that B. thuringiensis replicates within M. sexta cadavers at the expense of gut bacteria is in agreement with data from other studies that demonstrated such competition in lepidoptera (18, 33, 34) and diptera (1). The presence of gut bacteria may afford larvae some protection since aseptic larvae that had been exposed to antibiotics only during first instar were killed more rapidly by DiPel than were nonsterile larvae. The presence of gut bacteria was associated with a slight delay in mortality in synergism bioassays, which may be due to differences in bioassay methods. Alternatively, the presence of gut bacteria may have different effects on B. thuringiensis HD73 and the DiPel strain, HD1. The basis of the protective effect is unclear; however, direct antagonism of B. thuringiensis by gut bacteria was previously reported for other species of lepidoptera (20, 34). The level of Cry toxicity may be increased (25), decreased (32), or unaffected (19) by the inclusion of antibiotics in lepidopteran bioassays, although the effects of antibiotics on gut bacteria were not reported. We found aseptic larvae to be approximately twice as susceptible to Cry1Ac compared to nonsterile larvae, which is in agreement with data from recent studies of P. gossypiella (7) and P. xylostella (27).
B. thuringiensis was found to proliferate in both lepidopteran and coleopteran plasma and to establish lethal infections after injection into the lepidopteran hemocoel. In contrast, B. thuringiensis is unable to grow in L. dispar plasma (6) and was previously suggested to cooperate with facultatively pathogenic gut bacteria to establish septicemia (6, 7). The work described previously by Zhang et al. (36) was cited in support of the suggestion that the inability of B. thuringiensis to infect the hemocoel may be a wider phenomenon (6). It is noteworthy that the work described previously Zhang et al. (36) concerns a pleiotropic avirulent B. thuringiensis mutant whose parent strain was able to establish lethal hemocoelic infections in the Lepidoptera (13). Additionally, there is considerable evidence that shows that B. thuringiensis is capable of establishing lethal hemocoelic infections in a wide range of insects including many lepidopterans (2, 9, 13, 29, 32, 35, 36). Plasma growth assays do not accurately predict intrahemocoelic growth since B. thuringiensis grew in T. molitor plasma but not after injection into T. molitor larvae. Similarly, E. coli grew in lepidopteran plasma but not in living larvae.
Our finding that prior immunization of M. sexta larvae with E. coli elicits an increase in immunity to subsequent B. thuringiensis infection agrees with data from similar studies (9, 13). Immunization is commonly employed in the functional characterization of putative B. thuringiensis virulence factors that may be involved in the circumvention of host immune defenses (9, 10, 29). However, it was previously suggested that immunization may promote the growth of B. thuringiensis by removing immunological barriers such as defensive enzymes (6). Here it is shown that immunization increases the level of at least one defense enzyme, PO, in larval hemolymph, although there is no evidence of a causal link between increased PO activity and immunity to B. thuringiensis.
The present findings suggest that B. thuringiensis is an entomopathogen that can kill lepidopteran larvae by breaching the intestinal epithelium and invading the hemocoel. As previously suggested (28), it is probable that the array of antibiotics (24), bacteriocins (4), and quorum quenchers (23) produced by B. thuringiensis may confer a competitive advantage over gut microbes that coinvade the hemocoel and compete for the consumption of the host cadaver. Facultatively pathogenic gut bacteria may synergize Cry protein toxicity or the toxicity of B. thuringiensis strains which are attenuated versus a particular species of insect. However, the present work does not support an obligate role for gut bacteria in the insecticidal activity of B. thuringiensis toward M. sexta.
Supplementary Material
Acknowledgments
We are indebted to Venkatasamy Balasubramani for establishing our S. litura population, to Ben Raymond for the provision of DiPel DF, to Stuart Reynolds for the provision of M. sexta eggs, to Michael Kanost for the provision of pro-PO antisera and to Douglas Strachan for the translation of reference 19.
P.R.J. was supported by a graduate teaching assistantship from the University of Sussex.
Footnotes
Published ahead of print on 12 June 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aly, C. 1985. Germination of Bacillus thuringiensis var. israelensis spores in the gut of Aedes larvae (Diptera: Culicidae). J. Invertebr. Pathol. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 2.Angus, T. A. 1954. A bacterial toxin paralysing silkworm larvae. Nature 173:545-546. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Short protocols in molecular biology, p. 2.4.1-2.4.5. Wiley, New York, NY.
- 4.Barboza-Corona, J. E., H. Vazquez-Acosta, D. K. Bideshi, and R. Salcedo-Hernandez. 2006. Bacteriocin-like inhibitor substances produced by Mexican strains of Bacillus thuringiensis. Arch. Microbiol. 187:117-126. [DOI] [PubMed] [Google Scholar]
- 5.Beegle, C. C., L. C. Lewis, R. E. Lynch, and A. J. Martinez. 1981. Interaction of larval age and antibiotic on the susceptibility of three insect species to Bacillus thuringiensis. J. Invertebr. Pathol. 37:143-153. [Google Scholar]
- 6.Broderick, N. A., K. F. Raffa, and J. Handelsman. 2006. Midgut bacteria required for Bacillus thuringiensis insecticidal activity. Proc. Natl. Acad. Sci. USA 103:15196-15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broderick, N. A., C. J. Robinson, M. D. McMahon, J. Holt, J. Handelsman, and K. F. Raffa. 2009. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dulmage, H. T., H. M. Graham, and E. Martinez. 1978. Interactions between the tobacco budworm, Heliothis virescens, and the δ-endotoxin produced by the HD-1 isolate of Bacillus thuringiensis var. kurstaki: relationship between length of exposure to the toxin and survival. J. Invertebr. Pathol. 32:40-50. [Google Scholar]
- 9.Edlund, T., I. Siden, and H. G. Boman. 1976. Evidence for two immune inhibitors from Bacillus thuringiensis interfering with the humoral defense system of saturniid pupae. Infect. Immun. 14:934-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedhila, S., P. Nel, and D. Lereclus. 2002. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 184:3296-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frobius, A. C., M. R. Kanost, P. Gotz, and A. Vilcinskas. 2000. Isolation and characterization of novel inducible serine protease inhibitors from larval hemolymph of the greater wax moth Galleria mellonella. Eur. J. Biochem. 267:2046-2053. [DOI] [PubMed] [Google Scholar]
- 12.Gilliland, A., C. E. Chambers, E. J. Bone, and D. J. Ellar. 2002. Role of Bacillus thuringiensis Cry1 δ-endotoxin binding in determining potency during lepidopteran larval development. Appl. Environ. Microbiol. 68:1509-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heierson, A., I. Siden, A. Kivaisi, and H. G. Boman. 1986. Bacteriophage-resistant mutants of Bacillus thuringiensis with decreased virulence in pupae of Hyalophora cecropia. J. Bacteriol. 167:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann, D., M. Brehelin, and J. A. Hoffmann. 1974. Modifications of the hemogram and of the hemocytopoietic tissue of male adults of Locusta migratoria (Orthoptera) after injection of Bacillus thuringiensis. J. Invertebr. Pathol. 24:238-247. [DOI] [PubMed] [Google Scholar]
- 15.Hunter, F. R., N. E. Crook, and P. F. Entwistle. 1984. Viruses as pathogens for the control of insects, p. 323-347. In J. M. Grainger and J. M. Lynch (ed.), Microbial methods for environmental biotechnology. Academic Press, New York, NY.
- 16.Ignoffo, C. M., C. Garcia, and T. L. Couch. 1977. Effect of antibiotics on the insecticidal activity of Bacillus thuringiensis. J. Invertebr. Pathol. 30:277-278. [Google Scholar]
- 17.Ignoffo, C. M., D. L. Hostetter, R. E. Pinnell, and C. Garcia. 1977. Relative susceptibility of six soybean caterpillars to a standard preparation of Bacillus thuringiensis var. kurstaki. J. Econ. Entomol. 70:60-63. [Google Scholar]
- 18.Isakova, N. P. 1962. Le rôle de la microflore intestinale de l'insecte dans la pathogénèse de l'affection provoquée par Bacillus cereus var. galleriae. Entomophaga Mem. 2:175-178. [Google Scholar]
- 19.Ishiguro, F., M. Miyasono, and J. Kobayashi. 1981. The effect of antibiotics on the insect-killing activity of Bacillus thuringiensis, p. 129. In Proceedings of the 25th Annual Meeting of the Japanese Society of Applied Entomology and Zoology. Japanese Society of Applied Entomology and Zoology, Tokyo, Japan. (In Japanese.)
- 20.Jarosz, J. 1979. Gut flora of Galleria mellonella suppressing ingested bacteria. J. Invertebr. Pathol. 34:192-198. [DOI] [PubMed] [Google Scholar]
- 21.Jarrett, P., and H. D. Burges. 1987. Importance of spores, crystals, and δ-endotoxins in the pathogenicity of different varieties of Bacillus thuringiensis in Galleria mellonella and Pieris brassicae. J. Invertebr. Pathol. 50:277-284. [Google Scholar]
- 22.Lane, D. J. 1991. 16S/23S rRNA sequencing. John Wiley & Sons, Inc., New York, NY.
- 23.Lee, S. J., S.-Y. Park, J.-J. Lee, D.-Y. Yum, B.-T. Koo, and J.-K. Lee. 2002. Genes encoding the N-acyl homoserine lactone-degrading enzyme are widespread in many subspecies of Bacillus thuringiensis. Appl. Environ. Microbiol. 68:3919-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair, J. R., G. Narasimman, and V. Sekar. 2004. Cloning and partial characterization of zwittermicin A resistance gene cluster from Bacillus thuringiensis subsp. kurstaki strain HD1. J. Appl. Microbiol. 97:495-503. [DOI] [PubMed] [Google Scholar]
- 25.Nishiitsutsuji-Uwo, J., and Y. Endo. 1980. Mode action of Bacillus thuringiensis δ-endotoxin: relative roles of spores and crystals in toxicity to Pieris, Lymantria and Ephestia larvae. Appl. Entomol. Zool. 15:416-424. [Google Scholar]
- 26.Porcar, M., L. Navarro, and R. Jimenez-Peydro. 2006. Pathogenicity of intrathoracically administrated Bacillus thuringiensis spores in Blatta orientalis. J. Invertebr. Pathol. 93:63-66. [DOI] [PubMed] [Google Scholar]
- 27.Raymond, B., P. R. Johnston, D. J. Wright, R. J. Ellis, N. Crickmore, and M. B. Bonsall. 2009. A mid-gut microbiota is not required for the pathogenicity of Bacillus thuringiensis to diamondback moth larvae. Environ. Microbiol. [Epub ahead of print.] doi: 10.1111/j.1462-2920.2009.01980.x. [DOI] [PubMed]
- 28.Raymond, B., R. S. Lijek, R. I. Griffiths, and M. B. Bonsall. 2008. Ecological consequences of ingestion of Bacillus cereus on Bacillus thuringiensis infections and on the gut flora of a lepidopteran host. J. Invertebr. Pathol. 99:103-111. [DOI] [PubMed] [Google Scholar]
- 29.Salamitou, S., F. Ramisse, M. Brehelin, D. Bourguet, N. Gilois, M. Gominet, E. Hernandez, and D. Lereclus. 2000. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology 146:2825-2832. [DOI] [PubMed] [Google Scholar]
- 30.Sayyed, A. H., R. Gatsi, M. S. Ibiza-Palacios, B. Escriche, D. J. Wright, and N. Crickmore. 2005. Common, but complex, mode of resistance of Plutella xylostella to Bacillus thuringiensis toxins Cry1Ab and Cry1Ac. Appl. Environ. Microbiol. 71:6863-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnepf, E., N. Crickmore, J. Van Rie, D. Lereclus, J. Baum, J. Feitelson, D. R. Zeigler, and D. H. Dean. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somerville, H. J., Y. Tanada, and E. M. Omi. 1970. Lethal effect of purified spore and crystalline endotoxin preparations of Bacillus thuringiensis on several lepidopterous insects. J. Invertebr. Pathol. 16:241-248. [Google Scholar]
- 33.Swiecicka, I., D. K. Bideshi, and B. A. Federici. 2008. Novel isolate of Bacillus thuringiensis subsp. thuringiensis that produces a quasicuboidal crystal of Cry1Ab21 toxic to larvae of Trichoplusia ni. Appl. Environ. Microbiol. 74:923-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takatsuka, J., and Y. Kunimi. 2000. Intestinal bacteria affect growth of Bacillus thuringiensis in larvae of the oriental tea tortrix, Homona magnanima Diakonoff (Lepidoptera: Tortricidae). J. Invertebr. Pathol. 76:222-226. [DOI] [PubMed] [Google Scholar]
- 35.Vago, C., and E. Kurstak. 1965. Mechanism of action of Bacillus thuringiensis introduced by a hymenopteran parasite into the hemolymph of a lepidopteran. Antonie van Leeuwenhoek 31:282-294. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, M. Y., A. Lovgren, M. G. Low, and R. Landen. 1993. Characterization of an avirulent pleiotropic mutant of the insect pathogen Bacillus thuringiensis: reduced expression of flagellin and phospholipases. Infect. Immun. 61:4947-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.